Summary
Ubiquitin (Ub) and ubiquitin-like (Ubl) proteins regulate a diverse array of cellular pathways through post-translational attachment to protein substrates. Ub/Ubl-mediated signaling is initiated through E1, E2, and E3-mediated conjugation, transduced by proteins that recognize Ub/Ubl-modified substrates, and terminated by proteases which remove the Ub/Ubl from the substrate. Recent structural studies have elucidated mechanisms pertinent to Ub/Ubl conjugation, recognition, and deconjugation, highlighting essential steps during Ub/Ubl modification that illustrate common and divergent mechanistic themes within this important process.
Introduction
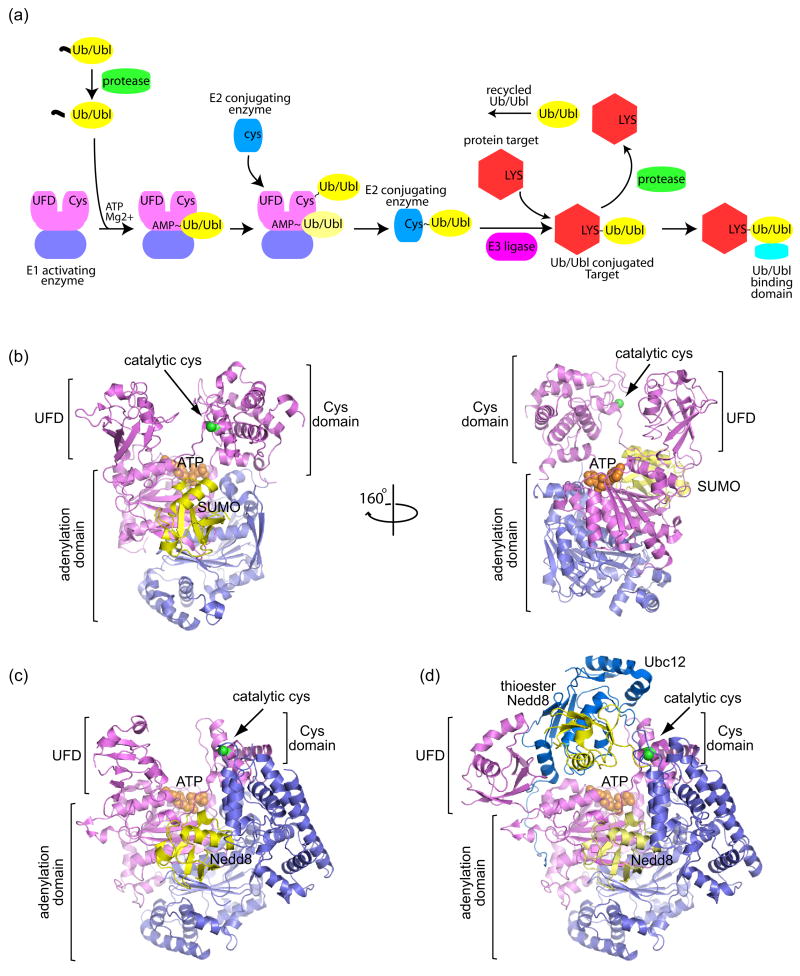
Post-translational protein modification by ubiquitin (Ub) and ubiquitin-like (Ubl) modifiers regulates a variety of cellular processes, including protein degradation, signaling, sorting, localization, activation and repression [1]. There are up to 14 distinct Ub/Ubl families, and while diverse at the primary sequence level, most exhibit similar three-dimensional folds. In instances where Ub/Ubls impart their activities through covalent attachment to substrates, Ub/Ubl pathways utilize analogous enzymes to facilitate covalent isopeptide bond formation between the Ub/Ubl C-terminus and substrate lysine (Figure 1a) [2].
Figure 1.
Schematic of the Ubiquitin/Ubiquitin-like (Ub/Ubl) protein modification pathway and structures of E1 activating enzymes. (a) The Ub/Ubl modification pathway begins with precursor Ub/Ubl (yellow) processing by proteases (green), exposing a C-terminal Gly-Gly motif. Ub/Ubl is activated by E1 enzymes (violet/pink) and transferred to a catalytic E1 cysteine residue to form a thioester bond (denoted by ‘~’). When the Ub/Ubl is linked to the E1 via a thioester, another Ub/Ubl may bind to the adenylation site (light yellow Ub/Ubl). E1 recruits a cognate E2 conjugating enzyme (blue) and the Ub/Ubl is transferred to the E2 via a thioester bond. Facilitated by an E3 ligase (magenta), the Ub/Ubl is conjugated to a Lys residue on a protein target (red), forming a stable isopeptide bond (denoted by ‘-‘). The Ub/Ubl conjugated protein target can be recognized by Ub/Ubl binding domains (cyan). The Ub/Ubl ‘signal’ can be terminated by deconjugating proteases. (b) Structure of the SUMO E1 Aos1/Uba2 (violet/pink) in complex with SUMO (yellow) and ATP (orange) (PDB code 1Y8R, [3]). The locations of the adenylation domain, Cys domain, and ubiquitin-fold domain (UFD) are indicated by labels and brackets. The SUMO E1 and Nedd8 E1 are heterodimeric. The adenylation domain is formed by both subunits, while the UFD and Cys domains are included within the Uba2 (for SUMO) and Uba3 (for Nedd8) subunits. ATP is shown in orange CPK within the adenylation active site. The catalytic cysteine on the Cys domain is denoted by a green sphere. The right panel shows the SUMO E1 rotated by 160° to illustrate the large distance between the ATP and E1 catalytic cysteine. (c) Structure of Nedd8 E1 APPBP1/Uba3 (violet/pink) in complex with Nedd8 (yellow) and ATP (orange) (PDB code 1R4N, [5]). (d) Structure of the double-loaded Nedd8~E1-Nedd8-E2 in which Nedd8 is captured in the adenylation site and as a thioester linked adduct to the E1 Cys domain (PDB code 2NVU, [6••]). The Nedd8 E2, Ubc12 (blue), is recruited by the UFD domain and interacts with the UFD, adenylation, and thioester linked Nedd8. The UFD is rotated 120° from that in (c) to accommodate the E2. All structural representations created with PyMOL (http://pymol.sourceforge.net/).
