Abstract
Mice deficient in interleukin (IL)-2 production or the IL-2 receptor α or β chains develop a lethal autoimmune syndrome. CD4+ regulatory T cells have been shown to prevent autoimmune diseases, allograft rejection, and to down-regulate antibody responses against foreign antigens. To assess the role of IL-2 in the generation and function of regulatory T cells, we transferred CD4+ T cells from mice genetically deficient in IL-2 or IL-2Rα (CD25) expression. A small number of splenic or thymic CD4+ T cells from IL-2 knockout mice can protect mice from spontaneous experimental autoimmune encephalomyelitis (EAE). In contrast, splenic or thymic CD4+ T cells from CD25 knockout donor mice conferred little or no protection. We conclude that T cells with regulatory potential can develop, undergo thymic selection, and migrate to the peripheral lymphoid organs in the absence of IL-2, and do not protect from disease by means of IL-2 secretion. However, IL-2 signaling in regulatory T cells is essential for their protective function. Altogether, our results favor a model whereby IL-2 induces regulatory T cell activity.
Keywords: CD4+ T lymphocytes, immune tolerance, encephalomyelitis, autoimmunity, hypersensitivity
Introduction
IL-2 has been known for many years as a T cell growth-promoting factor. It was therefore unexpected to find that mice deficient in IL-2 production or the IL-2 receptor α and β chains develop a lethal lymphoproliferative and autoimmune syndrome which has subsequently been referred to as “IL-2-deficiency syndrome” (1–4). The phenotype of the IL-2 and IL-2Rα and β KO mouse indicates that while the T cell survival/ growth promoting capacity of IL-2 can be exerted by other growth/survival factors, IL-2 is essential for the down-regulation of immune responses.
The role of IL-2 in immune tolerance appears to be twofold. First, it has been shown that IL-2 is critical in programming T cells for activation-induced cell death (AICD; references 5–7). It is likely that this function of IL-2 is dependent on its ability to increase surface expression of Fas ligand (FasL) and suppress expression of the inhibitor of apoptosis FLIP (8). Second, it is becoming increasingly apparent that besides contributing to AICD, IL-2 plays additional roles in immune regulation. For instance, Suzuki et al. showed that the lymphoproliferative syndrome in IL-2Rβ KO mice could be rescued by a bone marrow transplant from wild-type mice (9). In this case, there was no IL-2 signaling in the T cells of the recipient mice, thus the IL-2R+ donor-derived cells were contributing to immune tolerance by mechanisms other than IL-2-promoted AICD of the host cells. Klebb et al. (10) treated IL-2 KO mice with rhIL-2, and showed that splenocytes and thymocytes from IL-2–treated IL-2 KO mice could enhance survival of untreated IL-2 KO mice. In these experiments, however, transfer of large numbers of cells (2 × 107 splenocytes and 5 × 107 thymocytes) was required and the ensuing protection was partial. Malek et al. (11) showed that proximal lck promoter-driven transgenic expression of IL-2Rβ chain was sufficient to prevent the lymphoproliferation associated with lack of IL-2 signaling in IL-2Rβ KO mice, despite the fact that IL-2 signaling was greatly diminished in the peripheral T cells of the transgenic mice. Finally, Wolf et al. (12) showed that a small number (3 × 105) of CD4+CD25+ T cells from wild-type mice prevented the accumulation of antigen-stimulated TCR transgenic IL-2 KO T cells. As CD4+CD25+ T cells produce little or no IL-2 (12–14), it is unlikely that the CD4+CD25+ cells were impeding the accumulation of antigen-stimulated T cells through IL-2–promoted AICD. These results are consistent with a defect in the generation or function of regulatory T cells in the absence of IL-2.
We sought to determine the role of IL-2 using two experimental systems in which regulatory T cells (T-reg) have been shown to play a major role. In the first experimental system, mice that harbor a monoclonal myelin basic protein (MBP)-specific αβ T cell repertoire spontaneously develop experimental autoimmune encephalomyelitis (EAE; references 15 and 16). Spontaneous EAE can be prevented by a single administration of purified CD4+ splenocytes or thymocytes obtained from wild-type syngeneic mice (16, 17). In the second experimental system, a single immunization of mice that harbor monoclonal T and B cells leads to a hyper IgE response (18). This hyper antibody response against a foreign antigen can be down-modulated by CD4+ T cells from wild-type syngeneic donor mice (18). In both experimental systems, the role of IL-2 in regulatory T cell generation or function was studied by transferring CD4+ T cells from IL-2 KO mice.
We report here that CD4+ T cells from IL-2 KO donor mice efficiently protect mice from EAE (and hyper IgE responses), but CD4+ T cells from CD25 KO mice do not. We conclude that IL-2 signaling is essential for the appropriate function of regulatory T cells; however, regulatory T cells do not suppress EAE through secretion of IL-2. In addition, as splenic CD4+ T cells from IL-2 KO mice protected from EAE, we conclude that generation and selection of T cells with regulatory potential in the thymus does not require IL-2.
Materials and Methods
Mice.
All mice were housed under specific-pathogen-free (SPF) conditions in individually ventilated cages (Thoren) at the Skirball Institute Central Animal Facility, New York University Medical Center. T/α−β− mice have been described (16). In brief, T/α−β− mice are MBP-specific T cell receptor transgenic mice crossed to TCR α KO mice and TCR β KO mice. These H-2u/u mice have been extensively backcrossed into C57Bl/10.PL background, and develop EAE spontaneously (16).
