Abstract
Innate immune responses to pathogens critically impact the development of adaptive immune responses. However, it is not completely understood how innate immunity controls the initiation of adaptive immunities or how it determines which type of adaptive immunity will be induced to eliminate a given pathogen. Here we show that viral stimulation not only triggers natural interferon (IFN)-α/β–producing cells (IPCs) to produce vast amounts of antiviral IFN-α/β but also induces these cells to differentiate into dendritic cells (DCs). IFN-α/β and tumor necrosis factor α produced by virus-activated IPCs act as autocrine survival and DC differentiation factors, respectively. The virus-induced DCs stimulate naive CD4+ T cells to produce IFN-γ and interleukin (IL)-10, in contrast to IL-3–induced DCs, which stimulate naive CD4+ T cells to produce T helper type 2 cytokines IL-4, IL-5, and IL-10. Thus, IPCs may play two master roles in antiviral immune responses: directly inhibiting viral replication by producing large amounts of IFN-α/β, and subsequently triggering adaptive T cell–mediated immunity by differentiating into DCs. IPCs constitute a critical link between innate and adaptive immunity.
Keywords: dendritic cells, interferon α/β–producing cells, innate immunity, adaptive immunity, T cells
Introduction
Innate immunity has two functions: (i) directly killing pathogens and (ii) determining the initiation and type of adaptive immune responses 1 2. In most cases, cells in the innate immune system indirectly perform the second function by inducing maturation of dendritic cells (DCs) with proinflammatory cytokines such as IL-1 and TNF-α 3 4 5. The mature DCs then initiate adaptive immune responses by strongly activating antigen-specific naive T cells 6 and may also dictate the type of Th cell response to be Th1 or Th2 7 8 9 10. Thus, different cell types in the immune system are specialized to perform a particular function and cooperate to create the whole immune response.
IFN-α/β plays an essential role in antiviral innate immunity 11 by directly inhibiting viral replication in infected cells 12. Recent studies have shown that CD4+CD11c− type 2 DC precursors (pre-DC2s) in human blood are identical to natural IFN-α/β–producing cells (IPCs), which produce enormous amounts of IFN-α/β in response to viruses 13 14 15. Thus, IPCs/pre-DC2s may represent a crucial effector cell type in antiviral innate immunity. Interestingly, unlike other effector cell types in the innate immune system such as neutrophils, macrophages, and mast cells, IPCs have the potential to differentiate into DCs when cultured with IL-3 and CD40 ligand 16. However, these factors, mainly derived from activated T cells, may not represent the earliest physiological signals for DC differentiation in antiviral immune responses. Therefore, we asked (i) whether viruses can directly induce IPCs to differentiate into DCs after triggering the antiviral innate effector function of IPCs and (ii) what kind of T cell responses virus-induced DCs would initiate.
Materials and Methods
Isolation and Culture of Cells.
Monocytes, CD11c+ DCs, and IPCs were isolated from human peripheral blood as described 3 16. To isolate monocytes, total PBMCs were centrifuged on 52% Percoll (Amersham Pharmacia Biotech). The low-density cells were depleted of lymphocytes with a mixture of anti-CD2, anti-CD3, anti-CD8, anti-CD19, anti-CD20, and anti-CD56 mAbs and with magnetic beads coated with goat anti–mouse IgG (Dynabeads M-450; Dynal). To isolate CD11c+ DCs and IPCs, total PBMCs were depleted of lymphocytes and monocytes with a mixture of anti-CD3, anti-CD14, anti-CD16, anti-CD19, and anti-CD56 mAbs and with magnetic beads (Dynal). CD4+CD11c+lin− cells and CD4+CD11c−lin− cells were isolated as CD11c+ DCs and IPCs, respectively, by cell sorting. The cells were cultured in RPMI 1640 containing 10% FCS at 2 × 104 cells per 200 μl in round-bottomed 96-well culture plates in the presence of 106 PFU/ml HSV-1 (KOS strain) attenuated with gamma irradiation or 106 PFU/ml intact influenza virus (PR8 strain). The concentrations of viruses that induced optimal survival and IFN production were selected. Higher concentrations of viruses induced cell death, probably due to an overwhelming cytopathic effect (data not shown).
