Abstract
CD151 is a cell surface protein that belongs to the tetraspan superfamily. It associates with other tetraspan molecules and certain integrins to form large complexes at the cell surface. CD151 is expressed by a variety of epithelia and mesenchymal cells. We demonstrate here that in human skin CD151 is codistributed with α3β1 and α6β4 at the basolateral surface of basal keratinocytes. Immunoelectron microscopy showed that CD151 is concentrated in hemidesmosomes. By immunoprecipitation from transfected K562 cells, we established that CD151 associates with α3β1 and α6β4. In β4-deficient pyloric atresia associated with junctional epidermolysis bullosa (PA-JEB) keratinocytes, CD151 and α3β1 are clustered together at the basal cell surface in association with patches of laminin-5. Focal adhesions are present at the periphery of these clusters, connected with actin filaments, and they contain both CD151 and α3β1. Transient transfection studies of PA-JEB cells with β4 revealed that the integrin α6β4 becomes incorporated into the α3β1-CD151 clusters where it induces the formation of hemidesmosomes. As a result, the amount of α3β1 in the clusters diminishes and the protein becomes restricted to the peripheral focal adhesions. Furthermore, CD151 becomes predominantly associated with α6β4 in hemidesmosomes, whereas its codistribution with α3β1 in focal adhesions becomes partial. The localization of α6β4 in the pre-hemidesmosomal clusters is accompanied by a strong upregulation of CD151, which is at least partly due to increased cell surface expression. Using β4 chimeras containing the extracellular and transmembrane domain of the IL-2 receptor and the cytoplasmic domain of β4, we found that for recruitment of CD151 into hemidesmosomes, the β4 subunit must be associated with α6, confirming that integrins associate with tetraspans via their α subunits. CD151 is the only tetraspan identified in hemidesmosomal structures. Others, such as CD9 and CD81, remain diffusely distributed at the cell surface.
In conclusion, we show that CD151 is a major component of (pre)-hemidesmosomal structures and that its recruitment into hemidesmosomes is regulated by the integrin α6β4. We suggest that CD151 plays a role in the formation and stability of hemidesmosomes by providing a framework for the spatial organization of the different hemidesmosomal components.
Keywords: integrin α6β4, tetraspan CD151, hemidesmosome, focal adhesion, cross-talk
Introduction
CD151 has recently been characterized as a member of the tetraspan superfamily. This growing family comprises >20 highly conserved molecules, which intersect the plasma membrane four times (Wright and Tomlinson 1994; Maecker et al. 1997; Hemler 1998). Like other tetraspan molecules, CD151 contains one small and one large extracellular loop with short cytoplasmic carboxy- and amino-terminal domains. The large extracellular loop is thought to be involved in the binding to other molecules, such as integrins. Tetraspans probably bind to the α subunit of the heterodimeric integrins (Imai et al., 1995; Mannion et al. 1996; Lagaudrière-Gesbert et al. 1997; Yauch et al. 1998).
CD151 has been implicated in a wide variety of cell biological processes, including cell adhesion (Hasegawa et al. 1998; Fitter et al. 1999), cell motility (Yáñez-Mó et al. 1998; Yauch et al. 1998; Sincock et al. 1999; Sugiura and Berditchevski 1999; Berditchevski and Odintsova 1999), and the transport of integrins via vesicles (Sincock et al. 1999). Furthermore, because of an association with phosphatidylinositol-4-kinase, a role of CD151 in signal transduction and a link to the cytoskeleton has been suggested (Berditchevski et al. 1997; Yauch et al. 1998; Sugiura and Berditchevski 1999; Berditchevski and Odintsova 1999).
CD151 is expressed by a variety of epithelial and mesenchymal cells, including hematopoietic cells and myocytes. In the skin, it is expressed in the basal keratinocytes (Sincock et al. 1997). In addition to CD151 these keratinocytes express the tetraspans CD9, CD63, CD81, and CD82, and the integrins α2β1, α3β1, and α6β4 (Watt and Hertle 1994; Okochi et al. 1997). Both α3β1 and α6β4 are laminin-binding integrins, with a preference for laminin-5, the primary adhesive ligand present in the normal basal lamina of the adult skin (Carter et al. 1991; Rouselle et al. 1991).
Hemidesmosomes are specialized junctional complexes that function as cell attachment sites for binding to basement membranes, by linking intermediate filaments to the extracellular matrix (Jones et al. 1998; Borradori and Sonnenberg 1999). The integrin α6β4 is a major component of hemidesmosomes and it plays an essential role in their formation. In fact, patients who suffer from junctional epidermolysis bullosa associated with pyloric atresia (PA-JEB), due to mutations in the gene for α6 or β4, have rudimentary hemidesmosomes (Vidal et al. 1995; Ruzzi et al. 1997), while in α6- and β4-null mutant mice, these complexes are not formed at all (Dowling et al. 1996; Georges-Labouesse et al. 1996; van der Neut et al. 1996). Based on the hemidesmosomal components present, two subtypes of hemidesmosomes are distinguished: type II hemidesmosomes consist of α6β4 and plectin (Uematsu et al. 1994), while type I additionally contain BP180 and BP230 (bullous pemphigoid antigen 180 and 230, respectively). Type II hemidesmosomes are found in cylindrical epithelia, e.g., those lining the digestive tract (Fontao et al. 1997). Type I is the classical hemidesmosome, present in basal keratinocytes of multilayered squamous epithelium or urothelium (Borradori and Sonnenberg 1999; Nievers et al. 1999). Hemidesmosomes are defined at the ultrastructural level as electron dense structures, resembling half a desmosome. In certain epithelial cell cultures stained with antibodies against hemidesmosomal components, the typical Swiss cheese– or cauliflower-like pattern represents an equivalent structure (Riddelle et al. 1991). Focal adhesions are sites at the plasma membrane where integrins are clustered and associated with the actin cytoskeleton (Jockusch et al. 1995; Burridge et al. 1997). At these sites various cytoskeleton-associated and signaling molecules are also concentrated. Since plectin is found in both focal adhesions and hemidesmosomes, a functional link between these structures has been suggested (Sánchez-Aparicio et al. 1997; Nievers et al. 1998; Geerts et al. 1999). Contradictory results have been reported about the presence of tetraspans and particularly CD151 in focal adhesions (Nakamura et al. 1995; Berditchevski et al. 1997; Indig et al. 1997; Berditchevski and Odintsova 1999).
In this study, we focus on the role of CD151 in hemidesmosome formation and the cross talk between focal adhesions and hemidesmosomes. We show that CD151 is an as yet unrecognized novel component of hemidesmosomes. In β4-deficient keratinocyte cultures, CD151 is colocalized with α3β1 in pre-hemidesmosomal structures together with laminin-5. Upon transfection with β4, the surface expression of CD151 is enhanced and the protein becomes recruited into hemidesmosomes. This process only occurs when the α6 subunit is associated with β4, indicating that CD151 is probably bound to this subunit. These results provide new information on the function of CD151, the interaction of tetraspans with integrins in general and the process of hemidesmosome assembly.
Materials and Methods
Cell Lines
The human erythroleukemic cell line K562 was maintained in RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (GIBCO BRL), 100 U/ml penicillin and 100 U/ml streptomycin. K562 cells stably expressing α3β1 and α6β4 were established as described previously (Delwel et al. 1993; Niessen et al. 1994). Immortalized keratinocytes derived from a PA-JEB patient were isolated as published elsewhere (Schaapveld et al. 1998). Keratinocytes were grown in keratinocyte serum-free medium (SFM; GIBCO BRL), supplemented with 50 μg/ml bovine pituitary extract, 5 ng/ml epidermal growth factor, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cells were grown at 37°C in a humidified, 5% CO2 atmosphere.