17/9 DO11.10 RAG−/− BALB/c mice have been described (18). In brief, these mice were generated through the cross of 17/9 influenza hemagglutinin (HA)-specific immunoglobulin heavy and light chain knocked-in mice, DO11.10 TCR transgenic mice and RAG-1 KO mice. These H-2d/d mice have been extensively backcrossed into BALB/c background, and display a monoclonal OVA 323–339–specific T cell repertoire and a monoclonal HA-specific B cell repertoire (18).
IL-2 KO mice (19) and CD25 KO mice (3) backcrossed to C57BL/6 background were purchased from The Jackson Laboratory and crossed to C57BL/10.PL mice to incorporate H-2u. IL-2 KO mice (19) backcrossed to BALB/c background were purchased from The Jackson Laboratory.
Disease Evaluation.
EAE was scored as follows: level 1, limp tail; level 2, weak or partial leg paralysis; level 3, total hind leg paralysis; level 4, hind leg paralysis and weak or partial front leg paralysis; level 5, moribund. The weight of the mice was monitored twice a week for the duration of the experiments. All protocols involving mice were approved by New York University's Institutional and Animal Care Use Committee (IACUC).
Immunization for Hyper IgE Response and Serum IgE Determination by ELISA.
These procedures were performed as described (18). Briefly, 17/9 DO11.10 RAG−/− BALB/c mice were immunized with glutaraldehyde-cross-linked OVA-HA antigen. Mice were injected by intraperitoneal route with 100 μg of cross-linked OVA-HA adsorbed onto 1 mg of alum. A single immunization was performed in all experiments. The concentration of IgE in serum was determined by ELISA. Plates were coated with anti-IgE monoclonal antibody LO-ME (Caltag), and bound IgE was detected using biotinylated anti-IgE monoclonal antibody R35–118 (BD Biosciences). Purified anti-TNP IgE/κ monoclonal antibody IgE-3 (BD Biosciences) was used as IgE standard.
Antibodies and FACS® Analysis.
Anti-MBP TCR clonotypic antibody (3H12) was generated in our laboratory as described (16). Anti-OVA TCR (anti DO11.10) clonotypic antibody KJ1–26 was purchased from Caltag Labs. All other antibodies were purchased from BD Biosciences or Caltag.
Single cell suspensions in staining buffer (PBS containing 2% fetal calf serum and 0.1% NaN3), were incubated for 45 min at 4°C with the antibody cocktails. Samples were analyzed in a FACSCalibur™ instrument (Becton Dickinson).
Intracellular staining of IL-2 production was performed according to the protocol recommended by BD Biosciences. Briefly, cells were treated with 10 ng/ml PMA (Sigma-Aldrich) and 200 ng/ml ionomycin (Sigma-Aldrich) for 2 h followed by 10 μg/ml brefeldin-A addition (Sigma-Aldrich) for 2 additional hours. Cells were then surface stained with anti-CD4 and anti-MBP–specific TCR (3H12) for 20 min at 4°C in staining buffer, treated for 20 min with Cytofix/Cytoperm solution (BD Biosciences), washed, and incubated with Perm/wash buffer (BD Biosciences) for 10 min. Cells were washed and incubated in staining buffer for 45 min with anti–IL-2 (BD Biosciences), and analyzed on a FACSCalibur™ (Becton Dickinson).
Cell Purification and Transfer.
Cell purifications were performed by magnetic sorting using a Miltenyi Biotec VarioMACS apparatus. CD4+ single-positive thymocytes were prepared by magnetic depletion using anti-CD8 and anti-B220–coupled magnetic beads (Miltenyi Biotec). CD4+ splenocytes were prepared by positive sorting with anti-CD4–coupled beads using positive sorting columns. Cell purity was checked by FACS® analysis: CD8 depletion was >98% effective, whereas CD4 and B220 depletion were both >99% effective. Positively sorted cells were >95% pure. Cells were resuspended in PBS and injected intravenously at the indicated doses.
Online Supplemental Material.
The online supplemental data contains three figures, Figs. S1, S2, and S3. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20020190/DC1.
Results
Splenic and Thymic CD4+ Cells from IL-2 KO Mice Prevent Spontaneous Encephalomyelitis.
To determine the role of IL-2 in the control of spontaneous EAE by regulatory lymphocytes, we transferred 3 × 105 CD4+ T cells from 3–4-wk-old IL-2 KO mice and IL-2+ littermates into 4-wk-old T/α−β− IL-2+ recipient mice. The number of transferred cells (3 × 105) is among the smallest number of wild-type cells that reproducibly confers full protection against spontaneous EAE (20). We used a low dose of cells because this would facilitate the detection of a partial loss in regulatory T cell function. As shown in Fig. 1, A and B , splenic cells from IL-2 KO mice very effectively prevented spontaneous EAE. Recipient mice showed no weight loss or any signs of lymphoproliferative or wasting disease for as long as they were observed. In contrast, all monoclonal T/α−β− mice that did not receive donor-derived cells developed EAE spontaneously (Fig. 1, A and B).
Figure 1.
