Abstract
The house dust mite Dermatophagoides pteronyssinus allergen Der p 1 is the most immunodominant allergen involved in the expression of dust mite–specific immunoglobulin (Ig)E–mediated hypersensitivity. The reason for this potent IgE-eliciting property of Der p 1 remains unknown, but there is mounting in vitro evidence linking the allergenicity of Der p 1 to its cysteine protease activity. Here we demonstrate for the first time that immunization of mice with proteolytically active Der p 1 results in a significant enhancement in total IgE and Der p 1–specific IgE synthesis compared with animals immunized with Der p 1 that was irreversibly blocked with the cysteine protease inhibitor E-64. We conclude that the proteolytic activity of Der p 1 is a major contributor to its allergenicity.
Keywords: cysteine protease, Der p 1, IgE
Immunoglobulin E plays a central role in the sequence of events leading to allergic hypersensitivity disorders such as asthma that afflict ∼20% of the world population 1. The house dust mite Dermatophagoides pteronyssinus allergen Der p 1 has long been recognized as the most immunodominant allergen involved in the expression of dust mite–specific IgE–mediated hypersensitivity 2. The reason for this potent IgE-eliciting property of Der p 1 remains unknown, but there is mounting evidence linking the allergenicity of Der p 1 to its cysteine protease activity 3.
Recent in vitro work has established that Der p 1 selectively cleaves human CD25, the 55-kD α subunit of the T cell IL-2 receptor (the high-affinity form of which consists of α, β, and γ subunits) 4. As a result of cleavage of surface CD25, peripheral blood T cells show markedly diminished proliferation and IFN-γ secretion in response to stimulation by anti-CD3 antibody. It is known that CD4 Th cells undergo a cytokine-driven process of polarization and that IL-2, along with IFN-γ, and IL-4 are considered to be autocrine growth factors for the Th1 and Th2 subsets, respectively 5. The Th1 and Th2 cell populations promote the development of cells of the same subset while suppressing the propagation of those of the other subset. Therefore, Der p 1–induced cleavage of CD25 is likely to lead to impaired growth of cells of the Th1 subset and consequent augmentation of those of the Th2 subset.
These observations raise the question of whether the cysteine protease activity of Der p 1 would bias the immune response in favor of IgE. In this paper, we demonstrate that immunization of mice with proteolytically active Der p 1 results in a significant enhancement in total IgE and Der p 1–specific IgE synthesis compared with animals immunized with Der p 1 that was irreversibly blocked with the cysteine protease inhibitor E-64.
Materials and Methods
Der p 1 Preparation.
Der p 1 was isolated from house dust mite fecal pellets (Allergon) by a multistep procedure 6 involving immunoaffinity chromatography on immobilized anti–Der p 1 mAb (clone 4C1; Indoor Biotechnologies), removal of contaminating serine proteases on immobilized soybean trypsin inhibitor (Sigma Chemical Co.), and finally fast protein liquid chromatography (FPLC) to remove low-molecular-mass contaminants. The purity of the preparation was confirmed by NH2-terminal sequencing on an automatic amino acid sequencer (Applied Biosystems, Inc.), SDS-PAGE analysis (15% gel), and demonstration that enzymatic activity was completely dependent on preactivation with cysteine and totally inhibited by E-64 (l-trans-epoxysuccinyl-leucylamido [4-guanidino]butane). Protein concentration was determined using a bicinchoninic acid (BCA) microtiter plate assay and confirmed spectrophotometrically using the empirical absorption coefficient value for Der p 1 of E1% (280 nm) = 16.4.
Before use, Der p 1 was preactivated with 5 mM cysteine (Sigma Chemical Co.) to regenerate its thiol group, which becomes oxidized during purification. The catalytic activity of Der p 1 was ascertained in a continuous rate (kinetic) assay using the fluorogenic peptide substrate N-tert-butoxy-carbonyl (Boc)-Gln-Ala-Arg–7-amino-4-methyl-coumarin (AMC; reference 6). To block the proteolytic activity of cysteine-activated Der p 1, 1,000-fold molar excess of E-64 (Sigma Chemical Co.) was used; a similar molar ratio of the cysteine protease inhibitor iodoacetamide (Sigma Chemical Co.) was used as another sulfhydryl reactive agent.
CD25 Cleavage.
Spleen T cells were obtained from C57BL/6J mice using standard procedures. The cells (2 × 106) were suspended in RPMI (GIBCO Life Technologies) and stimulated for 3 d at 37°C with Con A (5 μg/ml final concentration) in the presence of IL-2 (100 U/ml) in a humidified atmosphere of 5% CO2. CD25 cleavage was performed by incubating 105 cells with up to 10 μg/ml Der p 1 (preactivated with 5 mM cysteine) for 1 h at 37°C in a total volume of 200 μl serum-free AIM V medium (GIBCO Life Technologies). The cells were then resuspended in RPMI containing 2% FCS, stained for 45 min at room temperature in the dark with FITC-labeled anti–mouse CD25 mAb (clone AMT-13; Sigma Chemical Co.), and fixed with 5% formaldehyde. Cells were analyzed on a FACScan™ (Becton Dickinson) as described 4. The expression of other T cell surface markers, namely CD3, CD4, and CD8, was monitored in the same way using appropriate PE- or FITC-labeled antibodies (clone KT3, Beckman Coulter; and clones YTS191.1 and KT15 [Serotec Ltd.], respectively).
