Abstract
The functional properties of dendritic cells (DCs) are strictly dependent on their maturational state. To analyze the influence of the maturational state of DCs on priming and differentiation of T cells, immature CD83− and mature CD83+ human DCs were used for stimulation of naive, allogeneic CD4+ T cells. Repetitive stimulation with mature DCs resulted in a strong expansion of alloreactive T cells and the exclusive development of T helper type 1 (Th1) cells. In contrast, after repetitive stimulation with immature DCs the alloreactive T cells showed an irreversibly inhibited proliferation that could not be restored by restimulation with mature DCs or peripheral blood mononuclear cells, or by the addition of interleukin (IL)-2. Only stimulation of T cells with mature DCs resulted in an upregulation of CD154, CD69, and CD70, whereas T cells activated with immature DCs showed an early upregulation of the negative regulator cytotoxic T lymphocyte–associated molecule 4 (CTLA-4). These T cells lost their ability to produce interferon γ, IL-2, or IL-4 after several stimulations with immature DCs and differentiated into nonproliferating, IL-10–producing T cells. Furthermore, in coculture experiments these T cells inhibited the antigen-driven proliferation of Th1 cells in a contact- and dose-dependent, but antigen-nonspecific manner. These data show that immature and mature DCs induce different types of T cell responses: inflammatory Th1 cells are induced by mature DCs, and IL-10–producing T cell regulatory 1–like cells by immature DCs.
Keywords: dendritic cells, regulatory T cells, T helper type 1 cells, interleukin 10, T cell differentiation
Introduction
Dendritic cells (DCs) are professional APCs specialized for the initiation of T cell immunity 1 2 3. Depending on their maturational state and their location, DCs perform different functions within the immune system. DCs normally reside in nonlymphoid tissues such as the skin in an immature form, where they are specialized for antigen capture. Activation of DCs and subsequent migration from nonlymphoid tissues to regional lymph nodes have been shown to be early steps during inflammatory processes and critical events in the generation of cell-mediated immune responses against various pathogens. After antigen uptake, inflammatory stimuli are necessary to switch DCs to a T cell stimulatory mode. This process has been called “maturation” and is associated with changes in the phenotype and function of DCs, including upregulation of costimulatory molecules and adhesion molecules, expression of chemokine receptors, and migration to the regional lymph nodes where DCs interact with recirculating T cells and initiate T cell immunity 1 2 3 4 5 6 7.
The activation of CD4+ T cells by antigen-presenting DCs leads to their differentiation into distinct populations of effector cells differing in their cytokine secretion pattern 8 9. The induction of a polarized T cell phenotype occurs at an early stage of the immune response and is influenced by the cytokine milieu during the priming process, the nature and intensity of TCR-mediated activation, costimulatory signals, the genetic background, and the type of APC involved 10 11 12 13. DCs play a critical role in initiating primary T cell responses and are promising as “nature's adjuvant” for therapeutic applications in humans 14. Therefore, it is important to define the influence of different DC subpopulations on the generation of different T cell phenotypes. In this study, we investigated whether the differentiation state of DCs can influence the polarization of the T cell response. Therefore, we generated two well-defined populations of monocyte-derived DCs: immature DCs (iDCs) cultured only with GM-CSF and IL-4 15, and mature CD83+ DCs (mDCs) generated by additional stimulation with a defined maturation cocktail 16. Both DC populations generated from the same donors were used for repetitive stimulation of allogeneic, naive cord blood–derived CD4+ T cells. We analyzed the phenotype, proliferative capacity, cytokine profile, and functional properties of polarized T cells. Here we report that iDCs and mDCs induce completely different T cell phenotypes: inflammatory Th1 responses induced by mDCs versus the polarization-forward, nonproliferative, IL-10–producing regulatory T (Tr) cells by iDCs.
Materials and Methods
Culture Medium.
X-VIVO-15 supplemented with 1% autologous plasma was used for culture of DCs. T cells were cultured and stimulated in X-VIVO-20 (both from BioWhittaker).
Cytokines.
All cytokines used in this study were recombinant human proteins and were used at plateau concentrations to induce the optimal generation of DCs. The final concentrations were: 800 U/ml GM-CSF (Leukomax; Sandoz), 1,000 U/ml IL-4 and IL-6 (Strathmann Biotech GmbH), 10 ng/ml IL-1β and TNF-α (Strathmann Biotech GmbH), and 1 μg/ml PGE2 (Minprostin; Amersham Pharmacia Biotech). 50 U/ml IL-2 (Proleukin; Chiron Corp.) was used for the culture and expansion of T cells.
Abs.
For immunostaining, the mouse IgGs used were CD1a, CD2, CD14, CD19, CD40, CD58, CD80, CD86, CD83, and CD152 (Coulter/Immunotech); the rat IgG used was anti–HLA-DR (YE2/36HLK; Serotec/Camon). Mouse and rat subclass–specific isotypes were from Coulter/Immunotech. The conjugated secondary reagents used were: FITC-conjugated goat anti–mouse IgG, and PE-conjugated goat anti–rat IgG (Jackson ImmunoResearch Laboratories). For the staining of MACS-sorted T cells, the secondary reagents used were: FITC- or PE-conjugated CD3, CD4, CD8, CD25, CD28, CD69, CD70, CD152, and CD154 FITC- and PE-conjugated mouse IgG (Coulter/Immunotech). PE-conjugated anti–inducible costimulator (ICOS) mAb F44 was provided by Dr. Kroczek (Robert Koch Institute, Berlin, Germany). The anti–TGF-β (R&D Systems; used according to the instructions of the manufacturer) and anti–IL-10 mAbs (JES-19F1.1.1; American Type Culture Collection; blocking capacity tested in proliferation assays using IL-10R–transfected Baf3 cells) were used for blocking experiments.
Cytokine Assays.
T cells were stimulated with allogeneic DCs (106 T cells plus 105 DCs) in 24-well plates in 1 ml X-VIVO-20. Cytokine synthesis was determined by the analysis of supernatants 48 h after stimulation. The commercially available ELISA kits for the human cytokines IFN-γ, IL-2, IL-4, IL-5, and IL-10 (BD PharMingen) were used as indicated by the manufacturer. For intracellular analysis of cytokine production, anti–IL-2–PE, anti–IL-4–PE, anti–IL-5–PE, anti–IL-10–PE, anti–IFN-γ–PE mAbs, and FITC/PE-conjugated isotypic mAb (BD PharMingen) were used according to the manufacturer's instructions. In brief, 6 d after restimulation, 106 T cells were activated with 2.4 μg/ml PHA plus 1 ng/ml PMA for 6 h. Monensin (1.3 μM/ml) was added for the last 4 h, and cells were collected, washed, fixed/saponin permeabilized (perm/fix solution; BD PharMingen), and stained with 0.5 μg/test of cytokine-specific Abs.
