Abstract
T cell activation by specific antigen results in a rapid and long-lasting downregulation of triggered T cell receptors (TCRs). In this work, we investigated the fate of downregulated TCR– CD3-ζ complexes. T cells stimulated by peptide-pulsed antigen-presenting cells (APCs) undergo an antigen dose-dependent decrease of the total cellular content of TCR-β, CD3-ε, and ζ chains, as detected by FACS® analysis on fixed and permeabilized T–APC conjugates and by Western blot analysis on cell lysates. The time course of CD3-ζ chain consumption overlaps with that of TCR downregulation, indicating that internalized TCR–CD3 complexes are promptly degraded. Inhibitors of lysosomal function (bafilomycin A1, folimycin) markedly reduced ζ chain degradation, leading to the accumulation of ζ chain in large Lamp1+ vesicles. These results indicate that in T cell–APC conjugates, triggered TCRs are rapidly removed from the cell surface and are degraded in the lysosomal compartment.
Tlymphocytes are activated by the engagement of their clonally expressed TCR with peptide–MHC complexes displayed on the APC surface. The antigen receptor of T cells is a multimeric protein complex composed of the clonotypic αβ heterodimer, the CD3 γδε chains, and the ζ homodimer. Whereas the αβ heterodimer is responsible for specific recognition, the associated CD3 chains and ζ homodimer are necessary for signal transduction (1). The assembly of this multichain receptor complex is highly regulated in T cells, because only correctly assembled receptors can reach the cell surface (2).
Upon conjugation with APCs, T cells undergo a sustained [Ca2+]i increase (3, 4) that results from the serial engagement and triggering of many TCRs by a small number of peptide–MHC complexes (5). A key feature of T cell antigen recognition is that the process of TCR/peptide–MHC interaction is self-limited by the downregulation of triggered TCR complexes (5, 6). Indeed, downregulation of triggered TCRs leads to extinction of sustained signaling in T–APC conjugates and affects T cell responsiveness to further antigenic stimulation (6).
The mechanisms that lead to downregulation of triggered TCRs are presently unknown and, in particular, it is not clear whether these TCRs are indeed degraded or simply internalized. In this study we investigated the fate of triggered TCR–CD3 components in human T cells interacting with peptide-pulsed APCs. We report that stimulation by the specific antigen results in rapid and profound loss of TCR-β, CD3-ε, and ζ chain due to degradation in the lysosomal compartment.
Materials and Methods
T Cell Clones and APCs.
Two DRBI*1104-restricted T cell clones (KS140 and KS70) specific for the tetanus toxin peptide (TT830– 843; QYIKANSKFIGITE) were used. Autologous Epstein–Barr virus (EBV)-B cells were used as APCs (4).
Intracellular Staining for CD3-ε and ζ Chain.
EBV-B cells were pulsed for 2 h at 37°C with various concentrations of TT830–843 in RPMI, 5% FCS. During the last 10 min, 1 μM BCECF-AM (2′,7-bis-(carboxyethyl)-5(6′)-carboxyfluorescin; Calbiochem, San Diego, CA) was added and the cells were washed four times. T cells were mixed with EBV-B cells at a 1:2 ratio in 200 μl RPMI, 5% FCS in U-bottomed microplates, centrifuged 1 min at 1,500 rpm to allow conjugate formation, and incubated at 37°C. In some experiments, T cells were pretreated for 1 h with 10 μg/ml cycloheximide and the drug was present throughout the assay. At different times, the cells were resuspended, washed in PBS, 0.5 mM EDTA and fixed for 10 min with 