Flow Cytometric Analysis.
IPCs were cultured for 3 d with 106 PFU/ml HSV, 10 ng/ml IL-3 (R&D Systems), 500 IU/ml IFN-α2b (Schering-Plough), 10 ng/ml TNF-α (R&D Systems), 10 ng/ml IL-6 (DNAX), 20 μg/ml mouse anti–human TNF-α mAb (MP9-20A4; a gift from J. Abrams, DNAX), or 20 μg/ml mouse anti–human IL-6 mAb (MQ2-39C3; DNAX). The resulting cells were stained with FITC-conjugated anti–HLA-A,B,C (G46-2.6; PharMingen), FITC-conjugated anti–HLA-DR (L243; Becton Dickinson), PE-conjugated anti-CD80 (L307.4; Becton Dickinson), PE-conjugated anti-CD86 (2331; PharMingen), or an isotype control Ab and were analyzed with a FACScan™ flow cytometer (Becton Dickinson). Dead cells were excluded by staining with propidium iodide.
T Cell Proliferation Assay.
Naive CD4+ T cells were isolated from umbilical cord blood (Advanced Bioscience Technologies) using a CD4 Positive Isolation Kit (Dynal). 5 × 104 naive CD4+ T cells were cocultured with fresh IPCs, HSV-induced DCs (HSV-DCs), or IL-3–induced DCs (IL-3–DCs) in round-bottomed 96-well culture plates in Yssel's medium (Irvine Scientific) containing 10% FCS. After 5 d, cells were pulsed with 1 μCi [3H]thymidine (Amersham Pharmacia Biotech) for 16 h before harvesting.
DC–T Cell Coculture for Cytokine Assays.
5 × 104 allogeneic naive CD4+ T cells were cocultured with 104 DCs for 6 d in round-bottomed 96-well culture plates in Yssel's medium containing 10% FCS. In some experiments, 10 μg/ml neutralizing mouse anti–human IL-12 mAb C.8.6.22 17 and/or a mixture of 1,000 neutralization U/ml rabbit anti–human IFN-α Ab (PBL), 2,000 neutralization U/ml rabbit anti–human IFN-β Ab (PBL), and 10 μg/ml mouse anti–human IFN-α/β receptor mAb IFNaRβ1 18 was added during the coculture. Alternatively, T cells were cultured for 6 d with plate-bound anti-CD3 mAb SPV-T3b 19 and 1 μg/ml soluble anti-CD28 mAb L293.1 (Becton Dickinson). After 6 d of priming, T cells were washed and restimulated at 106 cells/ml with anti-CD3 and anti-CD28 for 24 h for ELISA or for 5 h for intracellular cytokine staining. 10 μg/ml brefeldin A (Epicentre Technologies) was added at 2.5 h for intracellular staining.
Quantitation of Cytokine Secretion by ELISA.
ELISA kits from the following companies were used to analyze cytokine production: IFN-α (Biosource International), IFN-β (FUJIREBIO), IL-18 (MBL Medical and Biological Lab. Co.), IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-10, IL-12, IL-15, IFN-γ, lymphotoxin α, and GM-CSF (R&D Systems).
Flow Cytometric Analysis of Intracellular Cytokines.
Intracellular cytokines produced by T cells were analyzed as described 8.
Results
Viruses Directly Induce IPCs to Differentiate into DCs.