Generation of DCs.
DCs were generated from leukapheresis products as described previously 15 17. In brief, PBMCs were isolated by Ficoll density gradient centrifugation and stored frozen in aliquots. For each DC preparation, frozen PBMCs were thawed, and monocytes were isolated by plastic adherence and cultured in X-VIVO-15 plus 1% heat-inactivated autologous plasma including 800 U/ml GM-CSF and 1,000 U/ml IL-4. At day 5, nonadherent cells were rinsed off, washed once in PBS, and transferred to 6-well plates at 7 × 105 cells in 3 ml/well. For differentiation into mDCs, iDCs were additionally stimulated on day 6 with 10 ng/ml IL-1β, 10 ng/ml TNF-α, 1,000 U/ml IL-6, and 1 μg/ml PGE2 16. Nonadherent iDCs and mDCs at day 7 were used for T cell stimulation.
Flow Cytometric Analysis.
Immunofluorescence staining was performed after washing the cells twice with PBS plus 0.5% human serum albumin. Cells were incubated for 20 min at 4°C with each mAb diluted to the optimal concentration for immunostaining. After washing with cold PBS/human serum albumin, the cells were incubated with FITC- and PE-conjugated second step mAb for 20 min at 4°C, washed three times, and analyzed by flow cytometry (FACSCalibur™ and CELLQuest™ software; Becton Dickinson). Necrosis versus apoptosis was determined by propidium iodide and annexin V staining according to the instructions of the manufacturer (BD PharMingen).
Induction of Alloreactive T Cell Lines.
Naive CD4+ T cells were purified from cord blood using CD4 MACS beads as indicated by the manufacturer (Miltenyi Biotec; purity: >98% CD4+, >90% CD45RA+). Alternatively, naive CD4+ T cells were positive selected from the peripheral blood of healthy adults using a CD4/CD45RA Multisort kit (Miltenyi Biotec). 106 T cells were cultured with 105 iDCs or mDCs in 1 ml X-VIVO-20 in 24-well plates. Alloreactive T cells were expanded from day 6 in the presence of 50 U/ml IL-2. 2 wk after priming, T cells were restimulated with iDCs or mDCs from the same donor as in primary culture under identical culture conditions and expanded from day 3 on with IL-2 followed by weekly repetitive stimulations under identical culture conditions. Aliquots of the cultures were used for proliferation assays carried out in X-VIVO-20 with 5 × 104 T cells/well and different numbers of allogeneic iDCs/mDCs in 96-well plates. T cell proliferation was measured after 4 d of incubation and an additional 16-h pulse with [3H]TdR (37 kBq/well) using a liquid scintillation counter. For some experiments, the T cells were depleted from 48-h cocultures with mDCs using CD2 beads (10 beads/cell; Dynal). After depletion of T cells, the mDCs were used for stimulation of Th1 cells.
Transwell Experiments.
Transwell experiments were done in 24-well plates. 106 Th1 or Tr1 cells were stimulated with 105 mDCs or iDCs. Additionally, 106 Tr1 cells plus 105 mDCs were either added directly to the cultures of activated Th1 cells or were placed in Transwell chambers (Millicell, 0.4 μm; Millipore) in the same well. After 4 d of culture, activated T cells (105 cells/well) were transferred to 96-well plates in triplicates. Proliferation was measured after an additional 16-h pulse with [3H]TdR using a liquid scintillation counter.
Results
Suppressed Proliferation of Alloreactive T Cells Primed with iDCs.
To analyze the influence of the maturational state of DCs on the priming and differentiation of naive T cells, we generated alloreactive CD4+ T cell lines by repetitive stimulations with iDCs or mDCs from the same donor. Two well-defined DC populations were used: CD83− iDCs generated in the presence of GM-CSF and IL-4, and terminally differentiated CD83+ mDCs after an additional stimulation with a defined cytokine cocktail (TNF-α, IL-1β, IL-6, and PGE2). Although both populations expressed the costimulatory molecules CD80 and CD86, the expression of these molecules by mDCs was more homogeneous and significantly higher compared with iDCs (Fig. 1). Furthermore, the expression of HLA-DR was 10 times higher on mDCs, and only mDCs showed a homogeneous expression of CD58.
Figure 1.
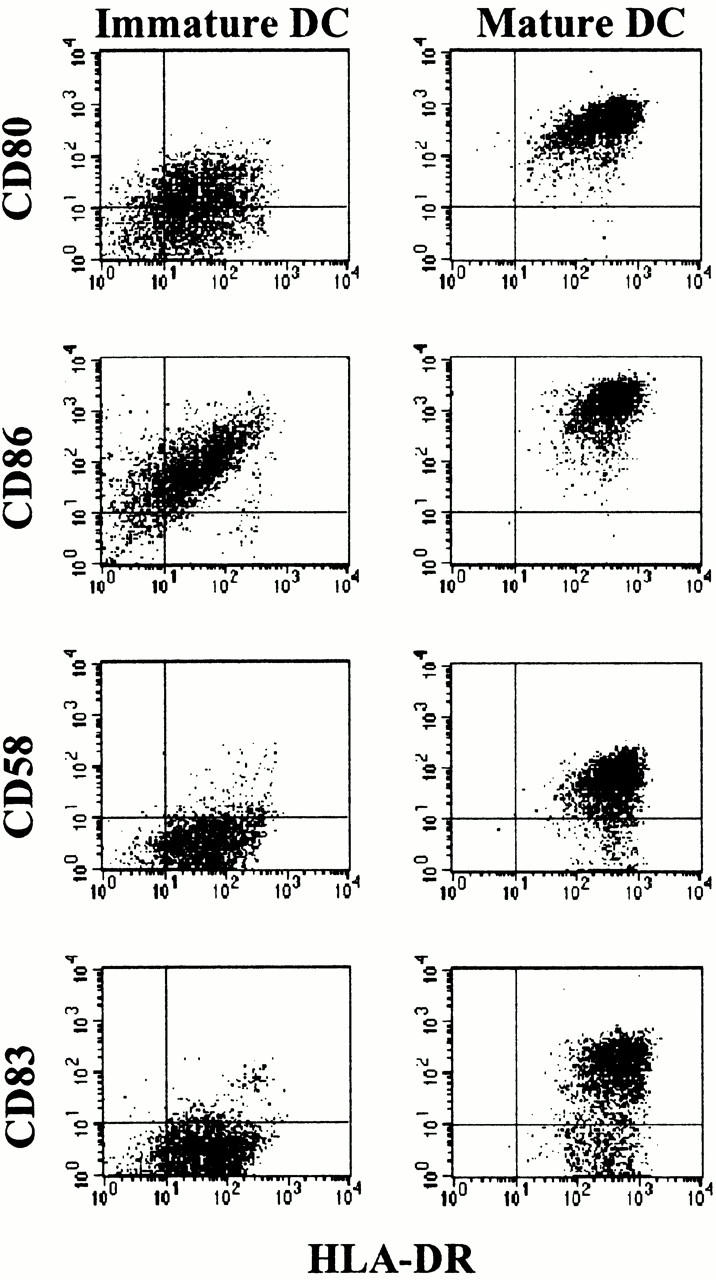
Phenotype of iDCs and mDCs. iDCs were generated from leukapheresis products by culture of adherent monocytes for 6 d in X-VIVO-15 plus 1% autologous plasma in the presence of GM-CSF and IL-4 (left panels); maturation of DCs was induced by stimulation with a cocktail of proinflammatory cytokines and PGE2 for 24 h (right panels). Surface expression of both populations was analyzed at day 7. The results were similar in 10 independent experiments.