3% paraformaldehyde. The cells were permeabilized for 10 min at room temperature with washing buffer (Hepes-buffered PBS containing 0.1% saponin) and stained with anti-CD3 (TR66; reference 4), anti-Vβ2 (Immunotech, Marseille, France), or anti-ζ (Santa Cruz Biotechnology, Santa Cruz, CA) in Hepes-buffered PBS containing 0.1% saponin and 2% BSA (or 5% FCS), followed by a goat anti–mouse PE-labeled Ab (SBA, Birmingham, AL). The TCR, CD3, and ζ fluorescence were analyzed on a FACScan® (Becton Dickinson, Mountain View, CA). EBV-B cells were gated out using both forward and side scatter (FSC/SSC) parameters and green BCECF fluorescence. In some experiments, T cells were conjugated with unstained peptidepulsed EBV-B cells; at different times of incubation at 37°C, the cells were gently resuspended and laid on poly-l-lysine–coated slides for 10 min at 37°C. The cells were fixed for 10 min with 3% paraformaldehyde, permeabilized for 10 min at room temperature with washing buffer, and stained with an anti-ζ (Santa Cruz) in Hepes-buffered PBS containing 0.1% saponin and 4% BSA, followed by FITC-labeled goat anti–mouse (SBA) and anti– human Lamp-1 (provided by Dr. S. Carlsson, Umea University, St. Louis, MO) followed by Texas Red–labeled goat anti–rabbit antibody (SBA). The samples were mounted in 90% glycerol–PBS containing 2.5% 1-4-diazabicyclo (2.2.2) octane (DABCO; Fluka AG, Buchs, Switzerland). In some experiments, T cells were pretreated with 0.5 μM bafilomycin A1 (Calbiochem) or with the vehicle of the drug only (DMSO 0.5%) for 1 h at 37°C before conjugate formation. The samples were examined using a BioRad MRC 1024 confocal microscope (Bio-Rad, Richmond, CA).
ζ Chain Detection by Western Blot.
EBV-B cells were pulsed with various concentrations of TT830–843. 5 × 105 T cells were pretreated for 1 h with 10 μg/ml cycloheximide mixed with 106 EBV-B cells in 500 μl RPMI 5% FCS in U-bottomed tubes, centrifuged to allow conjugate formation, and incubated at 37°C for 2 h in the presence of cycloheximide. In some experiments, T cells were pretreated with 1 μM bafilomycin A1 or with 1 μM folimycin (Calbiochem) or with the vehicle of the drugs only (DMSO 1%) for 1 h at 37°C before conjugate formation. The drugs were present in the culture throughout the assay. At different timepoints, the cells were mixed with PBS, lysed in either ice-cold RIPA buffer containing 1 mM NaVO4 or in prewarmed Laemmli buffer, sonicated, and boiled. After separation on 12.5% SDS-PAGE and transfer to nitrocellulose, membranes were blocked 1 h at room temperature with blocking buffer (5% nonfat dry milk, 0.05% Tween-20 in Tris-buffered saline) and incubated for 1 h with 1 μg/ml anti-ζ (Santa Cruz) in blocking buffer. After washing, the membranes were incubated for 1 h with HRP-labeled goat anti–mouse Ig (SBA) in blocking buffer. Filters were developed using an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL). Densitometric analysis was performed using a Computing Densitometer 300A (Molecular Dynamics, Sunnyvale, CA).
TCR Downregulation, IFN-γ Production, and [Ca2+] Measurement.
TCR downregulation, IFN-γ production, and [Ca2+]; were measured as previously described (4, 5). In some experiments, T cells were pretreated with 1 μM bafilomycin A1 or with 1 μM folimycin for 1 h at 37°C before conjugate formation and the drugs were kept in the culture through all the assay. Control cultures were done in the presence of vehicle only (1% DMSO).
Results
Reduced Content of TCR-β, CD3-ε, and ζ Chains in T Cells after Antigen Stimulation.