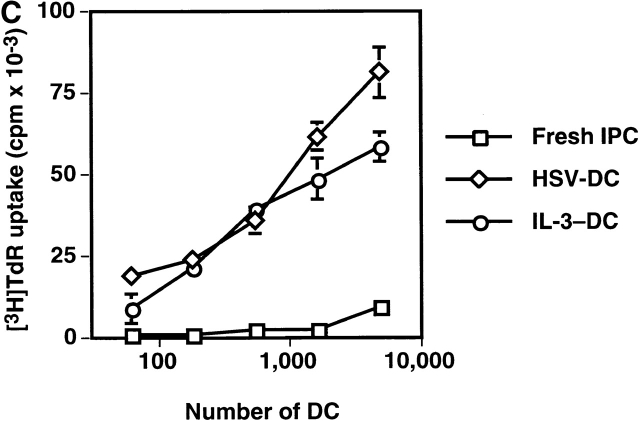
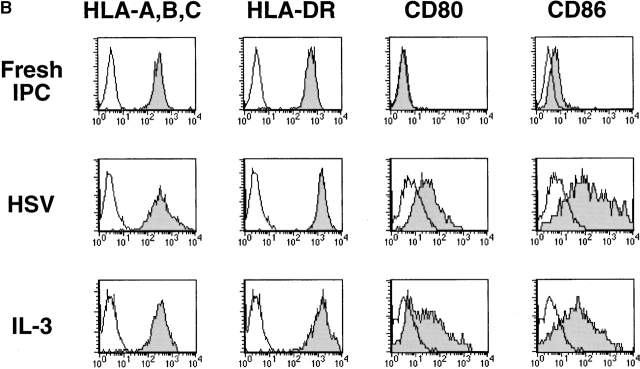
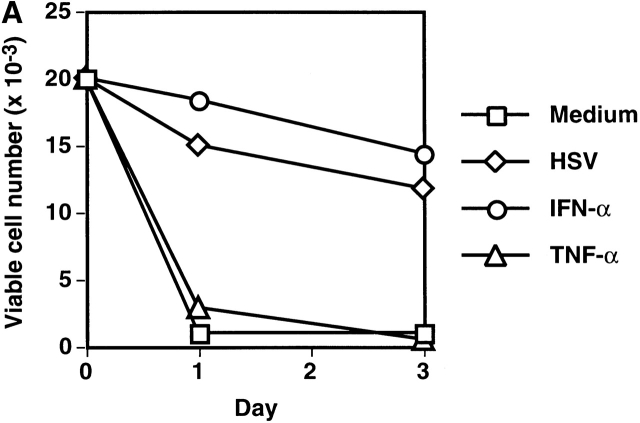
We and others previously showed that IPCs die rapidly and that few viable cells are found after 3 d of culture in medium alone (reference 16, 20, 21; Fig. 1 A). However, ∼50% of the initial numbers of the cells were viable after 3 d of culture with either HSV or IL-3. IPCs differentiate into immature DC2s when cultured with IL-3 16. Similarly, IPCs acquired DC morphology after 3 d of culture with HSV (data not shown). They also increased the expression of MHC class I and class II antigens as well as costimulatory molecules CD80 and CD86 (Fig. 1 B). IPCs stimulated with HSV for 3 d (HSV-DCs) were as potent as IPCs stimulated with IL-3 for 3 d (IL-3–DCs) in inducing proliferation of allogeneic naive CD4+ T cells (Fig. 1 C). Influenza virus also induced IPCs to differentiate into DCs (data not shown). These data demonstrate that viruses can directly induce differentiation of the DC precursors into DCs in the absence of exogenous cytokines.
Figure 1.
HSV induces differentiation of IPCs into DCs. (A) Cell numbers of IPCs cultured in different conditions. Viable cells were counted by trypan blue exclusion. The data shown are representative of three experiments. (B) Phenotype of fresh IPCs, HSV-stimulated IPCs, and IL-3–stimulated IPCs. Open histograms represent cells stained with isotype-matched control mAbs. The data shown are representative of four experiments. (C) Allogeneic mixed lymphocyte reaction. Naive CD4+ T cells from cord blood were cocultured with different numbers of fresh IPCs, HSV-DCs, or IL-3–DCs for 6 d. Error bars indicate SD. The data shown are representative of five experiments.
