Abstract
Regulation by transforming growth factor (TGF)-β plays an important role in immune homeostasis. TGF-β inhibits T cell functions by blocking both proliferation and differentiation. Here we show that TGF-β blocks Th1 differentiation by inhibiting the expression of T-bet, the apparent masterregulator of T helper (Th)1 differentiation. Restoration of T-bet expression through retroviral transduction of T-bet into developing Th1 cells abrogated the inhibitory effect of TGF-β. In addition, we show that, contrary to prior suggestions, downregulation of interleukin 12 receptor β2 chain is not key to the TGF-β–mediated effect. Furthermore, we show that the direct inhibitory effect of TGF-β on T cells is responsible, at least in part, for the inability of BALB/c mice to mount a Leishmania-specific Th1 response and to clear Leishmanial infection.
Keywords: T cell, differentiation, TGF-β, T-bet, Leishmania
Introduction
TGF-β is a pleitropic cytokine which performs multiple regulatory functions in the immune system (1). One such role of TGF-β in regulating immune homeostasis is the inhibition of naive T cell differentiation into effector cells. Specifically, addition of TGF-β to T cell cultures blocks T cell differentiation into either the Th1 or Th2 subset (2). This inhibition of T cell differentiation is separable from the antiproliferative action of TGF-β as it can be manifested in the presence of exogenous IL-2, which overcomes the antiproliferative action of TGF-β.
Although the phenomenon of TGF-β blockade of T cell differentiation has been widely addressed, the mechanisms through which TGF-β leads to such inhibition have only recently begun to be examined. Thus, we and others have recently demonstrated that TGF-β achieves the inhibition of T cell differentiation into Th2 effector cells through the inhibition of the expression of GATA-3 (3, 4), a transcription factor crucial for Th2 development (5, 6). Although a key mechanism of TGF-β inhibition of Th2 differentiation has been revealed by those studies, the mechanisms of inhibition of Th1 differentiation remained unclear. In this paper we focus on the mechanism of TGF-β induced inhibition of Th1 differentiation. It has been previously demonstrated that TGF-β inhibits the expression of IL-12Rβ2 in differentiating T cells (7).
Materials and Methods
Mice.
CD4-dnTGF-βRII mice were generated as described previously (8) and were backcrossed onto BALB/c genetic background. After the n4 generation, mice homozygous for BALB/c loci reported to be important for BALB/c susceptibility to Leishmania and IL-4 bias (9, 10), were selected for further breeding. Selection was based on D11MIT2, D11MIT2, D7MIT101, D16MIT34, and D5MIT145 markers. The transgene positive and negative progeny of these mice was used for the experiments. C57BL/6 and BALB/c mice were purchased from the National Cancer Institute.
Parasites and Infection Protocol.
Mice were infected in the right hind foot with 2 × 106 stationary phase Leishmania major promastigotes of the WR309 substrain and monitored as described previously (11).
In Vitro Restimulation of Lymph Node Cells for Cytokine Production.
CD4+ T cells were isolated 8 wk after infection from pooled popliteal and inguinal lymph nodes by negative selection using mAbs to CD8, MHC class II, B220 and FcR, followed by incubation with anti–mouse and anti–rat Ig-coated magnetic beads (PerSeptive Biosystems). T cell–depleted splenocytes obtained from uninfected C57BL/6 or BALB/c mice were used as a source of APCs. CD4+ T cells from the various infected animals groups were cultures in 96-well plates at 2 × 105 cells per well together with 2 × 105 APCs in the presence of various concentrations of parasite lysate (prepared by sonication of L. major promastigotes). After 4 d of culture, supernatants were collected for cytokine analysis.
Assaying for Cytokines in Culture Supernatants.
Supernatants were assayed for the presence of cytokines using kits purchased from Endogen for IFN-γ, IL-4, and IL-5, and from R&D Systems for IL-13.
Retroviral Constructs and Retroviral Transduction.
GFP-RV, IL-12Rβ2-RV, and T-bet-RV retroviral vectors were described previously (12, 13). Phoenix-Eco packaging cell line (gift of G. Nolan, Stanford University, Stanford, CA) was transfected according to Dr. Nolan's protocol. CD4+ T cells from BALB/c were transduced using the above retroviral constructs as described previously (3).
Western Blot Analysis.
Total T cell lysates were prepared as described previously (8), resolved by 10% SDS-PAGE, transferred to a PVDF membrane (Millipore), probed with anti–T-bet rabbit antisera (gift of L. Glimcher, Harvard University, Boston, MA), and developed using the ECL system (Pierce Chemical Co.). Membranes was subsequently stripped of antibodies with 0.1 M Glycine-HCl for 30 min at room temperature, and reprobed with anti–β-tubulin specific mAb (Sigma-Aldrich).