Ub/Ubl modifiers are translated as precursors, and proteases must process Ub/Ubls to expose a C-terminal Gly-Gly motif which is then adenylated by E1. The adenylate is attacked by a conserved E1 cysteine to form a thioester bond, releasing AMP. The E1~Ub/Ubl (where ‘~’ denotes a thioester bond) recruits an E2 conjugating enzyme whereupon the Ub/Ubl thioester is transferred from the E1 cysteine to a conserved E2 cysteine. E3 ligases facilitate Ub/Ubl transfer by coordinating both E2~Ub/Ubl and substrate to ensure specificity and to enhance conjugation. Once conjugated, the ‘Ub/Ubl signal’ can be recognized by proteins via Ub/Ubl binding domains. To terminate the signal, Ub/Ubl proteases deconjugate Ub/Ubl from the substrate.
We will present recent structural insights into each step of Ub/Ubl modification – including those relevant to conjugation, Ub/Ubl-substrate recognition, and deconjugation. Although each step in the Ub/Ubl pathway could sustain a comprehensive review in Current Opinion in Structural Biology, we will limit our discussion to selected structural studies involving ubiquitin, Nedd8, or SUMO to illuminate mechanisms pertinent to Ub/Ubl modification.
E1 Activating Enzymes
Structures are available for two heterodimeric E1s that facilitate Ub/Ubl activation and transfer to E2 conjugating proteins, including the Nedd8 E1 (APPBP1/Uba3) and the SUMO E1 (Aos1/Uba2 or SAE1/SAE2) [3–5]. These structures revealed three essential E1 domains, the Ub/Ubl adenylation domain, the Cys domain which contains the cysteine required for thioester transfer, and a ubiquitin-fold domain (UFD) responsible for E2 recruitment (Figures 1b and c).
Structures of APPBP1/Uba3-Nedd8-ATP and Aos1/Uba2-SUMO-Mg·ATP revealed the basis for cognate Ub/Ubl recognition by E1 and the positions for Nedd8 and SUMO prior to adenylation [3,5]. Comparisons between these E1s in their apo- and Ub/Ubl-bound forms indicated conformational differences for the Cys and UFD domains with respect to the adenylation domain. More dramatic conformational rearrangements were observed in the double-loaded Nedd8~E1-Nedd8-E2 structure wherein one Nedd8 was trapped in the adenylation site, another Nedd8 was linked via a thioester bond to the E1 catalytic cysteine, and Nedd8’s cognate E2, Ubc12, contacted the E1 UFD domain, Nedd8, and the E1 adenylation domain (Figure 1d) [6••]. In this structure, the UFD undergoes a dramatic 120° rotation to orient the E1 and E2 catalytic sites, revealing two ‘cryptic’ E2 interaction surfaces that were concealed in previous Nedd8 E1 structures. This remarkable suite of structures has provided a wealth of information pertinent to Ub/Ubl and E2 recruitment, two essential steps during Ub/Ubl activation.
E1~Ub/Ubl thioester formation may not be a pre-requisite for E2 recruitment to all E1s. Under conditions of oxidative stress, the SUMO E1 and E2 undergo reversible inhibition via disulfide bond formation between their active site cysteines in the absence of SUMO [7]. In addition, the SUMO E2 can interact with an isolated SUMO E1 cysteine domain in the absence of SUMO [8]. These data suggest that the SUMO E1 may not require a ‘thioester-switch’ to facilitate interactions between the E2, UFD, and Cys domains. Unlike Nedd8 and SUMO E1s which utilize a single E2, the ubiquitin E1 utilizes numerous and structurally distinct E2s. As such, it remains unclear how the Ub E1 can accommodate such a vast array of E2 conjugating proteins. Clues to this process may lie in the recently determined crystal structure obtained for the Ub E1 (Hermann Schindelin & Imsang Lee, personal communication). In Nedd8 and SUMO E1s, the UFD domains must undergo a large conformational change between apo and E2-loaded states. In the Ub E1 structure, the UFD domain appears preconfigured in an open conformation, providing one plausible mechanism that would allow the Ub E1 to recognize and recruit a variety of E2s.
Several outstanding questions remain with respect to E1, including Ub/Ubl adenylation, adenylate transfer to the E1 cysteine, and thioester transfer between E1 and E2. Unmodified C-terminal Ub/Ubl tails were observed in proximity to ATP and magnesium in Nedd8 and SUMO E1s [3,6••], suggesting that adenylation may be coupled to conformational changes and/or adenylate transfer to the E1 cysteine. To form the E1~Ub/Ubl thioester, the Ub/Ubl-adenylate must be attacked by the catalytic E1 cysteine; but in currently available structures, the Ub/Ubl-adenylate is >30 Å from the E1 cysteine (Figure 1b, right panel). And finally, while the double-loaded Nedd8 E1 structure has revealed the architecture of an Ub/Ubl~E1–E2-Ub/Ubl complex that is poised to undergo thioester transfer, a 20 Å gap still remains between the E1 and E2 active sites [6••]. NMR guided docking experiments between the isolated SUMO E1 Cys domain and E2 also reveal a large gap between catalytic cysteine residues (14–17 Å) [8]. As such, the structural basis for these reaction intermediates will remain a continued focus for future studies.