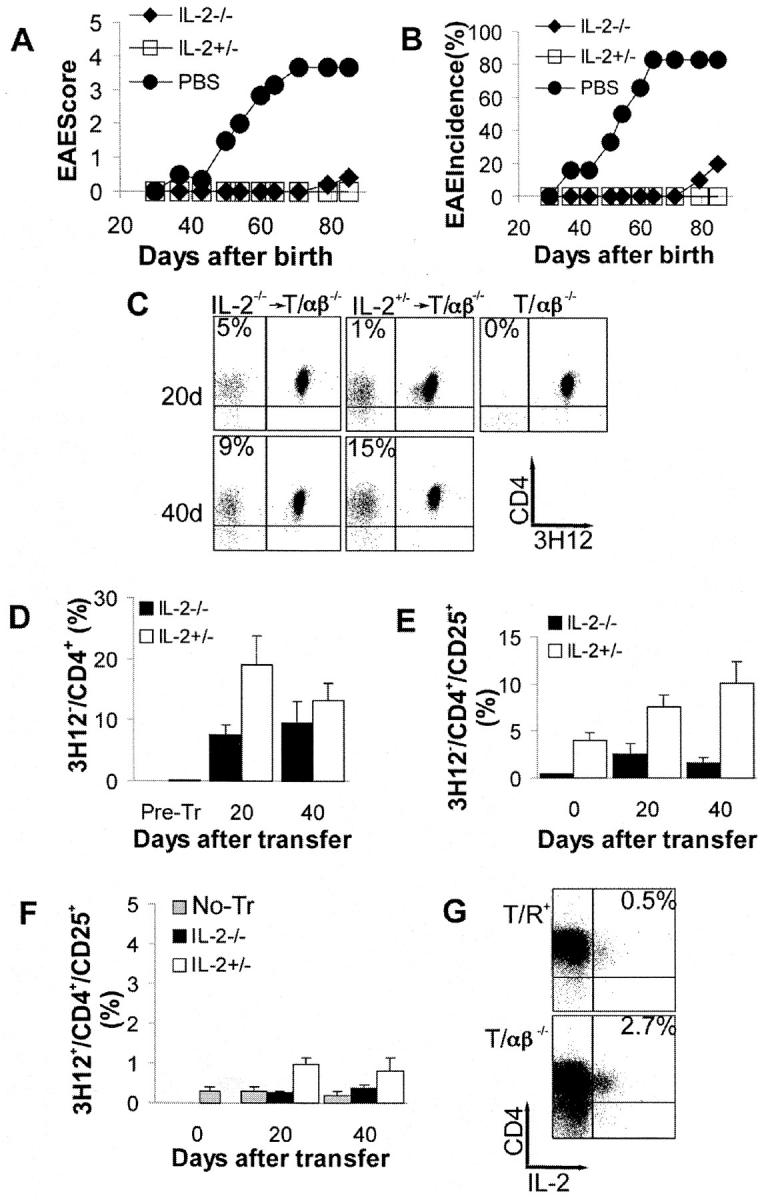
CD4+ splenocytes from IL-2 KO mice efficiently protect against spontaneous EAE. (A–G) 23–27-d-old T/α−β− IL-2+ mice were injected with PBS (n = 6) or with 3 × 105 purified CD4+ T cells from spleens of 3–4-wk-old IL-2−/− (n = 10) or IL-2+/− mice (n = 7). Mice were observed for 90 d and EAE was scored as described in Materials and Methods. (A) Average clinical EAE score. (B) Average EAE incidence. (C–G) Peripheral blood lymphocytes were collected from all recipient mice 20 and 40 d after cell transfer, stained with anti-MBP TCR (3H12) FITC, CD25 PE, CD4 PerCP, and CD3 APC and analyzed by FACS®. (C) Representative staining indicating the identification of donor-derived (3H12−) and recipient-generated (3H12+) T cells. Gated on CD3+ cells. (D) Expansion of donor-derived (3H12−) T cells. Cells were stained and gated as in C. Shown are the mean percentages of clonotype-negative cells from all mice in each group ± SD. (E) Average percentage of CD25 positive T cells (± SD) among the donor-derived CD4+ cells (CD4+3H12− gate). (F) Average percentage of CD25-positive T cells (± SD) among the recipient-generated CD4+ cells (CD4+3H12+ gate). (G) IL-2 production by MBP-specific T cells. Intracellular IL-2 staining of deep cervical lymph node cells from a healthy 7-wk-old T/R+ and a 7-wk-old T/α−β− mouse with early clinical signs of spontaneous EAE. Surface staining with anti-CD4 and 3H12. Gated on 3H12+ cells.
The reconstitution by donor-derived cells was monitored 20 and 40 d after cell transfer by analysis of peripheral blood from the recipient mice. Donor and host-derived T cells can be distinguished with the use of the anti-MBP TCR clonotypic antibody 3H12, which labels a negligible number of T cells from wild-type mice (16; Fig. 1 C). As shown in Fig. 1, C and D, there was no significant difference in the repopulation of donor-derived cells that were of IL-2 KO or IL-2+ origin in T/α−β− IL-2+ recipients. As expected given previous reports (13), the frequency of T cells expressing the IL-2R α chain (CD25) was greatly diminished in IL-2 KO donor mice; however, the expression of CD25 in donor-derived IL-2 KO T cells slightly increased after transfer (Fig. 1 E), likely indicating the paracrine effect of IL-2 produced by the host. Indeed, the production of IL-2 by MBP-specific T cells was confirmed by intracellular staining of CNS-draining deep cervical lymph node cells of T/α−β− and TCR Tg RAG+ (T/R+) mice (Fig. 1 G). A very low frequency (<1%) of circulating MBP-specific cells expressed CD25 at both time points irrespective of the IL-2 genotype of the donor-derived cells; expectedly, the frequency was higher in the presence of IL-2+ donor cells (Fig. 1 F).
To confirm the data obtained with splenocytes, we transferred 3 × 105 CD4+ single-positive thymocytes from IL-2 KO and IL-2+ mice. Similarly to what was observed with splenic T cells, thymic IL-2 KO CD4+ T cells conferred protection from EAE (online supplemental Fig. S1, A and B), and the expansion of donor-derived IL-2 KO cells after injection into IL-2+ T/α−β− mice was not significantly different from the expansion undergone by donor-derived IL-2+ cells (online supplemental Fig. S1 C).