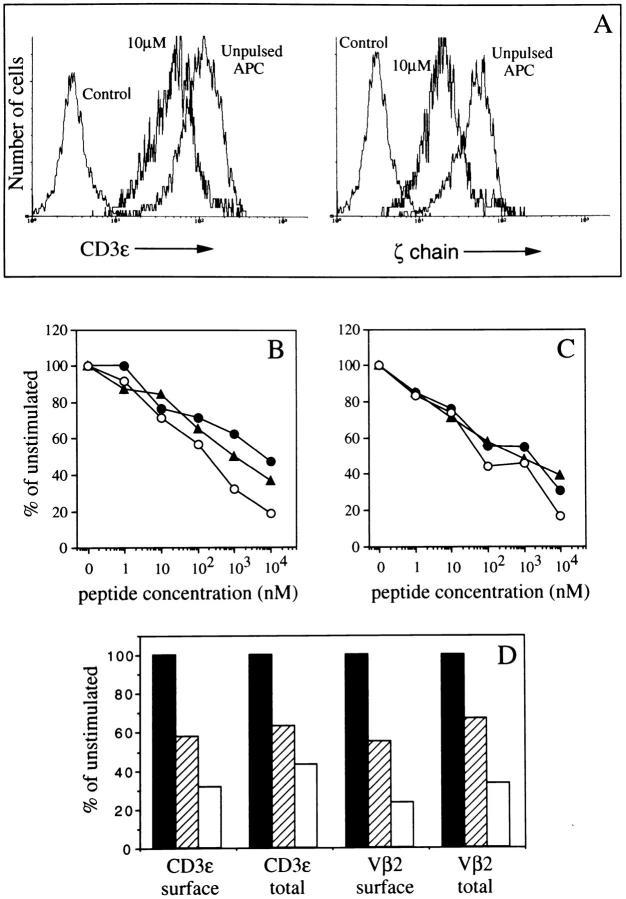
T cells conjugated with peptide-pulsed APCs undergo a rapid downregulation of triggered TCR–CD3 complexes that reaches a plateau in 1–2 h (5). To investigate whether downregulated receptors accumulate inside of T cells or are degraded, we measured the surface levels and the total cellular content of different TCR–CD3 components by FACS® analysis. As shown in Fig. 1, A–B, in T cells conjugated with peptide-pulsed APCs the surface level of CD3-ε and the total content of CD3-ε and ζ chains decreased in a parallel fashion with increasing doses of antigen. Blocking of protein synthesis did not affect the extent of CD3-ε and ζ chain decrease, demonstrating that disappearance of these components was not due to a decreased synthesis (Fig. 1 C ). Similar results were observed when levels of CD3-ε and TCR-β were measured in a different T cell clone (Fig. 1 D).
Figure 1.
Parallel decrease in surface expression and total cellular content of TCR-β, CD3-ε and ζ chains in T cells as a function of antigenic stimulation. T cells (clone KS140) were conjugated at 37°C with APCs pulsed with various doses of peptide. After 2 h the cells were either fixed and stained with anti-CD3-ε or anti-Vβ2, or fixed, permeabilized, and stained with anti-CD3-ε, anti-Vβ2, or anti ζ. (A) Staining for total CD3-ε (left) and ζ (right) in T cells conjugated with unpulsed or peptide-pulsed APCs. (B and C ) Levels of surface CD3-ε (○), total CD3-ε (•) and total ζ (▴) chains as a function of antigen concentration. In C, T cells were pretreated for 1 h with cycloheximide and the drug was present throughout the assay. (D) Levels of surface and total Vβ2 and CD3-ε in T cell clone KS70 stimulated with 100 nM (diagonal stripes) or 10 μM (empty) peptide expressed as percent of the staining of unstimulated cells (closed).
To investigate whether the reduced staining for CD3-ε and ζ chain could be due to the localization of triggered receptors in cellular compartments not accessible to antibodies, we measured ζ chain expression by Western blot. As shown in Fig. 2 A, the lysates of T–APC conjugates showed a dose-dependent decrease in the content of ζ chain. These results are comparable to those observed by FACS® analysis (Fig. 1). In addition, the time kinetics of ζ chain decrease parallels that of surface TCR downregulation (5, 6) reaching a plateau in about 2 h (Fig. 2 B).
Figure 2.