E2 Conjugating Enzymes
E2 conjugating enzymes accept Ub/Ubls from E1 through thioester bond exchange, resulting in a ‘charged’ E2~Ub/Ubl that can transfer the ‘donor’ Ub/Ubl to a target lysine to form an isopeptide bond between the Ub/Ubl C-terminus and the Nε atom of the target lysine. Isopeptide bond formation requires attack at the Ub/Ubl-thioester carbonyl carbon by the nucleophilic acceptor lysine. E2 catalytic cores share a conserved structure, but apart from the E2 cysteine, few discernable ‘catalytic’ residues have been identified. One notable exception was identification of an asparagine residue within the conserved E2 ‘HPN’ motif, a residue proposed by Pickart and co-workers to play a role in stabilizing the oxyanion intermediate during attack by the acceptor lysine [9]. Other E2 residues whose side chains facilitate conjugation have remained elusive.
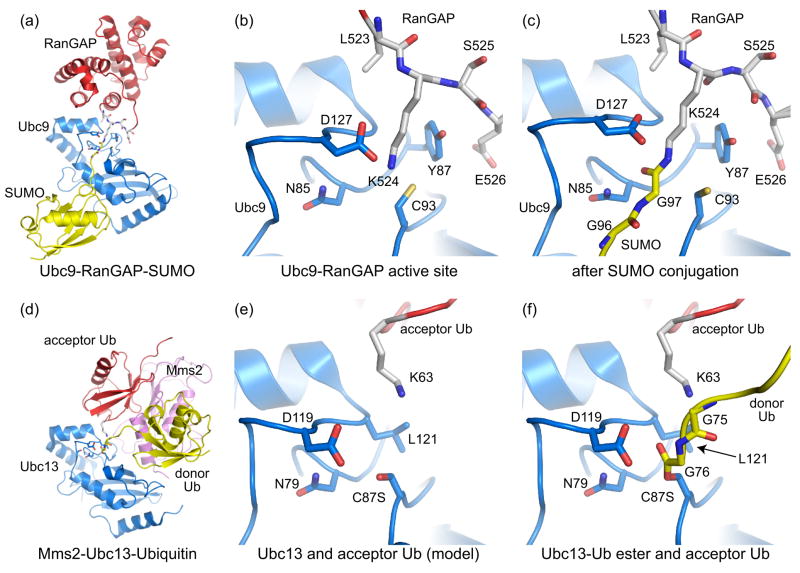
The structural basis for E2 interactions with substrate lysine residues have come from analysis of two systems, one involving Ubc9, the SUMO E2 [10,11•,12••]; and the other involving Mms2-Ubc13, the heterodimeric ubiquitin E2-ubiquitin E2 variant responsible for K63-linked poly-ubiquitin chain synthesis [13,14••]. Structures of Ubc9-RanGAP and Ubc9 in complex with SUMO conjugated RanGAP and the E3 Nup358 revealed the lysine orientation prior to and after isopeptide bond formation (Figure 2a–2c; [10,11•,12••]). In an Mms2-Ubc13-ubiquitin complex, the covalent Ub-ester-Ubc13 intermediate was trapped using an active site cysteine to serine mutant [14••]. In this structure, the ester-linked donor ubiquitin serendipitously contacted a symmetry related Mms2, inserting its K63 residue into the active site of the symmetry related Ubc13 partner (Figure 2d).
Figure 2.
Recognition of substrate acceptor lysine residues by E2 conjugating enzymes. (a) Structure depicting Ubc9 (blue) in complex with RanGAP (red) conjugated to SUMO (yellow) (PDB code 1Z5S, [11•]). Nup358 is not shown. (b) Ubc9-RanGAP active site in a pre-conjugation state (PDB code 2GRN, [12••]). (c) SUMO-conjugated RanGAP-Ubc9 complex showing the isopeptide bond and E2 active site in a post-conjugation state [11•]. Active site residues described in the text are shown. (d) Mms2-Ubc13-Ubiquitin complex containing Ubc13 (blue), Mms2 (pink), and ubiquitin (yellow) ester-linked to Ubc13 (PDB code 2GMI, [14••]). The donor Ub contacted a symmetry related Mms2, inserting its K63 residue into the active site of the symmetry related Ubc13 partner. Therefore, the acceptor Ub (red) originates from a symmetry related complex. (e) Ubc13 active site with the ester-linked Ub removed to facilitate comparisons between the E2 active sites and lysine positions in (b). (f) The Ubc13 active site indicating ester-linked donor Ub (yellow) and acceptor Ub (red).
Comparing E2 structures and lysine positions for RanGAP and acceptor Ub show that the lysine residues approach the E2 active site in a similar orientation (Figure 2b and 2e). In Ubc9-RanGAP, the distance between lysine Nε and cysteine Sγ was ~3.30 Å, and Ubc9 residues Y87 and D127 positioned the substrate lysine in both pre-conjugated and conjugated RanGAP (Figure 2b and 2c). In the Mms2-Ubc13-Ub complex, the lysine Nε and serine Oγ distance was greater than 5 Å, although the acceptor ubiquitin substrate lysine remains proximal to L121 and D119, two Ubc13 residues that correspond to Ubc9 Y87 and D127, respectively (Figure 2e). In this pre-conjugation state, the geometry of the Ub-ester linkage suggests an unfavorable orientation for catalysis as the carbonyl oxygen is pointing toward the lysine (Figure 2f).
Structural, biochemical, and computational studies on the SUMO E2 Ubc9 suggested that N85, Y87, and D127 contribute to E2 conjugation by optimally positioning the acceptor lysine and by collectively lowering its pK to facilitate nucleophilic activation [12••]. Computational analysis suggested that lysine deprotonation was facilitated through desolvation within the microenvironment of the E2 active site, not through removal of water from the active site, but by replacing optimal hydrogen-bonding interactions between the lysine and solvent with suboptimal interactions between the lysine and E2 side chains. Since these E2 residues are conserved at the level of property in other E2s, this may be a general mechanism for lysine positioning and activation. This hypothesis is strengthened by the observation that the Ub K63 side chain approaches the Ubc13 active site in a similar orientation to that observed for Ubc9-RanGAP1 (Figure 2e).