The fact that a limiting number of splenic CD4+ T cells were effective in preventing disease shows that IL-2 is not necessary for the thymic generation and selection of regulatory T cells, and also suggests that IL-2 is not necessary for the maintenance/survival of these cells in the periphery. If the latter were the case, we would have expected good protection from thymic IL-2 KO cells but impaired protection from splenic IL-2 KO cells. In addition, our results demonstrate that, at least in this experimental system, regulatory T cells do not protect from disease by means of IL-2 secretion (e.g., AICD induction), as CD4+ T cells incapable of IL-2 production are nevertheless effective in the prevention of spontaneous EAE. Thus, our results show that CD4+ T cells from IL-2 KO mice, a mouse strain which develops spontaneous autoimmune disease with 100% incidence, when transferred into T/α−β− mice, which also develop spontaneous autoimmune disease with 100% incidence, yield mice that appear to be completely healthy.
Splenic Cells from IL-2 KO Mice Prevent Hyper IgE Response.
Having shown that IL-2 KO CD4+ T cells confer protection against EAE, a spontaneous Th1 cell-mediated disease directed against a self antigen, we next assessed the capacity of IL-2 KO T cells to confer protection against hyper IgE. Hyper IgE is a Th2 cell-mediated condition that can be elicited in mice harboring a monoclonal T and B cell repertoire as a consequence of immunization with a foreign antigen (18). In this experimental system, it has been shown that purified CD4+ T cells from wild-type mice, but not CD8+ T cells or B cells, prevent the hyper IgE response (18).
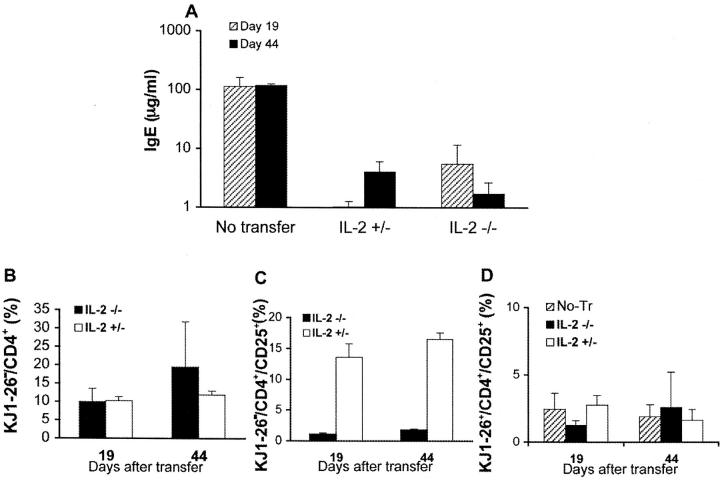
As shown in Fig. 2 A, singly immunized monoclonal T and B mice that received no cell transfer developed persistent serum IgE levels of ∼100 μg/ml. In contrast, immunized animals that had been transferred with 1.6 × 107 total spleen cells from wild-type or IL-2 KO syngeneic donor mice displayed IgE responses that were at least 10-fold reduced (Fig. 2 A). As was the case with the EAE experiment, peripheral expansion of donor-derived IL-2 KO and IL-2+ T cells was comparable (Fig. 2 B), and the frequency of CD25+ among circulating CD4+ donor-derived IL-2 KO cells raised to ∼2% upon transfer in both experimental systems (Figs. 1 F and 2 C). Also similarly to the EAE system, the frequency of CD25+ cells among circulating OVA-specific T cells was low (∼2%) 19 and 44 d after immunization (Fig. 2 D). We have previously shown that CD25 expression on OVA-specific T cells is quickly up-regulated after immunization, and is subsequently downregulated irrespective of the presence of regulatory T cells (18).
Figure 2.
IL-2 KO cells prevent hyper IgE response. 17/9 DO11.10 RAG−/− BALB/c mice were transferred with 1.6 × 107 spleen cells from 3-wk-old IL-2+/− or IL-2−/− BALB/c donor mice. The recipient mice were immunized by intraperitoneal route with OVA-HA in alum 24 h after the transfer of cells. n = 3 recipient mice per group. Data representative of one of two experiments performed (A). Serum IgE levels 19 and 44 d after immunization. Data presented as mean IgE concentration ± SD (B–D). Peripheral blood lymphocytes were stained with KJ1–26 FITC, CD25 PE, and CD4 PerCP and analyzed by FACS®. Data presented as mean percentage ± SD. (B) Expansion of CD4+ donor-derived cells (CD4+KJ1–26−) as a percentage of total lymphocytes (forward scatter × side scatter lymphocyte gate). (C) Average percentage of CD25 positive T cells (± SD) among the donor-derived CD4+ cells (CD4+KJ1–26− gate). (D) Average percentage of CD25 positive T cells (± SD) among the recipient's CD4+ cells (CD4+KJ1–26+ gate).
We have thus shown that IL-2 KO mice harbor peripheral CD4+ cells that can prevent spontaneous EAE and suppress hyper IgE responses.
Splenic and Thymic CD4+ Cells from CD25 KO Mice Fail to Prevent Spontaneous Encephalomyelitis.