IFN-α/β and TNF-α Function as Autocrine Survival and Differentiation Factors of IPCs, Respectively.
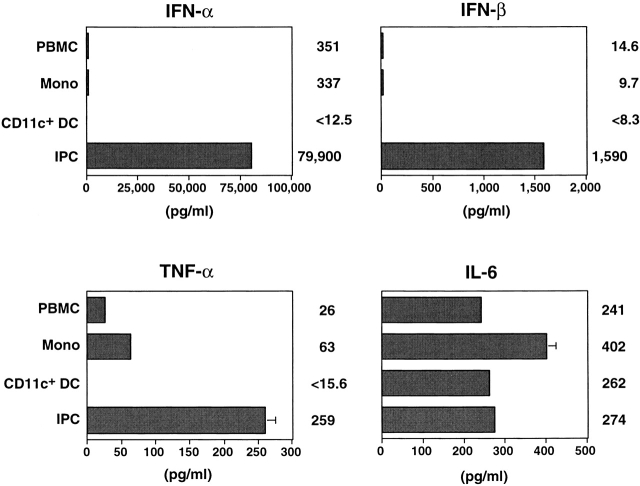
Next we tested whether endogenous cytokines from IPCs induced by viruses are responsible for the survival and differentiation of the cells. Consistent with previous reports 14 15, IPCs produced large amounts of IFN-α and IFN-β within 24 h in response to HSV, whereas monocytes and CD11c+ DCs produced only small or undetectable amounts (Fig. 2). IPCs also produced significant amounts of TNF-α and IL-6 (Fig. 2), but IL-1α, IL-1β, IL-3, IL-10, IL-12, IL-15, IL-18, IFN-γ, lymphotoxin α, and GM-CSF were undetectable (data not shown). IFN-α, but not TNF-α (Fig. 3 A) or IL-6 (data not shown), was found to maintain the survival of IPCs during 3 d of culture, suggesting that IFN-α is a virus-induced autocrine survival factor for IPCs. IFN-α did not upregulate CD80 and CD86 on IPCs (Fig. 3 B). However, TNF-α was found to upregulate CD86 and to a lesser extent CD80 on IPCs when added with IFN-α (Fig. 3 B). Anti–TNF-α diminished the expression of CD80 and CD86 on HSV-stimulated IPCs (data not shown). On the other hand, IL-6 or anti–IL-6 did not affect the expression of CD80 and CD86 (data not shown). Thus, IFN-α/β and TNF-α may represent virus-induced autocrine survival factors and a partial differentiation factor, respectively, for IPCs to become DCs.
Figure 2.
Cytokine production by different populations of blood cells stimulated with HSV. Cells were stimulated with HSV for 24 h, and cytokine concentrations in the supernatants were measured by ELISA. PBMC, total PBMCs; Mono, monocytes; CD11c+ DC, FACS-sorted CD11c+lin− immature DCs; IPC, FACS®-sorted CD4+ CD11c−lin− cells. Error bars indicate SD. The data shown are representative of four experiments.
Figure 3.