Since only two E2-substrate complexes have been resolved thus far, further investigation will be required to confirm the identity of residues involved in lysine positioning, activation, and isopeptide bond formation using other E2-substrate pairs. Several recent studies not described here have revealed non-covalent interactions between E2s and respective Ub/Ubls that modulate E1 activation, E3 recruitment, and Ub/Ubl chain elongation [15 and references therein, 16]. The basis for these and other relevant E2 activities will remain a focus of future inquiry.
E3 Ligases
E3 Ub/Ubl ligases promote conjugation by bringing charged E2~Ub/Ubls and substrates into close proximity. E3 ligases fall into two broad categories – the RING/U-box and HECT domain families. The RING/U-box E3s utilize a RING/U-box domain to recruit the E2~Ub/Ubl while the HECT domains recruit E2~Ub/Ubl to facilitate formation of an E3~Ub/Ubl thioester with a conserved HECT cysteine prior to substrate conjugation. Structural studies have been focused on resolving E3-substrate interactions and the mechanisms by which E3s facilitate E2-mediated Ub/Ubl conjugation to the substrate lysine.
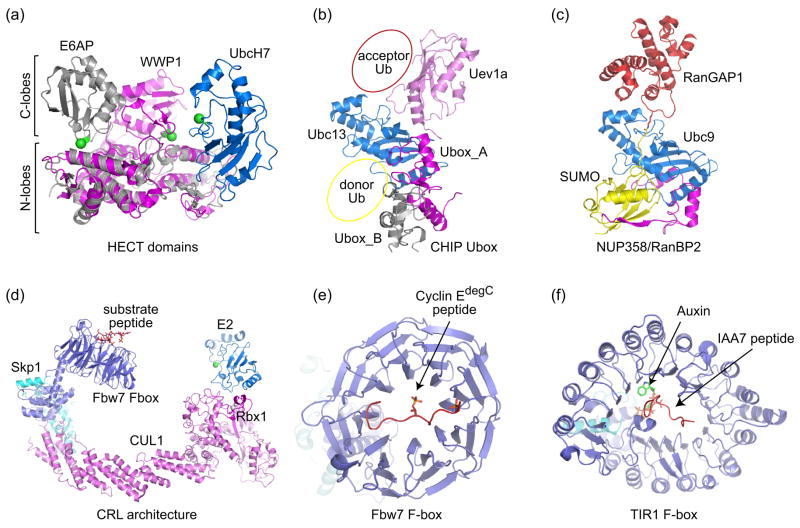
Structures of U-box domains verified their relationship to RING domains, despite the lack of metal-binding residues [17]. The recent structure of the CHIP U-box in complex with Ubc13-Uev1a revealed that U-box-E2 interactions resemble those observed in other RING-E2 complexes (Figure 3b; [18]). CHIP is homodimeric and includes two functional domains, an N-terminal TPR domain that interacts with chaperones, and a C-terminal domain that contains the U-box. Structures of full-length homodimeric CHIP revealed an asymmetric architecture and one available site for E2 binding. Based on the acceptor Ub position obtained from the structure of Mms2-Ubc13-Ub [14••] and donor positions inferred from the Ubc9/RanGAP1-SUMO/Nup358 [11•] and Ubc1~Ub thioester complexes [19], we have indicated positions for both donor and acceptor Ub within the CHIP U-box structure to illustrate the potential organization of this complex (Figure 3b).
Figure 3.
E3 Ub/Ubl ligases. (a) Structure of the HECT domain E6AP (grey) in complex with the E2 UbcH7 (blue) (PDB code 1C4Z, [20]). The HECT domain WWP1 (magenta) (PDB code 1ND7, [21]) is superimposed onto the E6AP HECT domain via their N-terminal lobes to demonstrate the relative movement of the C-terminal lobe towards the E2. The N- and C-lobes are indicated by labels and brackets. The catalytic cysteines for both HECT domains and UbcH7 are denoted by green spheres. (b) CHIP U-box homodimer (grey and magenta) bound to Ubc13/Uev1a (blue/pink) (PDB code 2C2V, [18]). Donor Ub and acceptor Ub positions are suggested based on Ubc1~Ub [19], SUMO-RanGAP-Ubc9 [11•], and Mms2-Ubc13-Ub structures [14••]. (c) Complex between SUMO-conjugated-RanGAP, the SUMO E2 Ubc9, and an E3 Nup358 (magenta) (PDB code 1Z5S, [11•]). Contacts between SUMO and N-terminal IR1 are mediated by an anti-parallel β-strand that contacts an exposed SUMO β-sheet and α-helix, an interaction now recognized as a common feature in proteins that contain SUMO interaction motifs (see Figure 4c and 4d). (d) Model of a Cullin-RING ligase (CRL) illustrating positions for the E2 and substrate receptors. The model was constructed by docking the F-box Fbw7 (PDB code 2OVQ, [31]) complex onto the structure of Cul1-Rbx1-Skp1 SCF (PDB code 1LDK, [29]). The E2 (catalytic cysteine denoted by a green sphere) is modeled based on the Cbl-RING-UbcH7 structure (PDB code 1FBV, [50]). (e) Structure of the Fbw7 F-box bound to phosphorylated cyclin E C-terminal degron [31]. (f) TIR1 F-box in complex with Auxin and IAA7 peptide (PDB code 2P1Q, [32••]).