In the experimental setups described in Figs. 1 and 2, IL-2 is produced by the host cells and is able to influence the donor-derived IL-2 KO cells. We subsequently wished to establish whether IL-2 signaling is required for T-reg function. To accomplish this goal, we used IL-2R α chain (CD25) KO mice as a source of putative T-reg cells, as CD25 KO cells display a greatly impaired response to IL-2. CD25's only known function is as an essential component of the high affinity IL-2 receptor; thus, the transfer of IL-2R α (CD25) KO CD4+ cells into IL-2R α chain+ T/α−β− mice directly examines the role of IL-2 in the function of regulatory T cells. We therefore transferred 3 × 105 CD4+ T cells from syngeneic IL-2R α chain (CD25) KO mice into CD25+ T/α−β− mice. In stark contrast to IL-2 KO T cells, neither thymic nor splenic T cells from IL-2R α chain KO mice conferred a level of disease protection that was comparable to the one conferred by similar number of T cells from CD25+ littermate donors (Fig. 3, A and B) , although thymic CD4+ cells from CD25 KO mice did provide a partial protection. Failure to confer protection was not due to poor expansion of donor-derived cells in the T/α−β− recipients, which are genetically competent to express CD25. To the contrary, thymic and splenic donor-derived CD25 KO cells expanded to up to 40% of the circulating CD4+ pool (Fig. 3 C), the remaining 60% being host-derived MBP-specific T cells. Failure to confer protection was also not due to improper homing of the donor-derived cells (online supplemental Fig. S2).
Figure 3.
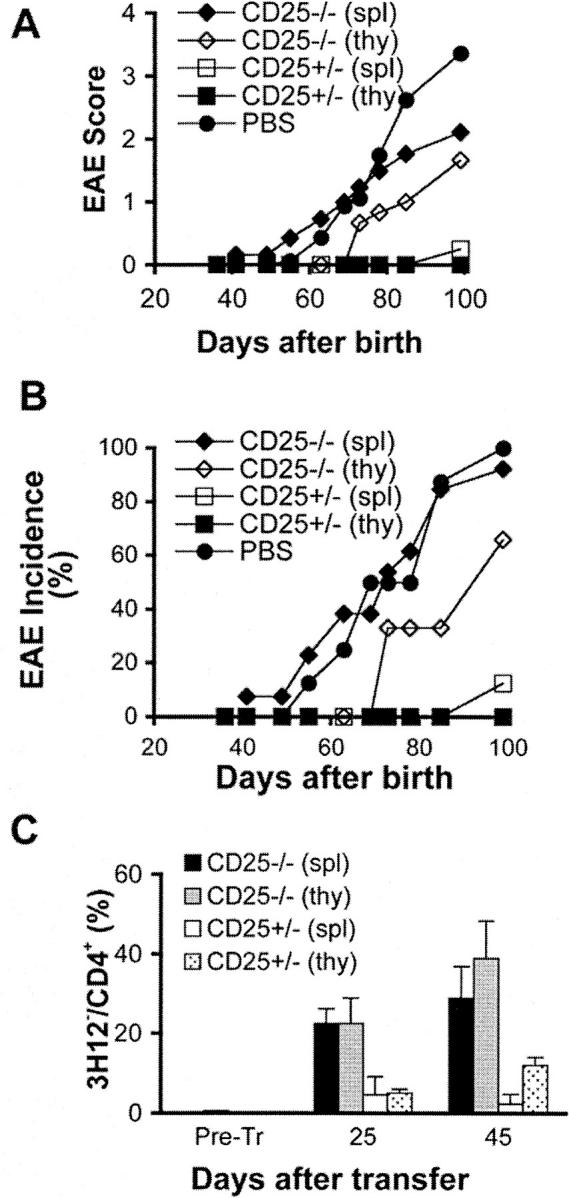
CD25 KO cells do not protect efficiently against spontaneous EAE. 23–27 d-old T/α−β− mice were injected with PBS (n = 8) or with 3 × 105 CD4+ T cells from spleen (spl) of CD25− / − mice (n = 13), spleen of CD25+/− mice (n = 8), thymus (thy) of CD25− / − mice (n = 3), or thymus of CD25+/− mice (n = 3) purified from 3–4-wk-old CD25−/− and CD25+/− donor mice. Mice were observed for 100 d and EAE were scored as described in Materials and Methods. (A) Average clinical EAE score. (B) Average EAE incidence. (C) The expansion of donor-derived (3H12−) CD4+ T cells in each experimental group was assessed by peripheral blood staining 25 and 45 d after cell transfer (CD4+ lymphocyte gate). Data presented as mean percentage ± SD.
MBP-specific TCR Transgenic RAG+ Mice Spontaneously Develop EAE.
To assess the regulatory potential of IL-2R α chain KO T cells under constant thymic output of putative regulatory T cells, we crossed the MBP-specific T cell receptor transgenic RAG+ mice (T/R+), which do not develop EAE spontaneously, with IL-2R α chain KO mice. As shown in Table I, all T/R+ CD25 KO mice developed EAE spontaneously, whereas none of the T/R+ CD25+ littermates did. At the time of severe EAE onset, the recipient animals displayed no signs of wasting disease. As in this experimental system the protection from EAE depends on T cells expressing TCR encoded by the endogenous TCR αβ loci, we used mice of H-2b/u MHC, which harbor a proportion of T cells escaping allelic exclusion about threefold larger than H-2u/u T/R+ mice (Table I and online supplemental Fig. S3). However, even a larger fraction of IL-2R α chain KO T cells that expressed endogenous TCRs was insufficient to confer protection. Thus, regardless of whether the MBP-specific T cells were CD25+ or CD25 KO, CD4+ T cells of CD25 KO origin failed to confer adequate protection against spontaneous EAE.
Table I.