Antibodies
Mouse mAbs J8H against the extracellular domain of the human α6 integrin subunit and 29A3 against the cytoplasmic domain of the human α3A subunit have been described previously (Hogervorst et al. 1993; de Melker et al. 1997). The mouse mAb J143, recognizing an extracellular epitope on the human α3 integrin subunit (Kantor et al. 1987), was from the American Type Culture Collection. Rat mAb B1E5 against the human α5 extracellular domain (Hall et al. 1990) was a kind gift from Dr. C.H. Damsky (University of California San Francisco, San Francisco, CA) and the mouse mAbs 4.3E1 (Hessle et al. 1984) and 450-11A (Kennel et al. 1990) against the extracellular and cytoplasmic domains of human β4 were donated by Drs. E. Engvall (The Burnham Institute, La Jolla, CA) and S.J. Kennel (Oak Ridge National Laboratory, Oak Ridge, TN). Rabbit sera against the cytoplasmic domains of the human α6A and β4 integrin subunits were prepared as described (Delwel et al. 1993; Niessen et al. 1994). The anti-α6 polyclonal antibody, A33, was raised in rabbits by immunizing with a GST-fused recombinant protein of the extracellular domain of α6 (amino acids [aa] 1–576). The rabbit polyclonal antibodies against the cytoplasmic domains of α3A (DiPersio et al. 1995), α5 (Defilippi et al. 1991) and β1A were gifts from Drs. M. DiPersio (Albany Medical College, Albany, NY), G. Tarone (Università di Torino, Italy) and U. Mayer (Max-Planck-Institut für Biochemie, Martinsried, Germany), respectively.
The mouse mAbs 121 and 815 directed against plectin (Okumura et al. 1999) and BP230 (Nishizawa et al. 1993) were generously provided by Dr. K. Owaribe (Nagoya University, Nagoya, Japan) and the rabbit sera against vinculin (Geiger 1979) and the carboxy-terminal domain of BP230 (Tanaka et al. 1990) were gifts form Drs. B. Geiger (The Weizmann Institute of Science, Rehovat, Israel) and J.R. Stanley (University of Pennsylvania, Philadelphia, PA). The anti-vinculin mAb VII-F9 was purchased from Sigma. Drs. P. Rouselle (Institut de Biologie et Chimie des Protéines, Lyon, France) and R.E. Burgeson (Harvard Medical School, Boston, MA) kindly provided the polyclonal laminin-5 antibody (Marinkovich et al. 1992) and the mAb BM165 against the α3 chain of laminin-5 (Rouselle et al. 1991).
The mouse mAb P48, also known as 11B1.G4, was clustered as CD151 in the VI International Leukocyte Typing Workshop (Ashman et al. 1997; Sincock et al. 1997). The mouse mAb 8C3 (CD151) was kindly provided by Dr. K. Sekiguchi (Osaka University, Osaka, Japan) and the mouse mAb Sfa-1 (CD151, Hasegawa et al. 1996) by Dr. H. Hasegawa (Ehime University, Ehime, Japan). The mouse mAbs MEM62 (CD9, Hořejší and Vlček 1991), MEM53 (CD53; Angelisová et al. 1994), M38 (CD81, Imai and Yoshie 1993) and C33 (CD82, Imai and Yoshie 1993), were generously provided with by Dr. V. Hořejší (Institute of Molecular Genetics, Prague, Czech Republic). Dr. F. Berditchevski (The University of Birmingham UK) kindly supplied the mouse mAb 6H1 (CD63, Berditchevski et al. 1995). Actin was stained by phalloidin labeled with TRITC (Sigma). The sheep anti–mouse and donkey anti–rabbit horseradish peroxidase–coupled secondary antibodies, and Texas red–conjugated donkey anti–rabbit antibody were purchased from Amersham Pharmacia Biotech. FITC-conjugated goat anti–mouse was obtained from Rockland.
Analysis of Integrin and Tetraspan Surface Expression
The surface expression of integrin subunits and tetraspan molecules on PA-JEB and β4-transduced PA-JEB/β4 cells was assessed by flow cytometry. 5 × 105 cells were preincubated for 30 min with PBS containing 2% BSA, followed by incubation with the primary antibody for 1 h. The cells were washed three times and then incubated with goat anti–mouse IgG coupled to FITC fluorescein for another hour. After a further three washes, cells were resuspended and analyzed with a FACScan® flow cytometer (Becton Dickinson).
Transient Transfection
The cDNAs encoding full-length β4Α and IL2R/β4, which consists of the extracellular and transmembrane domain of the IL-2 receptor and the cytoplasmic domain of the integrin β4 subunit, were subcloned into the pRc/CMV expression vector (Niessen et al. 1997; Nievers et al. 1998). PA-JEB keratinocytes were seeded onto coverslips 1–2 d before use. Cells were transfected using a cationic lipid-based DNA delivery protocol, Lipofectin Reagent® from Life Technologies. After a 16-h incubation period, the lipofectin reagent was replaced with keratinocyte-SFM medium for 12 h and then subsequently replaced with HAMF12/DMEM 1:3 for an additional 24 h, after which gene expression was assessed.
Stable Cellular Transduction
The full-length β4Α cDNA was released from pUC18-β4A (Niessen et al. 1997) by digesting with EcoRI and the resulting fragment was ligated into the retroviral LZRS-IRES-zeo expression vector, a modified LZRS retroviral vector conferring resistance to zeocin (Kinsella and Nolan 1996; van Leeuwen et al. 1997) to give the LZRS-IRES-zeo-β4A vector. This construct was then introduced into the Phoenix packaging cells (Kinsella and Nolan 1996) by the calcium phosphate precipitation method and virus containing supernatant was collected. PA-JEB cells were infected with the recombinant virus by the DOTAP method (Boehringer). After incubation for 6–8 h at 37°C, infected cells were selected with 0.2 mg/ml zeocin (Invitrogen). Cells expressing α6β4 at their surface were isolated by FACS®, expanded, and analyzed.
Immunofluorescence
Cells were seeded onto glass coverslips, cultured overnight, and then fixed with 1% (wt/vol) paraformaldehyde in PBS for 5 min at room temperature. Subsequently, cells were permeabilized in 0.3% (vol/vol) Triton X-100 in PBS for 5 min at room temperature and blocked for 30 min in 2% (wt/vol) BSA in PBS. Coverslips were inverted onto parafilm containing 25-μl drops of primary antibodies, diluted in PBS containing 2% BSA, and incubated for 60 min at room temperature. After washing twice with PBS, the coverslips were incubated a further 45 min at room temperature in the presence of goat anti–mouse-FITC or donkey anti–rabbit Texas red, diluted 1:100 and 1:400, respectively. To localize F-actin filaments, cells were incubated with TRITC-labeled phalloidin for 45 min. After washing twice with PBS, coverslips were mounted onto slides using Vectashield (Vector Laboratories Inc.) and viewed under a Leica TCS NT confocal laser-scanning microscope, equipped with an Ar/Kr laser (Leica).