The structure of the E6AP HECT domain revealed an L-shaped architecture consisting of an N-lobe and C-lobe [20]. Solved in complex with UbcH7, the structure revealed determinants in the N-lobe required for E2 binding, however the catalytic HECT cysteine was separated by a 40 Å gap from the E2 active site (Figure 3a). A more recent structure of the WWP1 HECT domain revealed that the two lobes formed an inverted T shape, repositioning the C-lobe closer to the active site, and closing the gap between E2 and E3 cysteine residues to within 16 Å (Figure 3a) [21]. Future structural studies will be required to reveal mechanisms employed by HECT, RING, and U-box domains with respect to their interactions with ubiquitin, substrates, and E2 conjugating enzymes.
SUMO E3s identified thus far include RING-type Siz/PIAS E3s [22,23] and Nup358/RanBP2 [24,25], an E3 that shares no similarity to either HECT or RING-type E3 ligases. An internal 30 kDa Nup358 domain consisting of two repetitive sequence elements (IR1-M-IR2) and parsed segments within were shown to exhibit E3 ligase activity for several SUMO substrates [24–28]. The crystal structure of SUMO conjugated RanGAP1 in complex with Ubc9 and the Nup358 IR1-M domain revealed the only structure to date for an E3 in complex with a conjugated substrate and E2 (Figure 3c) [11•]. Unlike most E3s which are postulated to make contacts to the E2 and substrate, the Nup358 E3 domain made no contacts to the substrate, but made numerous contacts to Ubc9 and SUMO.
The E3 ligase activity for Nup358 was explained with the following model. In the absence of E3, the Ubc9~SUMO thioester samples non-productive orientations that inhibit substrate interactions and misalign the thioester and E2 active site for chemistry. By tethering Ubc9 and SUMO, Nup358 organizes the complex to facilitate substrate interactions and to align the thioester bond within the E2 active site for attack by the lysine (see above). This model suggests one plausible mechanism by which isolated E3 RING domains ‘activate’ E2-mediated conjugation, namely by organizing their respective E2~Ub/Ubl thioester adducts in a similar manner to that observed for Nup358. Additional structures will be required to confirm or refute this hypothesis.
Cullin-RING Ub ligases (or CRLs) are modular E3 ligases built upon cullin, an elongated scaffold consisting of a rigid stalk that separates N- and C-terminal domains responsible for substrate and E2 recruitment, respectively (Figure 3d, [29,30]). The first structure of a CRL revealed a large gap between N- and C-terminal domains which must be bridged to facilitate substrate interaction with the E2 [29]. One unifying hypothesis is that CRLs function by simply increasing the effective concentrations of both substrate and E2~Ub thioester [29,31]. CRLs exhibit a number of distinct mechanisms for substrate recruitment [30], and two such cases are highlighted here.
In the first example, substrate interaction is integrated with upstream kinase signaling. The structural basis for differential recognition of phosphorylated cyclin E motifs was illustrated in recent structures of the Skp1-Fbw7 complex bound to a high-affinity doubly phosphorylated cyclin E motif and a low-affinity singly phosphorylated cyclin E motif (Figure 3e; [31]). These structures and accompanying biochemical data suggest that Fbw7 can also dimerize to facilitate degradation of weakly associated cyclin E substrates, suggesting alternative mechanisms that contribute to positioning the substrate proximal to the activated E2.
In the second example, the structure of an Arabidopsis F-box protein TIR1-auxin-Aux/IAA substrate complex illustrated the basis by which a small molecule can promote substrate interaction [32••]. In this complex, auxin is coordinated deep within the TIR1 leucine-rich repeat, providing additional surfaces for substrate interaction through formation of a composite binding site for the Aux/IAA peptide substrate (Figure 3f). This unprecedented result raises the possibility that other CRL adaptor proteins may function in concert with small molecules or second messengers to facilitate substrate recruitment.
Ub/Ubl Recognition
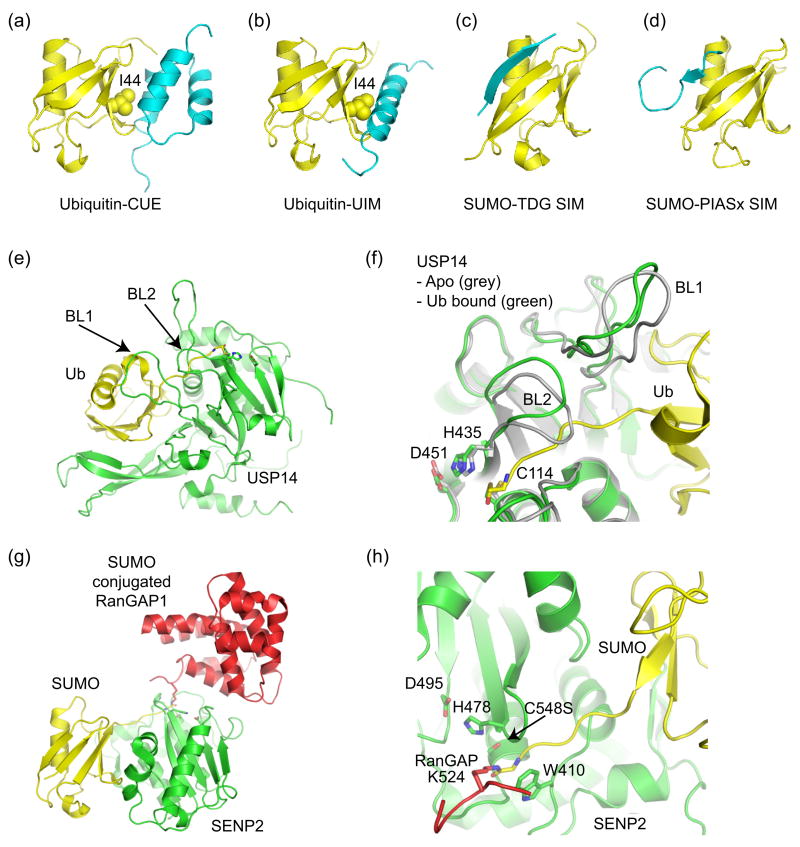
There are over 16 classes of Ub recognition domains [33,34]. While the majority is composed of α-helices, a few are known to contain zinc-binding motifs. Most of these interact with a hydrophobic patch on the ubiquitin surface surrounding Ile44, although they do so using different strategies. For example, UIM domains recognize the hydrophobic patch using a single α-helix (Figure 4b, [35]), while CUE domains consist of a 3 helix bundle which interacts with the hydrophobic patch through contacts to the first and third helices (Figure 4a, [36,37]).