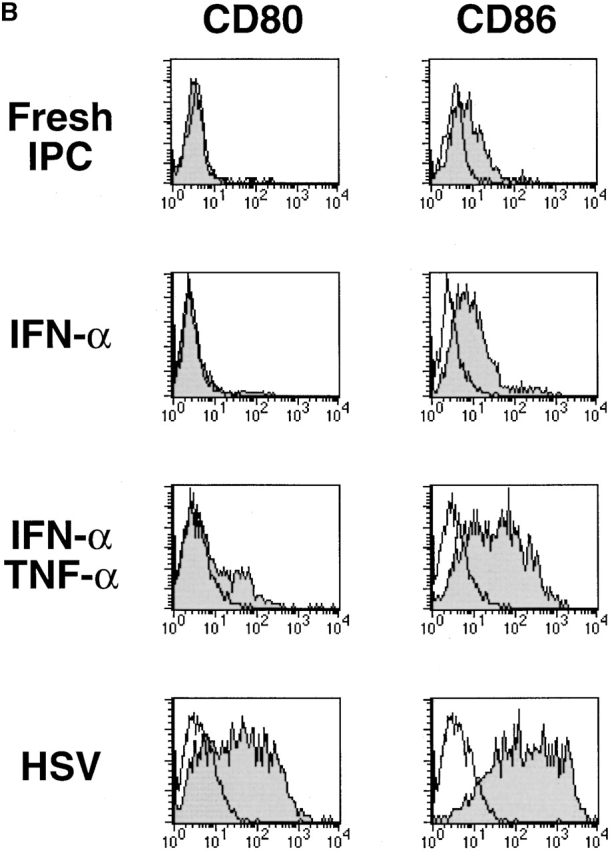
IFN-α and TNF-α function as autocrine survival and differentiation factors of IPCs, respectively. (A) Cell numbers of IPCs cultured without stimulation or with HSV, IFN-α, or TNF-α. Viable cells were counted by trypan blue exclusion. The data shown are representative of three experiments. (B) Expression of CD80 and CD86 on fresh IPCs and IPCs cultured with IFN-α, IFN-α and TNF-α, or HSV for 3 d. Few cells remained viable in culture with TNF-α alone, as shown in A. Open histograms represent cells stained with isotype-matched control mAbs. The data shown are representative of four experiments.
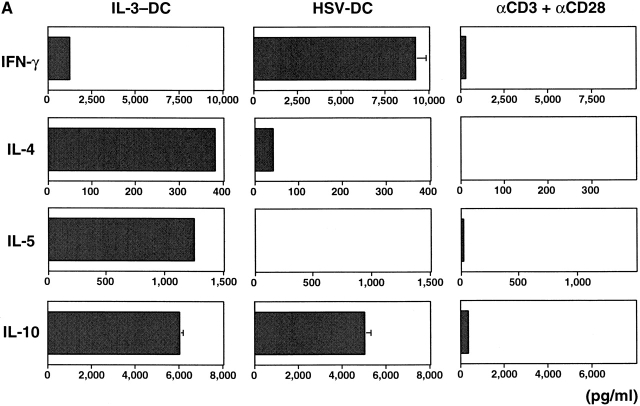
IL-3–DCs and HSV-DCs Induce Distinct T Cell Differentiation.
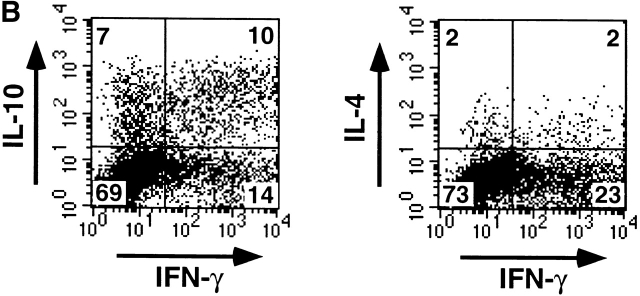
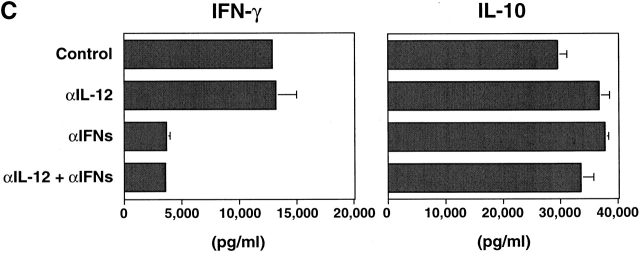
To examine the role of HSV-DCs in naive T helper cell differentiation, allogeneic naive CD4+ T cells were cultured for 6 d with IL-3–DCs, HSV-DCs, or anti-CD3 and anti-CD28. The activated T cells were restimulated with anti-CD3 and anti-CD28 for either 5 h for intracellular cytokine staining or 24 h for cytokine ELISA of the culture supernatants. Whereas IL-3–DCs induced naive CD4+ T cells to produce high levels of IL-4, IL-5, and IL-10 and a low level of IFN-γ as reported 8, HSV-DCs induced naive CD4+ T cells to produce high levels of IFN-γ and IL-10 and a low level of IL-4 (Fig. 4 A). Intracellular cytokine staining showed that HSV-DCs induced CD4+ T cells to differentiate into three subpopulations with respect to IFN-γ and IL-10 expression: (i) IFN-γ+IL-10−, (ii) IFN-γ−IL-10+, and (iii) IFN-γ+IL-10+ (Fig. 4 B). IL-12 22 and IFN-α (de Waal Malefyt, R., personal communication and reference 23) were shown to induce T cells to produce IFN-γ and IL-10. However, IPCs did not produce a detectable level of IL-12 during 3 d of culture with HSV (data not shown), and anti–IL-12 mAb did not inhibit IFN-γ or IL-10 production by T cells cultured with HSV-DCs (Fig. 4 C), suggesting that IL-12 is not involved in IFN-γ and IL-10 induction in this system. A mixture of anti-IFN-α, anti-IFN-β, and anti-IFN-α/β receptor Abs significantly diminished the induction of IFN-γ but not IL-10 in the T cell–HSV-DC coculture (Fig. 4 C), indicating that IFN-α/β from HSV-DCs is responsible for T cell production of IFN-γ but not IL-10. HSV-DCs may stimulate T cells to produce a high level of IL-10 by an IL-12– and IFN-α/β–independent mechanism.