Immunization Protocol.
Five groups of 10 female CBA/J mice were given six weekly intraperitoneal injections of 10 μg of proteolytically active Der p 1, 10 μg of E-64–blocked Der p 1, 10 μg of iodoacetamide-blocked Der p 1, 10 μg of OVA (as proteolytically inactive antigen; Sigma Chemical Co.), or 10 μg of OVA with E-64, respectively. All immunizations were given in 200 μg of Al(OH)3 as adjuvant. A tail bleed was obtained 1 wk before the start of immunization (prebleed), and a total bleed was obtained by cardiac puncture 1 wk after the last injection (final bleed). The proteolytic activity of Der p 1 and its inhibition with E-64 or iodoacetamide in the immunization mixture were ascertained as described above.
Antibody Detection.
Serum samples were initially titrated to determine the optimal dilution for testing each antibody isotype and subclass. The optimal dilutions used here were 1/10 for detecting total IgE, Der p 1–specific IgE, and OVA-specific IgE and 1/20,000, 1/40,000, and 1/250 for detecting Der p 1–specific IgG, IgG1, and IgG2b, respectively. Total IgE was detected by a sandwich ELISA using one monoclonal anti–mouse IgE (clone R35-72; PharMingen) as capture antibody and a second biotinylated monoclonal anti–mouse IgE (clone R35-118; PharMingen) as a detection antibody. Der p 1–specific IgE, OVA-specific IgE (measured using samples that have been depleted of IgG on a protein G column [Pharmacia]), and Der p 1–specific IgG, IgG1, and IgG2b were detected on microtiter plates coated with a 4 μg/ml solution of either Der p 1 or OVA and developed with biotinylated (for IgE clone R35-118 and IgG1 clone A85-1 [PharMingen] and for IgG2b clone AB275 [The Binding Site]) or alkaline phosphatase–conjugated isotype-specific antibodies (for IgG; Sigma Chemical Co.). Alkaline phosphatase–conjugated Extravidine (Sigma Chemical Co.) was used in conjunction with biotinylated antibodies. Unpaired Student's t test was used to compare levels of antibody responses between the different immunization groups; P < 0.05 was considered significant.
Results and Discussion
Der p 1 is a 25-kD cysteine protease whose structure has been modeled 7 on the crystal structure of papain, with which it shows considerable sequence similarities, most notably for residues involved in the enzyme active site 8. The proteolytic activity of Der p 1 can be inhibited by E-64, the class-specific inhibitor of microbial origin 9. This inhibition is brought about when cysteine within the Der p 1 active site forms a thioether covalent bond with the epoxy group of E-64. This is an irreversible process that does not lead to significant structural changes, as evidenced by crystallographic studies of a papain–E-64 complex 10.
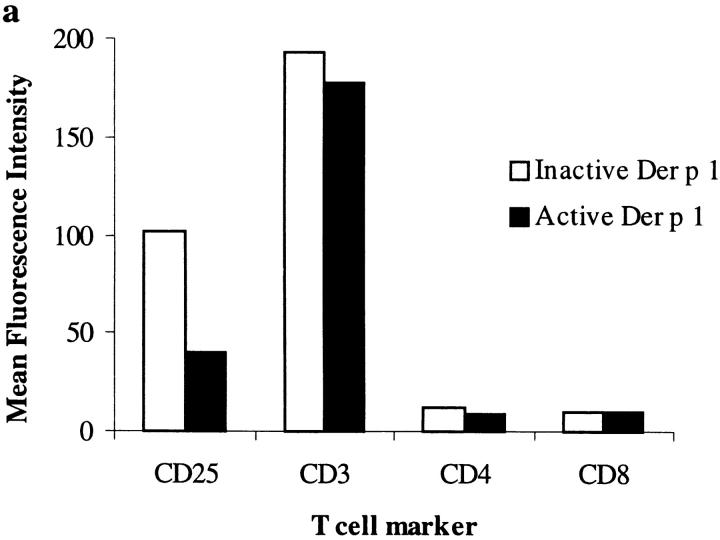
We have purified Der p 1 from fecal pellets using a multistep procedure and confirmed its purity by NH2-terminal sequencing, SDS-PAGE analysis, and demonstration that enzymatic activity was completely dependent on preactivation with cysteine and inhibited by E-64 and iodoacetamide (Fig. 1). We have recently shown that Der p 1 selectively cleaves human CD25 from the surfaces of peripheral blood T cells 4. Here we demonstrate that Der p 1 also selectively cleaves CD25 from cultured mouse spleen T cells (Fig. 2), which is not surprising given the high degree of sequence homology that exists between human 11 and mouse 12 CD25. This observation has therefore provided the justification for using this animal species for testing our hypothesis, namely that the proteolytic activity of Der p 1 is a major contributor to its allergenicity.