Immunoprecipitation and Western Blotting
Wild-type and transfected K562 cells, stably expressing α3β1 or α6β4, were lysed in 1% (wt/vol) CHAPS (Sigma) buffer (5 mM MgCl2, 150 mM NaCl, and 25 mM Hepes, pH 7.5) containing proteinase inhibitors (1 mM phenylmethylsulfonylfluoride, 10 μg/ml soybean trypsin inhibitor, and 10 μg/ml leupeptin). Alternatively, cells were lysed in 1% (vol/vol) NP-40 (Fluka) buffer (50 mM Tris-HCl, 150 mM NaCl, and 1 mM EDTA). Lysates were incubated for 1 h at 4°C with Gamma Bind-G Sepharose beads (Amersham Pharmacia Biotech), previously incubated overnight at 4°C with the precipitating antibodies. The beads carrying the immune complexes were washed three times with lysis buffer and twice with PBS. The immune complexes were eluted by addition of sample buffer, heated at 95 or 65°C, and separated by SDS-PAGE on 6 or 12% gels under reducing (2% β-mercaptoethanol) or nonreducing conditions. After electrophoresis, gels were electrophoretically transferred to a PVDF membrane (Immobilon-P; Bedford). The blots were stained with Coomassie blue to indicate the markers, destained (45% methanol and 5% acidic acid in demineralized water), and blocked for 30 min at 37°C with 2% dry milk in TBST-buffer (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.3% Tween-20). Subsequently, blots were incubated with primary antibodies in 0.2% dry milk in TBST for 90 min at room temperature. Primary antibodies were mAb 450-11A (1:500 dilution), 8C3 (1:5,000), pAb A33 (1:500), 29A3 (neat supernatant), and B1E5 (neat supernatant). After three washes with TBST with 0.2% dry milk, blots were incubated an additional hour at room temperature with horseradish peroxidase–conjugated sheep anti–mouse IgG or donkey anti–rabbit IgG, diluted 1:5,000 in 0.2% dry milk in TBST. The blots were again washed three times with TBST and the bound antibodies were detected by enhanced chemiluminescence as described by the manufacturer (ECL; Amersham Pharmacia Biotech).
Immunoelectron Microscopy
Biopsies of human skin were fixed with 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 2 h and then processed for ultrathin cryosectioning as previously described (Calafat et al. 1997). 45-nm cryosections were cut at −125°C using diamond knives (Drukker Cuijk) in an Utracryomicrotome (Leica) and transferred with a mixture of sucrose and methyl cellulose onto formvar-coated copper grids (Liou et al. 1996). The grids were placed on 35-mm petri dishes containing 2% gelatin. For immunolabeling, the sections were incubated with mAb P48 (dilution 1:100) for 45 min, followed by a 45-min incubation with rabbit anti–mouse IgG (dilution 1:10) and the incubated with 10-nm colloidal gold–labeled protein-A for 30 min. After washing, the cryosections were embedded in a mixture of methyl cellulose and uranyl acetate and examined with a Philips CM 10 electron microscope. For controls, the primary antibody was replaced by a nonrelevant mouse mAb.
Results
Identification of CD151 as a Novel Constituent of Hemidesmosomes
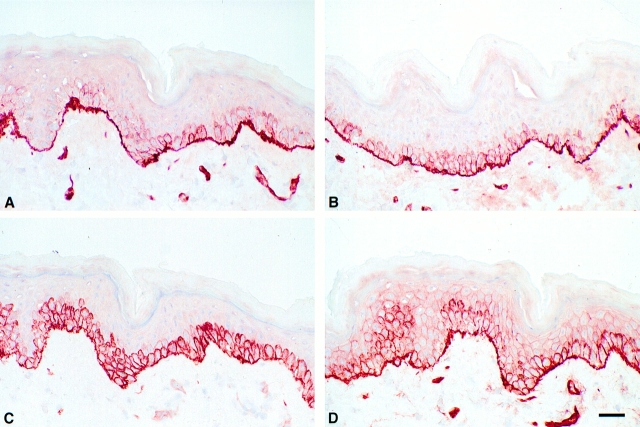
Immunohistochemical staining of frozen sections of the skin revealed that the laminin-binding integrins α6β4 (Fig. 1A and Fig. B) and α3β1 (Fig. 1 C) are present at the basal and basolateral surfaces of the basal keratinocytes, respectively. In addition, the α3β1 integrin is present in some suprabasal cells. The combined staining patterns of α3β1 and α6β4 are very similar to that of CD151 (Fig. 1 D). We next determined the exact distribution of CD151 in basal keratinocytes by immunoelectron microscopy. The results show that CD151 is concentrated in electron-dense hemidesmosomes (Fig. 2B and Fig. C). The protein was also detected along the lateral membranes, but not in desmosomes (Fig. 2 A). The ultrastructural location of CD151 is consistent with the results of the immunoperoxidase staining and suggests that CD151 is codistributed with α6β4 in hemidesmosomes. An association between CD151 and α3β1 is suggested by their similar patterns of staining in other parts of the cell.
Figure 1.
Codistribution of laminin-binding integrins and CD151 in the skin. Frozen sections of human skin are stained by immunoperoxidase reaction with (A) anti-α6 (J8H), (B) anti-β4 (4.3E1), (C) anti-α3 (J143), and (D) CD151 (P48). Anti-α6 and β4 produce strong staining of the basal region of basal keratinocytes, while anti-α3 reacts with basal and lateral surfaces of the basal cells and some suprabasal cells. The distribution of CD151 overlaps with that of both α3β1 and α6β4. Bar, 100 μm.
Figure 2.
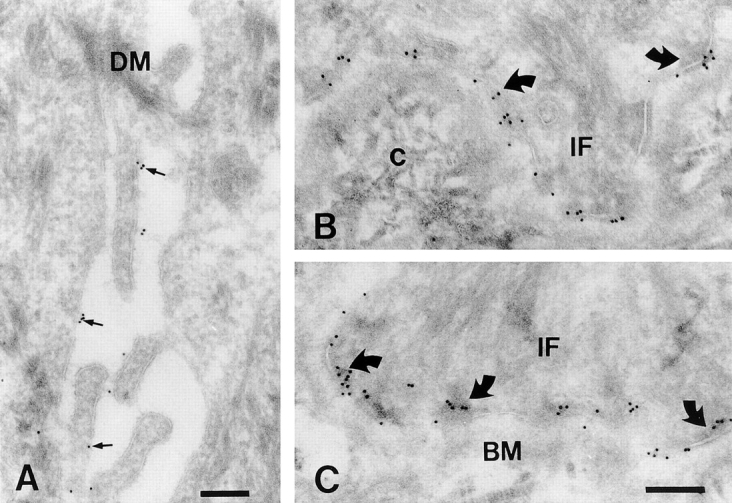
Localization of CD151 in hemidesmosomes. Ultrathin sections of human skin were labeled with P48 (CD151), followed by incubation with second antibodies and gold-conjugated protein A. In A an area of the basal epidermal cell layer is shown where two opposed cells meet. The lateral membranes of these cells show clear staining for CD151. Desmosomes were not stained. In B and C it is shown that CD151 is preferentially localized in hemidesmosomes (curved arrows). IF, intermediate filaments; DM, desmosome; BM, basement membrane; c, collagen. Bars, 200 nm.