Figure 4.
IL-3–DCs and HSV-DCs induce different types of differentiation of naive CD4+ T cells. Allogeneic naive CD4+ T cells were cultured for 6 d with IL-3–DCs, HSV-DCs, or anti-CD3 and anti-CD28. T cells were restimulated with anti-CD3 and anti-CD28 for 24 h (A) or 48 h (C) for ELISA or for 5 h for intracellular staining (B). (A) Quantitation of IFN-γ, IL-4, IL-5, and IL-10 by ELISA. Error bars indicate SD. The data shown are representative of four experiments. (B) Intracellular IFN-γ, IL-4, and IL-10 staining of T cells cultured with HSV-DCs. The percentages in each quadrant are indicated on the plot. The data shown are representative of three experiments. (C) IFN-γ and IL-10 production by T cells cultured with HSV-DCs in the presence of neutralizing anti–IL-12 mAb and/or a mixture of anti–IFN-α, anti–IFN-β, and IFN-α/β receptor Abs. The same concentration of anti–IL-12 mAb inhibited IFN-γ production by T cells cultured with anti-CD3, anti-CD28, and 10 ng/ml IL-12 (data not shown). Error bars indicate SD. The data shown are representative of three experiments.
Discussion
DC precursors develop into mature DCs in two steps: first, differentiation into immature DCs, and second, development from immature into mature DCs 6. Recent studies have shown that whereas microbial stimuli trigger the final maturation of DCs 24, differentiation of DC precursors into immature DCs is dependent on cytokines such as GM-CSF, TNF-α, and IL-4 3 25 26. However, the physiological relevance of these cytokines in DC differentiation in vivo remains to be established 27. Our study demonstrated that viral stimulation alone is capable of inducing DC precursors to differentiate into DCs in the absence of exogenous cytokines. This, together with the ability of monocytes to differentiate into DCs after undergoing transendothelial migration and phagocytosis 28 29, suggests that microbes, as well as cytokines, may directly trigger differentiation of DC precursors into DCs.