Figure 1.
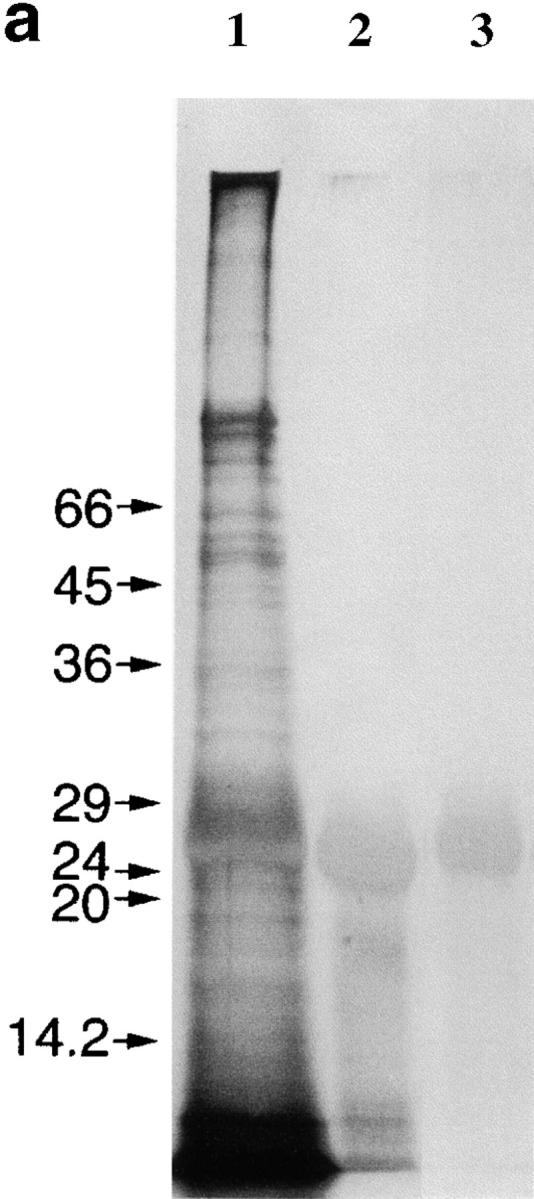
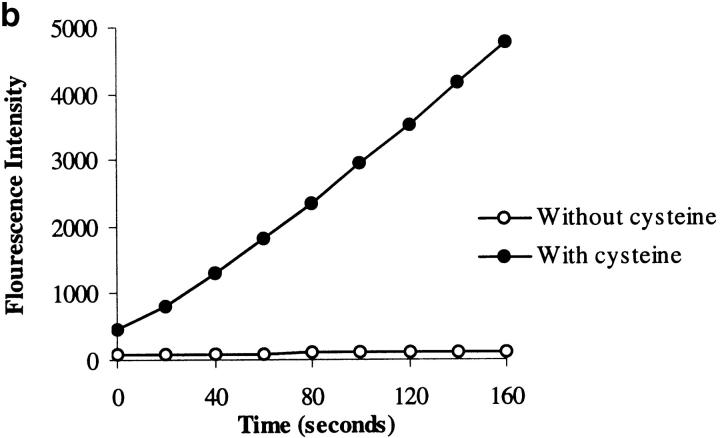
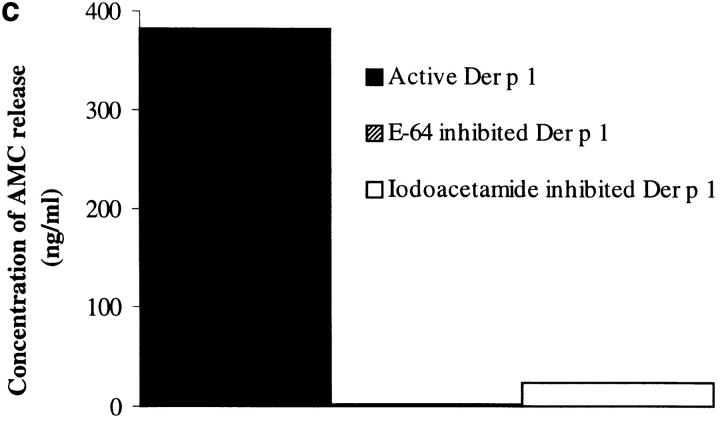
Assessment of the purity of the Der p 1 preparation. NH2-terminal sequencing showed that the sequence obtained (TNACSINGNA) matches the published sequence of Der p 1 8. (a) Silver-stained SDS-PAGE analysis showing crude extract of fecal pellets (lane 1), fraction obtained after immunoaffinity chromatography on immobilized anti–Der p 1 mAb 4C1 followed by removal of contaminating serine proteases on immobilized soybean trypsin inhibitor (lane 2), and the final product after FPLC (lane 3). Molecular mass standards are indicated at left. (b) Progression curves of the catalytic activity of Der p 1, measured in a continuous rate (kinetic) assay using the fluorogenic peptide substrate Boc-Gln-Ala-Arg-AMC 6, with or without preactivation with cysteine. (c) The addition of E-64 and iodoacetamide results in 100 and 94% inhibition of the enzymatic activity of Der p 1, respectively. Data presented in b and c are the means of duplicate experiments; SE was <5%.