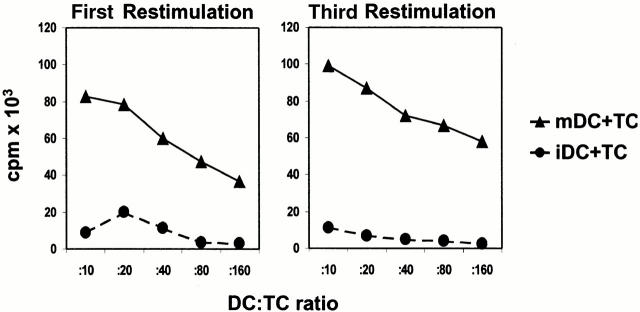
mDCs as well as iDCs were used for repetitive stimulations of naive cord blood–derived CD4+ T cells. Priming and repetitive restimulations with mDCs resulted in a strong proliferation of alloreactive T cells (Fig. 2) and a 30–50-fold expansion after two rounds of restimulation. In contrast, iDCs induced only a weak proliferation of alloreactive T cells in the primary culture, and the antigen-driven proliferation further decreased in the successive restimulations (Fig. 2), finally resulting in nonproliferative CD4+ T cells. Therefore, it was not possible to induce stable alloreactive T cell lines or a significant expansion of these T cells with allogeneic iDCs. The number of T cells also decreased slowly after repetitive stimulations. 40–60% of the initial T cell numbers could be recovered after two rounds of restimulation. However, neither significant necrosis nor apoptosis was observed in the cultures by propidium iodide and annexin V staining (data not shown).
Figure 2.
Proliferation of alloreactive CD4+ T cells induced by iDCs or mDCs. Naive CD4+ T cells purified by MACS sorting from cord blood were primed and restimulated (every week) with allogeneic iDCs or mDCs from the same donor (DC/T cell ratio of 1:10) in serum-free X-VIVO-20. Proliferation of alloreactive T cells (5 × 104/well, triplicate cultures) was determined by [3H]TdR incorporation. A representative result of four independent experiments is shown.
Irreversibly Impaired Proliferation of Alloreactive T Cells Primed with iDCs.
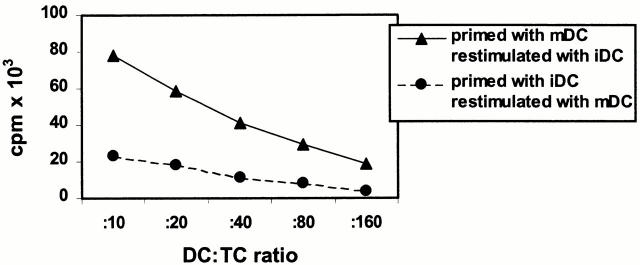
To analyze the proliferative capacity of alloreactive T cell lines induced by iDCs or mDCs in more detail, primed alloreactive T cells were restimulated with different APCs from the same donor. As shown in Fig. 3T cells primed with mDCs also expanded in the presence of iDCs or allogeneic PBMCs (data not shown). This suggests that iDCs do not inhibit the proliferation of primed alloreactive T cells or induce T cell death. In contrast, alloreactive T cells primed with iDCs showed only a marginal proliferation even after optimal restimulation with mDCs. In addition, this suppressed capacity for antigen-specific proliferation induced by repetitive restimulation with iDCs could not be restored in the presence of allogeneic PBMCs or by addition of 100 U/ml IL-2 (data not shown). Thus, priming and repetitive restimulation with iDCs induced an irreversibly inhibited capacity of antigen-driven proliferation in alloreactive CD4+ T cells.
Figure 3.
Cross-stimulation of alloreactive T cells induced with iDCs or mDCs. Naive CD4+ T cells were primed with allogeneic iDCs or mDCs from the same donor at a DC/T cell ratio of 1:10. T cells were restimulated two times under the same conditions as in the primary culture. 7 d after the second restimulation, alloreactive T cells primed with iDCs were cultured with mDCs, and alloreactive T cells primed with mDCs were cultured with iDCs (5 × 104 T cells/well, triplicate cultures). Proliferation of T cells was determined by the addition of [3H]TdR after 4 d of coculture. Similar results were obtained in three independent experiments.
Different Phenotype of Alloreactive T Cells After Activation with iDCs or mDCs.
To further characterize the alloreactive T cell lines, the expression of various surface molecules on activated T cells was analyzed. Both T cell populations primed with iDCs or mDCs showed a homogeneous expression of CD3 and CD4, and no contaminating cells such as CD14+ monocytes were detectable. Both T cell populations expressed CD25, CD95, and CD28, and showed an upregulation of ICOS (18; Fig. 4). In contrast, only T cell lines induced by iDCs showed a significant upregulation of CTL-associated molecule (CTLA)-4 (CD152), the high affinity counterreceptor of B7-1 and B7-2 that delivers a negative signal and inhibits T cell proliferation, IL-2 production, and cell cycle progression 19 20 21. This CTLA-4 expression was constantly detectable even 14 d after restimulation, suggesting that these T cells expressed CTLA-4 constitutively (data not shown). In contrast, no upregulation of CD152 on T cells primed with mDCs was detected. Furthermore, both T cell populations upregulated CD25, but only T cell lines activated by mDCs showed an enhanced expression of the activation molecules CD69, CD70, and CD154 (CD40 ligand). CD70 costimulates CD4+ T cells to produce IL-2 and IFN-γ, and cross-linking of CD70 also upregulates the expression of CD154 on activated T cells 22 23. Furthermore, CD154–CD40 interaction was shown to be a critical signal for T cell and DC activation 24 25 26. Thus, the different phenotypes of activated T cells induced by iDCs or mDCs correlate with the different rates of expansion. Furthermore, the phenotype of differentiated T cells was strictly dependent on the stimulator cells used for priming. After the second restimulation with iDCs, the irreversibly differentiated T cells induced by iDCs expressed the same phenotype described in Fig. 4 even upon restimulation with mDCs (data not shown). Therefore, phenotypic alterations of T cells after priming with iDCs cannot be easily explained as the direct result of an insufficient stimulation.