The finding that IL-3–DCs and HSV-DCs induce distinct types of T cell differentiation illustrates the critical role of innate immunity in determining the type of adaptive immune responses, depending on the nature of pathogens 30. Upon invasion of certain parasites or allergens, IL-3 produced by activated mast cells 31 may cause IPCs to differentiate into Th2-inducing DCs, which may contribute to the establishment of T cell–mediated allergic responses. On the other hand, HSV-DCs induce naive CD4+ T cells to produce IFN-γ and IL-10, different from a classical Th1 or Th2 type. Existence of a T cell population producing IFN-γ and IL-10 during intracellular infection with viruses 32, bacteria 33, and parasites 34 suggests that IFN-γ– and IL-10–producing T cells may play an important role in immune responses to intracellular pathogens. In fact, it has been suggested that IL-10 protects the host against detrimental effects of excessive cellular immune responses elicited during acute infection 35.
This study, together with previous studies 8 14 15, indicates that IPCs represent a unique cell lineage within the immune system, which performs the two master functions of innate immunity during their lifetime: (i) killing of viruses and (ii) initiation and dictation of adaptive immune responses. Importantly, the functional transition from IPCs to DCs occurs with viral stimulation alone in the absence of any exogenous factors. In addition to viruses, bacterial stimuli such as heat-killed Staphylococcus aureus also induce IPCs to produce large amounts of IFN-α/β 36 and to upregulate CD80 and CD86 (data not shown). These findings, together with pleiotropic effects of IFN-α/β on various cell types in the immune system such as macrophages 37, NK cells 38, and T cells 39 40 41, suggest that IPCs represent a crucial cell type in the immune system that, upon recognizing various types of pathogens, promptly alerts the immune system to “dangers” 42 by producing vast amounts of IFN-α/β and subsequently initiates adaptive immune responses by differentiating into DCs. Furthermore, IPCs may be involved in allergic responses by differentiating into Th2-inducing DCs in response to IL-3. This marked versatility of IPCs distinguishes them from other cell types in the immune system that have only limited functions and suggests that IPCs may play a key role in integrating the innate and adaptive aspects of various immune responses.
In conclusion, this study offers an account of the cellular and molecular mechanisms by which innate immunity connects with and shapes adaptive immunity. Owing to the apparent involvement of IPCs in a variety of immune responses, enhancing or suppressing functions of these cells may serve as potential therapies for infectious diseases, cancers, and allergic diseases.
Acknowledgments
We thank T.L. Nagabhushan for IFN-α2b, R. Chase for HSV-1, R.L. Coffman for influenza virus, O.R. Colamonici for anti–IFN-α/β receptor mAb, J. Cupp for cell sorting, and B. Blom, R. de Waal Malefyt, H. Kanzler, L.L. Lanier, A. O'Garra, and D. Wylie for critical reading of the manuscript.
DNAX Research Institute of Molecular and Cellular Biology is supported by Schering-Plough Corporation.
Footnotes
N. Kadowaki's present address is Dept. of Hematology and Oncology, Graduate School of Medicine, Kyoto University, 54 Shogoin Kawara-cho, Sakyo-ku, Kyoto 606-8507, Japan.
Abbreviations used in this paper: DCs, dendritic cells; IPCs, natural IFN-α/β–producing cells.
