Abstract
1. The effects of NG-nitro-L-arginine methyl ester (L-NAME) and NG-monomethyl-L-arginine (L-NMMA), their D-isomers, and dexamethasone on noradrenaline (NA)-induced contractions and antagonism by alpha-adrenoceptor antagonists, have been investigated in rat isolated thoracic aortic rings with/without endothelium. 2. NA produced concentration-dependent contractions of isolated aortic rings with EC50 values of 2.41 +/- 0.54 (n = 21) and 28.00 +/- 8.50 (n = 25) nM for endothelium-denuded and -intact preparations respectively. Acetylcholine (ACh) relaxed NA-precontracted rings with intact, but not those denuded of endothelium. 3. Treatment with L-NAME (1-30 microM), or L-NMMA (10-500 microM), but not their D-isomers, resulted in an endothelium-dependent enhancement of NA-induced contractions. Pre-treatment, in vitro, with 0.5 microM dexamethasone neither directly potentiated, nor influenced L-NAME-induced potentiation of NA-mediated contractions in endothelium-intact rings; however, dexamethasone pretreatment reduced EC50 values for NA, and also prevented L-NAME-induced potentiation, in denuded rings equilibrated for 5 h under resting tension. 4. In both intact and denuded rings, phentolamine, prazosin and WB 4101 shifted NA concentration-response curves to the right; L-NAME, and also L-NMMA, but not their D-isomers, reversed the blockade as indicated by significant decreases in NA dose-ratios. In denuded rings, reversal by L-NAME or L-NMMA was prevented following pretreatment with dexamethasone.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