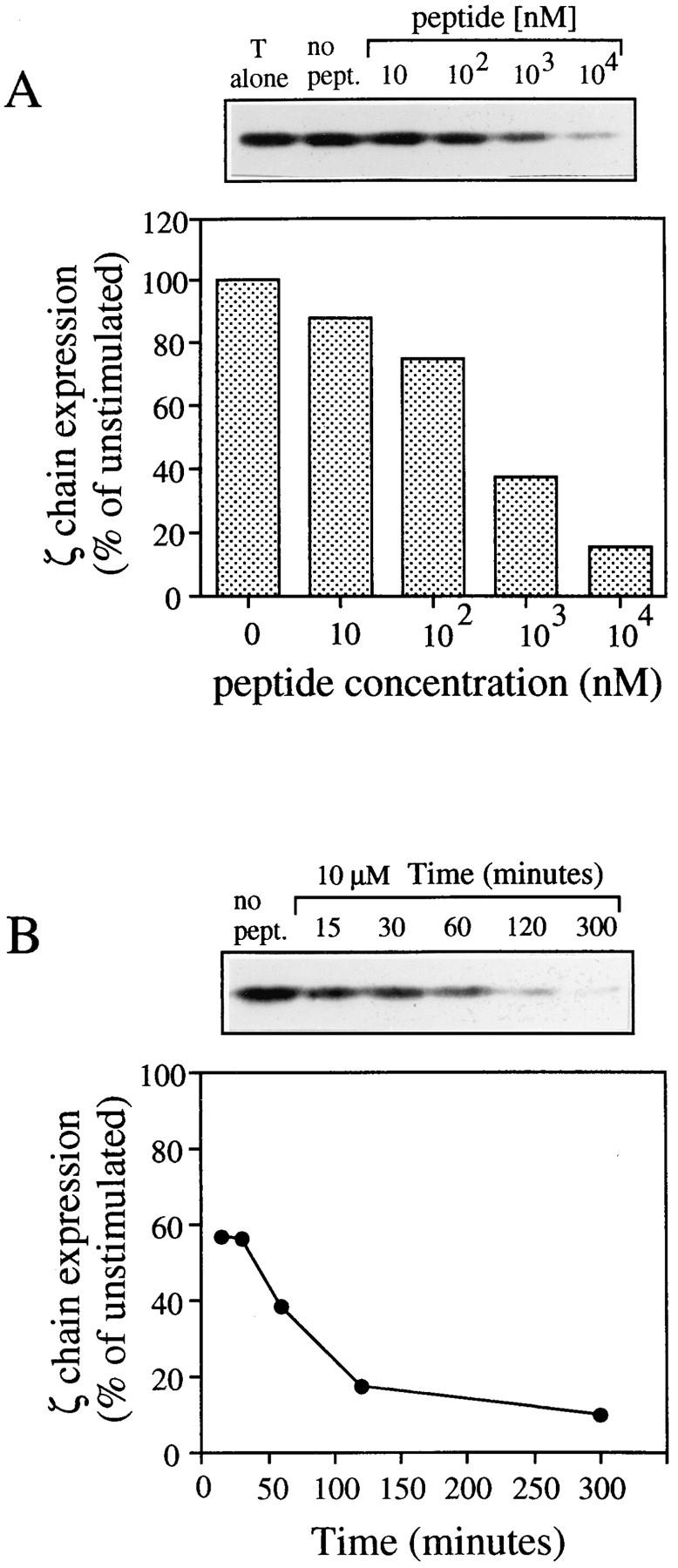
Dose response and kinetics of antigen-induced ζ chain loss. T cells were conjugated in the presence of cycloheximide with APCs pulsed with various peptide concentrations. At the indicated time points the cells were lysed and the amount of ζ chain was measured by Western blot. (A) ζ chain loss as a function of antigen concentration. (B) Time course of ζ chain loss in T cells conjugated with APC pulsed with 10 μM peptide.
The above results demonstrate that TCR triggering by specific antigen results in a net decrease in the cellular content of TCR and CD3-ε and ζ molecules. This indicates that downregulated TCR–CD3-ζ complexes are promptly degraded.
Lysosomal Degradation of CD3-ζ in Activated T Cells.