References
- Fearon D.T., Locksley R.M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunityimpact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Cella M., Danieli C., Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartmentdownregulation by cytokines and bacterial products. J. Exp. Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- Rissoan M.-C., Soumelis V., Kadowaki N., Grouard G., Briere F., de Waal Malefyt R., Liu Y.-J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R., De Smedt T., Michel P., Godfroid J., Pajak B., Heirman C., Thielemans K., Leo O., Urbain J., Moser M. CD8α1 and CD8α2 subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., Smith J.L., Caspary G., Brasel K., Pettit D., Maraskovsky E., Maliszewski C.R. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U., Steinhoff U., Reis L.F., Hemmi S., Pavlovic J., Zinkernagel R.M., Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Isaacs A., Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. 1957;147:258–267. [PubMed] [Google Scholar]
- Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol. Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Cella M., Jarrossay D., Facchetti F., Alebardi O., Nakajima H., Lanzavecchia A., Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.C., Filgueira L., Durand I., Banchereau J., Liu Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A., Rengaraju M., Valiante N.M., Chehimi J., Kubin M., Aste M., Chan S.H., Kobayashi M., Young D., Nickbarg E. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J. Exp. Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamonici O.R., Domanski P. Identification of a novel subunit of the type I interferon receptor localized to human chromosome 21. J. Biol. Chem. 1993;268:10895–10899. [PubMed] [Google Scholar]
- Spits H., Keizer G., Borst J., Terhorst C., Hekman A., de Vries J.E. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R.M., Peng M., Cameron P.U., Gezelter S., Kopeloff I., Swiggard W.J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J. Exp. Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus J., BitMansour A., Warnke R., Thompson P.A., Carballido J., Picker L.J., Lund-Johansen F. Dendritic cell ontogenya human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F., Paganin C., Peritt D., Paiola F., Scupoli M.T., Aste-Amezaga M., Frank I., Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeure C.E., Wu C.Y., Shu U., Schneider P.V., Heusser C., Yssel H., Delespesse G. In vitro maturation of human neonatal CD4 T lymphocytes. II. Cytokines present at priming modulate the development of lymphokine production. J. Immunol. 1994;152:4775–4782. [PubMed] [Google Scholar]
- Reis e Sousa C., Sher A., Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr. Opin. Immunol. 1999;11:392–399. doi: 10.1016/S0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kampgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P.O., Steinman R.M., Schuler G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Vremec D., Lieschke G.J., Dunn A.R., Robb L., Metcalf D., Shortman K. The influence of granulocyte/macrophage colony-stimulating factor on dendritic cell levels in mouse lymphoid organs. Eur. J. Immunol. 1997;27:40–44. doi: 10.1002/eji.1830270107. [DOI] [PubMed] [Google Scholar]
- Randolph G.J., Beaulieu S., Lebecque S., Steinman R.M., Muller W.A. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- Randolph G.J., Inaba K., Robbiani D.F., Steinman R.M., Muller W.A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- Abbas A.K., Murphy K.M., Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J.H., Watson C.J., Hanley-Hyde J., Nordan R.P., Paul W.E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Sarawar S.R., Doherty P.C. Concurrent production of interleukin-2, interleukin-10, and gamma interferon in the regional lymph nodes of mice with influenza pneumonia. J. Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F., Nisii C., Righetti S., Micciolo R., Marchesini M., Cazzadori A., Trinchieri G. CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin. Immunol. 1999;92:224–234. doi: 10.1006/clim.1999.4752. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Channon J.Y., Matsuura T., Schwartzman J.D., Shin D.W., Kasper L.H. Functional and quantitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp. Parasitol. 1999;91:212–221. doi: 10.1006/expr.1998.4359. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R.T., Wysocka M., Hieny S., Scharton-Kersten T., Cheever A., Kuhn R., Muller W., Trinchieri G., Sher A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 1996;157:798–805. [PubMed] [Google Scholar]
- Svensson H., Cederblad B., Lindahl M., Alm G. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus . J. Interferon Cytokine Res. 1996;16:7–16. doi: 10.1089/jir.1996.16.7. [DOI] [PubMed] [Google Scholar]
- Ortaldo J.R., Mantovani A., Hobbs D., Rubinstein M., Pestka S., Herberman R.B. Effects of several species of human leukocyte interferon on cytotoxic activity of NK cells and monocytes. Int. J. Cancer. 1983;31:285–289. doi: 10.1002/ijc.2910310306. [DOI] [PubMed] [Google Scholar]
- Biron C.A. Activation and function of natural killer cell responses during viral infections. Curr. Opin. Immunol. 1997;9:24–34. doi: 10.1016/s0952-7915(97)80155-0. [DOI] [PubMed] [Google Scholar]
- Sun S., Zhang X., Tough D.F., Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J., Mitchell T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousens L.P., Peterson R., Hsu S., Dorner A., Altman J.D., Ahmed R., Biron C.A. Two roads divergedinterferon α/β– and interleukin 12–mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]