FACS® Analyses.
Intracellular cytokine staining was performed as described previously (8). 20,000 events were collected, and after gating on GFP+ or GFP− cells, intracellular cytokine staining was analyzed. Gates for cytokine staining were set using isotype matched control antibody staining. Gates for GFP (FL1) positive cells were determined using nontransduced controls.
RNA Preparation and Semiquantitative RT-PCR.
RNA and cDNA were prepared as described previously (3). Subsequently, each sample was subjected to PCR with sense and antisense primers for β-actin and T-bet. Primers for β-actin were described previously (3) and for T-bet are: 5′ TTC CCA TTC CTG TCC TTC ACC G (sense) and 5′ GGA AGG TCG GGG TAA AAA C (antisense). To amplify mRNA specifically, and not contaminating genomic DNA, primers for both β-actin and T-bet were designed to span an intron. PCR were performed at different numbers of cell cycles to ensure that comparison of PCR products for various samples is performed in the linear part of an amplification curve.
Results
TGF-β Blockade of Th1 Differentiation Is Independent of Inhibition of IL-12 Receptor β2 Chain.
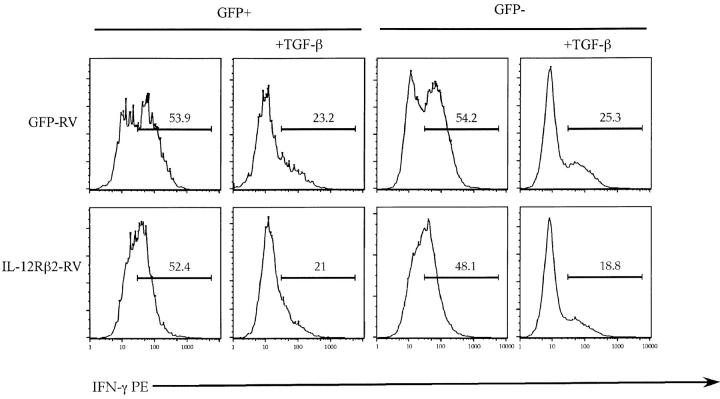
Since IL-12 and IL-12 induced signal transducer and activator of transcription (STAT)-4 activation has been shown to be crucial for Th1 differentiation (14), it was suggested that TGF-β inhibits Th1 differentiation through the inhibition of IL-12 receptor expression. To test this hypothesis we used a retroviral vector in order to introduce exogenous IL-12Rβ2 chain into differentiating Th1 cells and subjected these cells to TGF-β treatment. We reasoned that if TGF-β inhibited the development of Th1 cells through the inhibition of IL-12 receptor expression then the cells transduced with the retrovirus, and hence expressing exogenous IL-12Rβ2 chain, would be resistant to TGF-β inhibition. Infection of T cells with this retroviral vector enabled IL-12 signaling in Th2 cells (12), which do not normally express this chain of IL-12 receptor and thus do not respond to IL-12 signaling. The retroviral construct contains a bicistronic element carrying the GFP gene, thereby distinguishing IL-12Rβ2–expressing T cells on the basis of green fluorescence. As control retroviral vector, we used an “empty” vector containing only the GFP gene. As can be seen from Fig. 1 , in agreement with previously reported data addition of TGF-β to the cultures of developing Th1 cells inhibited the percentage of IFN-γ1 cells. Viral transduction of IL-12 receptor β2 chain did not, however, provide any protective benefit against inhibitory effects of TGF-β to the developing Th1 cells (Fig. 1). TGF-β inhibited Th1 differentiation in cells expressing retrovirally transduced IL-12Rβ2 chain to the same degree as in cells transduced with the control vector (52 ± 16% inhibition by TGF-β for IL-12Rβ2-RV transduced cells vs. 56 ± 3% in GFP-RV transduced cells; n = 3 experiments).
Figure 1.
Ectopic expression of IL-12Rβ2 does not reverse the inhibitory effect of TGF-β on number of differentiating Th1 cells. Primary CD4+ T cells were stimulated, infected with GFP-RV (top) or IL-12Rβ2-RV (bottom), and cultured under Th1 conditions in the presence or absence of rhuTGF-β1 (3 ng/ml). On day 5 after primary activation T cells were stimulated with PMA/ionomycin for 5 h. Cells were analyzed by flow cytometry for IFN-γ expression in either infected GFP+ or uninfected GFP− T cells. Results of one representative experiment out of three experiments done are shown.