Figure 4.
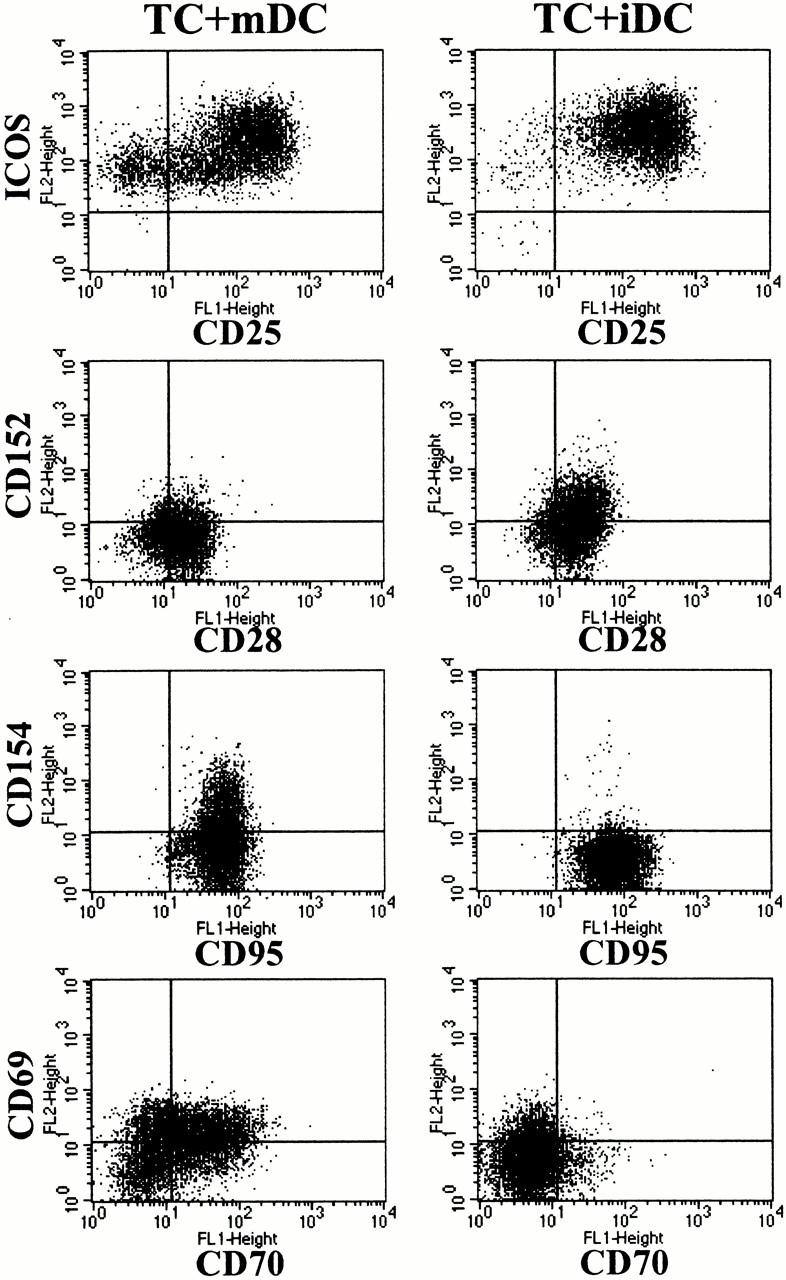
Phenotype of alloreactive T cells after stimulation with allogeneic DCs. Naive CD4+ T cells were primed and restimulated with allogeneic iDCs or mDCs from the same donor. The phenotype of CD4+ T cells 42 h after coculture with allogeneic DCs (first restimulation) is shown (representative result of seven independent experiments).
Induction of IL-10–producing T Cells by iDCs and Th1 Cells by mDCs.
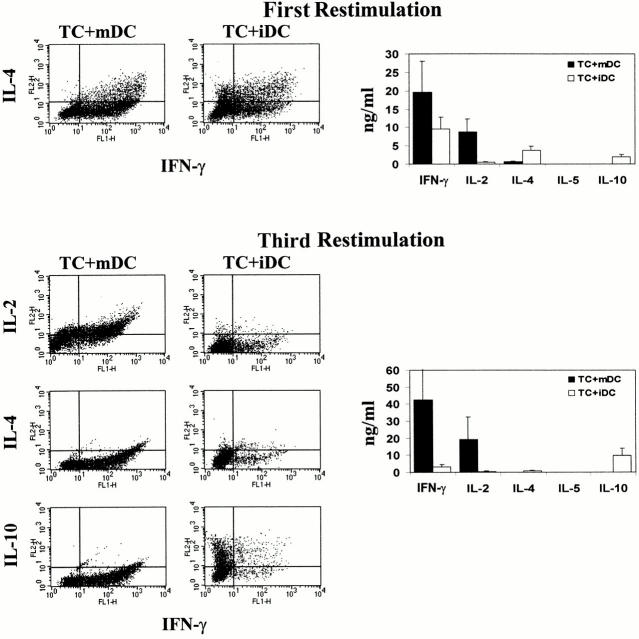
CD4+ T cell subsets can be distinguished according to their ability to produce distinct patterns of cytokines. To analyze the cytokine profiles of alloreactive T cell lines after repetitive stimulation with iDCs or mDCs, supernatants of activated T cells were tested by ELISA. In addition, intracellular cytokine staining of CD4-sorted T cells was performed to determine the percentage of cells releasing a certain cytokine. As shown in Fig. 5, mDCs induced a strong differentiation of naive CD4+ T cells into Th1 cells. Such T cells produce large amounts of IFN-γ after priming with mDCs and showed a typical Th1 cytokine profile after several restimulations (large amounts of IFN-γ and IL-2 but no production of IL-4, IL-5, or IL-10). In contrast, naive T cells primed and restimulated with allogeneic iDCs showed a Th0 cytokine profile after the first restimulation (synthesis of intermediate amounts of IFN-γ and IL-4). After repetitive stimulation with iDCs, the alloreactive T cells lost their capacity to synthesize IFN-γ, IL-2, and IL-4, indicating that these T cells did not differentiate into Th2 cells, but showed, however, an enhanced production of IL-10. Thus, the respective T cell progeny exhibited a cytokine profile characteristic of Tr1 cells, i.e., synthesis of high amounts of IL-10 and no or negligible production of IFN-γ, IL-2, IL-4, or IL-5.