Coprecipitation of CD151 with α3β1 and α6β4
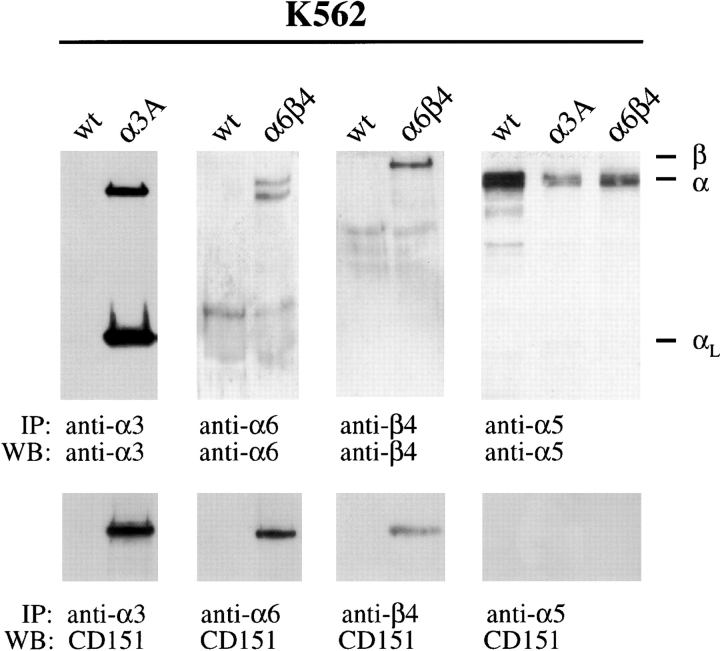
Initial attempts using keratinocytes to show that CD151 forms a complex with α6β4 all failed because α6β4, when present in hemidesmosomes, could not be extracted with a buffer containing 1% CHAPS. We therefore used K562 cells instead, which stably express the integrin α6β4, but do not contain hemidesmosomes. Control K562 cells, which only express α5β1, and K562 cells transfected with α3 were also included in the analyses. Cells were lysed in 1% CHAPS and integrins were immunoprecipitated with integrin subunit-specific antibodies. The presence of CD151 in the precipitates was assessed by immunoblotting with specific antibodies (Fig. 3). Protein bands corresponding to CD151 could be detected in the immunoprecipitates containing α3β1 and α6β4, but not in those that contain α5β1. When cells were lysed in 1% NP-40, CD151 was only detected in the immunoprecipitates containing α3β1 (not shown).
Figure 3.
CD151 is associated with α3β1 and α6β4 in transfected K562 cells. Lysates of wild-type (wt) K562 and of α3A- and α6β4-transfected K562 cells, stably expressing α3β1 or α6β4, respectively, were immunoprecipitated with anti-integrin antibodies. Immunoprecipitates were analyzed by Western blotting using anti-integrin subunit antibodies to monitor the amount of integrins immunoprecipitated (upper half, reduced gel) and using CD151 to detect possible association with integrin subunits (lower half, nonreduced gel). Antibodies used for immunoprecipitation were polyclonal antibodies against α3 and α5, and the mAbs J8H and 4.3E1 against α6 and β4, respectively. For the detection of integrin subunits on blots, polyclonal antibodies against α6 (A33) and the mAbs 29A3, B1E5, and 450-11A and 8C3 against α3, α5, β4, and CD151 were used. The anti-α5 (B1E5) and α6 (A33) subunit antibodies are directed against extracellular domains and proteins bands stained with these antibodies correspond to the unprocessed and heavy chains of the corresponding α subunits. The antibody 29A3 is directed against the cytoplasmic domain of the α3A subunit and on blot it reacts with the light chain of α3 (αL) and the unprocessed α3 precursor (α). CD151 is coprecipitated with anti-α3 from K562 cells expressing α3β1 and with anti-α6 and anti-β4 from K562 cells expressing α6β4. No coprecipitation is seen with anti-α5 from K562 cells.
Localization of CD151 in Pre-Hemidesmosomal and Hemidesmosomal Structures in Cultured Keratinocytes
Cultured keratinocytes were used to further investigate the localization of CD151 and its possible role in hemidesmosome formation. Previously, we have shown that in PA-JEB keratinocytes, which lack β4, no hemidesmosomes are formed (Schaapveld et al. 1998). However, hemidesmosome formation is induced by transfection with β4, a process that can occur in both a ligand-dependent and -independent manner, the latter requiring the β4 cytoplasmic domain and the presence of plectin (Schaapveld et al. 1998; Nievers et al. 2000).
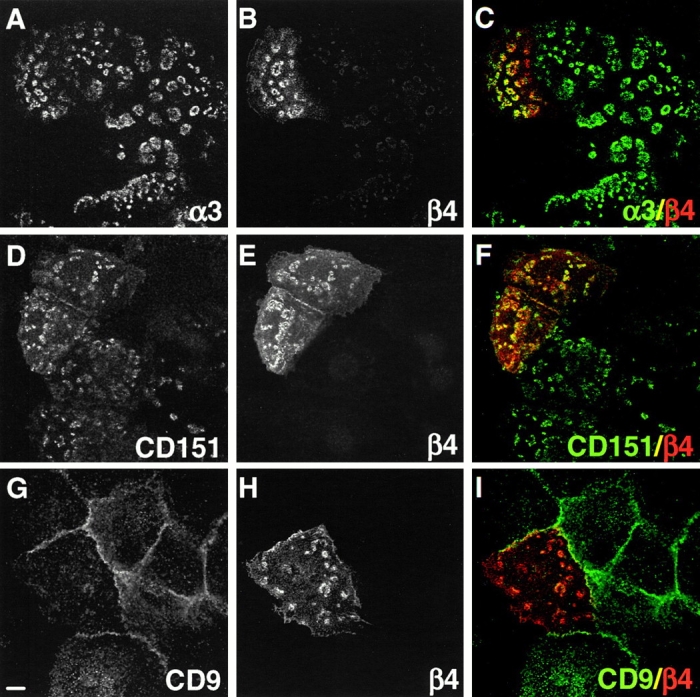
Staining of untransfected PA-JEB cells showed complete colocalization of CD151 and α3β1 in clusters at the basal surface of the cell (Fig. 4, A–C). The ligand for α3β1, laminin-5, was concentrated beneath these basal clusters (Fig. 4, D–F). Only CD151 is colocalized with α3. The other tetraspans CD9 and CD81 are diffusely distributed over the plasma membrane, while the distribution pattern of CD63 is granular in the cytoplasm (data not shown). The laminin-5 patches, which were left in the tracks of migrated cells, do not contain α3β1 or α6β4.
Figure 4.
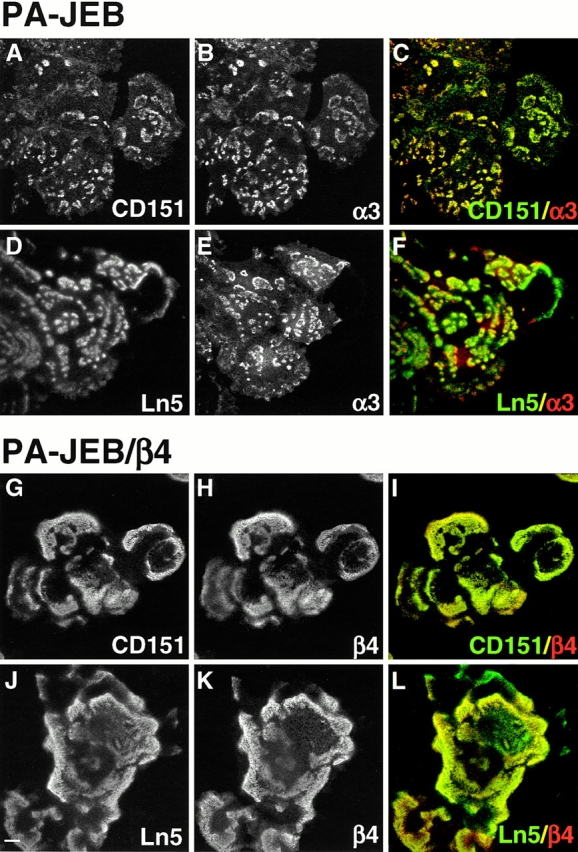
Colocalization of α3β1 and α6β4 with CD151 on patches of laminin-5. PA-JEB (A–F) and α6β4-expressing PA-JEB/β4 cells (G–L) were processed for double immunofluorescence confocal microscopy using mAb CD151 (A and G) or mAb BM165 against laminin-5 (D and J), together with polyclonal antibodies against α3 (B and E) or β4 (H and K). Composite images (C, F, I, and L) were generated by superimposition of the green and red signals, with areas of overlap appearing as yellow. The distribution patterns of α3β1 and CD151 in PA-JEB cells and of α6β4 and CD151 in PA-JEB/β4 cells completely overlap. The integrins α3β1 and α6β4, both receptors for laminin-5, are associated with patches of laminin-5 that are deposited by the cells at sites of cell-substrate contact. Cells that had migrated have left patches of laminin-5 that do not contain α3 or β4. Bar, 10 μm.