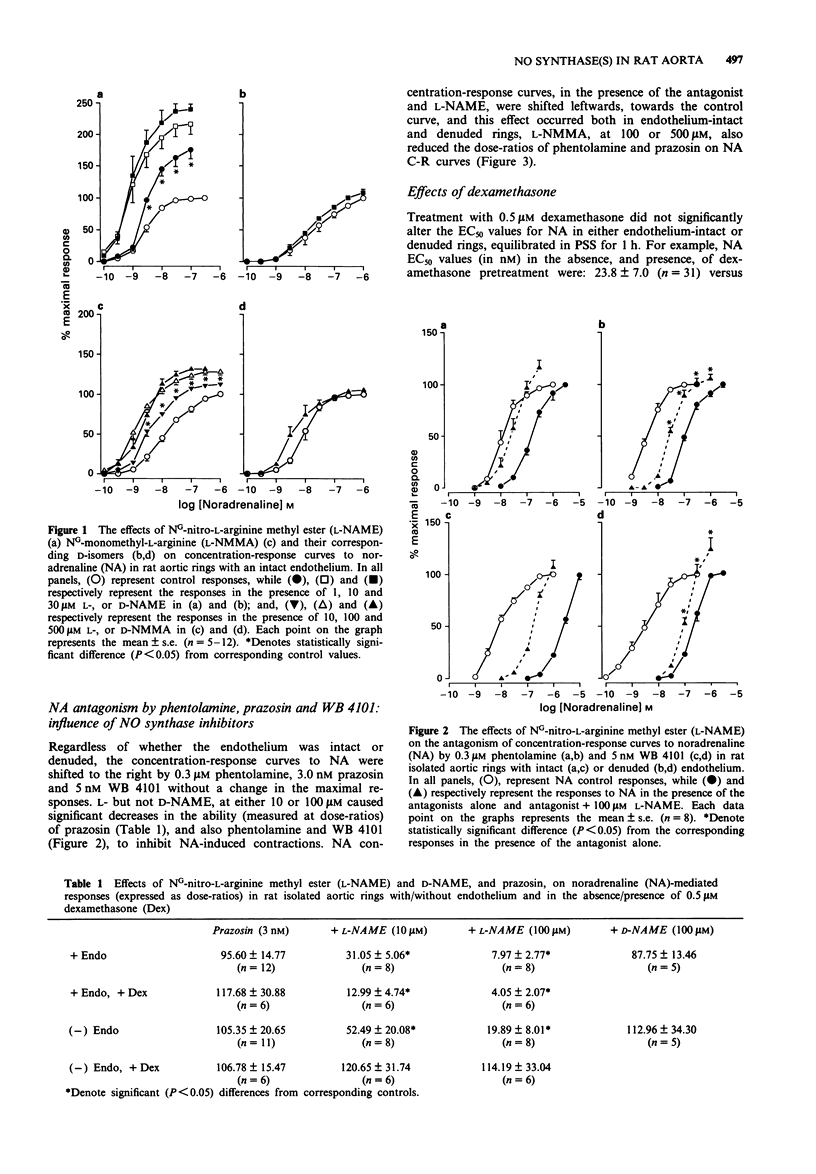
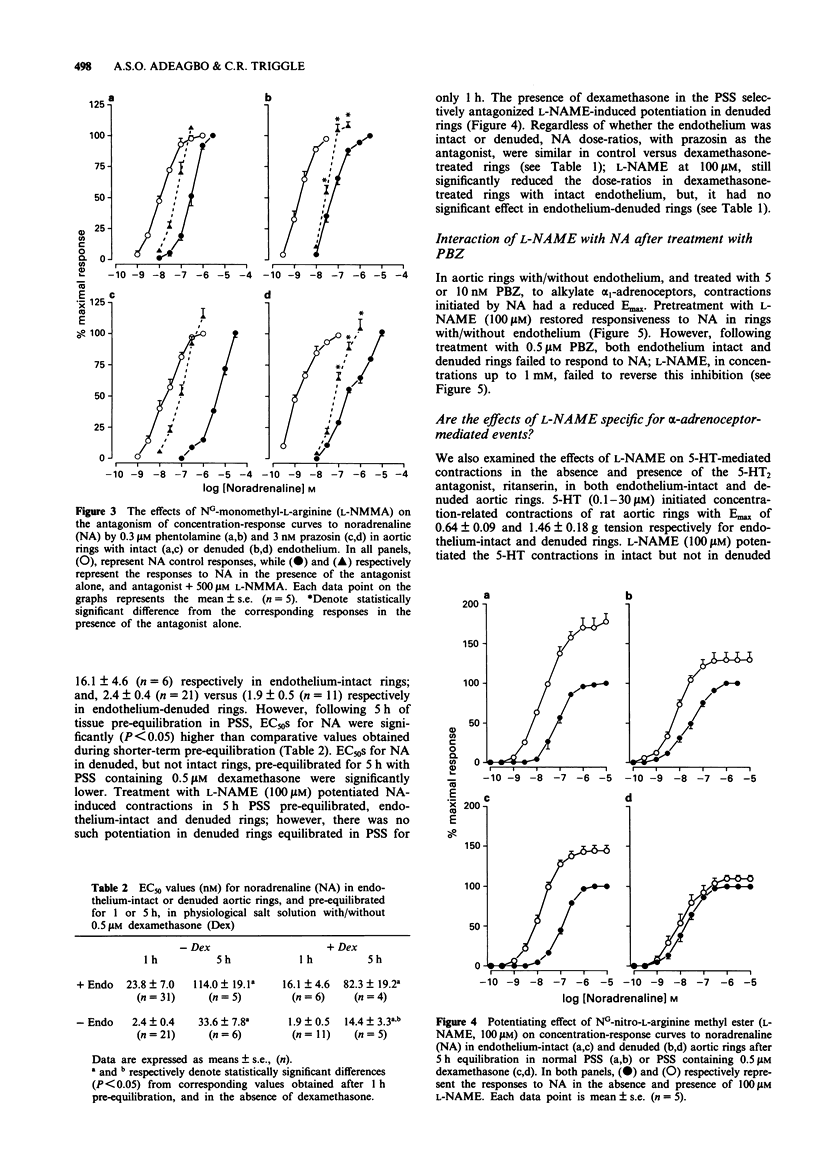
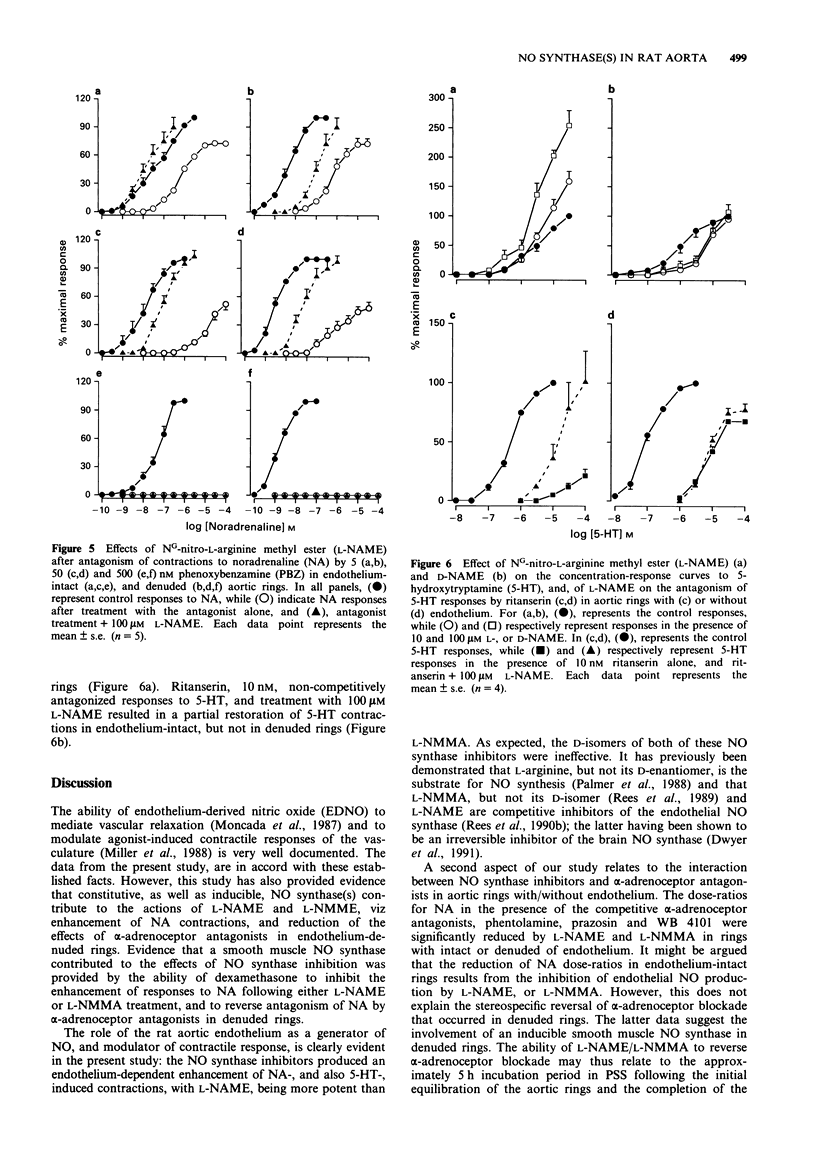


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alosachie I., Godfraind T. Role of cyclic GMP in the modulation by endothelium of the adrenolytic action of prazosin in the rat isolated aorta. Br J Pharmacol. 1986 Nov;89(3):525–532. doi: 10.1111/j.1476-5381.1986.tb11152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosachie I., Godfraind T. The modulatory role of vascular endothelium in the interaction of agonists and antagonists with alpha-adrenoceptors in the rat aorta. Br J Pharmacol. 1988 Oct;95(2):619–629. doi: 10.1111/j.1476-5381.1988.tb11684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman-Krzan M. Role of endothelium in the binding of [3H]prazosin to the aortic alpha 1-adrenergic receptors. Eur J Pharmacol. 1985 Apr 23;111(1):137–138. doi: 10.1016/0014-2999(85)90124-4. [DOI] [PubMed] [Google Scholar]
- Carrier G. O., White R. E. Enhancement of alpha-1 and alpha-2 adrenergic agonist-induced vasoconstriction by removal of endothelium in rat aorta. J Pharmacol Exp Ther. 1985 Mar;232(3):682–687. [PubMed] [Google Scholar]