Figure 2.
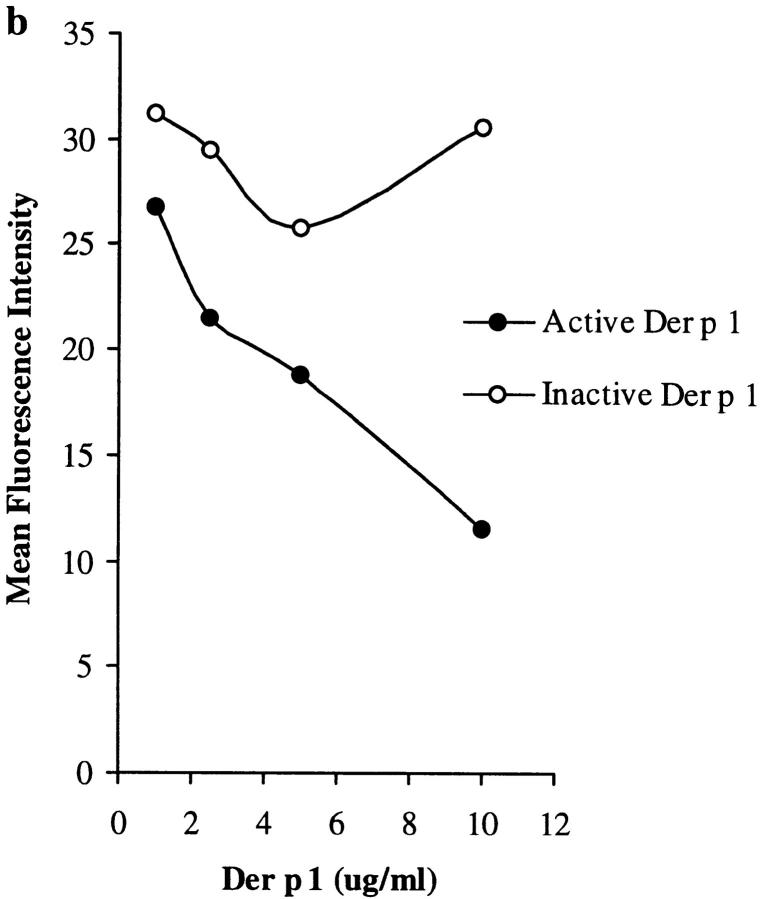
Proteolytically active Der p 1 (10 μg/ml) cleaves mouse CD25, but not CD3, CD4, or CD8, from spleen T cells as monitored by flow cytometry (a); cleavage of CD25 is dose dependent (b). No such effect was demonstrable when Der p 1 was inactivated by E-64. Data presented are the means of duplicate experiments; SE was <5%.
Intraperitoneal immunization of groups of 10 CBA/J mice with either proteolytically active or inactive (E-64–blocked) Der p 1 over a 6-wk period showed a statistically significant enhancement in total IgE (P < 0.01) and Der p 1–specific IgE (P < 0.02) responses in animals immunized with proteolytically active Der p 1. This effect was IgE specific, as Der p 1–specific IgG, IgG1, and IgG2b responses increased to the same extent with proteolytically active or inactive Der p 1 (Fig. 3). We are not sure why the IgG1 response did not follow that of IgE, as these two isotypes are considered to be coregulated in the mouse. However, the IgE-restricted enhancement seen in response to immunization with proteolytically active Der p 1 does suggest a mechanism that is unique to IgE isotype switching/synthesis. Furthermore, our control experiments clearly show that the IgE-specific effect observed here is not due to E-64 exerting a suppressive influence on IgE production by a mechanism that is independent of its binding to the Der p 1 enzyme active site (Fig. 4). First, suppression of total IgE (P < 0.04) and Der p 1–specific IgE (P < 0.05) productions was also obtained when the proteolytic activity of Der p 1 was blocked with iodoacetamide, another sulfhydryl reactive agent. Second, the IgE antibody response to OVA, a proteolytically inactive antigen, was not suppressed when the animals were immunized with OVA plus E-64.
Figure 3.
