Abstract
Although researchers have noted high level activation of rodent mononuclear phagocytes for nitric oxide (NO) synthase type 2 (S2) expression and NO production with a variety of agents such as interferon (IFN) γ and endotoxin, it has been difficult to demonstrate activation of human mononuclear phagocytes. The purpose of this study was to determine if IFN-α serves as an activator in vitro and in vivo in humans. Treatment of normal monocytes or mononuclear cells in vitro with IFN-α caused a dose-dependent increase in monocyte NOS2 activity and NO production, and increased expression of NOS2 protein and mRNA expression. To determine if in vivo administration of IFN-α also modulated NOS2, we studied blood cells from patients with hepatitis C before and after IFN-α therapy. Untreated patients with chronic hepatitis C virus infection had levels of NOS activity and NOS2 antigen in freshly isolated mononuclear cells similar to those of healthy subjects, and they expressed minimal or no NOS2 mRNA. However, IFN-α treatment of patients with hepatitis C infection was associated with a significant elevation in mononuclear cell NOS activity, NOS2 antigen content, and NOS2 mRNA content. IFN-α–treated patients had significant decreases in levels of serum alanine aminotransferase and plasma hepatitis C mRNA. The degree of IFN-α–enhanced mononuclear cell NOS2 antigen content correlated significantly with the degree of reduction in serum alanine aminotransferase levels. Thus, IFN-α treatment of cells in vitro or administration of IFN-α to hepatitis C patients in vivo increases expression of mononuclear cell NOS2 mRNA expression, NOS activity, NOS2 antigen expression, and NO production. Since NO has been reported to have antiviral activity for a variety of viruses, we speculate that induced NO production may be related to the antiviral action(s) of IFN-α in hepatitis C infection.
Nitric oxide (NO)1 is a simple chemical mediator produced endogenously from l-arginine by the action of NO synthase (NOS), a family of related enzymes encoded by separate genes (1, 2). The inducible (or immune) form of the enzyme, NOS2, is found prominently in mononuclear phagocytes and hepatocytes, and is capable of high level NO production. Its expression is regulated primarily by transcription, and it is modulated by various cytokines and microbial products (1). NO has potent antimicrobial activities in vitro and in vivo against a wide array of organisms including certain viruses (3–5).
IFN-γ, endotoxin, and TNF treatment in vitro can potently increase NOS2 expression and NO production by rodent macrophages, but it has been difficult to show comparable activation in vitro of normal human mononuclear phagocytes for high level expression (6–8). However, cells from patients with illnesses such as malaria (9), rheumatoid arthritis (7, 10), and tuberculosis (11) have mononuclear phagocytes that clearly express NOS2 and produce NO. In rodent macrophages, exogenous IFN-α cannot activate macrophages for NO production (12), but macrophage-synthesized IFN-α can augment NO production in an autocrine fashion (13). IFN-α had not previously been investigated thoroughly in human mononuclear phagocytes for this function.
The purpose of this study was to determine whether IFN-α could activate human monocytes in vitro and in vivo for NOS2 expression and NO production. We demonstrate here that IFN-α treatment of normal mononuclear cells in vitro induces increased expression of monocyte NOS activity, NOS2 antigen, and mRNA content, and production of NO, and that treatment of patients with chronic hepatitis C virus infection with IFN-α in vivo causes increased mononuclear cell NOS activity, NOS2 antigen content, and mRNA content.
Materials and Methods
Subjects.
Control normal subjects were recruited locally. Patients with chronic hepatitis C (diagnosed by second generation recombinant immunoblot assay and/or hepatitis C virus RNA measurement and liver biopsy) were recruited consecutively from the hepatology outpatient clinics at Duke University Medical Center (DUMC). The study protocol was approved by the DUMC Institutional Review Board. Informed consent was obtained from each subject before participation. Patients and controls were excluded if they had a coexisting chronic inflammatory condition, active allergy or infection, malignancy, or if they were receiving ribavirin, nitroglycerin, or other nitrate-containing medications. Pregnant women were excluded because of reports of elevated NO production in pregnancy. All subjects and patients were abstinent of alcohol. Patients had no identifiable cause(s) for chronic hepatitis other than hepatitis C; all had confirmed hepatitis C infection and hepatitis as determined by blood studies and liver biopsies. Patients received 3–10 million U of recombinant IFN-α2b (INTRON®A; 2 × 108 U/mg; Schering-Plough, Kenilworth, NJ) subcutaneously three times per week.
Blood Mononuclear Cell Preparation.
30 ml of blood was drawn into lithium heparin. Mononuclear cells were prepared using Ficoll/Hypaque as previously noted (7) and stored at −70°C until use. For some in vitro experiments, monocytes were prepared by sequential Ficoll/Hypaque-Percoll/adherence technique (6). Freshly isolated monocytes were cultured at 3 × 105 cells or mononuclear cells 5 × 105 cells/6-mm diameter microtiter plate well in 0.2 ml of DMEM with 10% heated (56°C for 30 min) normal, pooled human serum.
NOS Enzyme Activity and Antigen Analyses.
Cellular extracts were prepared and analyzed for NOS activity (14C-l-arginine conversion to 14C-l-citrulline) and antigen content by immunoblot as previously described (7). In some enzyme assays, 2 mM NG-monomethyl-l-arginine was included to determine if the conversion to l-citrulline was NOS-mediated. Immunoblots were done using either a monoclonal anti-NOS2 antibody from Transduction Laboratories (anti-macNOS; Lexington, KY) or from Research and Diagnostic Antibodies (1E8-B8; Richmond, CA). We used untreated human colon cancer cell line cells (DLD-1; reference 14) as negative controls. DLD-1 cells treated with human recombinant IFN-γ (100 U/ml), and TNF (100 U/ml), IL-1 (0.5 ng/ml), and IL-6 (200 U/ml) for 3 d were used as positive controls for demonstrating NOS2 antigen. 25–50 μg protein extract of the cells was used in the individual lanes. A positive immunoblot for NOS2 was one in which a clear band was visible at 130– 131 kD.