EAE Incidence and Lymphocyte Repertoire in H-2b/u T/R+ CD25 KO and CD25+/− Littermates
| Mouse genotype |
CD4+/Vβ8.1.2+ | CD4+/Vβ8.1.2− | Mice with EAE/mice observed |
|---|---|---|---|
| % | % | ||
| T/R+CD25−/− | 13.3 ± 3.7 | 1.1 ± 0.6 | 4/4 |
| T/R+CD25+/− | 16.5 ± 4.2 | 1.0 ± 0.6 | 0/10 |
Peripheral blood was obtained from mice of the indicated genotype at age 4 wk, and stained with anti-CD4, anti-B220, and anti-Vβ8.1/8.2 (stains the transgenic chain) antibodies. n = 7 for CD25 knockout mice and n = 10 for CD25+ mice. Samples of the staining are shown in online supplemental Fig. S3. Four T/R+ CD25 KO mice and all the CD25+ littermates were monitored for EAE development for 4 mo.
Taken together with all prior observations on IL-2 and CD25 KO mice, our results support the notion that the immunostimulatory role of IL-2 on effector T cells can be exerted by other cytokines, but IL-2 signaling is necessary for regulatory T cell function.
Discussion
We have shown that CD4+ T cells from IL-2 KO mice were able to protect from spontaneous EAE. In the experimental systems that we used, the T cells from the recipient mice were capable of producing IL-2; however, IL-2 itself is not protective, as spontaneous EAE developed in untransferred IL-2+ T/α−β− mice. T/α−β− mice develop spontaneous EAE because they cannot generate the TCR specificity of regulatory T cells (15, 16). Our present data indicate that IL-2 KO mice select T cells displaying the appropriate T-reg cell repertoire, as T cells with regulatory potential can be found in the spleen of donor IL-2 KO mice. The crucial role of IL-2 is evident by the failure of IL-2R α chain KO T cells to confer protection to CD25+ T/α−β− mice, and by the development of spontaneous disease in T/R+ CD25 KO mice. Although it is formally possible that CD25 has additional roles that are IL-2 independent, this possibility seems highly unlikely given the extensive research in the field and the overwhelming similarities between IL-2 KO and CD25 KO mice.
In prior work we showed that full protection against spontaneous EAE could be achieved by CD4+CD25− cells prepared from wild-type donor mice (20, 21). In this report, we showed that CD4+ T cells from IL-2 KO mice, which, like the sorted CD25− cells, are largely CD25-negative but genetically CD25+, also protect very effectively against spontaneous EAE. In contrast, T cells genetically deficient in CD25 protect poorly against spontaneous EAE. It has been known for many years that IL-2 induces the up-regulation of the IL-2 receptor (22, 23). Thus, it is very likely that CD25-negative cells from IL-2 KO mice respond to IL-2 (in these experimental scenarios produced only by the recipients' cells) via the intermediate affinity IL-2R βγ, and upregulate the expression of CD25 to form the high affinity IL-2R αβγ. High affinity response to IL-2 then effects the regulatory cells in one of three nonexclusive ways: (a) induces T-reg precursors to become regulatory in the periphery, (b) induces T-regs to expand and reach numbers that can be effective in the control of autoimmunity or allergy, and/or (c) triggers their regulatory functions, which could be mediated via the secretion of down-modulatory cytokines such as IL-10 and/or TGF-β (Fig. 4) .
Figure 4.
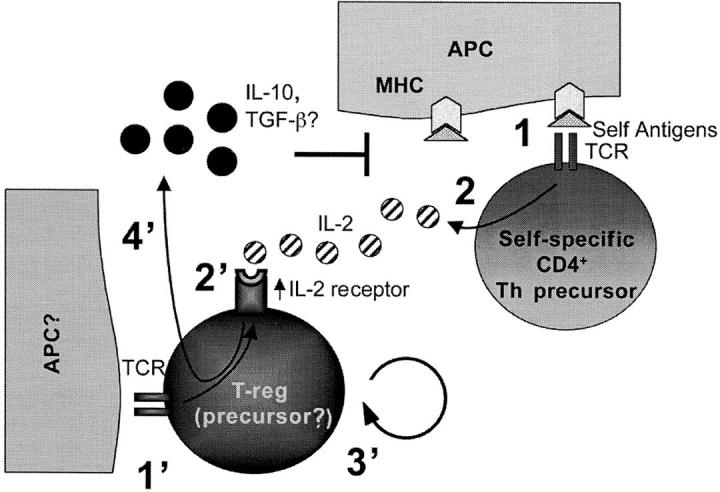
Model depicting the possible effect of IL-2 on regulatory T cells based on the spontaneous EAE data. (reference 1). Self-specific Th precursor cells encounter self antigen and (reference 2) secrete IL-2. Although costimulatory molecules are not drawn in the model due to lack of space, in the spontaneous EAE system the generation of MBP-specific Th effector cells is CD28-dependent (reference 34). (1') Regulatory T cells or their precursors interact via TCR with yet unknown ligands, and (2') up-regulate the IL-2 receptor. (3') IL-2 promotes regulatory T cell differentiation or expansion in the periphery, and/or (4') triggers regulatory functions such as secretion of down-modulatory cytokines. In this scheme, the source of IL-2 is the self-specific T cell population, although other activated host cell types could also produce IL-2 (reference 35).
We have described that CD4+ T cells from IL-10 KO mice are less effective in preventing spontaneous EAE than CD4+ T cells from IL-10+ mice (20), although the IL-10 dependence of regulatory T cells is much less noticeable in the spontaneous EAE model than in IBD models (24, 25).