TGF-β Inhibits T-bet Expression during Th1 Development.
The above results indicate that although TGF-β inhibits IL-12Rβ2 chain expression, this inhibition is likely secondary to the inhibitory effect of TGF-β on Th1 cell development, since the introduction of exogenous IL-12Rβ2 molecule did not restore Th1 differentiation inhibited by TGF-β. Therefore, as a next step we investigated whether TGF-β inhibits T-bet, a factor which expression seems to be limiting for Th1 development (13), and the one that is preceding IL-12Rβ2 chain expression (15). T-bet expression is also independent of IL-12 and STAT-4 signaling (15).
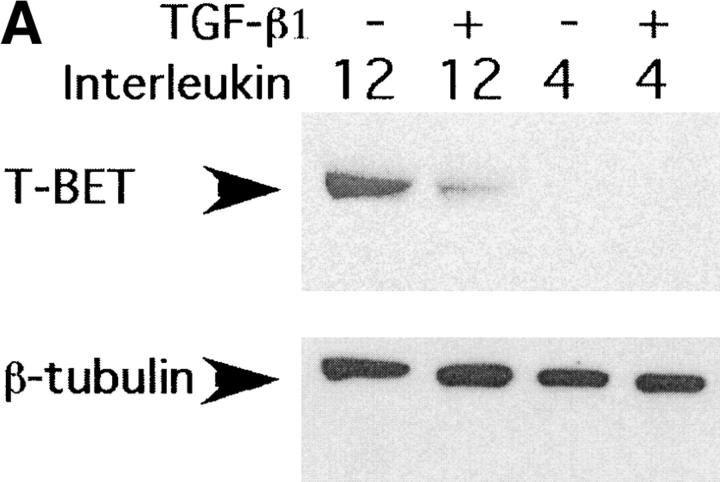
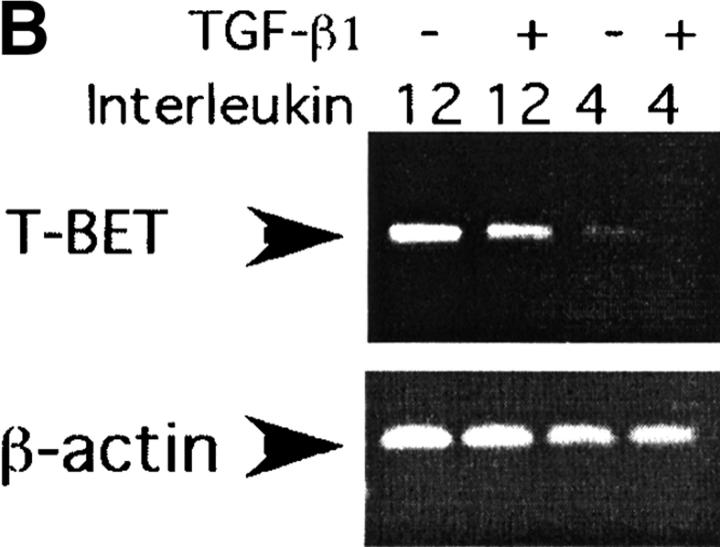
As can be seen from the results of Western blot analysis (Fig. 2 A) TGF-β inhibited T-bet expression in Th1 cultures 20 h after T cell activation. To test whether TGF-β inhibits T-bet expression at the transcriptional or translational level we investigated T-bet mRNA expression after TGF-β treatment by RT-PCR analysis. As can be seen from Fig. 2 B, activation of T cells under Th1 culture conditions induced expression of T-bet mRNA and TGF-β inhibited T-bet induction under those conditions. Thus, TGF-β inhibits expression of a critical Th1 differentiating factor, at the mRNA and protein levels.
Figure 2.
TGF-β inhibits T-bet expression in activated primary T cells. (A) CD4+ T cells were stimulated with anti-CD3/CD28 mAbs (5 μg/ml each) in the presence or absence of rmIL-4 (1,000 U/ml), IL-12 (3.5 ng/ml), and/or rhuTGF-β1 (3 ng/ml). After 20 h of culture, total cell lysates were normalized for protein level and resolved by 10% SDS-PAGE followed by Western immunoblotting with an anti–T-bet–specific rabbit antiserum. Results of one representative experiment out of three experiments done are shown. (B) CD4+ T cells were stimulated like in A, after 20 h of culture, total cell RNA was prepared, reverse transcribed into cDNA, and amplified using β-actin and T-bet specific primers. Experiment was conducted twice and results of a representative experiment are shown.
Reversal of TGF-β Inhibition on Th1 Differentiation by Ectopic Expression of T-bet in T Cells.