Reverse Transcriptase-PCR Analyses.
Total RNA was isolated by the method of Chomczynski and Sacchi (15), resuspended in diethyl pyrocarbonate–treated water, and reprecipitated overnight with cold isopropanol at −20°C. 1 ug of RNA was reverse transcribed with random hexamers and murine leukemia virus reverse transcriptase (RT; Perkin Elmer, Branchburg, NJ) for 15 min at 42°C. Reactions were stopped by heating for 5 min at 99°C. The final product was then amplified with 2.5 U of AmpliTaq® DNA polymerase (Perkin Elmer) and 0.15 μM of sense and antisense primers in PCR buffer containing 100 mM Tris-HCl, 50 mM KCl, 25 mM MgCl2, and 10 μM deoxyribonucleotide triphosphate. Mixtures were overlaid with mineral oil and amplified for 40 cycles. The primers for NOS2 were 5′-CCT GAG CTC TTC TTC GAA ATC C-3′ (sense) and 5′-AGG ATG TTG TAG CGC TGG AC-3′ (antisense). The expected product is 229 bp. As a control, we used the following primers for the glyceraldehyde-3-phosphate dehydrogenase: 5′-CTA CTG GCG CTG CCA AGG CTG T-3′ (sense) and 5′-GCC ATG AGG TCC ACC ACC CTG T-3′ (antisense). The expected product is 390 bp. Final PCR products were separated on a 1% Tris-borate/ EDTA agarose gel and visualized by ethidium bromide staining.
Measurement of Serum IFN-α Levels.
Serum IFN-α levels were done using a previously reported cytokine-specific sandwich ELISA (Endogen, Cambridge, MA); levels were calculated using standard curves generated with recombinant IFN-α. The lower limit of the detection by the assay was 5 pg/ml.
Statistical Analysis.
Continuous variables were compared using the Student's t test or ANOVA as appropriate. Categorical variables were compared using the Fisher's exact test. The Bonferroni correction was used to control the type I error rate when multiple comparisons were performed. Since the NOS activity data were not normally distributed, we used logarithmic transformation to allow for parametric analysis (ANOVA testing) of the results. The Kruskal-Wallis test was performed on nontransformed data to confirm these results. To test for an increasing dose– response relationship between IFN-α dose in vitro and expression of NOS activity and production of nitrite/nitrate, the Page test for ordered alternatives was used. We used the Statistical Analysis System (SAS Institute, Inc., Cary, NC). P values are two-sided using α = 0.05 as the reference standard for determining significance.
Results
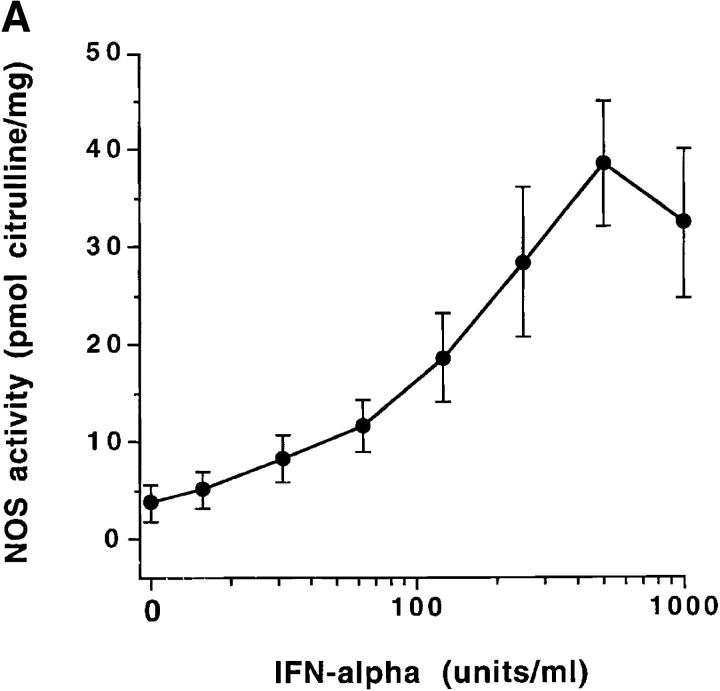
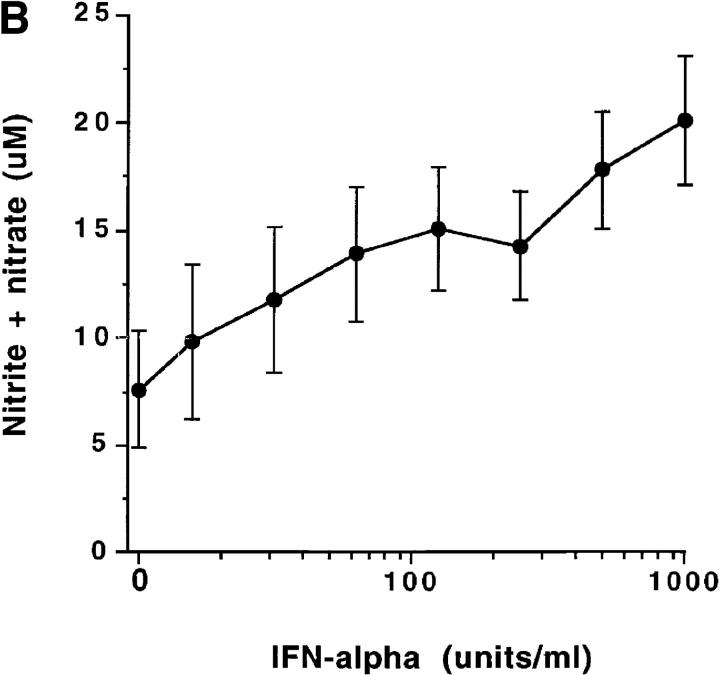
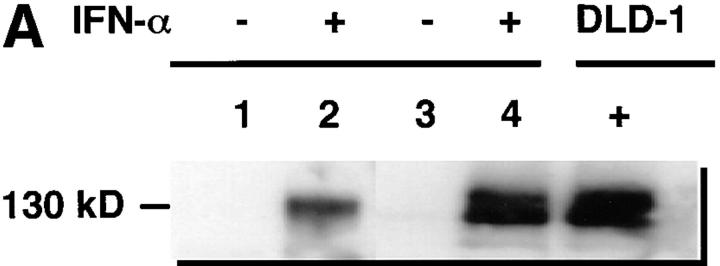
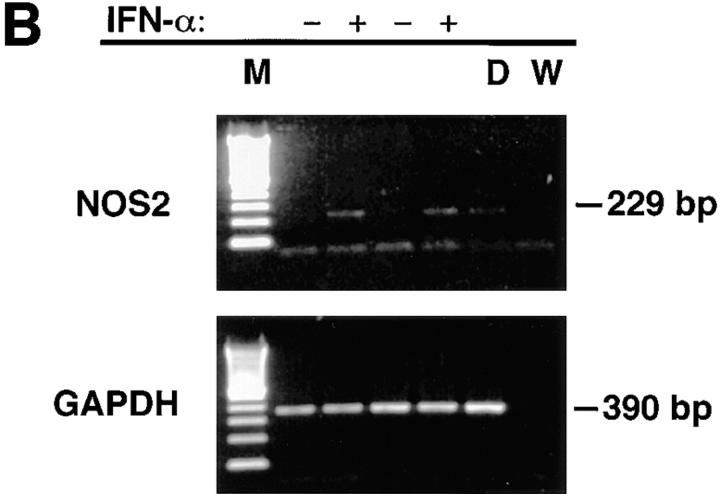