We have previously shown that, in vivo, T-reg cells prevent the generation of fully differentiated effector Th cells but do not appreciably block the initial activation and expansion of Th precursor cells (18). We showed here that the initial activation of the recipient's cells may be necessary to produce IL-2, which is key to the function of regulatory T cells. One question arises as to how immune responses could occur if the initial activation of Th precursor cells sets in motion the T-reg–mediated downregulation of the responses. Although we do not know the answer to this question, it is possible that strong stimuli mediated by innate immunity signals overwhelm the T-reg loop leading to Th-mediated immune responses, whereas responses to weak stimuli are thwarted. For instance, MBP-specific T/R+ mice, which do not develop EAE spontaneously due to the presence of T-reg cells, succumb to a fulminant disease when immunized with MBP in the presence of complete Freund's adjuvant and pertussis toxin (15). We have also shown that regulatory T cells expand less than conventional T cells when placed together in TCR transgenic/RAG KO recipient mice (20), providing one possible explanation for the dominance of Th immune response over downregulation when stimuli are strong. In the absence of regulatory T cells, however, even weak stimuli lead to Th-mediated responses.
A population of CD4+CD25+ T cells protects against a number of experimental autoimmune conditions (25–28). In vitro studies have shown that CD4+CD25+ T cells block the proliferation and reduce IL-2 mRNA levels of CD4+CD25− T cells (14, 29). CD4+CD25+ T cells proliferate in vitro in the presence of IL-2 and, during the expansion period, temporarily lose their capacity to block the proliferation of CD4+CD25− cells. The significance of in vitro proliferation data to in vivo regulation of autoimmune or allergic conditions remains to be clarified. First, in vitro, CD25+ T cells block proliferation only at CD25+/CD25− ratios that exceed those found in vivo. Second, we have recently shown that regulatory T cell–mediated protection against a hyper IgE response in vivo occurred without inhibition of early activation and without blockade of the initial proliferation of antigen-specific T cells (18). Third, whereas the control of in vitro proliferation by CD25+ T cells appears to be cytokine independent, in vivo protection from IBD by CD25+ T cells has been shown to be cytokine dependent (24, 25).
The immunostimulant properties of IL-2 are the basis for its use in cancer clinical trials. Thus far, however, the response rates have been modest in some studies and extremely low in other studies (30–32). Of interest, in a 14 patient phase II study to evaluate IL-2 treatment in nasopharyngeal carcinoma, Chi et al. found that IL-2 was clinically ineffective, and that, among other parameters, the treatment led to increased seric levels of IL-10 (33). In light of the data presented in this manuscript, it is possible that one of the reasons for the below expectation immunostimulatory responses to IL-2 in vivo could lay on its role in regulatory T cell function.
In conclusion, IL-2 stimulation may be an important physiological stimulus for triggering regulatory T cell function, with potential to enhance the activity of regulatory T cells in a variety of autoimmune and allergic conditions.
Acknowledgments
We wish to dedicate this paper to the memory of Dr. G. Jeanette Thorbecke. We thank Allen K. Wensky for critically reading the manuscript.
Gláucia C. Furtado was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and is supported by the National Multiple Sclerosis Society. Work in J.J. Lafaille lab is supported by the National Institutes of Health, the National Multiple Sclerosis Society, the Hirschl-Caulier Trust, and the Charles A. Dana Foundation.
The online version of this article contains supplemental material.
References
- 1.Sadlack, B., H. Merz, H. Schorle, A. Schimpl, A.C. Feller, and I. Horak. 1993. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 75:253–261. [DOI] [PubMed] [Google Scholar]
- 2.Sadlack, B., J. Lohler, H. Schorle, G. Klebb, H. Haber, E. Sickel, R.J. Noelle, and I. Horak. 1995. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25:3053–3059. [DOI] [PubMed] [Google Scholar]
- 3.Willerford, D.M., J. Chen, J.A. Ferry, L. Davidson, A. Ma, and F.W. Alt. 1995. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 3:521–530. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki, H., T.M. Kundig, C. Furlonger, A. Wakeham, E. Timms, T. Matsuyama, R. Schmits, J.J. Simard, P.S. Ohashi, H. Griesser, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 268:1472–1476. [DOI] [PubMed] [Google Scholar]
- 5.Lenardo, M.J. 1991. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 353:858–861. [DOI] [PubMed] [Google Scholar]
- 6.Kneitz, B., T. Herrmann, S. Yonehara, and A. Schimpl. 1995. Normal clonal expansion but impaired Fas-mediated cell death and anergy induction in interleukin-2-deficient mice. Eur. J. Immunol. 25:2572–2577. [DOI] [PubMed] [Google Scholar]
- 7.Wang, R., A.M. Rogers, B.J. Rush, and J.H. Russell. 1996. Induction of sensitivity to activation-induced death in primary CD4+ cells: a role for interleukin-2 in the negative regulation of responses by mature CD4+ T cells. Eur. J. Immunol. 26:2263–2270. [DOI] [PubMed] [Google Scholar]