In PA-JEB/β4 cells, α6β4 was found to be concentrated in hemidesmosome-like structures at sites of cell–substrate contact. These hemidesmosomal structures appear to be much larger than the clusters formed by α3β1 in the untransfected PA-JEB cells, but, like the α3β1 clusters, they contain CD151 and are associated with laminin-5 (Fig. 4, G–L).
In transient transfection experiments, which enabled us to visualize expression of the different components in transfected and untransfected cells in a single confocal high power field, it could be demonstrated that α6β4 becomes localized at sites, where the α3β1-CD151 clusters are present (Fig. 5). Thus, the formation of these clusters seems to precede the formation of mature hemidesmosomes, which requires expression of α6β4. Therefore, we refer to the α3β1-CD151 clusters as pre-hemidesmosomal structures. It is also shown that the amount of CD151 in the clusters increased after expression of β4 (Fig. 5, A–C and D–F). In contrast, that of α3 seems to become reduced, compared with that in pre-hemidesmosomal structures in untransfected cells (Fig. 5, G–I, see also Fig. 8). The increased reaction with anti-CD151 was not due to cross-reactivity of the antibody with epitopes unrelated to CD151, since two other antibodies against CD151, 8C3 and Sfa-1, gave similar results. Tetraspans, other than CD151, are absent from hemidesmosomes, as shown for CD81 (Fig. 5, J–L).
Figure 5.
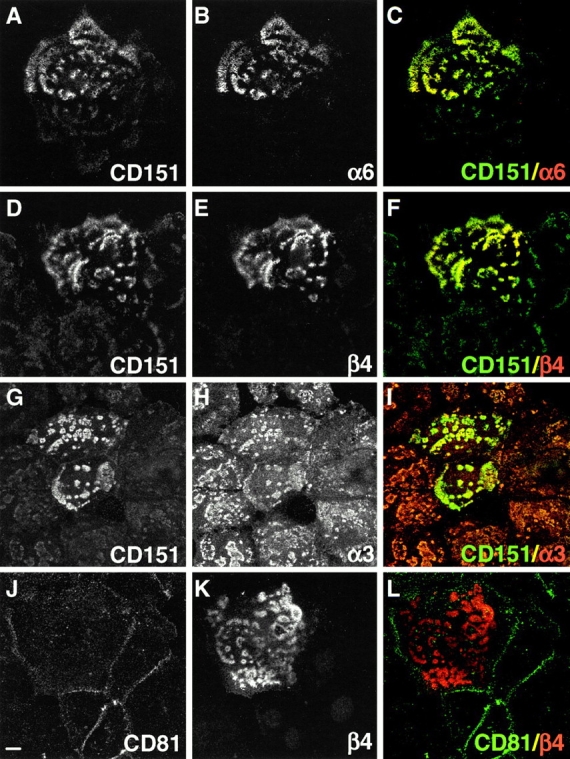
Expression of α6β4 induces recruitment of CD151 into hemidesmosomal structures. PA-JEB cells were transiently transfected with β4, fixed, and double-labeled with mAbs CD151 (A, D, and G) or CD81 (J) and polyclonal antibodies against α6 (B), β4 (E and K), or α3 (H). Merged images are shown in the right panels (C, F, I, and L). Sections are focused at the cell–substrate interface. Upon transfection, expression of β4 results in the formation of hemidesmosome-like structures in which α6β4 is concentrated and codistributed with CD151. These hemidesmosomal structures are formed at sites where complexes of α3β1 and CD151 are also present. Some of the hemidesmosomal structures lack α3β1, while in others the amount of α3β1 is reduced; they may represent intermediate steps in the maturation of hemidesmosomes. Mature hemidesmosomes do not contain α3β1 (see also Fig. 8). Bar, 10 μm.
Figure 8.
CD151 is not recruited by IL-2R/β4. PA-JEB cells were transiently transfected with IL-2R/β4 chimera, fixed, and double-labeled with mAbs 4.3E1 against β4 (B), CD151 (D), or CD9 (G) and polyclonal antibodies against α3 (A) or β4 (E and H). Merged images are shown in the right panels (C, F, and I). Expression of the IL2R/β4 chimera induces the formation of hemidesmosomal structures, but not the recruitment of CD151 into these structures. The IL2R/β4 also fails to recruit CD9 which is diffusely distributed over the plasma membrane. Bars, 10 μm.
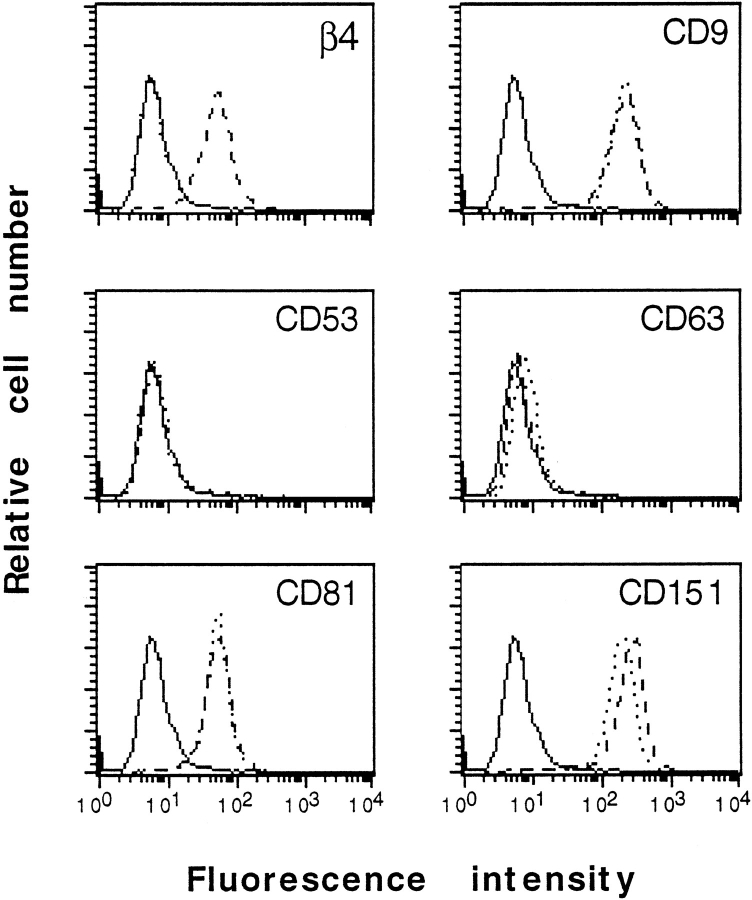
Recently, Yauch et al. 1998 have shown that CD151 expression is upregulated after transfection of K562 cells with α3. Similarly, we observed that CD151 surface expression increased by 35% when PA-JEB keratinocytes are transfected with β4 (Fig. 6). Thus, the clustering of CD151 in hemidesmosomes may result from the recruitment of intracellular pools of CD151, although additional redistribution of surface CD151 is not excluded.