Freshly isolated monocytes from eight healthy male volunteers showed an increase in NOS activity in response to IFN-α2b treatment in vitro. The increase peaked at 500 U/ml (Fig. 1 A). Similarly, production of NO (measured as nitrate and nitrite, the stable catabolites of NO) increased with IFN-α2b treatment (Fig. 1 B). Increasing doses of IFN-α2b were associated with significantly increased NOS activity and nitrite/nitrate production (P <0.001). Studies also demonstrated that treatment of monocytes in vitro with IFN-α2a (Roferon®; Roche Laboratories, Nutley, NJ) augmented NOS activity and NO production (data not shown). In immunoblot studies, normal subject monocytes from four out of six treated in vitro with IFN-α2b had increased expression of NOS2 antigen (Fig. 2 A). We did RT-PCR analysis of RNA from blood mononuclear cells to determine if IFN-α induced increased levels of NOS2 mRNA. Mononuclear cells from normal subjects were cultured for 3 d without or with 500 U/ml IFN-α. Untreated cells from zero out of six normal individuals had NOS2 mRNA expression, whereas all those treated with IFN-α in vitro expressed NOS2 mRNA (six out of six) (Fig. 2 B).
Figure 1.
(A) Enhancement of normal blood monocyte NOS enzyme activity by treatment with IFN-α in vitro. Purified blood monocytes from eight separate normal individuals were cultured for 3 d with the indicated amounts of IFN-α, and analyzed for NOS activity. The points show the mean ± one SEM. (B) Enhancement of normal blood monocyte NO production by treatment with IFN-α in vitro. Purified blood monocytes from eight separate normal individuals were cultured as noted in Fig. 3 A, and then supernatant media samples were assessed for content of nitrite/nitrate. The points show the mean ± one SEM.
Figure 2.
(A) Immunoblot analyses of extracts of mononuclear cells treated in vitro with IFN-α. Cells were cultured with (+) or without (−) 500 U/ml of IFN-α for 3 d. Extracts were then made as described in the Methods section, and equivalent amounts of cellular protein were analyzed. Antibody anti-MacNOS was used. Cells from two separate, normal individuals were analyzed. Analysis of the first is shown in lanes 1 and 2, and that of the second is in lanes 3 and 4. DLD-1 + signifies extracts from the human colon cancer cell line DLD-1 treated with IFN-γ, IL-1, and TNF in vitro. Results demonstrate that IFN-α treatment induces NOS2 antigen expression. (B) RT-PCR analysis of mRNA of mononuclear cells treated in vitro with IFN-α. Cells from two separate, normal individuals were cultured with (+) or without (−) 500 U/ml of IFN-α for 12 h. RNA was extracted and analyzed as described in the Methods section for NOS2 and glyceraldehyde-3-phosphate dehydrogenase mRNA. M, molecular weight markers; D, cells of the human colon cancer cell line DLD-1 treated with IFN-γ, IL-1, and TNF; and W, distilled water.