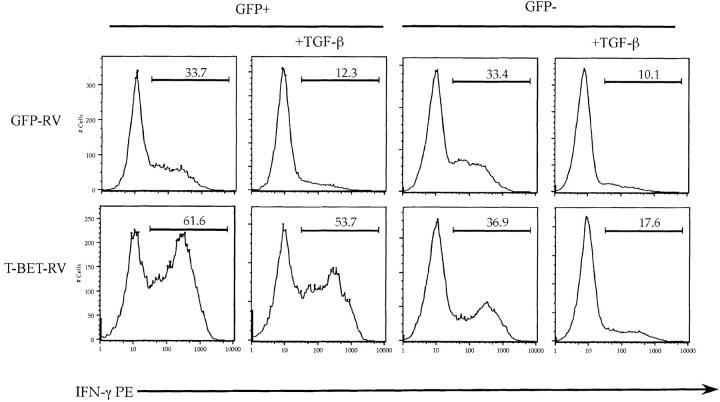
To test whether inhibition of T-bet expression by TGF-β constitutes the mechanism whereby TGF-β inhibits Th1 differentiation we took advantage of the retroviral system to introduce exogenous T-bet into differentiating CD4 T cells (13). Introduction of T-bet into differentiating Th1 cells increased the percentage of IFN-γ1 cells in Th1 differentiation cultures (Fig. 3) . In accordance with the previous data, cells that were transduced with the control vector or cells that were not transduced with T-bet (GFP− cells from T-bet-RV group) were inhibited by TGF-β. Remarkably, exogenous expression of T-bet rendered differentiating Th1 cells completely resistant to TGF-β inhibition (Fig. 3). The results of this experiment demonstrated that inhibition of T-bet by TGF-β explains the inhibition of Th1 differentiation by TGF-β, since the restoration of T-bet expression alone through retroviral transduction completely reversed this inhibition (4 ± 7% inhibition by TGF-β for T-bet-RV transduced cells vs. 56 ± 3% in GFP-RV transduced cells; n = 3 experiments).
Figure 3.
Ectopic expression of T-bet abrogates the inhibitory effect of TGF-β on number of differentiating Th1 cells. Primary CD4+ T cells were stimulated, infected with GFP-RV (top) or T-bet-RV (bottom), and cultured under Th1 conditions in the presence or absence of rhuTGF-β1 (3 ng/ml). On day 5 after primary activation, T cells were stimulated with PMA/ionomycin for 5 h. Results of one representative experiment out of three experiments done are shown.
Direct Effect of TGF-β on T Cells Is Responsible for the Failure of BALB/c Mice to Develop a Th1 Response to L. Major.
We considered that the inhibition of Th1 development by TGF-β might play an important physiological role in many pathophysiological immune responses. Thus, we investigated the well-known inability of BALB/c mice to mount a protective Th1 response to the intracellular parasite L. major (16). Several mechanisms have been proposed to explain this defect. One popular hypothesis is based on observations of the decreased expression of IL-12Rβ2 chain on T cells from infected BALB/c mice (17, 18) and the ability of IL-4 to inhibit the expression of the β2 chain of the IL-12 receptor on T cells of BALB/c origin (19). This hypothesis proposes that defective Th1 response in these mice results from the decreased expression of IL-12 receptor and therefore signaling by IL-12. However, recent experiments demonstrated that BALB/c mice expressing a transgenic IL-12Rβ2 chain on T cells were as susceptible to L. major and equally incapable of mounting a Th1 response to the parasite as nontransgenic BALB/c mice (20).
These experiments demonstrated that Leishmania did not inhibit Th1 differentiation through the inhibition of IL-12 signaling in T cells. Since we demonstrated that TGF-β did not inhibit Th1 differentiation through the inhibition of IL-12 signaling in T cells we tested whether TGF-β is involved in the inhibition of the anti-Leishmanial Th1 response. It has been shown previously that infection with Leishmania leads to increased TGF-β production, and that TGF-β contributes to the direct inhibition of macrophage anti-Leishmanial function (21, 22). To test our hypothesis that L. major inhibits the generation of the protective anti-Leishmania Th1 response through TGF-β–mediated direct inhibition of Th1 differentiation, we challenged BALB/c mice expressing a transgenic dominant-negative TGF-β receptor type II in T cells with L. major.
We have previously described the generation of transgenic mice expressing dominant negative TGF-β receptor II (CD4-dnTGFβRII) exclusively on T cells by way of a T cell–specific promoter (8). In these mice TGF-β signaling is blocked in T cells, but not other cells. For the L. major study, we backcrossed these mice (originally maintained on the C57BL/6 background) to BALB/c mice. At the n4 generation we selected and used for experiments mice homozygous for the BALB/c alleles at the loci demonstrated to be important for Leishmania susceptibility as well as for IL-4 skewing (9, 10) (data not shown).