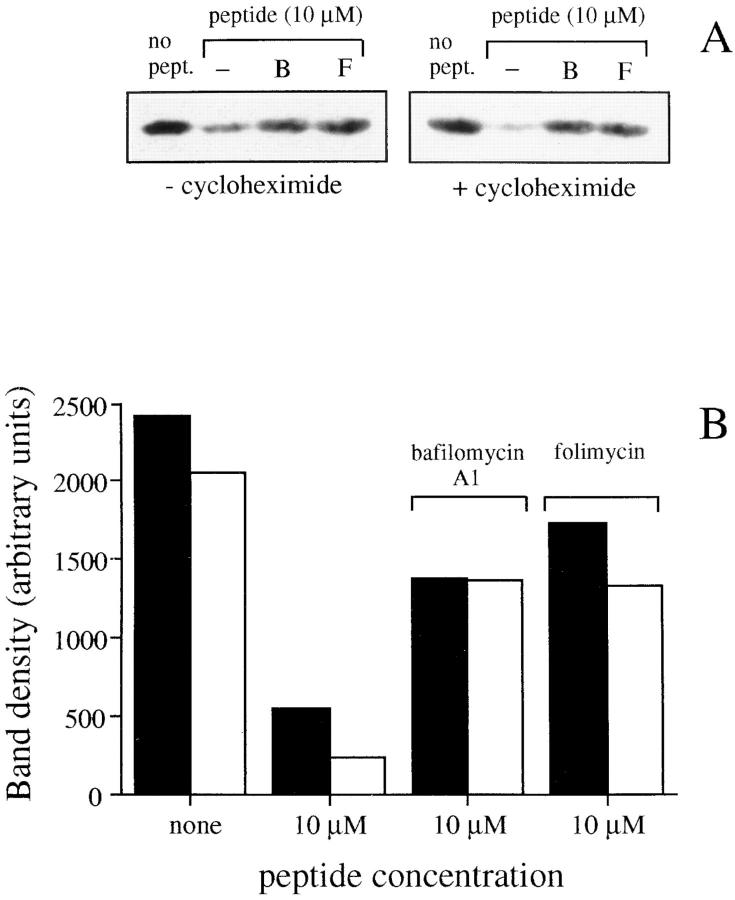
A wellknown mechanism for the inactivation of hormone and growth factor receptors is their internalization upon ligand binding followed by their degradation in lysosomes (7). To find whether triggered TCRs are degraded via a similar mechanism we stimulated T cells with peptide-pulsed APCs in the presence of bafilomycin A1 or folimycin, drugs that affect lysosomal function by increasing lysosomal pH (8, 9). These drugs do not interfere with TCR downregulation, sustained [Ca2+]i increase, and IFN-γ production (Fig. 3), indicating that they are not toxic for the T cell biological response. However, bafilomycin A1 and folimycin dramatically inhibited ζ chain degradation as detected by Western blot analysis on total cell lysates (Fig. 4). The inhibition of ζ chain degradation by bafilomycin A1 and folimycin was also observed when protein synthesis was blocked by pretreating T cells with cycloheximide (Fig. 4). This result indicates that the effect of bafilomycin A1 and folimycin is not due to the accumulation of newly synthesized unassembled components, but actually results from a block of the degradation of triggered receptors.
Figure 3.
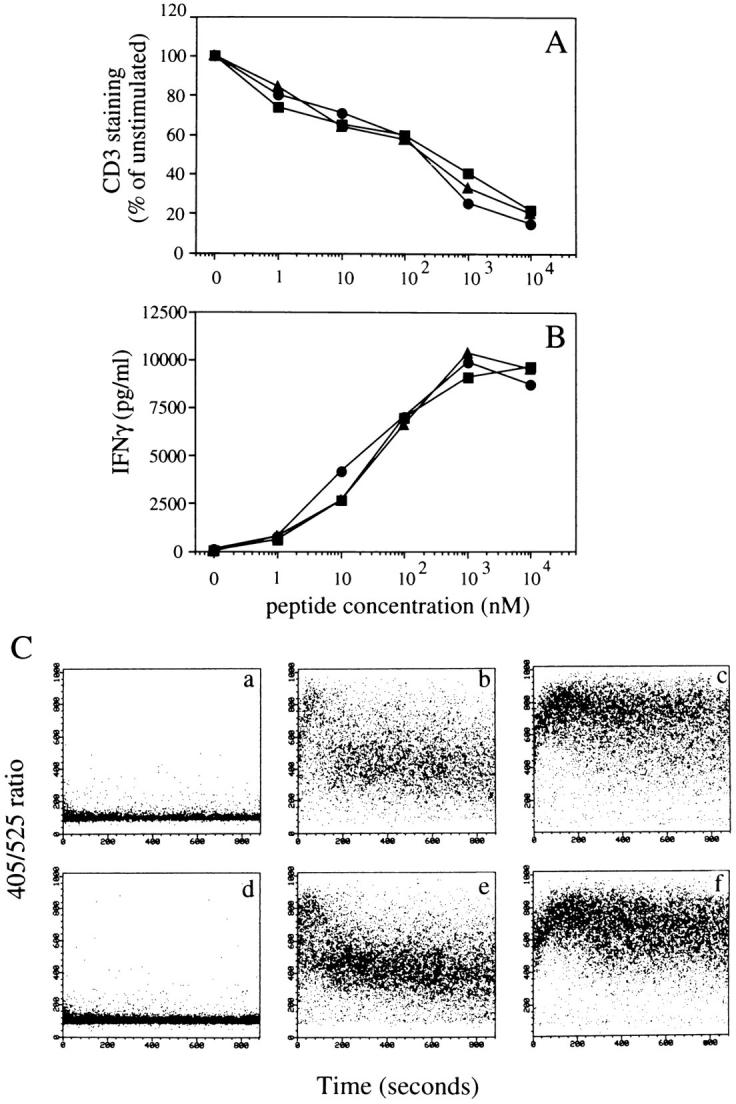
Bafilomycin A1 and folimycin do not interfere with T cell activation induced by specific antigen. T cells pretreated for 1 h with bafilomycin A1 (▪), folimycin (▴), or vehicle only (•) were conjugated with APCs pulsed with various peptide concentrations and the drugs were left in culture throughout the assay. (A) CD3 downregulation after 4 h; (B) IFN-γ production after 4 h; (C ) [Ca2+]i increase in T cells conjugated with unpulsed APC (a and d ) with APC pulsed with 25 nM (b and e), or 10 μM peptide (c and f ). T cells were either pretreated with bafilomycin A1 (d, e, f ) or with the vehicle only (a, b, c).