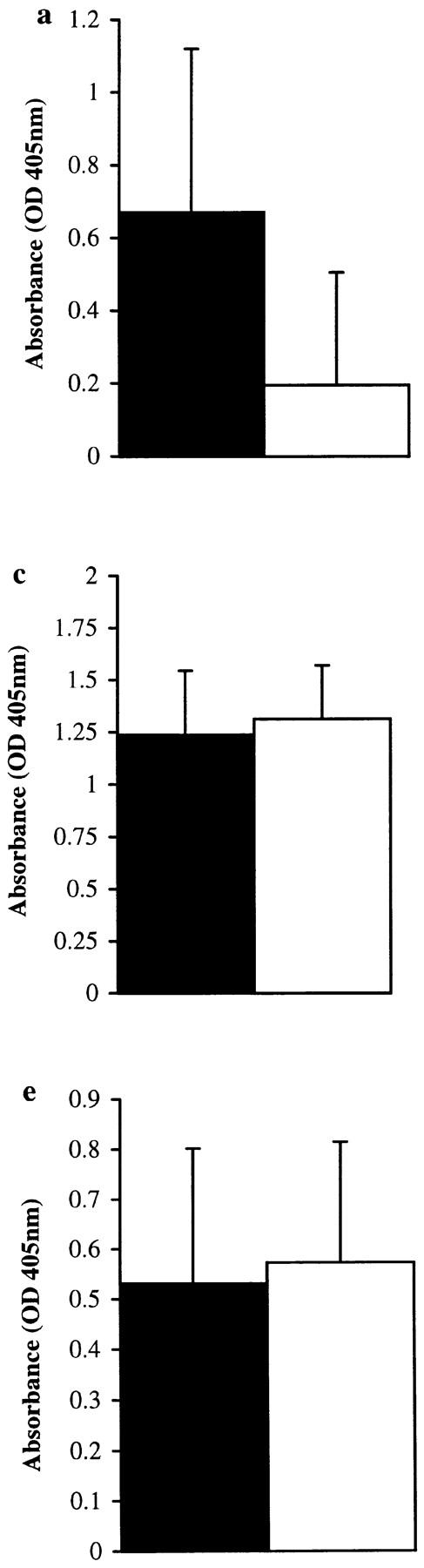
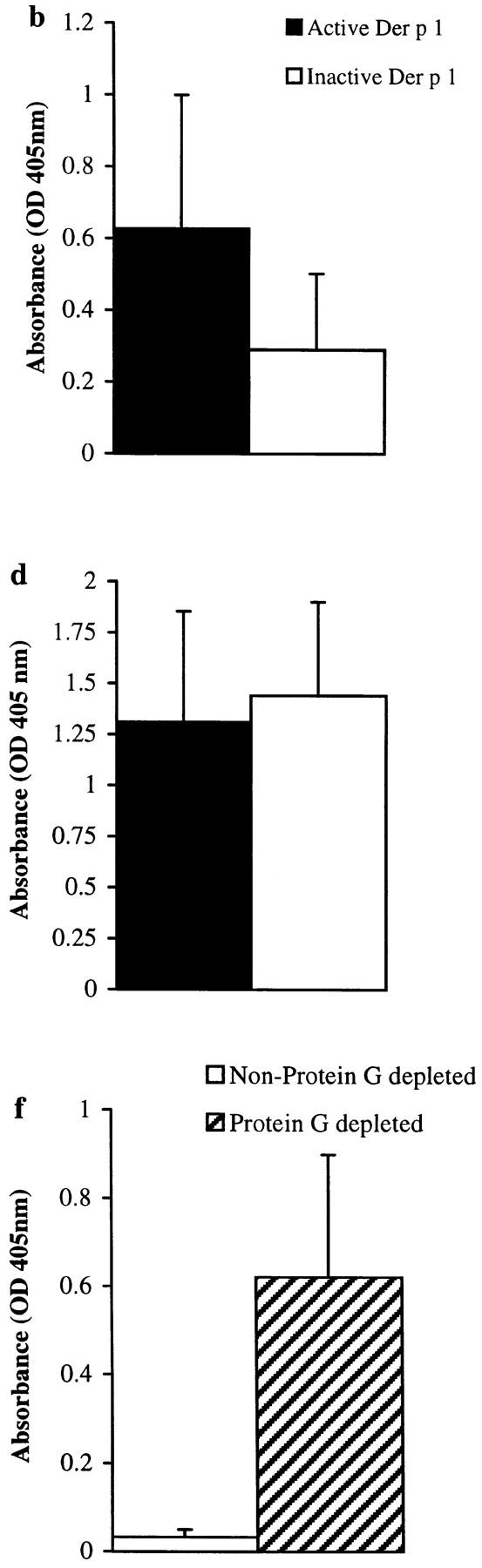
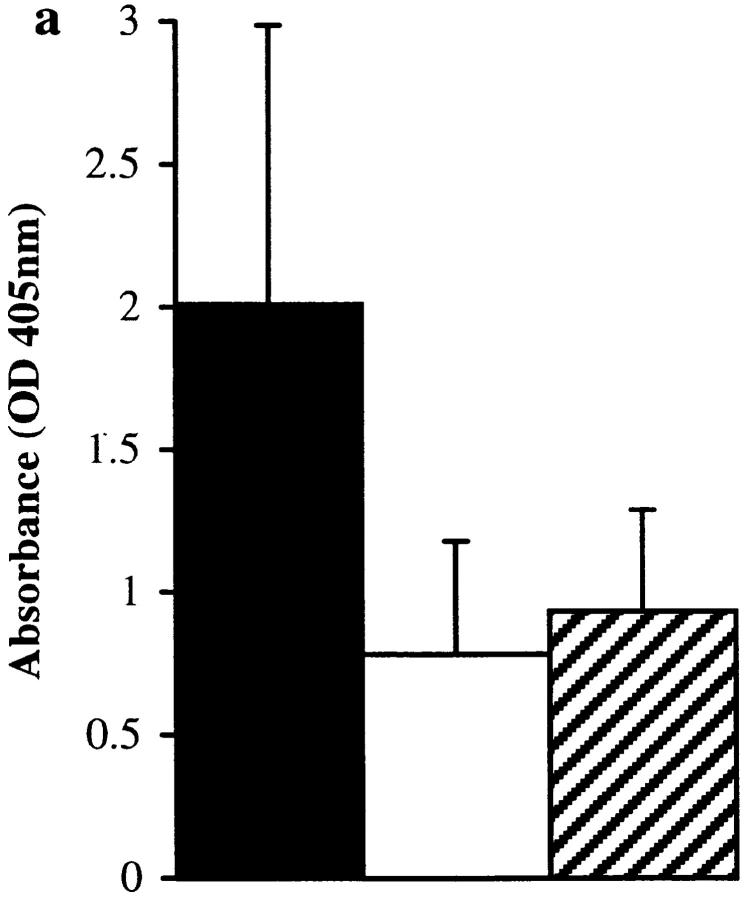
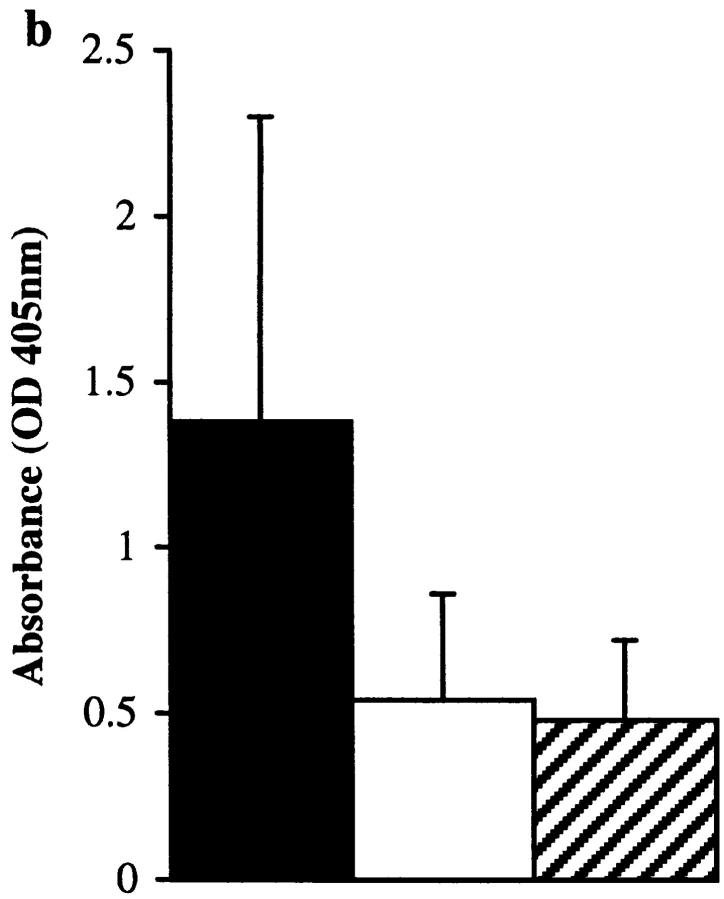
Total IgE (a) and Der p 1–specific IgE (b), IgG (c), IgG1 (d), and IgG2b (e) levels in final bleeds obtained from groups of 10 mice immunized with proteolytically active or inactive (E-64–blocked) Der p 1 measured by ELISA. There was a statistically significant enhancement in total IgE (P < 0.01) and Der p 1–specific IgE (P < 0.02) responses in animals immunized with proteolytically active Der p 1; prebleeds showed no measurable levels of antibody (except for baseline levels of total IgE). Depleting serum samples of IgG by protein G treatment (to overcome competition) from animals immunized with proteolytically active Der p 1 enhances the detection of Der p 1–specific IgE (f); this effect was also observed with animals immunized with proteolytically inactive Der p 1. Error bars represent 95% confidence intervals, and the results are representative of two immunization experiments.