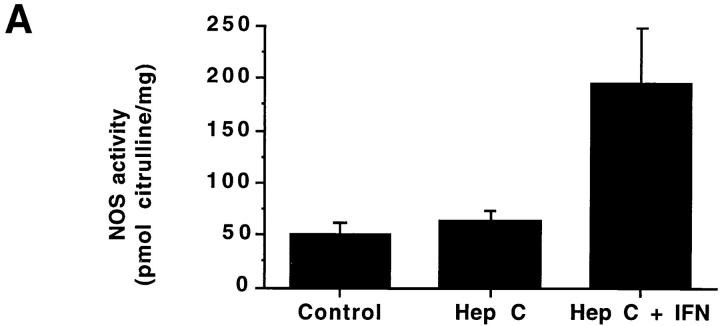
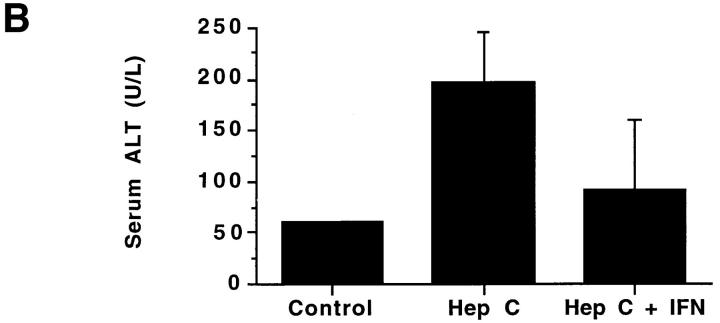
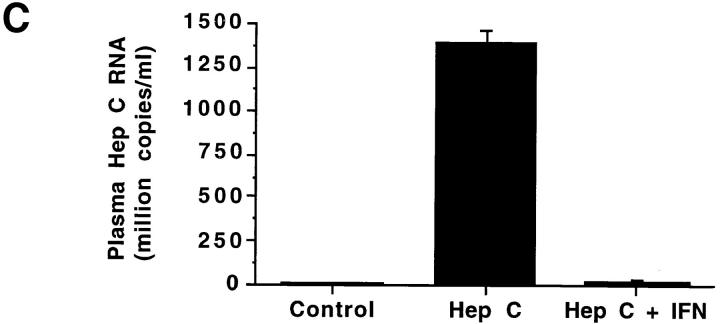
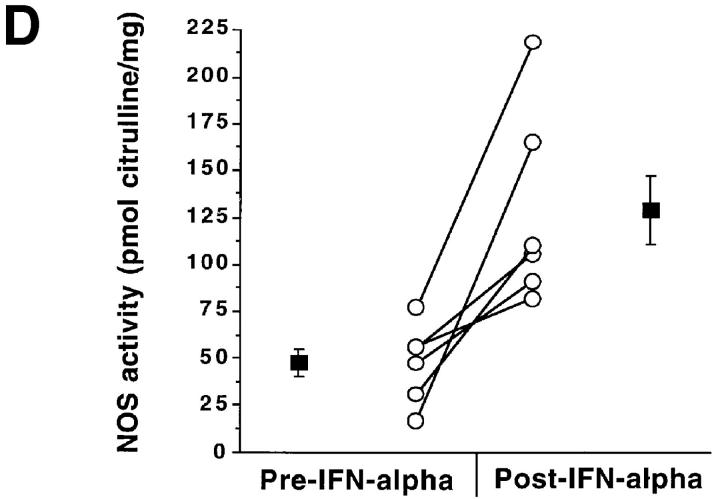
In an attempt to determine if IFN-α treatment in vivo augmented NOS2 expression, we studied patients with hepatitis C before and after IFN-α therapy. Table 1 displays characteristics of the subjects and details of their treatments. As expected, hepatitis C patients receiving IFN-α treatment had higher levels of IFN-α than did normal subjects or hepatitis C patients not receiving IFN-α. There was an overall difference in the blood mononuclear cell NOS activity levels among the three groups (P <0.004; Fig. 3 A). Untreated patients with chronic hepatitis C and healthy controls had comparable NOS activity (ability to convert l-arginine to l-citrulline) in freshly isolated blood mononuclear cells. The activity was inhibited by >80% by inclusion of N-monomethyl-l-arginine in the assaying (data not shown), indicating that the activity was mediated by NOS. Patients receiving IFN-α2b therapy had significantly higher NOS activity levels than did healthy controls (adjusted P <0.05) and hepatitis C patients not receiving IFN-α2b therapy (adjusted P <0.05). Although IFN-α2b treatment caused increases in NOS2 levels, levels of serum alanine aminotransferase (ALT; an indicator of active hepatitis) and plasma hepatitis C RNA decreased with IFN-α2b therapy (Fig. 3, B and C; P = 0.002 and 0.02, respectively, by paired Students t test). When we analyzed samples from individuals both before and after receiving IFN-α2b, there was a significant increase in NOS activity after the IFN-α2b treatment in six patients in whom paired samples were available at baseline and during IFN-α2b therapy (2–8-wk time interval) (P <0.02, paired t test; Fig. 3 D).
Table 1.
Characteristics of Subjects
| Normal subjects | n = 9 (5 M, 4 F) | |||
| Age 39.4 ± 5.8 yr* | ||||
| Total number of hepatitis C patients | n = 26 (17 M, 9 F) | |||
| Chronic hepatitis (18) | ||||
| Chronic hepatitis with cirrhosis (8) | ||||
| Hepatitis C patients not on IFN-α treatment (never treated or blood sampled before started on IFN-α treatment) | n = 18 (14 M, 4 F) | |||
| Age 43.8 ± 10.5 yr | ||||
| Hepatitis C patients on IFN-α treatment | n = 15 (10 M, 5 F) | |||
| Age 40.7 ± 10.5 yr | ||||
| 3 to 10 million units IFN-α three times/wk | ||||
| 1–40 wk of treatment | ||||
| 26.4 ± 12.0 h from last injection until blood draw | ||||
| Serum IFN-α levels | Normal subjects: | 13.8 ± 0.2 pg/ml | ||
| Hepatitis C: | 14.5 ± 0.8 pg/ml | |||
| Hepatitis C on IFN-α: | 34.0 ± 13.9 pg/ml‡ |
Mean ± SD.
P <0.04. M, male; F, female.
Figure 3.
(A–C) NOS enzyme activity in extracts of blood mononuclear cells (A), serum ALT (B), and plasma hepatitis C RNA levels (C). The bars show the mean + one SEM for samples taken from the different subject groups. Control, normal control subjects; Hep C, patients with hepatitis C not on therapy; Hep C + IFN, patients with hepatitis C on IFN-α therapy. For NOS measurements, n = 9 for Control, 18 for Hep C, and 15 for Hep C + IFN; for serum ALT, n = 15; and for plasma hepatitis C RNA, n = 4. (D) NOS enzyme activity in extracts of blood mononuclear cells from six individual patients before and after IFN-α treatment. The lines connect the values from an individual patient's samples before and after IFN-α therapy. The solid squares show the means ± one SEM of the groups.