Figure 6.
Expression of α6β4 results in an increase in the surface levels of CD151. Flow cytometry was used to analyze the surface expression of β4 (4.3E1) and different tetraspan molecules CD9 (MEM62), CD53 (MEM53), CD63 (6H1), CD81 (M38), and CD151 (P48) on PA-JEB (dotted lines) and PA-JEB/β4 cells (interrupted lines). Negative control (solid lines) staining with secondary FITC-conjugated anti–mouse IgG is shown for comparison. The expression of β4 on the surface of PA-JEB cells (PA-JEB/β4) was selectively accompanied by an increase in the surface levels of CD151; the levels of CD9 and CD81 remain unaltered. Both PA-JEB and PA-JEB/β4 cells are negative for CD53 and CD63.
Localization of CD151 in Type I and Type II Hemidesmosomes
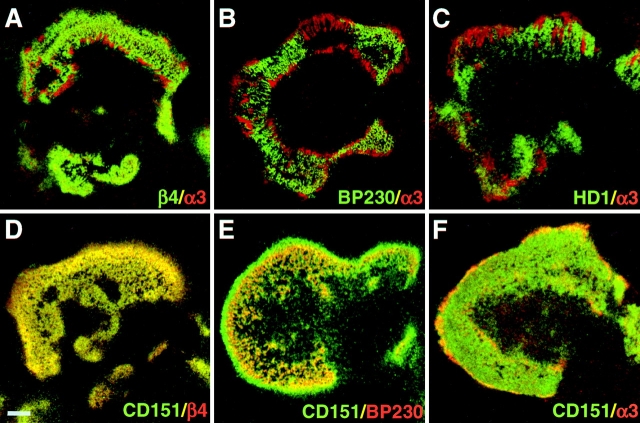
Based on their components, hemidesmosomes can be divided into two types, type I and II hemidesmosomes. Type II hemidesmosomes, which contain α6β4 and plectin, are considered to be precursors of the classical type I hemidesmosomes, which in addition contain BP180 and BP230 (Nievers et al. 1999). We investigated whether CD151 is expressed in both types. In PA-JEB/β4 cells that stably express the integrin α6β4, type II hemidesmosomes are more abundant than type I hemidesmosomes and in some cells only type II hemidesmosomes are present. As shown in Fig. 7 D, in PA-JEB/β4 cells, the staining pattern of hemidesmosomes for CD151 and α6β4 is very similar. This is also true in those cells that only contain type II hemidesmosomes. Similar results were obtained when we compared the distribution of CD151 with that of plectin (not shown). In contrast, CD151 is more widely distributed than BP230 (Fig. 7 E). Together, these data show that CD151 is a component of both types of hemidesmosomes. The α3 subunit is expressed at the periphery of the hemidesmosomes and is not colocalized with α6β4, BP230, or plectin (Fig. 7, A–C). Importantly, while in untransfected PA-JEB cells, the expression pattern of α3 completely overlaps with that of CD151 (Fig. 4), in PA-JEB/β4 cells that stably express the integrin α6β4, the integrin α3β1 and CD151 are only partially colocalized in the vicinity of hemidesmosomes (Fig. 7 F, see also A) and in some cells are not colocalized at all. This suggests that when β4 is expressed in the hemidesmosomal structures the α3β1-CD151 complexes are replaced by α6β4-CD151.
Figure 7.
CD151 is localized in type I and II hemidesmosomes. α6β4-expressing PA-JEB/β4 cells were fixed and double-labeled with mAb 4.3E1 against β4 (A), mAb 815 against BP230 (B), mAb 121 against plectin (C) or mAb CD151 (F), and polyclonal antibodies against α3 (A, B, C and F). Cells were also double-labeled with mAb CD151 (D, E) and polyclonal antibodies against β4 (D) or BP230 (E). Only dual-labeled images are shown. In cells that express both type I and II hemidesmosomes, the areas containing CD151 are larger than that containing BP230 (E), whereas those containing CD151 and β4 are identical (D). Focal adhesions that surround the hemidesmosomal structures contain α3β1 (A–C). CD151 is partially codistributed with α3β1 (F). Bar, 5 μm.
The β4 Cytoplasmic Domain Does Not Support the Recruitment of CD151 into Hemidesmosomes
The integrin α and β subunits associate noncovalently via their extracellular domains. Using an IL-2R/β4 chimera, which consists of the extracellular and transmembrane domains of the IL-2 receptor and the β4 integrin cytoplasmic domain, we have shown that dimerization of β4 with α6 is not required for hemidesmosome formation (Nievers et al. 1998). Because the extracellular domain of the IL-2 receptor is unable to bind laminin-5, the process is entirely driven by the cytoplasmic domain of β4.
It is generally assumed that integrins are associated with tetraspans by their α subunit. To determine whether association of β4 with the α6 subunit is necessary for the recruitment of CD151 into hemidesmosomes, PA-JEB cells were transfected with the IL-2R/β4 chimera (Fig. 8). In transfected cells, colocalization of the IL-2R/β4 construct is seen with laminin-5 (not shown), α3 (Fig. 8, A–C), and CD151 (Fig. 8, D–F). However, the redistribution of CD151 to the hemidesmosomes, as seen in cells transfected with full-length β4, did not occur (compare results in Fig. 5). This demonstrates that for the recruitment of CD151 into hemidesmosomes, heterodimerization of the β4 subunit with the α6 subunit is required. Furthermore, these data indicate that CD151 clustering is not essential for the formation of hemidesmosomes.
Localization of α3β1-CD151 in Focal Adhesions Is Affected by the Expression of α6β4
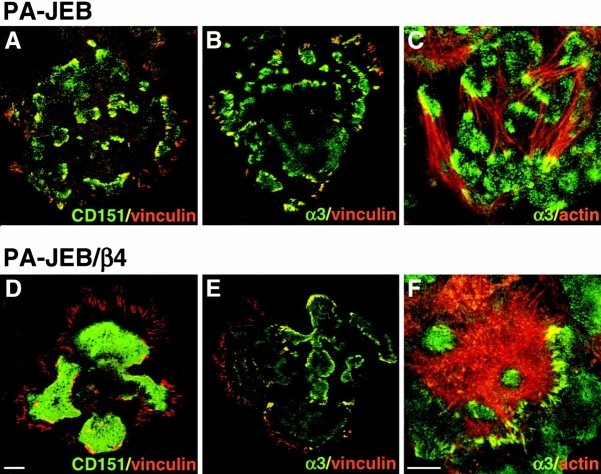
Previously, we have shown that hemidesmosomes are surrounded by focal adhesions (Schaapveld et al. 1998). Focal adhesions are specialized sites at the plasma membrane where integrins are clustered and connected to the actin cytoskeleton. Fig. 9 shows the compartmentalization of, and the close link between hemidesmosomal structures and focal adhesions, as well as the components shared between them. In both untransfected PA-JEB cells and cells transfected with β4, actin filaments are shown to terminate in focal adhesions containing α3β1 and vinculin (Fig. 9C and Fig. F). Focal adhesions are also present at the cell periphery, but these lack α3β1 and CD151. As expected, because of the near perfect colocalization of CD151 and α3 in PA-JEB cells (Fig. 4), CD151 is a component of the focal adhesions that surround the pre-hemidesmosomal structures. However, in β4-transfected PA-JEB cells, CD151 was mainly restricted to the hemidesmosomal structures and was only partially colocalized with vinculin in the surrounding focal adhesions. This suggests that when hemidesmosomes are formed upon transfection with β4, the distribution of CD151 over hemidesmosomes and focal adhesions changes.