- Collier J., Vallance P. Second messenger role for NO widens to nervous and immune systems. Trends Pharmacol Sci. 1989 Nov;10(11):427–431. doi: 10.1016/s0165-6147(89)80001-x. [DOI] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Downie J. W., Slack B. E. Sensitivity to indomethacin of tetrodotoxin-resistant contractions of smooth muscle from the base of rabbit bladder. Br J Pharmacol. 1983 Jun;79(2):334–336. doi: 10.1111/j.1476-5381.1983.tb11005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval D., Funder J. W., Devynck M. A., Meyer H. Arterial glucocorticoid receptors: the binding of tritiated dexamethasone in rabbit aorta. Cardiovasc Res. 1977 Nov;11(6):529–535. doi: 10.1093/cvr/11.6.529. [DOI] [PubMed] [Google Scholar]
- Dwyer M. A., Bredt D. S., Snyder S. H. Nitric oxide synthase: irreversible inhibition by L-NG-nitroarginine in brain in vitro and in vivo. Biochem Biophys Res Commun. 1991 May 15;176(3):1136–1141. doi: 10.1016/0006-291x(91)90403-t. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Kalsner S. Mechanism of hydrocortisone potentiation of responses to epinephrine and norepinephrine in rabbit aorta. Circ Res. 1969 Mar;24(3):383–395. doi: 10.1161/01.res.24.3.383. [DOI] [PubMed] [Google Scholar]
- Lues I., Schümann H. J. Effect of removing the endothelial cells on the reactivity of rat aortic segments to different alpha-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1984 Dec;328(2):160–163. doi: 10.1007/BF00512066. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Mostaghim R., Thomas G., Ramwell P. W. Endothelial potentiation of relaxation response to phentolamine in rat thoracic aorta. J Pharmacol Exp Ther. 1988 Feb;244(2):475–478. [PubMed] [Google Scholar]