- 8.Refaeli, Y., L. Van Parijs, C.A. London, J. Tschopp, and A.K. Abbas. 1998. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 8:615–623. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki, H., Y.W. Zhou, M. Kato, T.W. Mak, and I. Nakashima. 1999. Normal regulatory alpha/beta T cells effectively eliminate abnormally activated T cells lacking the interleukin 2 receptor beta in vivo. J. Exp. Med. 190:1561–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klebb, G., I.B. Autenrieth, H. Haber, E. Gillert, B. Sadlack, K.A. Smith, and I. Horak. 1996. Interleukin-2 is indispensable for development of immunological self- tolerance. Clin. Immunol. Immunopathol. 81:282–286. [DOI] [PubMed] [Google Scholar]
- 11.Malek, T.R., B.O. Porter, E.K. Codias, P. Scibelli, and A. Yu. 2000. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J. Immunol. 164:2905–2914. [DOI] [PubMed] [Google Scholar]
- 12.Wolf, M., A. Schimpl, and T. Hunig. 2001. Control of T cell hyperactivation in IL-2-deficient mice by CD4(+)CD25(-) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur. J. Immunol. 31:1637–1645. [DOI] [PubMed] [Google Scholar]
- 13.Papiernik, M., M.L. de Moraes, C. Pontoux, F. Vasseur, and C. Penit. 1998. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int. Immunol. 10:371–378. [DOI] [PubMed] [Google Scholar]
- 14.Thornton, A.M., and E.M. Shevach. 1998. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafaille, J.J., K. Nagashima, M. Katsuki, and S. Tonegawa. 1994. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 78:399–408. [DOI] [PubMed] [Google Scholar]
- 16.Olivares-Villagomez, D., Y. Wang, and J.J. Lafaille. 1998. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188:1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van de Keere, F., and S. Tonegawa. 1998. CD4+ T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti-myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 188:1875–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curotto de Lafaille, M.A., S. Muriglan, M.J. Sunshine, Y. Lei, N. Kutchukhidze, G.C. Furtado, A.K. Wensky, D. Olivares-Villagomez, and J.J. Lafaille. 2001. Hyper immunoglobulin E response in mice with monoclonal populations of B and T lymphocytes. J. Exp. Med. 194:1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schorle, H., T. Holtschke, T. Hunig, A. Schimpl, and I. Horak. 1991. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 352:621–624. [DOI] [PubMed] [Google Scholar]
- 20.Furtado, G.C., D. Olivares-Villagomez, M.A. Curotto De Lafaille, A.K. Wensky, J.A. Latkowski, and J.J. Lafaille. 2001. Regulatory T cells in spontaneous autoimmune encephalomyelitis. Immunol. Rev. 182:122–134. [DOI] [PubMed] [Google Scholar]
- 21.Olivares-Villagomez, D., A.K. Wensky, Y. Wang, and J.J. Lafaille. 2000. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J. Immunol. 164:5499–5507. [DOI] [PubMed] [Google Scholar]
- 22.Smith, K.A., and D.A. Cantrell. 1985. Interleukin 2 regulates its own receptors. Proc. Natl. Acad. Sci. USA. 82:864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bismuth, G., J.L. Moreau, G. Somme, M. Duphot, A. Dautry-Varsat, R.J. Robb, and J. Theze. 1985. Regulation of interleukin 2 (IL2) receptor expression: IL2 as an inducing signal for the expression of its own receptor on a murine T helper cell line. Eur. J. Immunol. 15:723–727. [DOI] [PubMed] [Google Scholar]
- 24.Asseman, C., S. Mauze, M.W. Leach, R.L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190:995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annacker, O., R. Pimenta-Araujo, O. Burlen-Defranoux, T.C. Barbosa, A. Cumano, and A. Bandeira. 2001. CD25(+) CD4(+) T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J. Immunol. 166:3008–3018. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151–1164. [PubMed] [Google Scholar]
- 27.Sakaguchi, S. 2000. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 101:455–458. [DOI] [PubMed] [Google Scholar]
- 28.Shevach, E.M. 2000. Regulatory T cells in autoimmmunity. Annu. Rev. Immunol. 18:423–449. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, T., Y. Kuniyasu, M. Toda, N. Sakaguchi, M. Itoh, M. Iwata, J. Shimizu, and S. Sakaguchi. 1998. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10:1969–1980. [DOI] [PubMed] [Google Scholar]
- 30.Atkins, M.B., M.T. Lotze, J.P. Dutcher, R.I. Fisher, G. Weiss, K. Margolin, J. Abrams, M. Sznol, D. Parkinson, M. Hawkins, et al. 1999. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17:2105–2116. [DOI] [PubMed] [Google Scholar]
- 31.Bukowski, R.M. 1997. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer. 80:1198–1220. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani, G., V. Gebbia, M. Airoldi, C. Bumma, P. Contu, A. Bianchi, M. Ghiani, D. Dessi, E. Massa, L. Curreli, et al. 1998. Neo-adjuvant chemo-(immuno-)therapy of advanced squamous-cell head and neck carcinoma: a multicenter, phase III, randomized study comparing cisplatin + 5-fluorouracil (5-FU) with cisplatin + 5-FU + recombinant interleukin 2. Cancer Immunol. Immunother. 47:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi, K.H., J.N. Myers, K.C. Chow, W.K. Chan, Y.W. Tsang, Y. Chao, S.H. Yen, and M.T. Lotze. 2001. Phase II trial of systemic recombinant interleukin-2 in the treatment of refractory nasopharyngeal carcinoma. Oncology. 60:110–115. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira-dos-Santos, A.J., A. Ho, Y. Tada, J.J. Lafaille, S. Tonegawa, T.W. Mak, and J.M. Penninger. 1999. CD28 costimulation is crucial for the development of spontaneous autoimmune encephalomyelitis. J. Immunol. 162:4490–4495. [PubMed] [Google Scholar]
- 35.Granucci, F., C. Vizzardelli, N. Pavelka, S. Feau, M. Persico, E. Virzi, M. Rescigno, G. Moro, and P. Ricciardi-Castagnoli. 2001. Inducible IL-2 production by dendritic cells revealed by global gene expression analysis. Nat. Immunol. 2:882–888. [DOI] [PubMed] [Google Scholar]