Figure 5.
Induction of IL-10–producing CD4+ T cells by allogeneic iDCs. 106 naive cord blood CD4+ T cells were cultured with allogeneic iDCs or mDCs at a DC/T cell ratio of 1:10 in serum-free medium. Repetitive stimulations under the same conditions were repeated weekly. Cytokines in the supernatants (right) were detected by ELISA 48 h after restimulation. The induced cytokine profiles (left) were also analyzed by intracellular staining of activated T cells. Cells were collected and washed 6 d after coculture with allogeneic DCs and activated with 2.4 μg/ml PHA plus 1 ng/ml PMA for 6 h. Monensin (1.33 μM/ml) was added for the last 4 h of culture. The cells were fixed and stained for detection of intracellular cytokines using FITC- or PE-conjugated specific mAbs. Cytokine profiles and production after the first and after the third restimulation are shown. One of six experiments with similar results is shown.
IL-10–producing T Cells Induced by iDCs Suppress Antigen-driven Proliferation of Th1 Cells.
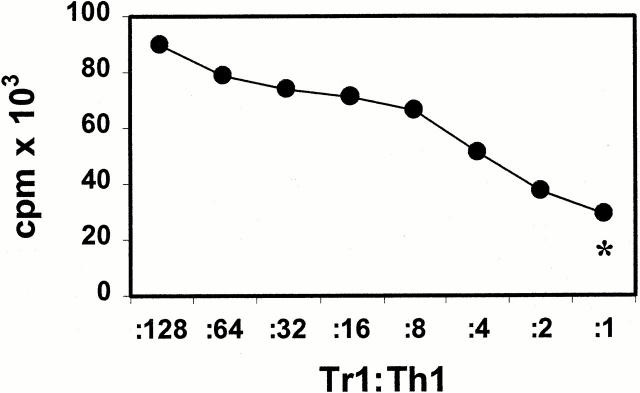
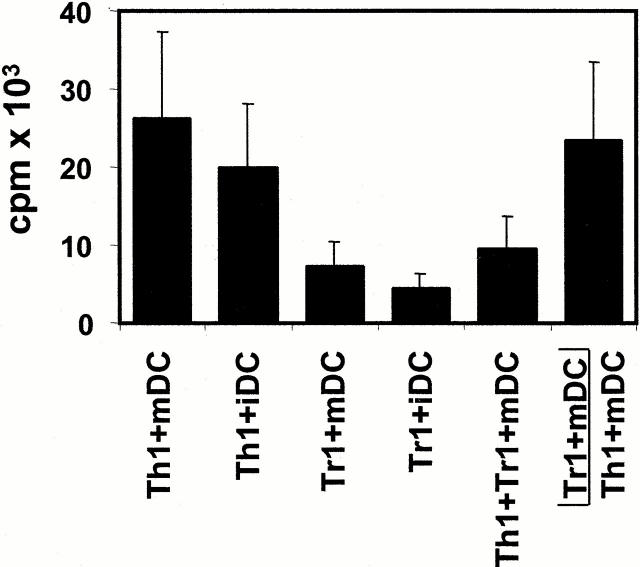
To analyze the functional properties of IL-10–producing Tr1-like cells, coculture experiments with alloreactive Th1 cell lines were performed. To this end, alloreactive Th1 cell lines were restimulated with mDCs in the presence of different numbers of Tr1-like cells (also stimulated with mDCs) from the same donor. As shown in Fig. 6, Tr1-like cells suppressed the proliferation of syngeneic Th1 cells in response to allogeneic mDCs in a dose-dependent manner. At a Th1/Tr1 ratio of 1:1, the proliferation of Th1 cells is reduced to nearly the level of proliferation of the activated Tr1-like cells alone (Tr1-like cells plus mDCs, ratio of 1:10; Fig. 6). The observation that activated Tr1-like cells produce high levels of the immunosuppressive cytokine IL-10 and no T cell growth–promoting cytokines such as IL-2 and IL-4 suggests that the inhibitory effect of Tr1-like cells on the proliferation of Th1 cells might be a direct result of soluble inhibitory factors produced by activated Tr1-like cells. To test this hypothesis, alloreactive Th1 and Tr1-like cells were stimulated with allogeneic iDCs or mDCs. Additionally, alloreactive Tr1-like cells were either added directly to cocultures of activated Th1 cells or placed in Transwell chambers in the same well and activated with mDCs. The semipermeable polycarbonate membrane allows the free exchange of soluble factors but excludes direct cell contact of Th1 and Tr1-like cells. As shown in Fig. 7, alloreactive Th1 cell lines proliferated in the presence of allogeneic mDCs as well as in the presence of allogeneic iDCs. In contrast, Tr1-like cells with low proliferative capacity showed only weak rates of expansion, independent of the antigen-presenting population used. Furthermore, in coculture, Tr1-like cells suppressed the proliferation of Th1 cells almost to the level of Tr1-like cells induced by mDCs. This suggests that the proliferation of the cocultured Th1 cells is almost completely blocked. The separation of both T cell lines in Transwell chambers virtually abolished this immunosuppressive effect of Tr1-like cells. These results suggest that direct cell contact of Tr1 and Th1 cells is essential for the inhibitory capacity of Tr1-like cells. The comparatively low counts in Transwell experiments resulted from the experimental procedure (preculture in 24-well Transwell plates and transfer to 96-well plates at day 4) and is not a result of weak stimulatory capacity of mDCs in these assays.
Figure 6.
Inhibition of antigen-specific proliferation of Th1 cells after coculture with Tr1-like cells. Th1 and Tr1 cell lines were induced by repetitive stimulation of naive CD4+ T cells with allogeneic mDCs (Th1 cytokine profile) or iDCs (Tr1 cytokine profile) at DC/T cell ratios of 1:10. 7 d after the third restimulation, the Th1 cells (5 × 104 cells/well) were restimulated with mDCs (5 × 103 cells/well) in the presence of different numbers of Tr1-like cells from the same cord blood fraction induced with iDCs from the same DC donor. Proliferation of T cells was determined by [3H]TdR incorporation after 4 d of culture. *Background proliferation of Tr1-like cells plus mDCs. One of three experiments is shown.