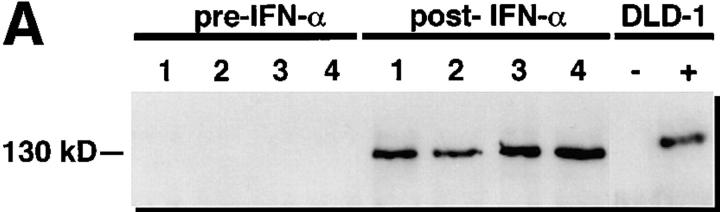
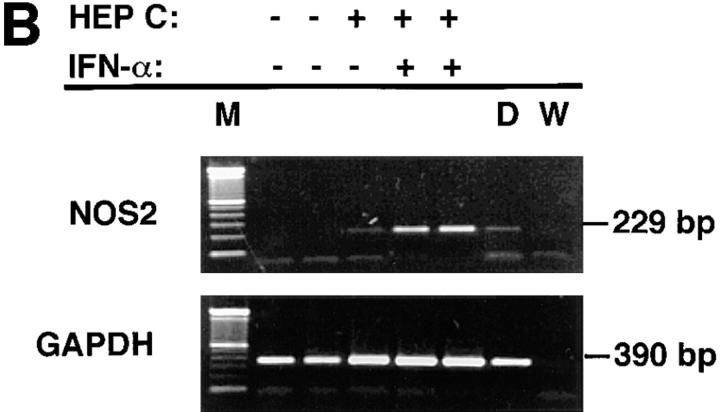
To determine if the increase in NOS activity after IFN-α treatment was accompanied by an increase in NOS2 protein, we analyzed cells for NOS2 antigen content. NOS2 antigen in extracts of blood mononuclear cells was detectable by immunoblot analysis in zero out of seven of the untreated hepatitis C patients and in zero out of five of the healthy control subjects tested. However, eight out of eight samples from hepatitis C patients treated with IFN-α2b had cells with detectable NOS2 antigen. These differences in NOS2 antigen expression among the groups were significant (P <0.00001, Fisher's exact test). Similarly, analysis of matched sets of cells from hepatitis C patients before and after IFN-α2b treatment revealed zero out of four with detectable NOS2 before treatment and four out of four with detectable NOS2 after treatment (Fig. 4 A). Using RT-PCR analyses with cells isolated from six normal individuals and examined without any in vitro culture, we could find no NOS2 mRNA (zero out of six) (Fig. 4 B). In two out of two patients with hepatitis C not on IFN-α treatment, we noted relatively low level expression of NOS2 mRNA, while three out of three hepatitis C patients on IFN-α treatment had relatively higher levels of NOS2 mRNA expression. Fig. 4 B shows representative results.
Figure 4.
(A) Immunoblot analyses of extracts of blood mononuclear cells from hepatitis C patients before and after IFN-α treatment. Equivalent amounts of cellular protein were analyzed in each lane. Antibody 1E8-B8 was used. Samples from patients one through four were collected before IFN-α treatment (pre– IFN-α) or after receiving IFN-α in vivo (post–IFN-α). Controls for human NOS2 were from the human colon cancer cell line DLD-1 without (−) or with (+) treatment with IFN-γ, IL-1, and TNF in vitro. (B) RT-PCR analysis of mononuclear cells from normal subjects, patients with hepatitis C, and patients with hepatitis C treated in vivo with IFN-α. Cells were isolated, frozen, extracted, and analyzed as noted in the Methods section. Cells from the two normal subjects (HEP C − and IFN-α −), one patient with hepatitis C (HEP C + and IFN-α −), and two patients with hepatitis C on treatment with IFN-α (HEP C + and IFN-α +) were analyzed. M, molecular weight markers; D, cells of the human colon cancer cell line DLD-1 treated with IFN-γ, IL-1, and TNF; and W, distilled water.
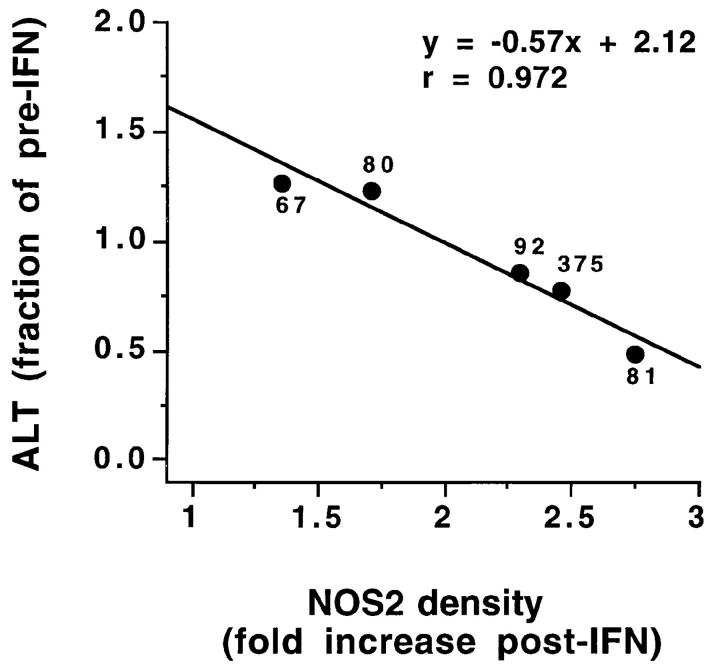
There was a statistically significant correlation between the fold increase in NOS2 antigen immunoblot band density and the degree of reduction of the serum ALT level (fraction of pretreatment ALT level after IFN-α therapy); with increasing NOS2 antigen expression, there was a greater treatment-associated decrease in serum ALT (Fig. 5). The correlation was statistically significant (P <0.001 by ANOVA, and <0.05 by Spearman testing). Because of a lack of certain simultaneous determinations, we could analyze data from only five of the eight subjects before and after treatment. While the NOS2 antigen immunoblot band density and the degree of reduction of the serum ALT level correlated, the correlation between NOS activity (pico moles citrulline/mg protein) and ALT reduction was not significant. Nevertheless, these data suggest that the augmentation in NOS2 levels may be causally related to the observed decreases in serum ALT levels and decrease in liver inflammation associated with IFN-α therapy.