Figure 4.
Ub/Ubl binding domains and Ub/Ubl proteases. (a) Ub in complex with CUE (PDB code 1OTR, [36,37]). (b) Ub in complex with UIM (PDB code 1Q0W, [35]). CUE and UIM domains recognize the Ub hydrophobic patch centered about Ile44. (c) SIM from thymine DNA glycosylase (TDG) in complex with SUMO indicating an anti-parallel orientation for the SIM β-strand (PDB code 1WYW, [39]), similar to that observed for the Nup358 IR1 SIM domain (Figure 3c). (d) SIM from PIASx adopts a parallel β-strand orientation in complex with SUMO (PDB code 2ASQ, [40]). (e) Structure of Ub protease USP14 in complex with Ub-aldehyde (PDB code 2AYO, [43]). Blocking loops (BL1 and BL2) are denoted. (f) In Apo-USP14 (grey) (PDB code 2AYN), BL1 and BL2 occlude access to the preformed catalytic site. In USP14-Ub-aldehyde (green), Ub induces a conformational change wherein BL1 and BL2 move away to provide access to the catalytic site. Active site residues are shown. (g) Structure of SUMO protease SENP2 in complex with SUMO conjugated RanGAP (PDB code 2IO2, [47•]). (h) Close-up of the SENP2 active site to illustrate the 90° kink and isopeptide bond between RanGAP and SUMO. Similar structures were observed for SENP1 [48•]. Active site residues are shown.
SUMO interaction motifs (SIMs) were recently identified in several proteins that bind non-covalently to SUMO [38]. Although SIMs lack a uniform consensus motif, most contain a stretch of four hydrophobic residues flanked by acidic residues. Structures of SIM-SUMO complexes suggested that SIMs adopt a β-strand conformation that contacts a hydrophobic groove on the SUMO surface composed of an exposed SUMO β-sheet and α-helix. The orientation of SIMs can vary. In crystal structures of SUMO complexes with Nup358 and TDG [11•,39], the SIM binds in an anti-parallel fashion (Figure 3c and 4c). However in the NMR structure of PIASx bound to SUMO, the strand adopts a parallel orientation (Figure 4d, [40]).
It is intriguing that SUMO and ubiquitin recognition occurs on distinct surfaces, although this may only indicate that we have not yet uncovered all of the motifs capable of recognizing respective Ub/Ubls. To transduce specific signals, the Ub/Ubl must be recognized in the context of a conjugated substrate. As such, some major issues remain unresolved, namely how Ub/Ubl recognition occurs in the context of a particular substrate, and how particular chain topologies are recognized by single or tandem Ub/Ubl binding domains.
Ub/Ubl Proteases
Most Ub/Ubl proteases fall within the papain-like protease family, and catalysis is achieved via a catalytic-triad composed of side chains from a nucleophilic cysteine, adjacent histidine, and stabilizing asparagine/aspartic acid residue. Recent structures for several different classes of Ub/Ubl deconjugating enzymes have revealed mechanisms pertinent to protease regulation and Ub/Ubl substrate recognition [41].
The structures of two UBP Ub proteases illustrate discreet mechanisms whereby Ub binding induces conformational changes that lead to formation of a productive active site. In the structure of apo-HAUSP, the catalytic Cys and His residues were separated by 9.7 Å [42]. In contrast, the HAUSP-Ub-aldehyde complex suggested that Ub binding induced a conformational change that brings the Cys and His residues to within 3.6 Å, aligning the catalytic-triad residues into a productive conformation. The structure of apo-USP14 revealed a pre-formed active site [43], although two ‘blocking’ loops occluded entry into the catalytic cleft. In this instance, Ub binding induced a conformational change which causes the two loops to move away from and allow access to the catalytic cleft (Figures 4e and 4f). These mechanisms, coupled with specific substrate interactions, provide a means to keep the protease in an inactive form until Ub is presented in the context of an appropriate ubiquitinated substrate.
SENP/Ulp SUMO proteases recognize SUMO in a manner similar to that observed for Ub recognition by the UCH Yuh1 Ub protease [44–46]. Unlike the Ub proteases discussed above, SENP/Ulp active sites are not occluded by surface loops and appear preformed. Recent structures of SENP1 and SENP2 revealed the first examples for any Ub/Ubl protease in complex with conjugated substrates and precursors, revealing additional determinants required for hydrolysis of isopeptide and peptide bonds during deconjugation and processing, respectively [47•,48•]. In structures of SENP1 and SENP2 in complex with SUMO conjugated RanGAP or SUMO precursors, the isopeptide or peptide bond kinks by 90° in the active site, respectively (Figure 4g and 4h). The structures and accompanying biochemical data suggest that the protease can establish additional specificity for the substrate by making contacts between the protease and kinked substrate C-terminal to the scissile peptide bond.
As structures are not yet available for other Ub/Ubl proteases in complex with their bona fide substrates, future efforts will be directed toward understanding structural determinants that dictate substrate preferences – whether a protease is involved in Ub/Ubl deconjugation, processing, or both; and whether a protease can differentiate between Ub/Ubl chains, mono-Ub/Ubl substrates, or in the case for SUMO, between SUMO isoforms.