Figure 7.
The inhibitory effect of Tr1-like cells on the proliferation of Th1 cells requires direct cell contact. T cells with a Th1 or Tr1 cytokine profile were induced by repetitive stimulations of naive cord blood–derived CD4+ T cells with allogeneic mDCs or iDCs from the same donor. 7 d after the third restimulation, 106 Th1/Tr1 cells were stimulated with 105 allogeneic iDCs or mDCs in 24-well Transwell plates. Additionally, 106 Tr1 cells were added directly to the coculture of Th1 plus mDCs or were placed in Transwell chambers in the same well with 105 mDCs. After 4 d of culture, 105 activated T cells/well were transferred in 96-well plates to measure incorporation of [3H]TdR for the final 16 h. Results representative of three independent experiments are presented as mean cpm of triplicate determinations.
The Inhibitory Effect of Tr1-like Cells Is Antigen Nonspecific and Can Be Partially Reversed by Addition of High Doses of IL-2.
The Th1-inhibitory properties of Tr1-like cells could be mediated either directly via a Tr1-like/Th1 interaction via surface or soluble molecules or, alternatively, via the downregulation of the costimulatory capacity of target mDCs by Tr1-like cells. To test the latter possibility, we stimulated Th1 cells with mDCs preincubated with Tr1-like cells for 48 h. Fig. 8 A illustrates that such mDCs exerted the same stimulatory capacity as untreated mDCs. This finding clearly argues against the assumption that the Th1-suppressive properties of Tr1-like cells are the consequence of a suppression of the costimulatory capacity of mDCs by Tr1-like cells.
Figure 8.
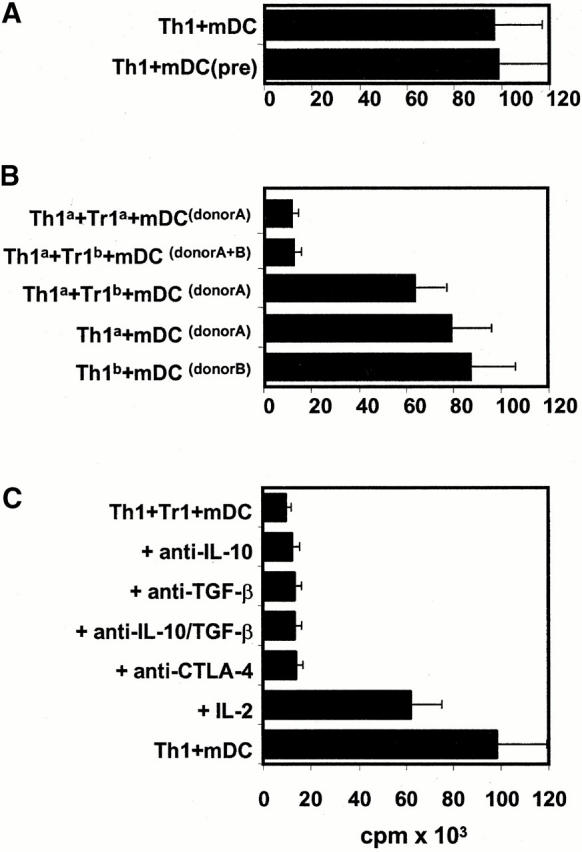
The inhibitory effect of Tr1-like cells was antigen nonspecific and partially reversible by the addition of IL-2. (A) Day 7 mDCs were cultured for 48 h alone or in the presence of Tr1-like cells. Thereafter, T cells were depleted using CD2 beads and the resulting mDCs (mDC[pre]; 104/well) were used for stimulation of alloreactive Th1 cells (105 cells/well) compared with untreated mDCs. (B) Polarized Th1 cells and Tr1-like cells from the same donor were induced by repetitive stimulation of naive T cells with either allogeneic iDCs/mDCs from donor A (Tr1a/Th1a) or donor B (Tr1b/Th1b). Both T cell populations (105 cells/well) were cocultured after the second restimulation in the presence of mDCs (104 cells/well) from donor A and/or donor B as indicated. (C) 10 μg/ml anti–IL-10, 2 μg/ml anti–TGF-β, and 10 μg/ml anti–CTLA-4 were added at the onset of the cultures that were prepared as described under B. After 4 d of culture, [3H]TdR was added for an additional 16 h. Results representative of three independent experiments are presented as mean cpm of triplicate determinations.
In the next series of experiments, we investigated whether Tr1-like and Th1 cells have to interact with target structures expressed by the same mDCs. Therefore, we used Th1 and Tr1-like cells generated from the same donor by priming with mDCs/iDCs from two different donors, A and B. As shown above, donor A–specific Tr1-like cells suppressed the proliferation of donor A–specific Th1 cells in coculture. Furthermore, donor B–specific Tr1 cells also suppressed the proliferation of donor A–specific Th1 cells in the presence of donor A and donor B mDCs (Fig. 8 B). The results suggest that the Th1-inhibitory capacity of Tr1-like cells does not require the simultaneous presentation of the target antigens on the same mDCs. Nevertheless, activation of Tr1-like cells by the respective target mDCs is an essential prerequisite, as the incubation of donor A–specific Th1 cells with donor B–specific Tr1-like cells solely in the presence of donor A–derived mDCs did not lead to an inhibition of Th1 proliferation.
Transwell chamber experiments indicated that direct cell contact is essential for the Th1-inhibitory properties of Tr1-like cells (Fig. 7). However, this experiment cannot exclude the involvement of membrane-bound molecules like CTLA-4 and cytokines like IL-10 and TGF-β, which might be upregulated as a consequence of such a direct cell contact. Fig. 8 C demonstrates that neither neutralizing mAbs directed against IL-10 and TGF-β nor blocking mAbs binding to CTLA-4 could reverse the inhibitory capacity of Tr1-like cells. By contrast, the addition of relatively high amounts of IL-2 (100 U/ml) restored the proliferation of Th1 cells at least partially (∼70%). As the proliferation of Tr1-like cells could not be induced with such amounts of IL-2, this result suggests that Tr1-like cells suppress the proliferation of Th1 cells, possibly via the inhibition of endogenous IL-2 production.