Figure 5.
Correlation between mononuclear cell NOS2 immunoblot band density and degree of reduction of serum ALT in response to IFN-α treatment. Immunoblots were processed using an antibody against NOS2. The NOS2 density was calculated by densitometry and expressed as the ratio of NOS2 band density/background density using a densitometry. The “fold increase post-IFN” for band densities and “fraction of pre-IFN” ALT values were calculated by dividing the pretreatment values by the posttreatment values. The ALT values before IFN-α treatment are shown for each point. A normal ALT level was 10–60 U/liter.
Discussion
IFNs are a family of proteins with established antiviral and immunomodulatory properties (16). While much is known regarding the possible molecular and cellular modes of action of IFNs, the precise mechanism(s) by which IFN-α mediates its anti–hepatitis C virus effect in vivo is not known. Our study shows that IFN-α2b treatment of patients with hepatitis C increases their blood mononuclear cell NOS enzyme activity and their NOS2 antigen and mRNA expression. In addition, we demonstrate that treatment of purified monocytes or mononuclear cells from healthy donors with IFN-α2b or IFN-α2a in vitro enhances their NO production, and NOS enzyme activity, and their NOS2 antigen and mRNA expression. Among isolated mononuclear cells, monocytes are those most likely to express NOS2 and produce NO (6, 7). Our work provides good evidence that IFN-α serves to activate human mononuclear cells (most likely mononuclear phagocytes) for NOS2 expression and NO production both in vitro and in vivo. More than 90% of patients with chronic hepatitis C have circulating immune complexes (17), and immune complexes may enhance NO formation by rodent mononuclear phagocytes (18, 19); thus, immune complexes and IFN-α could have cooperated in vivo to enhance NOS2 expression.
NO has potent antimicrobial activities against a wide array of organisms. These include protozoa, fungi, and bacteria (including Leishmania major, Mycobacterium leprae, Mycobacterium tuberculosis, Toxoplasma gondii, Cryptococcus neoformans, and Schistosoma mansoni; references 3, 4). Also, NO is a critical effector for macrophage-mediated tumor cell cytotoxicity (20). In rodent systems, pharmacological inhibition of NOS or genetic disruption of NOS2 reduces host resistance to infection (3, 4, 21, 22). NO can inhibit infection with DNA viruses (e.g., ectromelia, Herpes simplex virus, vaccinia, and Epstein-Barr virus) and RNA viruses (e.g., vesicular stomatitis virus and Coxsackie virus; references 5, 23–29).
Based on our results, we think that IFN-α–induced NOS2 expression and NO production may be responsible (at least in part) for the anti–hepatitis C effects of IFN-α in vivo. There was a statistically significant correlation between the degree of increase in NOS2 antigen in blood mononuclear cells and the degree of improvement in hepatitis as reflected by a decrease in serum ALT. We do not know the precise mechanism of this possible NO-mediated inhibition; NO likely affects both cellular and viral factors that modify the infectivity. With vaccinia virus, NO-mediated interference with ribonucleotide reductase function appears to be important (30, 31). NO may also damage nucleic acids, alter cellular growth and differentiation, and modify a variety of transcription factors (2, 5, 27, 32, 33); all of these effects might alter viral infectivity. There have been no demonstrations of NO antiviral activity for hepatitis C. There is currently no efficient and reliable cell culture system for growth of hepatitis C virus in vitro, and chimpanzees are the only suitable nonhuman hosts for hepatitis C virus growth in vivo.
In a subset of patients with hepatitis C virus infection, treatment with IFN effectively inhibits viral replication and reduces liver injury, but the effect is usually brief, with an overall response rate of 10–25%. Predictors of a poor response to IFN include viral factors such as high serum hepatitis C virus RNA levels, viral genotype 1, and the absence of sequence mutations in the NS5 region of the viral genome; host-specific factors such as age, weight, duration of infection, and immune status also are apparently important factors (34). The responses of patients to IFN-α treatment correlate inversely with plasma iron saturation and liver iron content (the response is worse with more iron). In some cases, depletion of iron by repeated phlebotomy is associated with reduction in serum ALT abnormalities, reduction in plasma hepatitis C virus RNA levels, and improved responsiveness to IFN-α treatment (35–37). We speculate that iron in these patients may blunt IFN-α– induced NOS2 expression (38), or quench NO antiviral effects (26). NO targets iron- and thiol-containing proteins such as hemoglobin, guanylate cyclase, ribonucleotide reductase, aconitase, and mitochondrial electron transport enzymes, and glyceraldehyde phosphate dehydrogenase (2, 20, 31, 39). Excess iron decreases NOS2 mRNA transcription, and reduction of iron (e.g., by treatment with the iron chelator deferoxamine) can increase NOS2 mRNA levels in vitro (38).
Patients receiving IFN-α for a variety of indications (e.g., hepatitis B or C and malignancies) may develop “autoimmune” illnesses with inflammation similar to rheumatoid arthritis (RA) and SLE (40–43). Our work and that by others has indicated that NO may be a mediator of inflammation in human autoimmune diseases such as RA and SLE. NO is increased in synovial fluid and serum of patients with RA (44). Synovial tissues from patients with RA contain increased amounts of NOS2 and overproduce NO (10, 45), and RA patients overproduce NO systemically and have blood mononuclear cells with increased NOS2 expression and NO production (7, 46). It is possible that the IFN-α treatment-related inflammatory illnesses are due (at least in part) to an IFN-α–mediated increase in NO production.