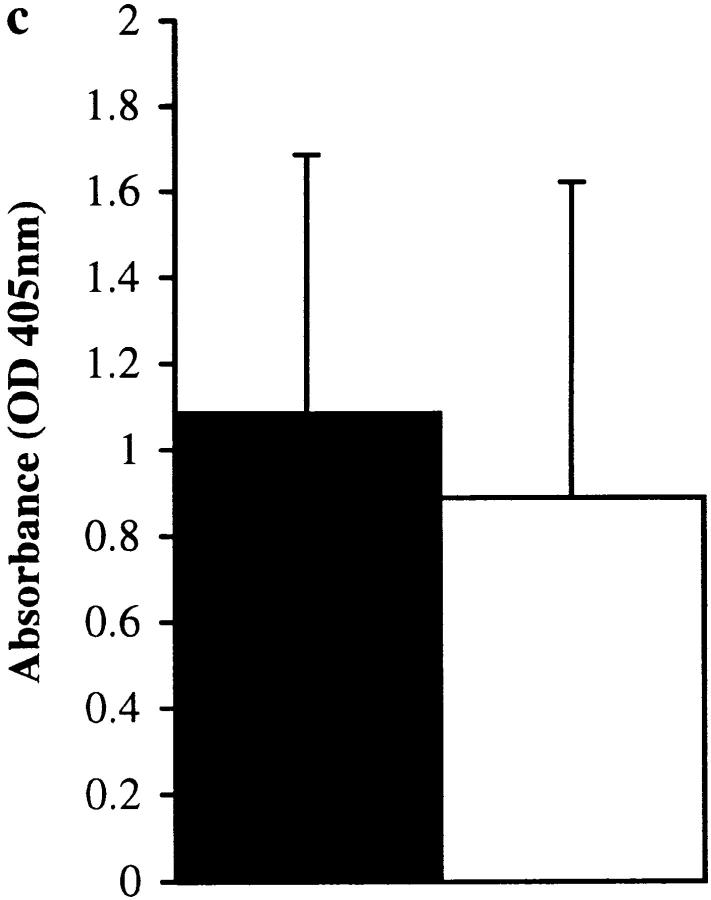
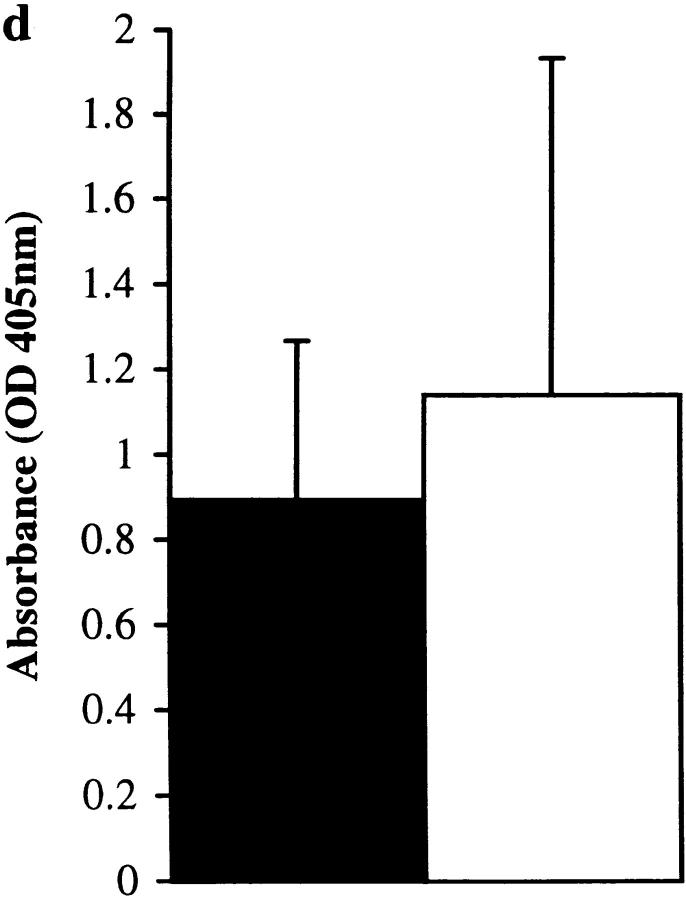
Figure 4.
Control experiments showing that the IgE-specific effect observed in Fig. 3 is not due to E-64 exerting a suppressive influence on IgE production by a mechanism that is independent of its binding to the Der p 1 enzyme active site. Total IgE (a) and Der p 1–specific IgE (b) levels in final bleeds obtained from groups of 10 mice immunized with proteolytically active (black bar) or inactive (iodoacetamide [hatched bar]- or E-64–blocked [white bar]) Der p 1 measured by ELISA. There was a statistically significant suppression of total IgE and Der p 1–specific IgE responses in animals immunized with Der p 1 that was rendered proteolytically inactive by either iodoacetamide (P < 0.04 for total IgE and P < 0.05 for Der p 1–specific IgE) or E-64 (P < 0.01 for total IgE and P < 0.02 for Der p 1–specific IgE). Total IgE (c) and OVA-specific IgE (d) levels in final bleeds obtained from groups of 10 mice immunized with OVA (black bar) or OVA plus E-64 (white bar) were measured by ELISA. Prebleeds showed no measurable levels of antibody (except for baseline levels of total IgE). Error bars represent 95% confidence intervals.