Conclusions and future directions
A remarkable explosion of new structures in the Ub/Ubl field has occurred since the last article appeared in Current Opinion in Structure Biology in 2002 [49]. While many pertinent issues have been resolved through structure determination of protein complexes involved in Ub/Ubl conjugation and deconjugation, much work remains to be done. We have attempted to construct models that illustrate mechanisms pertinent to Ub/Ubl pathways by cobbling together results from studies in ubiquitin, Nedd8, or SUMO pathways. However, is not always clear when mechanistic comparisons are warranted since Ub/Ubl pathways are not strictly conserved. To resolve both general and pathway-specific issues pertinent to respective Ub/Ubl modification pathways, it will remain a continued focus to develop reagents that facilitate analysis of physiologically relevant complexes between substrates and Ub/Ubl pathway components.
Acknowledgments
A.D.C. and C.D.L. are supported in part by a grant from the National Institutes of Health (GM65872). A.D.C. acknowledges support from the NIH (GM075695).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 2.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 3.Lois LM, Lima CD. Structures of the SUMO E1 provide mechanistic insights into SUMO activation and E2 recruitment to E1. EMBO J. 2005;24:439–451. doi: 10.1038/sj.emboj.7600552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walden H, Podgorski MS, Schulman BA. Insights into the ubiquitin transfer cascade from the structure of the activating enzyme for NEDD8. Nature. 2003;422:330–334. doi: 10.1038/nature01456. [DOI] [PubMed] [Google Scholar]
- 5.Walden H, Podgorski MS, Huang DT, Miller DW, Howard RJ, Minor DL, Jr, Holton JM, Schulman BA. The structure of the APPBP1-UBA3-NEDD8-ATP complex reveals the basis for selective ubiquitin-like protein activation by an E1. Mol Cell. 2003;12:1427–1437. doi: 10.1016/s1097-2765(03)00452-0. [DOI] [PubMed] [Google Scholar]
- 6.Huang DT, Hunt HW, Zhuang M, Ohi MD, Holton JM, Schulman BA. Basis for a ubiquitin-like protein thioester switch toggling E1-E2 affinity. Nature. 2007;445:394–398. doi: 10.1038/nature05490. •• This structure revealed a double Nedd8 loaded E1 in complex with its cognate E2. One Nedd8 was coordinated within the adenylation active site, and the other Nedd8 thioester linked to the E1 catalytic cysteine. Positioning of the E2 is facilitated by a 120° rotation of the UFD domain with respect to its location in the E1-Nedd8 complex. This structure revealed the basis for selective E2 and Nedd8 recruitment, and that formation of the Nedd8-E1 thioester revealed cryptic E2 interaction surfaces that facilitate recognition of the cognate E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bossis G, Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. doi: 10.1016/j.molcel.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Hu W, Cai S, Lee B, Song J, Chen Y. The Intrinsic Affinity between E2 and the Cys Domain of E1 in Ubiquitin-like Modifications. Mol Cell. 2007;27:228–237. doi: 10.1016/j.molcel.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu PY, Hanlon M, Eddins M, Tsui C, Rogers RS, Jensen JP, Matunis MJ, Weisman AM, Wolberger C, Pickart CM. A conserved catalytic residue in the ubiquitin-conjugating enzyme family. EMBO J. 2003;22:5241–5250. doi: 10.1093/emboj/cdg501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell. 2002;108:345–356. doi: 10.1016/s0092-8674(02)00630-x. [DOI] [PubMed] [Google Scholar]
- 11.Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature. 2005;435:687–692. doi: 10.1038/nature03588. •This study revealed the only example to date for an E3 in complex with Ub/Ubl-conjugated substrate and E2. The kinetic analysis included in this study suggested that E3s can activate an E2 for conjugation in the absence of contacts to the substrate by establishing contacts between the E2 and Ub/Ubl that optimize substrate interaction and align the thioester for attack within the E2 active site. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yunus AA, Lima CD. Lysine activation and functional analysis of E2-mediated conjugation in the SUMO pathway. Nat Struct Mol Biol. 2006;13:491–499. doi: 10.1038/nsmb1104. •• This study and [14••] represent the only two systems for which we have structural data suggesting how E2 interacts with a substrate lysine. Both studies indicate that the acceptor lysine approaches the active site in a similar manner. Furthermore, [12] uncovered several E2 active site residues that play dual roles, namely positioning the target lysine and collectively lowering its pK for nucleophilic activation during catalysis. [DOI] [PubMed] [Google Scholar]
- 13.VanDemark AP, Hofmann RM, Tsui C, Pickart CM, Wolberger C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell. 2001;105:711–720. doi: 10.1016/s0092-8674(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 14.Eddins MJ, Carlile CM, Gomez KM, Pickart CM, Wolberger C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat Struct Mol Biol. 2006;13:915–920. doi: 10.1038/nsmb1148. •• See annotation for [12••] [DOI] [PubMed] [Google Scholar]
- 15.Capili AD, Lima CD. Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. J Mol Biol. 2007;369:608–618. doi: 10.1016/j.jmb.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007;26:2797–2807. doi: 10.1038/sj.emboj.7601711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohi MD, Vander Kooi CW, Rosenberg JA, Chazin WJ, Gould KL. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat Struct Biol. 2003;10:250–255. doi: 10.1038/nsb906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH. Chaperoned ubiquitylation--crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton KS, Ellison MJ, Barber KR, Williams RS, Huzil JT, McKenna S, Ptak C, Glover M, Shaw GS. Structure of a conjugating enzyme-ubiquitin thiolester intermediate reveals a novel role for the ubiquitin tail. Structure. 2001;9:897–904. doi: 10.1016/s0969-2126(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2–E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 21.Verdecia MA, Joazeiro CA, Wells NJ, Ferrer JL, Bowman ME, Hunter T, Noel JP. Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol Cell. 2003;11:249–259. doi: 10.1016/s1097-2765(02)00774-8. [DOI] [PubMed] [Google Scholar]