Discussion
The capacity of DCs to initiate or modulate immune responses appears to depend on their phenotype and functional maturation. In this study, we compared the influence of different maturational states of DCs on the differentiation of naive CD4+ T cells. To address this issue, we used monocyte-derived DCs, as this is a particularly well-defined model 15 17. iDCs can be generated from monocytes by culture in GM-CSF plus IL-4, and converted into mDCs by providing an appropriate stimulus 15 16 17. We used a defined cytokine cocktail that reliably induces fully mature, Th1-inducing DCs 16. We show that mDCs induce the strong expansion of T cells with a polarization towards Th1 cells in concordance with previous findings 16. In contrast, repetitive stimulation of naive cord blood–derived T cells with allogeneic iDCs resulted in the induction of nonexpanding, IL-10–producing T cells. These T cells suppressed the alloantigen-driven proliferation of syngeneic Th1 cells in cocultures in a contact and dose-dependent manner and were therefore termed Tr1-like cells. Remarkably, this downregulation of Th1 responses by these Tr1-like cells occurred even upon stimulation of Th1 lines by potent immunostimulatory mDCs. Detailed analyses revealed that the suppressor function of such Tr1-like cells is antigen nonspecific and is probably based on an APC-independent mechanism. Moreover, suppression does not require that the target antigens are presented by the same APCs. Finally, blocking of CTLA-4 or endogenous IL-10 and TGF-β has no effect, whereas the addition of IL-2 could at least partially overcome the Tr1-like–mediated suppression.
Regarding the development of IL-10–producing T cells, it has been shown that the addition of IL-10 to primary murine T cell cultures resulted in the generation of such T cells 27. In addition, Assenmacher et al. 28 have shown that the treatment of IFN-γ–producing murine CD4+ T cells with IL-4 within the first 3 wk after priming led to the development of IL-10–producing T cells that lost their capacity to produce IFN-γ or IL-4. To the best of our knowledge, this study is the first report that shows the reliable induction of human Tr cells in vitro. Remarkably, we find that a particular APC type, namely iDC, is able to induce IL-10–producing CD4+ T cells with regulatory properties in the absence of exogenous cytokines. Naive human T cells stimulated with allogeneic iDCs initially produced IFN-γ and IL-4 but lost their potential to produce either IFN-γ or IL-4 after the subsequent restimulations with iDCs (Fig. 5). Taking into account the data of Assenmacher et al. 28, one might speculate that a possible mechanism for the induction of IL-10–producing Tr1-like cells in the presence of iDCs is the conversion of human IFN-γ producers into IL-10 producers by transiently produced endogenous IL-4. Of note is that ongoing studies indicate that the iDCs we used do not produce significant amounts of IL-10 (data not shown).
The suppressive properties of the human IL-10–producing Tr1-like cells as outlined herein suggest that they are the human counterpart of a recently described population of murine Tr cells. These cells arise from the thymus of naive mice, express CD4, CD25, and CTLA-4, and their activation via the B7-CD28 pathway is thought to be essential for induction and survival in the periphery 29 30 31 32 33 34. They comprise ∼5–10% of the peripheral CD4+ T cells and could not be induced to proliferate in vitro upon polyclonal activation using plate-bound anti-CD3 mAb 29 30 31. Regarding their physiological function, it has been shown that such T cells are involved in the maintenance of peripheral tolerance 29. The data dealing with the mechanism of the suppressive properties of murine Tr cells are controversial. Groux et al. 27 have shown by using neutralizing Abs that the suppression appears primarily mediated by IL-10 and TGF-β, readily explaining why separating the Tr1 cells in Transwell chambers from the alloreactive T cells did not abolish the suppressive effect on the latter. In sharp contrast, Thornton and Shevach have shown that the suppression is cytokine independent but mediated through direct cell–cell contact 35. These discrepancies might be due to the existence of different types of Tr cells, but also to differences in the experimental design. Groux et al. 27, for example, have used allogeneic monocytes as APCs, which are sensitive to the inhibitory effects of IL-10 for stimulation of both populations, e.g., downregulation of the costimulatory capacity of monocytes by IL-10 led to an impaired T cell proliferation. Using IL-10–insensitive mDCs 36 37, we showed that the suppressive effect of human Tr1-like cells required cell–cell contact. The effect was virtually abolished when Tr1-like cells were separated from Th1 cells in Transwell chambers. In agreement with these data, Thornton and Shevach 38 have also observed a cell contact–dependent, cytokine-independent mechanism for the suppressive effect of their murine Tr cells stimulated with anti-CD3 mAb. In addition, we showed that the inhibitory properties of human Tr1-like cells were antigen nonspecific and did not require that the antigens recognized by Tr1-like cells and Th1 cells were presented on the same APCs. These findings are also in agreement with data shown by Thornton and Shevach for murine Tr cells 38. Thus, human IL-10–producing T cells induced by repetitive stimulations with iDCs showed many similarities to the murine CD4+CD25+ T cells described by Thornton and Shevach 38.
A characteristic marker of murine Tr cells is the constitutive expression of CTLA-4. This negative regulatory CD28 homologue on activated T cells is critical for the induction of Tr cells 30 31. In agreement with these reports, an early upregulation of CTLA-4 on activated T cells could be observed after stimulation with iDCs but not after stimulation with mDCs 39 40. It has been reported that the function of murine Tr cells is dependent on the expression of CTLA-4 and the production of TGF-β 32 33. Blockade of TGF-β in vivo led to an abrogation of T cell–mediated suppression and induction of severe colitis 33. In contrast, in our hands the suppressive properties of human Tr1-like cells could not be inhibited by blocking CTLA-4 or TGF-β in vitro. Therefore, we suggest that different molecular mechanisms involving additional surface molecules are responsible for the suppressive properties of human Tr1-like cells in vitro. Indeed, Takahashi et al. 32 also postulated that blockade of other surface molecules different from CTLA-4 could abrogate the suppressive activity of murine CD4+CD25+ T cells.
Additional regulatory elements that influence the interaction of T cells and DCs are the CD27/CD70 and CD40/CD154 pathways, members of the TNF family critical for T cell activation and differentiation. It was shown that CD27/CD70 costimulation of naive T cells (like B7/CD28) enhanced the production of IL-2 and IFN-γ and furthermore induced the upregulation of CD154 on T cells 22 23. Cross-linking of CD40 by CD154-expressing T cells is an efficient stimulus for DCs, leading to IL-12 production and upregulation of CD80, CD86, and other T cell stimulatory molecules 24 25 26. Furthermore, DC-derived IL-12 costimulates the proliferation of T cells, and IL-12 is also a key factor for directing the differentiation of naive T cells towards Th1 cells 11. In agreement with these data, we observed upregulation of CD70 and subsequent upregulation of CD154 on T cells exclusively after stimulation with mDCs but not in the presence of iDCs.