This study provides the first evidence that IFN-α can augment NOS2 expression and NO production by human blood mononuclear cells in vitro and in vivo. It is not known whether the magnitude of NOS expression will be predictive of response to IFN-α treatment in patients with hepatitis C virus infection; future studies may determine this. Amaro et al. recently showed that patients with hepatitis C had reduced levels of serum nitrite as compared to control subjects. However, the nitrite levels were higher in hepatitis C patients who responded to IFN-α therapy (47). In their study, serum nitrate levels were not measured, dietary intake of nitrite and nitrate was not controlled, and levels of NOS activity and NOS2 antigen were not measured (47).
Although in this study we focus on monocytes as the producers of NO, hepatocytes can also express NOS2 and produce large amounts of NO after activation (48). We did not test hepatocytes for the ability to produce NO in response to IFN-α. Kane et al. recently reported that tissue in 60% of liver biopsies from hepatitis C patients (but none of “normal” subjects) expressed NOS2 mRNA by RT-PCR analysis (49). The authors did not report whether the patients were receiving IFN-α treatment. IFN-α–induced NOS2 expression and NO production by hepatocytes would provide an efficient manner of delivering an antiviral effector molecule to the offending pathogen in its primary cellular target. In addition to indirectly stimulating endogenous NO production by treatment with agents such as IFN-α, delivery of NO per se, perhaps selectively to the liver (50), might be effective in inhibiting hepatitis C virus or other viruses in vivo.
Acknowledgments
We thank Dr. Robert Webber for the anti-NOS2 antibody 1E8-B8.
This study was supported in part by a Smith-Kline Beecham Research Clinical Award from the American Digestive Health Foundation (A.I. Sharara), Schering-Plough Pharmaceuticals (A.I. Sharara), the Veterans Affairs Research Service (J.B. Weinberg), the James R. Swiger Hematology Research Fund (J.B. Weinberg), National Institutes of Health grant AR-39162 (J.B. Weinberg), and National Institutes of Health training grant AI-07217 (D.J. Perkins).
Footnotes
Abbreviations used in this paper: ALT, alanine aminotransferase; DLD-1, human colon cancer cell line; NO, nitric oxide; RA, rheumatoid arthritis; RT, reverse transcriptase; S, synthase.
References
- 1.Nathan C, Xie Q-W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 2.Moncada S, Higgs A. The l-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 3.Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 4.Degroote MA, Fang FC. NO inhibitions. Antimicrobial properties of nitric oxide. Clin Infect Dis. 1995;21:S162–S165. doi: 10.1093/clinids/21.supplement_2.s162. [DOI] [PubMed] [Google Scholar]
- 5.Mannick JB. The antiviral role of nitric oxide. Res Immunol. 1995;146:693–697. doi: 10.1016/0923-2494(96)84920-0. [DOI] [PubMed] [Google Scholar]
- 6.Weinberg JB, Misukonis MA, Shami PJ, Mason SN, Sauls DL, Dittman WA, Wood ER, Smith GK, McDonald B, Bachus KE, et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS). Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- 7.St. Clair EW, Wilkinson WE, Lang T, Sanders L, Misukonis MA, Gilkeson GS, Pisetsky DS, Granger DL, Weinberg JB. Increased expression of blood mononuclear cell nitric oxide synthase type 2 in rheumatoid arthritis patients. J Exp Med. 1996;184:1173–1178. doi: 10.1084/jem.184.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albina JE. On the expression of nitric oxide synthase by human macrophages—why no NO [review] J Leukocyte Biol. 1995;58:643–649. doi: 10.1002/jlb.58.6.643. [DOI] [PubMed] [Google Scholar]
- 9.Anstey NM, Weinberg JB, Hassanali M, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL. Nitric oxide in Tanzanian children with malaria. Inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai H, Kohsaka H, Liu MF, Higashiyama H, Hirata Y, Kanno K, Saito I, Miyasaka N. Nitric oxide production and inducible nitric oxide synthase expression in inflammatory arthritides. J Clin Invest. 1995;96:2357–2363. doi: 10.1172/JCI118292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson S, Bonecinialmeida MDG, Silva LE, Jr, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 13.Zhou AQ, Chen ZF, Rummage JA, Jiang H, Kolosov M, Kolosova I, Stewart CA, Leu RW. Exogenous interferon-gamma induces endogenous synthesis of interferon-alpha and -beta by murine macrophages for induction of nitric oxide synthase. J Interferon Cytokine Res. 1995;15:897–904. doi: 10.1089/jir.1995.15.897. [DOI] [PubMed] [Google Scholar]
- 14.Sherman PA, Laubach VE, Reep BR, Wood ER. Purification and cDNA sequence of an inducible nitric oxide synthase from a human tumor cell line. Biochemistry. 1993;32:11600–11605. doi: 10.1021/bi00094a017. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Vilcek, J., and G.C. Sen. 1996. Interferons and other cytokines. In Virology. 3rd ed. Vol. 1. B.N. Fields, D.M. Knipe and P.M. Howley, editors. Lippincott-Raven, Philadelphia. 375–399.
- 17.Tsai JF, Jeng JE, Chang WY, Ho MS, Lin ZY, Tsai JH. Circulating immune complexes in chronic hepatitis C. J Med Virol. 1995;46:12–17. doi: 10.1002/jmv.1890460104. [DOI] [PubMed] [Google Scholar]
- 18.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB (Fed Am Soc Exp Biol) J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 19.Mulligan MS, Moncada S, Ward PA. Protective effects of inhibitors of nitric oxide synthase in immune complex-induced vasculitis. Br J Pharmacol. 1992;107:1159–1162. doi: 10.1111/j.1476-5381.1992.tb13423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibbs, J.B., Jr., R.R. Taintor, Z. Vavrin, D.L. Granger, J.-C. Drapier, I.J. Amber, and J.R. Lancaster, Jr. 1990. Synthesis of nitric oxide from a terminal guanidino nitrogen atom of l-arginine: a molecular mechanism regulating cellular proliferation that targets intracellular iron. In Nitric Oxide from l-arginine: A Bioregulatory System. S. Moncada and E.A. Higgs, editors. Elsevier Science Publishers B.V., New York. 189–223.