- 22.Johnson ES, Gupta AA. An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell. 2001;106:735–744. doi: 10.1016/s0092-8674(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 23.Sharrocks AD. PIAS proteins and transcriptional regulation--more than just SUMO E3 ligases? Genes Dev. 2006;20:754–758. doi: 10.1101/gad.1421006. [DOI] [PubMed] [Google Scholar]
- 24.Pichler A, Gast A, Seeler JS, Dejean A, Melchior F. The nucleoporin RanBP2 has SUMO1 E3 ligase activity. Cell. 2002;108:109–120. doi: 10.1016/s0092-8674(01)00633-x. [DOI] [PubMed] [Google Scholar]
- 25.Pichler A, Knipscheer P, Saitoh H, Sixma TK, Melchior F. The RanBP2 SUMO E3 ligase is neither HECT- nor RING-type. Nat Struct Mol Biol. 2004;11:984–991. doi: 10.1038/nsmb834. [DOI] [PubMed] [Google Scholar]
- 26.Matunis MJ, Wu J, Blobel G. SUMO-1 modification and its role in targeting the Ran GTPase-activating protein, RanGAP1, to the nuclear pore complex. J Cell Biol. 1998;140:499–509. doi: 10.1083/jcb.140.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 28.Saitoh H, Pu R, Cavenagh M, Dasso M. RanBP2 associates with Ubc9p and a modified form of RanGAP1. Proc Natl Acad Sci U S A. 1997;94:3736–3741. doi: 10.1073/pnas.94.8.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng N, Schulman BA, Song L, Miller JJ, Jeffrey PD, Wang P, Chu C, Koepp DM, Elledge SJ, Pagano M, et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 30.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 31.Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-Cyclin E complex: Multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2006;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 32.Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. •• This structure of the F-box TIR1 in complex with the hormone Auxin and IAA peptide illustrates a mechanism whereby auxin facilitates substrate recruitment. Auxin is located within a deep groove in the TIR1 F-box protein, forming a composite substrate binding surface. This study illuminated a basis by which small molecules confer specificity by bridging substrates to their substrate receptors. [DOI] [PubMed] [Google Scholar]
- 33.Hurley JH, Lee S, Prag G. Ubiquitin-binding domains. Biochem J. 2006;399:361–372. doi: 10.1042/BJ20061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harper JW, Schulman BA. Structural complexity in ubiquitin recognition. Cell. 2006;124:1133–1136. doi: 10.1016/j.cell.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 35.Swanson KA, Kang RS, Stamenova SD, Hicke L, Radhakrishnan I. Solution structure of Vps27 UIM-ubiquitin complex important for endosomal sorting and receptor downregulation. EMBO J. 2003;22:4597–4606. doi: 10.1093/emboj/cdg471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prag G, Misra S, Jones EA, Ghirlando R, Davies BA, Horazdovsky BF, Hurley JH. Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell. 2003;113:609–620. doi: 10.1016/s0092-8674(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 37.Kang RS, Daniels CM, Francis SA, Shih SC, Salerno WJ, Hicke L, Radhakrishnan I. Solution structure of a CUE-ubiquitin complex reveals a conserved mode of ubiquitin binding. Cell. 2003;113:621–630. doi: 10.1016/s0092-8674(03)00362-3. [DOI] [PubMed] [Google Scholar]
- 38.Kerscher O. SUMO junction-what's your function? New insights through SUMO-interacting motifs. EMBO Rep. 2007;8:550–555. doi: 10.1038/sj.embor.7400980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba D, Maita N, Jee JG, Uchimura Y, Saitoh H, Sugasawa K, Hanaoka F, Tochio H, Hiroaki H, Shirakawa M. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature. 2005;435:979–982. doi: 10.1038/nature03634. [DOI] [PubMed] [Google Scholar]
- 40.Song J, Zhang Z, Hu W, Chen Y. Small ubiquitin-like modifier (SUMO) recognition of a SUMO binding motif: a reversal of the bound orientation. J Biol Chem. 2005;280:40122–40129. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- 41.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041–1054. doi: 10.1016/s0092-8674(02)01199-6. [DOI] [PubMed] [Google Scholar]
- 43.Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- 45.Reverter D, Lima CD. A basis for SUMO protease specificity provided by analysis of human Senp2 and a Senp2-SUMO complex. Structure. 2004;12:1519–1531. doi: 10.1016/j.str.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Johnston SC, Riddle SM, Cohen RE, Hill CP. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J. 1999;18:3877–3887. doi: 10.1093/emboj/18.14.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reverter D, Lima CD. Structural basis for SENP2 protease interactions with SUMO precursors and conjugated substrates. Nat Struct Mol Biol. 2006;13:1060–1068. doi: 10.1038/nsmb1168. • This study, along with [48•], shows Ub/Ubl proteases SENP1 and SENP2 in complex with bona fide substrates. Structures of SENP1 and SENP2 in complex with SUMO conjugated RanGAP or SUMO-2/3 precursors show that the isopeptide or peptide bond kinks by 90° in the active site. These structures and accompanying biochemical data suggest that the kinked substrate contributes to substrate specificity by establishing contacts between protease and substrate C-terminal to the scissile bond. [DOI] [PubMed] [Google Scholar]
- 48.Shen L, Tatham MH, Dong C, Zagorska A, Naismith JH, Hay RT. SUMO protease SENP1 induces isomerization of the scissile peptide bond. Nat Struct Mol Biol. 2006;13:1069–1077. doi: 10.1038/nsmb1172. • See annotation for [47•] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanDemark AP, Hill CP. Structural basis of ubiquitylation. Curr Opin Struct Biol. 2002;12:822–830. doi: 10.1016/s0959-440x(02)00389-5. [DOI] [PubMed] [Google Scholar]
- 50.Zheng N, Wang P, Jeffrey PD, Pavletich NP. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]