Several models have provided evidence that the triggering of DC differentiation and maturation is essential for the effective activation of the immune system 41 42 43. In addition, it has been shown that resting donor–derived APCs induced prolonged allograft survival 42. Furthermore, defined populations of murine immature bone marrow–derived DCs were found to induce alloantigen-specific tolerance in an MLR system in vitro as well as in vivo in a heat transplant model, possibly via the induction of Tr cells 43 44. Therefore, the induction of tolerance versus immunity may be determined by resting/iDCs versus activated/mDCs. This would help to establish peripheral tolerance to self. Microbial invasion results in local inflammation and destruction of self-tissue and DC maturation mediated by inflammatory cytokines, viral or microbial constituents such as double-stranded RNA, LPS, and certain CpG oligonucleotides or CD40 ligand. Thus, the invading microorganisms regulate the T cell response by transforming a tolerance-inducing iDC via accessory signals in an immunostimulatory mDC.
In conclusion, we have found that repetitive stimulation of naive cord blood–derived CD4+ T cells by allogeneic iDCs generates T cells that display the typical properties of Tr cells including: (a) a characteristic cytokine production profile (IL-10+/+IL-4−IL-2+/−IFN-γ+/−); (b) intrinsic low proliferative capacity; and (c) suppression of antigen-specific proliferation of other T cells. Our data also point to the induction of Tr cells by iDCs as a peripheral tolerance mechanism to self in the steady state. Our observation has important implications for DC vaccination against infections and tumors 14, as the use of iDCs rather than mDCs might result in tolerance induction by inducing Tr cells.
Acknowledgments
The authors thank Dr. K. Steinbrink, Dr. A. Tüttenberg, and Dr. M. Maurer for critical reading of this manuscript and helpful discussions. We also thank Dr. R. Kroczek for providing anti-ICOS Abs.
This work was supported by Deutsche Forschungsgemeinschaft grants A6 SFB432 (to A.H. Enk) and C13 SFB263 (to G. Schuler).
Footnotes
H. Jonuleit and E. Schmitt contributed equally to this work.
Abbreviations used in this paper: CTLA, CTL-associated molecule; DC, dendritic cell; iDC, immature DC; mDC, mature DC; ICOS, inducible costimulator; Tr, regulatory T.
References
- Young J.W., Steinman R.M. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J. Exp. Med. 1990;171:1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Cella M., Sallusto F., Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997;9:10–16. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- De Smedt T., Pajak B., Muraille E., Lespagnard L., Heinen E., De Baetselier P., Urbain J., Leo O., Moser M. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 1996;184:1413–1424. doi: 10.1084/jem.184.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Koide S., Crowley M., Witmer Pack M., Livingstone A.M., Fathman C.G., Inaba K., Steinman R.M. Presentation of exogenous protein antigens by dendritic cells to T cell clones. Intact protein is presented best by immature, epidermal Langerhans cells. J. Exp. Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T.R., Sad S. The expanding universe of T-cell subsetsTh1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Murphy K. Role of cytokines in development of Th1 and Th2 cells. Chem. Immunol. 1996;63:1–13. doi: 10.1159/000319475. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Macatonia S.E., Hosken N.A., Litton M., Vieira P., Hsieh C.S., Culpepper J.A., Wysocka M., Trinchieri G., Murphy K.M., O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- Hosken N.A., Shibuya K., Heath A.W., Murphy K.M., O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-α/β-transgenic model. J. Exp. Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G.J., Boussiotis V.A., Anumanthan A., Bernstein G.M., Ke X.Y., Rennert P.D., Gray G.S., Gribben J.G., Nadler L.M. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R.M. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J. Exp. Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani N., Reider D., Heuer M., Ebner S., Kampgen E., Eibl B., Niederwieser D., Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- Jonuleit H., Kuhn U., Muller G., Steinbrink K., Paragnik L., Schmitt E., Knop J., Enk A.H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- Bender A., Sapp M., Schuler G., Steinman R.M., Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Krummel M.F., Boitel B., Hurwitz A., Sullivan T.J., Fournier S., Cassell D., Brunner M., Allison J.P. The role of CTLA-4 in the regulation and initiation of T-cell responses. Immunol. Rev. 1996;153:27–46. doi: 10.1111/j.1600-065x.1996.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Stuhler G., Zobywalski A., Grunebach F., Brossart P., Reichardt V.L., Barth H., Stevanovic S., Brugger W., Kanz L., Schlossman S.F. Immune regulatory loops determine productive interactions within human T lymphocyte-dendritic cell clusters. Proc. Natl. Acad. Sci. USA. 1999;96:1532–1535. doi: 10.1073/pnas.96.4.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen R.Q., Lens S.M., Lammers K., Kuiper H., Beckmann M.P., van Lier R.A. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer Lehmann K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F., Stanzl U., Jennewein P., Janke K., Heufler C., Kampgen E., Romani N., Schuler G. High level IL-12 production by murine dendritic cellsupregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Assenmacher M., Lohning M., Scheffold A., Richter A., Miltenyi S., Schmitz J., Radbruch A. Commitment of individual Th1-like lymphocytes to expression of IFN-gamma versus IL-4 and IL-10selective induction of IL-10 by sequential stimulation of naive Th cells with IL-12 and IL-4. J. Immunol. 1998;161:2825–2829. [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K., Wolfl M., Jonuleit H., Knop J., Enk A.H. Induction of tolerance by IL-10-treated dendritic cells. J. Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- Steinbrink K., Jonuleit H., Muller G., Schuler G., Knop J., Enk A.H. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8+ T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Tivol E.A., Borriello F., Schweitzer A.N., Lynch W.P., Bluestone J.A., Sharpe A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Thomson A.W., Lu L. Are dendritic cells the key to liver transplant tolerance? Immunol. Today. 1999;20:27–32. doi: 10.1016/s0167-5699(98)01378-4. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y.J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Khanna A., Morelli A.E., Zhong C., Takayama T., Lu L., Thomson A.W. Effects of liver-derived dendritic cell progenitors on Th1- and Th2-like cytokine responses in vitro and in vivo. J. Immunol. 2000;164:1346–1352. doi: 10.4049/jimmunol.164.3.1346. [DOI] [PubMed] [Google Scholar]
- Lutz M.B., Kukutsch N.A., Menges M., Rossner S., Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur. J. Immunol. 2000;30:1048–1052. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Lutz M.B., Suri R.M., Niimi M., Ogilvie A.L., Kukutsch N.A., Rossner S., Schuler G., Austyn J.M. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur. J. Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]