Figure 4.
Antigen-induced degradation of ζ chain is prevented by bafilomycin A1 and folimycin. T cells were pretreated for 1 h with bafilomycin A1 or folimycin in the presence or absence of cycloheximide. The cells were conjugated with APCs pulsed with 10 μM peptide and total content of ζ chain was determined after 2 h by Western blot. The drugs were present throughout the assay. (A) Western blot. (B) Densitometric analysis of the bands obtained in the absence (closed ) or in the presence (empty) of cycloheximide.
Morphological Evidence for Targeting of Triggered CD3-ζ to the Lysosomes.
To identify better the site of TCR–CD3 complex degradation, we investigated by confocal microscopy the fate of triggered TCRs. T cells were conjugated with peptide-pulsed or unpulsed APCs and after 2 h the conjugates were fixed, permeabilized, and stained with anti-ζ chain antibodies.
In unstimulated T cells most of the ζ chain is associated with the plasma membrane and this staining pattern is not affected by preincubation with bafilomycin A1 (Fig. 5, A, B, E ). In T cells that had been conjugated for 2 h with peptide-pulsed APCs, the staining was much weaker and did not show the ring-shaped pattern of ζ chain surface expression (Fig. 5 C ). Strikingly, when conjugates were formed in the presence of bafilomycin A1 a dramatic accumulation of ζ chain was observed in intracellular vesicles (Fig. 5 D). In these vesicles, most of the internalized ζ chain colocalized with the lysosomal marker LAMP-1 (Fig. 5 F). These results demonstrate that upon TCR/peptide– MHC interaction ζ chains are promptly removed from the cell surface and degraded in the lysosomes.
Figure 5.