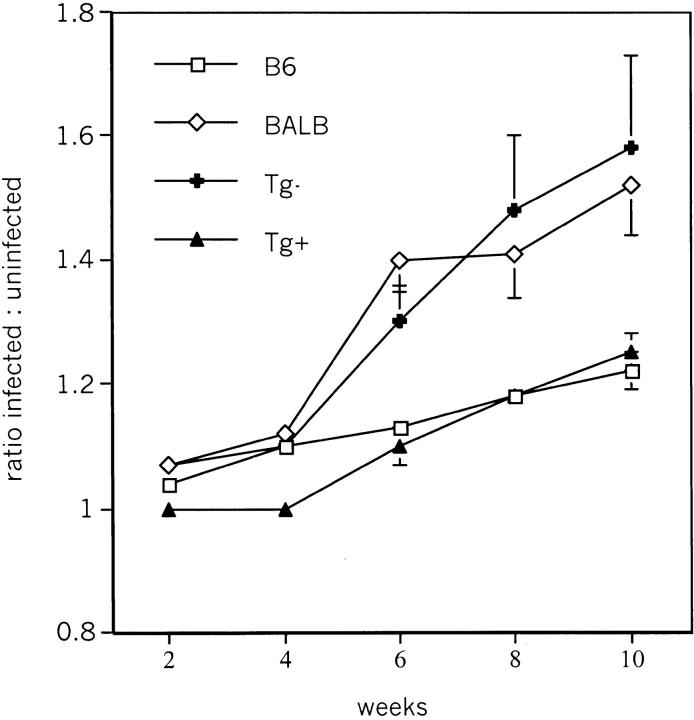
Footpad infection of BALB/c mice and CD4-dnTGF-βRII transgene negative littermates resulted in progressive proliferation of the parasites leading to footpad swelling (Fig. 4) , and eventually to large nonhealing lesions. Strikingly, CD4-dnTGF-βRII transgene positive BALB/c mice arrested Leishmania infection as effectively as genetically resistant C57BL/6 mice. Thus, susceptibility to Leishmania infection requires TGF-β signaling in T cells.
Figure 4.
CD4-dnTGF-βRII BALB/c mice are resistant to L. major infection. Mice were infected in the right foot with 2 × 106 stationary phase L. major promastigotes. The mean footpad thickness of infected and uninfected feet was determined for each group of mice, and the ratio of these means calculated and plotted. The plot shows data from four combined experiments, with total of 10 mice for each group.
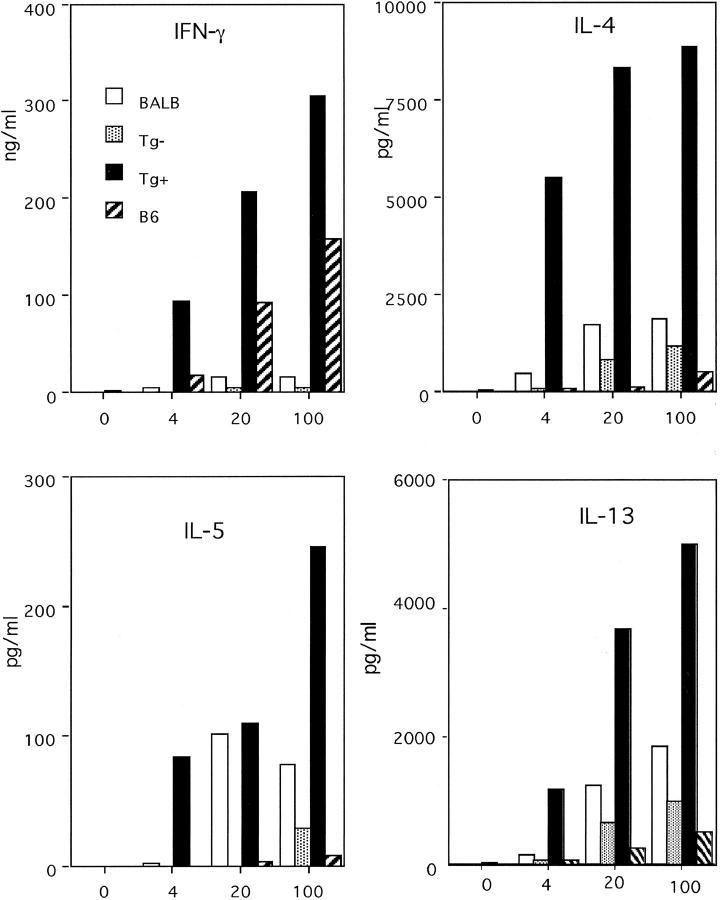
Since TGF-β inhibits Th1 differentiation and the susceptibility to Leishmania infection is associated with the lack of Th1 response we tested the ability of T cells from CD4−dnTGF-βRII BALB/c mice to mount a Leishmania-specific Th1 response. As shown in Fig. 5 , infection of transgene positive BALB/c mice resulted in a potent Leishmania antigen-specific Th1 response. In fact, the levels of IFN-γ produced by CD4+ T cells from CD4−dnTGF-βRII Tg+ mice in response to total Leishmania antigen were even higher than the levels made by the genetically resistant C57BL/6 strain. Transgene negative littermates displayed the strong Th2 response and lack of IFN-γ secretion typical to the BALB/c strain. Strikingly, Tg+ BALB/c mice also exhibited a marked Th2 response despite the development of a very strong Th1 response.