Figure 9.
Differential localization of CD151 and vinculin in PA-JEB compared with PA-JEB/β4 cells. Cells were fixed and double-labeled with mAb CD151 and polyclonal antibodies against vinculin (A and D), or with polyclonal antibodies against α3, and a mAb against vinculin (B and E) or to detect F-actin with TRITC-conjugated phalloidin (C and F). Only composite images (A–F), generated by superimposition of the green and red signals are shown. In PA-JEB cells, CD151 is codistributed with α3 in focal adhesions surrounding the pre-hemidesmosomal clusters, whereas in α6β4-expressing PA-JEB/β4 cells, the codistribution with α3 is only partial. In both PA-JEB and PA-JEB/β4 cells, the α3β1 is localized in focal adhesions together with vinculin and F-actin. Note that the focal adhesions at the cell periphery do not contain CD151 and α3. Bars: (A, B, D, and E) 10 μm; (F and C) 10 μm.
Discussion
In Vivo and In Vitro Association between CD151 and the Laminin-binding Integrins: CD151 Is Identified as a Component of Hemidesmosomes
An overlap in the expression patterns of CD151 and α5β1 in human tissues has previously been described (Sincock et al. 1997). The integrin α5β1, which is a receptor for fibronectin, is expressed by a variety of cells, such as endothelial and hematopoietic cells. In addition, immunohistochemical data indicated that the expression patterns of CD151 and the laminin-binding integrins α3β1, α6β1, and α7β1 also overlaps (Sincock et al. 1997). In this study, we analyzed the expression of CD151 in skin and show that CD151 is coexpressed with α3β1 and α6β4 at the basolateral surface of the basal keratinocytes. Furthermore, immunoprecipitation experiments using transfected K562 cells indicated that CD151 forms complexes with the α3β1 or α6β4 laminin-binding integrins, confirming previous findings (Yauch et al. 1998; Serru et al. 1999; Sincock et al. 1999). However, in contrast to earlier studies (Hasegawa et al. 1998; Fitter et al. 1999; Sincock et al. 1999), we could not confirm coimmunoprecipitation of CD151 with α5β1 from lysates of these cells. Finally, in line with the observation that CD151 is colocalized and associates with α6β4, the presence of CD151 in hemidesmosomes was demonstrated by immunoelectron microscopy.
CD151 and Sequential Stages in the Formation of Hemidesmosomes
The integrin α6β4, a receptor for laminin-5, is a major component of hemidesmosomes and crucial for initiating their formation since it forms a scaffold for the binding of the other hemidesmosomal components (Borradori et al. 1997; Rezniczek et al. 1998; Schaapveld et al. 1998; Geerts et al. 1999; Hopkinson and Jones 2000). The formation of type II hemidesmosomes, containing α6β4 and plectin, precedes the formation of type I hemidesmosomes that additionally contain BP230 and BP180. Type II hemidesmosomes are also considered to be immature hemidesmosomes, present in the early phase of wound healing and in the more dynamic kinds of epithelia. In contrast, type I hemidesmosomes are formed in the stabilizing phase of wound healing and in more stress-resistant epithelia (Uematsu et al. 1994; Goldfinger et al. 1999).
By comparing β4-deficient PA-JEB cells with such cells transfected with β4, the localization of CD151 could be studied before and after hemidesmosomes were formed. Our data demonstrate that hemidesmosomes are formed in defined, consecutive stages. At first, laminin-5 is deposited, followed by the recruitment of α3β1 and CD151 in what may be called pre-hemidesmosomal structures. At this stage, there is relatively little CD151 present. After β4 transfection in PA-JEB cells, hemidesmosomes are formed. The integrin α6β4 binds to the deposited laminin-5, and this is followed by the recruitment of plectin (Schaapveld et al. 1998). Ultimately, BP180 and BP230 become associated with the α6β4-plectin complexes (Borradori et al. 1998; Hopkinson and Jones 2000).
The localization of α6β4 in the pre-hemidesmosomal structures is associated with an increase in the amount of CD151 and a loss of α3β1 from these structures. In addition to the clustering of CD151 in hemidesmosome-like structures, we found that the levels of CD151 at the cell surface are increased in PA-JEB/β4 cells, that stably express the integrin α6β4. It has been suggested that CD151 has a role in endocytosis and subsequent recycling of β1 integrins to the cell surface because of their similar intracellular localization in endosomal structures (Sincock et al. 1999). Recycling of the integrin α6β4 from the plasma membrane to internal pools and back to the plasma membrane has also been observed (Bretscher 1992; Gaietta et al. 1994). Based on this knowledge and the data presented, it is tempting to speculate that CD151 is involved in the internalization of α6β4 into the cells and the sorting of it to hemidesmosomes in keratinocytes. Binding of α6β4 to an immobilized ligand may prevent the integrin from becoming internalized, thus resulting in an increased expression at the cell surface.
The mechanism responsible for the loss of α3β1 from hemidesmosomal structures is not known, but it might be explained by a higher affinity of α6β4 for laminin-5, thus preventing α3β1 from interacting with it. Both α3β1 and α6β4 bind to the same domain of their ligand (Delwel and Sonnenberg 1997; Aumailley and Rouselle 1999). Alternatively, by inside-out signaling, the activity of α3β1 might be downregulated so that the protein can no longer interact with laminin-5. The affinities of α3β1 and α6β4 for laminin-5 might also become different as a result from proteolytic processing of the α3 or γ2 chain of the laminin-5 molecule (Giannelli et al. 1997; Goldfinger et al. 1998, Goldfinger et al. 1999). It has been suggested that unprocessed laminin-5 is the primary ligand for α3β1, while α6β4 may preferentially interact with proteolytically processed laminin-5 (Burgeson and Christiano 1997).
Since complex formation between tetraspans is well established and since their expression patterns broadly overlap, they may compensate for each other if one of them is lacking (Berditchevski et al. 1996; Yáñez-Mó et al. 1998; Fitter et al. 1999). The localization of CD151 in pre-hemidesmosomal structures and its recruitment into hemidesmosomes in PA-JEB cells after transfection with β4, however, appears to be selective as the tetraspans CD9, CD63, and CD81, which are also expressed by keratinocytes, were not detected in these structures. The distinct distribution of CD151 suggests that at least one of its functions is different from those of the other tetraspans.
The α6 Chain Is Critical for the Recruitment of CD151 into Hemidesmosomes
Based on experimental data, it is generally assumed that integrins bind directly or indirectly to tetraspans by the extracellular domain of their α-subunit (Mannion et al. 1996; Yauch et al. 1998). This assumption is supported by our finding that the IL-2R/β4 chimera cannot recruit CD151 into hemidesmosomes. The mechanism by which this chimera becomes localized into the pre-hemidesmosomal structures is not clear. We consider a direct binding of the IL2R/β4 chimera to CD151 unlikely, because CD151 contains only three small intracellular domains. Previous studies have indicated that binding to plectin is required for the localization of the IL2R/β4 chimera at the basal side of PA-JEB keratinocytes (Nievers et al. 2000). However, it is unlikely that plectin by itself can mediate the localization of the chimera into the α3β1/CD151 clusters, because the protein is not concentrated at these sites (Schaapveld et al. 1998). Perhaps, plectin facilitates the interaction of β4 with an as yet unidentified component in the α3β1-CD151 clusters and thus contributes to the localization of the chimera at these sites.