- 21.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 22.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma–induced nitric oxide synthase. Science (Wash DC) 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 26.Karupiah G, Harris N. Inhibition of viral replication by nitric oxide and its reversal by ferrous sulfate and tricarboxylic acid cycle metabolites. J Exp Med. 1995;181:2171–2179. doi: 10.1084/jem.181.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannick JB, Asano K, Izumi K, Kieff E, Stamler JS. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994;79:1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein CJ, Hill SL, Lafond-Walker A, Wu J, Allen G, Landavere M, Rose NR, Herskowitz A. Nitric oxide inhibits viral replication in murine myocarditis. J Clin Invest. 1996;97:1837–1843. doi: 10.1172/JCI118613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin Y-L, Huang Y-L, Ma S-H, Yeh C-T, Chiou S-Y, Chen L-K, Liao C-L. Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol. 1997;71:5227–5235. doi: 10.1128/jvi.71.7.5227-5235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melkova Z, Esteban M. Inhibition of vaccinia virus DNA replication by inducible expression of nitric oxide synthase. J Immunol. 1995;155:5711–5718. [PubMed] [Google Scholar]
- 31.Lepoivre M, Fieschi F, Coves J, Thelander L, Fontecave M. Inactivation of ribonucleotide reductase by nitric oxide. Biochem Biophys Res Commun. 1991;179:442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- 32.Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 33.Tamir S, Burney S, Tannenbaum SR. DNA damage by nitric oxide. Chem Res Toxicol. 1996;9:821–827. doi: 10.1021/tx9600311. [DOI] [PubMed] [Google Scholar]
- 34.Sharara AI, Hunt CM, Hamilton JD. Hepatitis C [review] Ann Intern Med. 1996;125:658–668. doi: 10.7326/0003-4819-125-8-199610150-00006. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi H, Takikawa T, Nishimura N, Yano M, Isomura T, Sakamoto N. Improvement of serum aminotransferase levels after phlebotomy in patients with chronic active hepatitis C and excess hepatic iron [see comments] Am J Gastroenterol. 1994;89:986–988. [PubMed] [Google Scholar]
- 36.Ikura Y, Morimoto H, Johmura H, Fukui M, Sakurai M. Relationship between hepatic iron deposits and response to interferon in chronic hepatitis C. Am J Gastroenterol. 1996;91:1367–1373. [PubMed] [Google Scholar]
- 37.Piperno A, Sampietro M, Dalba R, Roffi L, Fargion S, Parma S, Nicoli C, Corbetta N, Pozzi M, Arosio V, et al. Iron stores, response to alpha-interferon therapy, and effects of iron depletion in chronic hepatitis C. Liver. 1996;16:248–254. doi: 10.1111/j.1600-0676.1996.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiss G, Werner-Felmayer G, Werner ER, Gruenewals K, Wachter H, Hentze MW. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994;180:969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drapier JC, Hibbs JB., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986;78:790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vial T, Descotes J. Immune-mediated side-effects of cytokines in humans. Toxicology. 1995;105:31–57. doi: 10.1016/0300-483x(95)03124-x. [DOI] [PubMed] [Google Scholar]
- 41.Wandl UB, Nagel-Hiemke M, May D, Kreuzfelder E, Kloke O, Kranzhoff M, Seeber S, Niederle N. Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clin Immunol Immunopathol. 1992;65:70–74. doi: 10.1016/0090-1229(92)90250-r. [DOI] [PubMed] [Google Scholar]
- 42.Ronnblom LE, Alm GV, Oberg K. Autoimmune phenomena in patients with malignant carcinoid tumors during interferon-alpha treatment. Acta Oncol. 1991;30:537–540. doi: 10.3109/02841869109092414. [DOI] [PubMed] [Google Scholar]
- 43.Nadir F, Fagiuoli S, Wright HI, Nadir A, Hopp E, Gavaler J, Van Thiel DH. Rheumatoid arthritis: a complication of interferon therapy. Journal of the Oklahoma State Medical Association. 1994;87:228–230. [PubMed] [Google Scholar]
- 44.Farrell AJ, Blake DR, Palmer RM, Moncada S. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis. 1992;51:1219–1222. doi: 10.1136/ard.51.11.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McInnes LB, Leung BP, Field M, Wei XQ, Huang FP, Sturrock RD, Kinninmonth A, Weidner J, Mumford R, Liew FY. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueki Y, Miyake S, Tominaga Y, Eguchi K. Increased nitric oxide levels in patients with rheumatoid arthritis. J Rheumatol. 1996;23:230–236. [PubMed] [Google Scholar]
- 47.Amaro MJ, Bartolome J, Pardo M, Cotonat T, Lopezfarre A, Carreno V. Decreased nitric oxide production in chronic viral hepatitis B and C. J Med Virol. 1997;51:326–331. doi: 10.1002/(sici)1096-9071(199704)51:4<326::aid-jmv11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 48.Nussler AK, Di Silvio M, Billiar TR, Hoffman RA, Geller DA, Selby R, Madariaga J, Simmons RL. Stimulation of the nitric oxide synthase pathway in human hepatocytes by cytokines and endotoxin. J Exp Med. 1992;176:261–264. doi: 10.1084/jem.176.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kane JM, III, Shears LL, II, Hierholzer C, Ambs S, Billiar TR, Posner MC. Chronic hepatitis C virus infection in humans: induction of hepatic nitric oxide synthase and proposed mechanisms for carcinogenesis. J Surg Res. 1997;69:321–324. doi: 10.1006/jsre.1997.5057. [DOI] [PubMed] [Google Scholar]
- 50.Saavedra JE, Billiar TR, Williams DL, Kim YM, Watkins SC, Keefer LK. Targeting nitric oxide (NO) delivery in vivo. Design of a liver-selective NO donor prodrug that blocks tumor necrosis factor-alpha–induced apoptosis and toxicity in the liver. J Med Chem. 1997;40:1947–1954. doi: 10.1021/jm9701031. [DOI] [PubMed] [Google Scholar]