Figure 5.
L. major–infected CD4-dnTGF-βRII BALB/c mice produce Th1-type cytokines. CD4+ T cells isolated from lymph nodes of mice infected with L. major promastigotes 8 wk earlier and were set up in the presence of different concentrations of L. major lysate and 4 d later culture supernatant was collected for cytokine analysis.
Discussion
Our results uncover an important molecular mechanism by which TGF-β mediates the inhibition of T cell differentiation into type 1 Th cells. First, we demonstrate that inhibition of T-bet, a recently discovered molecular switch which determines Th1 differentiation (13), is critical for TGF-β–induced inhibition of Th1 differentiation. Second, we show here that downregulation of IL-12 receptor β2 chain expression appears not to be important for the TGF-β–mediated affect. Interestingly, it has been shown recently that induction of T-bet in Th1 cells does not require IL-12 signaling, but rather that T-bet expression is required for IL-12Rβ2 expression (15). Since it was also shown that T-bet can directly transactivate IFN-γ gene expression without the need for IL-12 signaling, it is likely that the observed inhibition of IL-12Rβ2 chain by TGF-β is an event secondary to T-bet inhibition. In the same way that IL-12Rβ2 alone cannot restore Th1 inhibition by TGF-β, restoration of IL-12 signaling through expression of IL-12Rβ2 also did not reestablish Th1 differentiation inhibited by IL-4 (12, 23). Together, these data support the notion that IL-12 plays a limited role in the direct activation of Th1 differentiation. As suggested by Mullen et al. (15), the role of IL-12 is likely limited more to a supporting role as a growth factor for Th1 cells rather than a differentiation factor. The ability of retrovirally introduced T-bet to overcome TGF-β–induced inhibition of Th1 differentiation demonstrates that T-bet is the most critical and primary target for the inhibition of Th1 differentiation by TGF-β.
In addition to uncovering the mechanism through which TGF-β inhibits Th1 differentiation in vitro, we describe an important role for TGF-β in the inhibition of Th1 differentiation in vivo. Specifically, we show here that TGF-β signaling in T cells plays a critical role in the inhibition of Th1 responses to Leishmania and that TGF-β is at least partially responsible for the susceptibility to L. major of BALB/c mice. The susceptibility of BALB/c mice to Leishmania and the lack of a Leishmania-specific Th1 response has been previously linked to an early burst of IL-4 production by Vβ4Vα8 CD4+ T cells in the first 48 h after infection (24). Thus, early (but not late) depletion of IL-4 or the Vβ4Vα8 subset resulted in protective Th1 responses in BALB/c mice (25). Nevertheless, administration of IL-4 to the resistant mouse strain, C57BL/6, did not divert the Th1 response to Th2 and render these animals susceptible to parasite growth (26). These results suggest that IL-4 is necessary, but not sufficient for the inhibition of the Leishmania-specific Th1 response and susceptibility to infection. Importantly, even when blocking of IL-4 rendered BALB/c mice resistant to Leishmania it did not fully restore Th1 response in these mice (26, 27). Together these data indicate that IL-4 production in BALB/c mice is not the single factor which inhibits the Th1 response and causes susceptibility to infection. Therefore, our observation that abrogation of TGF-β signaling in T cells renders BALB/c mice resistant to Leishmania infection and permits the generation of the Leishmania-specific Th1 response provides an important piece of information to this puzzle.