Our results are direct evidence that the cysteine protease activity of Der p 1 induces a significant increase in IgE responses. Such an effect is clearly consistent with the ability of Der p 1 to proteolytically cleave mouse CD25 and induce a Th2 response by modulating the balance between IL-4 and IFN-γ 13. The recent demonstration in mice that Leishmania mexicana cysteine proteinase–deficient mutants potentiate a Th1 response, compared with the Th2 response normally seen in response to infection with wild-type parasite 14, is also of great relevance here. These findings suggest that immune deviation toward Th1 in dust mite–allergic individuals could potentially be achieved by administering Der p 1 in a catalytically inactive (mutant) form. On the other hand, exploring the potential Th2 adjuvant property of the proteolytic activity of Der p 1 would have important implications in defining principles for modulation of Th1-mediated pathological conditions.
Our demonstration that the cysteine protease activity of Der p 1 enhances total IgE production, apart from increasing Der p 1-specific IgE, suggests that this allergen may play a central role in destabilizing the microenvironment within target tissues to one that is proallergic and thus aids in the initiation and propagation of the allergic cascade. In other words, the proteolytic activity of Der p 1 may exert an IgE-specific adjuvant effect. The in vivo relevance of the proteolytic activity of Der p 1 is further highlighted by reports demonstrating that it increases the permeability of the human respiratory epithelium to macromolecules 15 16. Such observations, together with our current findings showing a direct effect on the immune system, indicate that the proteolytic activity of Der p 1 is a major contributor to its allergenicity.
Acknowledgments
This work was supported by a British Lung Foundation grant (P99/13).
References
- Sutton B.J., Gould H.J. The human IgE network. Nature. 1993;366:421–428. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- Tovey E.R., Chapman M.D., Platts-Mills T.A.E. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- Shakib F., Schulz O., Sewell H.F. A mite subversivecleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol. Today. 1998;19:313–316. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- Schulz O., Sewell H.F., Shakib F. Proteolytic cleavage of CD25, the α subunit of the human T cell interleukin 2 receptor, by Der p 1, a major mite allergen with cysteine protease activity. J. Exp. Med. 1998;187:271–275. doi: 10.1084/jem.187.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Schulz O., Sewell H.F., Shakib F. A sensitive fluorescent assay for measuring the cysteine protease activity of Der p 1, a major allergen from the dust mite Dermatophagoides pteronyssinus . Mol. Pathol. 1998;51:222–224. doi: 10.1136/mp.51.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham C.M., Srinivasan N., Thorpe C.J., Overington J.P., Kalsheker N.A. Comparative modelling of major house dust mite allergen Der p 1structure validation using an extended environmental amino acid propensity table. Protein Eng. 1994;7:869–894. doi: 10.1093/protein/7.7.869. [DOI] [PubMed] [Google Scholar]
- Chua K.Y., Stewart G.A., Thomas W.R., Simpson R.J., Dilworth R.J., Plozza T.M., Turner K.J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. Homology with cysteine proteases. J. Exp. Med. 1988;167:175–182. doi: 10.1084/jem.167.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A.J., Kembhavi A.A., Brown M.A., Kirschke H., Knight C.G., Tamai M., Hanada K. L-trans-epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Matsumoto K., Ohishi H., Ishida T., Inoue M., Kitamura K., Mizuno H. Refined x-ray structure of papain.E-64-c complex at 2.1-Å resolution. J. Biol. Chem. 1991;266:14771–14777. doi: 10.2210/pdb1pe6/pdb. [DOI] [PubMed] [Google Scholar]
- Cosman D., Cerretti D.P., Larsen A., Park L., March C., Dower S., Gillis S., Urdal D. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984;312:768–771. doi: 10.1038/312768a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Malek T.R., Leonard W.J., Greene W.C., Shevach E.M., Germain R.N. Nucleotide sequence and expression of a mouse interleukin 2 receptor cDNA. J. Immunol. 1985;134:4212–4217. [PubMed] [Google Scholar]
- Comoy E.E., Pestel J., Duez C., Stewart G.A., Vendeville C., Fournier C., Finkelman F., Capron A., Thyphronitis G. The house dust mite allergen, Dermatophagoides pteronyssinus, promotes type 2 responses by modulating the balance between IL-4 and IFN-γ. J. Immunol. 1998;160:2456–2462. [PubMed] [Google Scholar]
- Alexander J., Coombs G.H., Mottram J.C. Leishmania mexicana cysteine proteinase-deficient mutants have attenuated virulence for mice and potentiate a Th1 response. J. Immunol. 1998;161:6794–6801. [PubMed] [Google Scholar]
- Herbert C.A., King C.M., Ring P.C., Holgate S.T., Stewart G.A., Thompson P.J., Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p 1. Am. J. Respir. Cell Mol. Biol. 1995;12:369–378. doi: 10.1165/ajrcmb.12.4.7695916. [DOI] [PubMed] [Google Scholar]
- Winton H.L., Wan H., Cannell M.B., Thompson P.J., Garrod D.R., Stewart G.A., Robinson C. Class specific inhibition of house dust mite proteinases which cleave cell adhesion, induce cell death and which increase the permeability of lung epithelium. Br. J. Pharmacol. 1998;124:1048–1059. doi: 10.1038/sj.bjp.0701905. [DOI] [PMC free article] [PubMed] [Google Scholar]