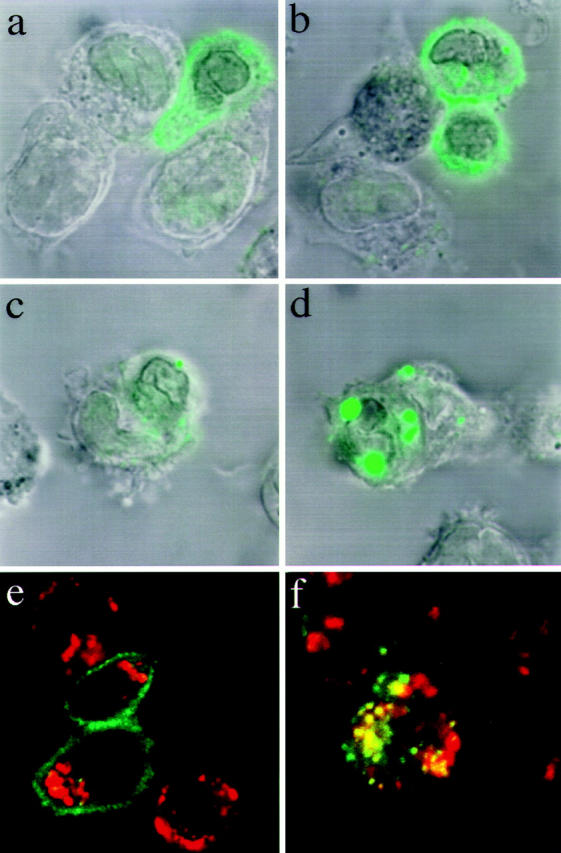
Triggered CD3-ζ is targeted to the lysosomes for degradation. T cells were conjugated with either unpulsed APCs (a, b, e) or peptide-pulsed (10 μM) APCs (c, d, f ). T cells were treated with bafilomycin A1 1 h before conjugate formation (b, d, e, f ) or with vehicle only (a and c) the drug was left in culture throughout the assay. After 2 h the conjugates were fixed, permeabilized, and stained with anti-ζ (green) and anti-Lamp-1 (red ).
Discussion
In specific T–APC conjugates TCRs are downregulated in an antigen dose- and time-dependent fashion (5). The reduction of TCR levels plays an important role in extinguishing the signaling process and reduces T cell responsiveness to antigenic stimulation (6). So far, TCR downregulation has been defined only as a long-lasting disappearance of TCRs from the cell surface, whereas their fate has not been investigated. In this work, we demonstrate that triggered TCR–CD3-ζ complexes are degraded in the lysosomes after antigenic stimulation. This conclusion is based on measurements of the total cellular content of TCR-β, CD3-ε, and ζ chains that are quantitatively lost in an antigen dependent fashion and on the effect of drugs that block lysosomal degradation.
Whereas CD3 and TCR are tightly associated, the ζ chain, which plays a dominant role in TCR-mediated signal transduction, is associated to the complex in a loose fashion. Indeed, whereas ζ is required for assembly and surface expression of the TCR (2, 10), it has been proposed that it may be exchanged on mature receptors (11). Our data demonstrate that in the process of antigen-induced downregulation TCR–CD3 and ζ chains are irreversibly linked. Recently, Cai et al. (12) showed that in mouse resting T cells TCR downregulation is due to internalization rather than shedding, but degradation of the TCR–CD3 complexes has not been investigated.
The mechanisms involved in TCR downregulation by specific antigen are presently unknown. It has been shown that phosphorylation of Ser 126 of the CD3-γ chain is both necessary and sufficient for TCR downregulation induced by pharmacological stimulation of PKC (13). However, the mechanisms of TCR downregulation induced by PMA or specific ligand are fundamentally different, because mutations of CD3-γ that abolish PMA-induced downregulation do not affect ligand-induced downregulation (Salio, M., S. Valitutti, and A. Lanzavecchia, manuscript in preparation).
As are many other surface receptors, TCR–CD3-ζ complexes are constitutively internalized and recycled to the plasma membrane (14, 15). Our results suggest that triggering by cognate ligand leads to the failure of TCRs to recycle back to the surface due to their targeting to lysosomes. Among possible signals for targeting triggered TCR complexes to lysosomes, it is interesting to consider ubiquitination (7, 16). Indeed, ubiquitination of TCR–CD3 subunits (17) and especially ζ chains (18), have been demonstrated following TCR cross-linking.