TGF-β has been previously demonstrated to play an important role in the progression of Leishmaniasis. Specifically, it was shown that Leishmania infection leads to TGF-β production (22). Importantly, administration of TGF-β exacerbated disease in C57BL/6 mice, while administration of anti–TGF-β antibodies ameliorated the disease in BALB/c mice (22). Since TGF-β can directly inhibit the anti-Leishmanial properties of macrophages (21, 28), it was suggested that TGF-β plays an important role in the progression of the disease through the direct inhibition of macrophage function. Since we specifically blocked TGF-β signaling in T cells in the current study as opposed to a general blockade achieved with antibodies in the former study (22), we conclude that TGF-β plays a critical role in susceptibility to Leishmania by directly inhibiting T cell function. Specifically, we demonstrate that T cell–specific abrogation of TGF-β signaling renders BALB/c mice resistant to Leishmania infection and permits the generation of parasite-specific Th1 cells. Th1 differentiation and resistance to Leishmania did not result from low IL-4 production and Th2 differentiation in the absence of TGF-β signaling in T cells. In fact, CD4-dnTGF-βRII BALB/c mice displayed increased Th2 cytokine production.
Early IL-4 production and Vβ4Vα8 CD4+ cells appear to be necessary to downregulate IL-12 receptor β2 chain expression, observations which led to the proposal that inhibiting this receptor blocked the IL-12 signal in T cells and consequently prevented Th1 differentiation. Nonetheless, IL-12Rβ2 downregulation as a result of Leishmania infection is not responsible for the lack of Th1 differentiation as BALB/c mice expressing a transgenic IL-12Rβ2 on T cells failed to mount Th1 response and lead to Leishmania resistance (20). Since downregulation of IL-12Rβ2 expression was neither required to inhibit Th1 differentiation by IL-4 (12, 23) nor by TGF-β it appears that downregulation of T-bet by TGF-β could be a mechanism for in vivo susceptibility of BALB/c mice to Leishmania. It is interesting in this regard that T-bet maps to Chromosome 11 (29), a chromosome that contains a Leishmania susceptibility gene (9).
Acknowledgments
We thank Laurie Glimcher and Ann O'Garra for generous gifts of reagents. We also thank Judy Miller for technical help and Fran Manzo for help with manuscript preparation.
L. Gorelik was an Associate and R.A. Flavell is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Institutes of Health grants AI-39158 (to S. Constant) and AI36529 (to R.A. Flavell).
L. Gorelik's current address is Biogen, Inc., Cambridge, MA 02139.
S. Constant's current address is Department of Microbiology and Tropical Medicine, George Washington University, Washington, D.C. 20037.
References
- 1.Letterio, J.J., and A.B. Roberts. 1998. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 16:137–161. [DOI] [PubMed] [Google Scholar]
- 2.Sad, S., and T.R. Mosmann. 1994. Single IL-2-secreting precursor CD4 T cell can develop into either Th1 or Th2 cytokine secretion phenotype. J. Immunol. 153:3514–3522. [PubMed] [Google Scholar]
- 3.Gorelik, L., P.E. Fields, and R.A. Flavell. 2000. Cutting edge: TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 165:4773–4777. [DOI] [PubMed] [Google Scholar]
- 4.Heath, V.L., E.E. Murphy, C. Crain, M.G. Tomlinson, and A. O'Garra. 2000. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 30:2639–2649. [DOI] [PubMed] [Google Scholar]
- 5.Zhang, D.H., L. Cohn, P. Ray, K. Bottomly, and A. Ray. 1997. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 272:21597–21603. [DOI] [PubMed] [Google Scholar]
- 6.Zheng, W., and R.A. Flavell. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 89:587–596. [DOI] [PubMed] [Google Scholar]
- 7.Gorham, J.D., M.L. Guler, D. Fenoglio, U. Gubler, and K.M. Murphy. 1998. Low dose TGF-β attenuates IL-12 responsiveness in murine Th cells. J. Immunol. 161:1664–1670. [PubMed] [Google Scholar]
- 8.Gorelik, L., and R.A. Flavell. 2000. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181. [DOI] [PubMed] [Google Scholar]
- 9.Beebe, A.M., S. Mauze, N.J. Schork, and R.L. Coffman. 1997. Serial backcross mapping of multiple loci associated with resistance to Leishmania major in mice. Immunity. 6:551–557. [DOI] [PubMed] [Google Scholar]