- Oriowo M. A., Nichols A. J., Ruffolo R. R., Jr Receptor protection studies with phenoxybenzamine indicate that a single alpha 1-adrenoceptor may be coupled to two signal transduction processes in vascular smooth muscle. Pharmacology. 1992;45(1):17–26. doi: 10.1159/000138968. [DOI] [PubMed] [Google Scholar]
- Oriowo M. A., Ruffolo R. R., Jr Activation of a single alpha-1-adrenoceptor subtype in rat aorta mobilizes intracellular and extracellular pools of calcium. Pharmacology. 1992;44(3):139–149. doi: 10.1159/000138906. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Frendo B., Eloy L., Grünfeld J. P. Methylprednisolone-induced hypertension in the rat: evidence against the role of plasma volume changes, vasopressin and renal prostaglandin E2. J Hypertens. 1985 Oct;3(5):461–467. [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schini V. B., Vanhoutte P. M. L-arginine evokes both endothelium-dependent and -independent relaxations in L-arginine-depleted aortas of the rat. Circ Res. 1991 Jan;68(1):209–216. doi: 10.1161/01.res.68.1.209. [DOI] [PubMed] [Google Scholar]
- Yard A. C., Kadowitz P. J. Studies on the mechanism of hydrocortisone potentiation of vasoconstrictor responses to epinephrine in the anesthetized animal. Eur J Pharmacol. 1972 Oct;20(1):1–9. doi: 10.1016/0014-2999(72)90209-9. [DOI] [PubMed] [Google Scholar]