What could be the physiological relevance of antigeninduced TCR degradation? It is interesting to note that the time kinetics of receptor degradation overlaps with the time kinetics of sustained [Ca2+]i increase and TCR downregulation (Fig. 2; references 5, 6). This indicates that serial TCR triggering and degradation are ongoing phenomena. We and others have shown that in T cells undergoing sustained signaling, treatments that terminate the process of TCR engagement result in the extinction of the signaling process within a few minutes (4, 19). A rapid degradation of triggered TCRs could be important to inactivate already triggered receptors as soon as they have been internalized while new receptors are interacting with the ligands. As a consequence, any single TCR would be triggered by a single peptide–MHC complex only once and the length of signaling would reflect the length of the serial receptor engagement. This mechanism could be at work to ensure a strict control of the extent of T cell activation by a defined number of antigenic determinants.
Acknowledgments
We thank M. Dessing for help in [Ca2+]i measurements; M. Dessing and S. Meyer for image processing; K. Campbell, K. Karjalainen and J. Pieters for discussion and critical reading of the manuscript.
Footnotes
The Basel Institute for Immunology was founded and is supported by Hoffman-La Roche Ltd. Co., Basel, Switzerland.
References
- 1.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 2.Klausner RD, Lippincott J, Schwartz, Bonifacino JS. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- 3.Donnadieu E, Cefai D, Tan YP, Paresys G, Bismuth G, Trautmann A. Imaging early steps of human T cell activation by antigen-presenting cells. J Immunol. 1992;148:2643–2653. [PubMed] [Google Scholar]
- 4.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valitutti S, Müller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature (Lond) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 6.Valitutti S, Müller S, Dessing M, Lanzavecchia A. Signal extinction and T cell repolarization in T helper cell-antigen-presenting cell conjugates. Eur J Immunol. 1996;26:2012–2016. doi: 10.1002/eji.1830260907. [DOI] [PubMed] [Google Scholar]
- 7.Strous GJ, van Kerkhof P, Govers R, Ciechanover A, Schwartz AL. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. EMBO (Eur Mol Biol Organ) J. 1996;15:3806–3812. [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)–ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem. 1991;266:17707–17712. [PubMed] [Google Scholar]
- 9.Woo JT, Shinohara C, Sakai K, Hasumi K, Endo A. Isolation, characterization and biological activities of concanamycins as inhibitors of lysosomal acidification. J Antibiot Tokyo. 1992;45:1108–1116. doi: 10.7164/antibiotics.45.1108. [DOI] [PubMed] [Google Scholar]
- 10.Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science (Wash DC) 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 11.Ono S, Ohno H, Saito T. Rapid turnover of the CD3 zeta chain independent of the TCR–CD3 complex in normal T cells. Immunity. 1995;2:639–644. doi: 10.1016/1074-7613(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 12.Cai Z, Kishimoto H, Brunmark A, Jackson MR, Peterson PA, Sprent J. Requirements for peptideinduced T cell receptor downregulation on naive CD8+T cells. J Exp Med. 1997;185:641–652. doi: 10.1084/jem.185.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dietrich J, Hou X, Wegener AM, Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO (Eur Mol Biol Organ) J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krangel MS. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987;165:1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minami Y, Samelson LE, Klausner RD. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. J Biol Chem. 1987;262:13342–13347. [PubMed] [Google Scholar]
- 16.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 17.Cenciarelli C, Hou D, Hsu KC, Rellahan BL, Wiest DL, Smith HT, Fried VA, Weissman AM. Activation-induced ubiquitination of the T cell antigen receptor. Science (Wash DC) 1992;257:795–797. doi: 10.1126/science.1323144. [DOI] [PubMed] [Google Scholar]
- 18.Cenciarelli C, Wilhelm KG, Jr, Guo A, Weissman AM. T cell antigen receptor ubiquitination is a consequence of receptor-mediated tyrosine kinase activation. J Biol Chem. 1996;271:8709–8713. doi: 10.1074/jbc.271.15.8709. [DOI] [PubMed] [Google Scholar]
- 19.Beeson C, Rabinowitz J, Tate K, Gutgemann I, Chien YH, Jones PP, Davis MM, McConnell HM. Early biochemical signals arise from low affinity TCR–ligand reactions at the cell–cell interface. J Exp Med. 1996;184:777–782. doi: 10.1084/jem.184.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]