- 10.Bix, M., Z.-E. Wang, B. Thiel, N.J. Schork, and R.M. Locksley. 1998. Genetic regulation of commitment to interleukin 4 production by a CD4+ T cell-intrinsic mechanism. J. Exp. Med. 12:2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Constant, S.L., C. Dong, D.D. Yang, M. Wysk, R.J. Davis, and R.A. Flavell. 2000. JNK1 is required for T cell-mediated immunity against Leishmania major infection. J. Immunol. 165:2671–2676. [DOI] [PubMed] [Google Scholar]
- 12.Heath, V.L., L. Showe, C. Crain, F.J. Barrat, G. Trinchieri, and A. O'Garra. 2000. Cutting edge: ectopic expression of the IL-12 receptor-β2 in developing and committed Th2 cells does not affect the production of IL- 4 or induce the production of IFN-γ. J. Immunol. 164:2861–2865. [DOI] [PubMed] [Google Scholar]
- 13.Szabo, S.J., S.T. Kim, G.L. Costa, X. Zhang, C.G. Fathman, and L.H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 100:655–669. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, M.H., Y.L. Sun, T. Hoey, and M.J. Grusby. 1996. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 382:174–177. [DOI] [PubMed] [Google Scholar]
- 15.Mullen, A.C., F.A. High, A.S. Hutchins, H.W. Lee, A.V. Villarino, D.M. Livingston, A.L. Kung, N. Cereb, T.P. Yao, S.Y. Yang, et al. 2001. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 292:1907–1910. [DOI] [PubMed] [Google Scholar]
- 16.Reiner, S.L., and R.M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151–177. [DOI] [PubMed] [Google Scholar]
- 17.Himmelrich, H., C. Parra-Lopez, F. Tacchini-Cottier, J.A. Louis, and P. Launois. 1998. The IL-4 rapidly produced in BALB/c mice after infection with Leishmania major down-regulates IL-12 receptor β2-chain expression on CD4+ T cells resulting in a state of unresponsiveness to IL-12. J. Immunol. 161:6156–6163. [PubMed] [Google Scholar]
- 18.Jones, D., M.M. Elloso, L. Showe, D. Williams, G. Trinchieri, and P. Scott. 1998. Differential regulation of the interleukin-12 receptor during the innate immune response to Leishmania major. Infect. Immun. 66:3818–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo, S.J., A.S. Dighe, U. Gubler, and K.M. Murphy. 1997. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishikomori, R., S. Gurunathan, K. Nishikomori, and W. Strober. 2001. BALB/c mice bearing a transgenic IL-12 receptor β2 gene exhibit a nonhealing phenotype to Leishmania major infection despite intact IL-12 signaling. J. Immunol. 166:6776–6783. [DOI] [PubMed] [Google Scholar]
- 21.Barral, A., M. Barral-Netto, E.C. Yong, C.E. Brownell, D.R. Twardzik, and S.G. Reed. 1993. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc. Natl. Acad. Sci. USA. 90:3442–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barral-Netto, M., A. Barral, C.E. Brownell, Y.A. Skeiky, L.R. Ellingsworth, D.R. Twardzik, and S.G. Reed. 1992. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 257:545–548. [DOI] [PubMed] [Google Scholar]
- 23.Nishikomori, R., R.O. Ehrhardt, and W. Strober. 2000. T helper type 2 cell differentiation occurs in the presence of interleukin 12 receptor β2 chain expression and signaling. J. Exp. Med. 191:847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launois, P., I. Maillard, S. Pingel, K.G. Swihart, I. Xenarios, H. Acha-Orbea, H. Diggelmann, R.M. Locksley, H.R. MacDonald, and J.A. Louis. 1997. IL-4 rapidly produced by Vβ4 Vα 8 CD4+ T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 6:541–549. [DOI] [PubMed] [Google Scholar]
- 25.Launois, P., K.G. Swihart, G. Milon, and J.A. Louis. 1997. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J. Immunol. 158:3317–3324. [PubMed] [Google Scholar]
- 26.Sadick, M.D., N. Street, T.R. Mosmann, and R.M. Locksley. 1991. Cytokine regulation of murine leishmaniasis: interleukin 4 is not sufficient to mediate progressive disease in resistant C57BL/6 mice. Infect. Immun. 59:4710–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noben-Trauth, N., W.E. Paul, and D.L. Sacks. 1999. IL-4- and IL-4 receptor-deficient BALB/c mice reveal differences in susceptibility to Leishmania major parasite substrains. J. Immunol. 162:6132–6140. [PubMed] [Google Scholar]
- 28.Nelson, B.J., P. Ralph, S.J. Green, and C.A. Nacy. 1991. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-β1. J. Immunol. 146:1849–1857. [PubMed] [Google Scholar]
- 29.Zhang, W.X., and S.Y. Yang. 2000. Cloning and characterization of a new member of the T-box gene family. Genomics. 70:41–48. [DOI] [PubMed] [Google Scholar]