The finding that the IL2R/β4 chimera is directed to pre-hemidesmosomal structures without clustering of CD151, whereas it remains capable of recruiting BP180 and BP230 (Nievers et al. 1998), suggests that CD151 is not essential for the formation of hemidesmosomes. However, it is important to mention that although the chimera becomes localized in pre-hemidesmosomal structures, these never seem to reach the size of the hemidesmosomal clusters as seen in the PA-JEB/β4 cells that stably express the integrin α6β4 (see Fig. 4). Thus, CD151 might have a role in stabilizing the hemidesmosomal structures and in this way in determining their size. However, definition of the exact role of CD151 in hemidesmosome formation awaits further analysis.
The Role of CD151 in the Spatial Organization of Hemidesmosomes and the Surrounding Focal Adhesions
Apart from their spatial proximity, a link between hemidesmosomes and focal adhesions has previously been suggested, since they share plectin (Sánchez-Aparicio et al. 1997; Nievers et al. 1998; Geerts et al. 1999). In this paper, we provide evidence that CD151 is another component shared by these structures. This protein, however, is not an essential component of focal adhesions, and its expression seems to be affected by α6β4. Only in β4-deficient PA-JEB keratinocytes did CD151 appear to be a resident protein of focal adhesions, while in β4-transfected PA-JEB cells, its distribution seems to be dynamically regulated, varying between cells and even between focal adhesions in the same cell. The absence of CD151 in focal adhesions in PA-JEB/β4 cells appeared not to affect the localization of α3β1 because this integrin is colocalized with vinculin in focal adhesions before and after β4 transfection. An explanation for the shift in the distribution of CD151 from focal adhesions to hemidesmosomes after β4 transfection could be that the protein binds more strongly to α6β4 than to α3β1. However, biochemical studies do not support this conclusion because complexes of α6β4 and CD151 could not be detected in cells that have been lysed in 1% NP-40, whereas under these conditions CD151 remains complexed with α3β1. CD151 cannot only associate with the integrin α3β1 but also with α6β1 (Yauch et al. 1998; Serru et al. 1999; our own unpublished results). We established previously that expression of α6β1 is upregulated and localized to focal adhesions in β4-deficient PA-JEB cells (Schaapveld et al. 1998). Thus, it is possible that downregulation of α6β1 after β4 is expressed contributes to the loss of CD151 from focal adhesions. However, as a result of prolonged culturing, the PA-JEB keratinocytes had lost most of their surface α6β1 and then synthesized more α5β1. At this stage, therefore, the effect of α6β1 on the localization of CD151 can only be limited.
A complete physical separation of focal adhesions and hemidesmosomes may take place after the removal of CD151 and α3β1 from these structures, respectively. Subsequently, the hemidesmosomal structures may mature and then contain BP180 and BP230. The actin cytoskeleton that is concentrically located around hemidesmosomes and associated with the focal adhesions may act as a physical barrier for hemidesmosomal components, thereby confining them to and thus maintaining hemidesmosomes.
Although the presence of α3β1 in the pre-hemidesmosomal structures suggests an important role for this integrin in the formation of hemidesmosomes, results with knockout mice revealed that hemidesmosome formation can occur in the absence of α3β1 (DiPersio et al. 1997). It is possible that in the absence of α3β1 other integrins form complexes with CD151 which, when clustered at the cell basis, can serve as nucleation sites for hemidesmosome assembly by α6β4. However, there is little support for this explanation, since CD151 seems to interact specifically with laminin-binding integrins. A more likely explanation for the presence of hemidesmosomes in the α3-null mice is that for the formation of hemidesmosomes, initial α3β1-mediated clustering of CD151 is not required, but that it only facilitates the subsequent recruitment of CD151/α6β4 into hemidesmosomal structures. In that case, hemidesmosomes can still be formed in the absence of α3β1, but the kinetics of their assembly might be different from that in wild-type mice. Alternatively, the structure and stability of hemidesmosomes may, in fact, be compromised in the α3-null mice in a way that it is not yet evident at the ultrastructural level when the mice die at birth.
The focal adhesions found at the cell periphery neither contain α3β1 nor CD151. These focal adhesions are probably assembled on fibronectin and vitronectin derived from serum. The integrins that interact with these adhesive ligands are α5β1 and αvβ3 and both may be involved in the initial adhesion and spreading of the cells. The absence of CD151 as well as of α3β1 in these peripheral focal adhesions is consistent with the finding that this tetraspan preferentially associates with the laminin-binding integrins α3β1 and α6β4 and provides further support for its role in hemidesmosome assembly and stability.
In summary, we demonstrate that CD151 is a newly detected hemidesmosomal component. We show that CD151 plays a role in the sequence of events, which take place in hemidesmosome assembly. An additional role for CD151 in the cross-talk with the surrounding focal adhesions is suggested.
Acknowledgments
We thank Ed Roos and Leo Price (both from the Division of Cell Biology, The Netherlands Cancer Institute, Amsterdam, the Netherlands), Paul Engelfriet (Central Laboratory of the Netherlands Red Cross, Amsterdam, the Netherlands), and Luca Borradori (Hôpitaux Universitaires de Genève, Switzerland) for helpful advice and critical reading of the manuscript. Lenny Brocks is acknowledged for assistance with the CLSM and Duco Kramer for the generation of the polyclonal anti-α6 antibody. We are indebted to our colleagues for their generous gift of antibodies.
This work was supported by grants from The Netherlands Kidney Foundation (C 96.1581), the Dutch Cancer Society (NKI 99-2039), the Dystrophic Epidermolysis Bullosa Research Association, and the Biomedical and Health program (BIOMED, BMH4-CT97-2062).
Footnotes
Abbreviations used in this paper: BP180, bullous pemphigoid antigen 180; BP230, bullous pemphigoid antigen 230; IL2R, interleukin-2α receptor; PA-JEB, pyloric atresia associated with junctional epidermolysis bullosa.
References
- Angelisová P., Hilgert I., Hōejπí V. Association of four antigens of the tetraspans family (CD37, CD53, TAPA- 1, and R2/C33) with MHC class II glycoproteins. Immunogenetics. 1994;39:249–256. doi: 10.1007/BF00188787. [DOI] [PubMed] [Google Scholar]
- Ashman, L.K., S. Fitter, P.M. Sincock, L.Y. Nguyen, and A.C. Cambareri. 1997. CD151 (PETA-3) workshop summary report. Leukocyte Typing VI. White Cell Differentiation Antigens. T. Kishimoto, H. Kikutani, A.E.G.Kr. von dem Borne, S.M. Goyert, D.Y. Mason, M. Miyasaka, L. Moretta, K. Okumura, S. Shaw, T.A. Springer, K. Sugamura, and H. Zola, editors. Garland Publishing Inc. 681–683.
- Aumailley M., Rouselle P. Laminins of the dermo-epidermal junction. Matrix Biol. 1999;18:19–28. doi: 10.1016/s0945-053x(98)00004-3. [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Bazzoni G., Hemler M.E. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J. Biol. Chem. 1995;270:17784–17790. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Zutter M.M., Hemler M.E. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol. Biol. Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F., Tolias K.F., Wong K., Carpenter C.L., Hemler M.E. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J. Biol. Chem. 1997;272:2595–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- Berditchevski F., Odintsova E. Characterization of integrin-tetraspanin adhesion complexesrole of tetraspanins in integrin signaling. J. Cell Biol. 1999;146:477–492. doi: 10.1083/jcb.146.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L., Koch P.J., Niessen C.M., Erkeland S., van Leusden M.R., Sonnenberg A. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the β4 integrin subunit. J. Cell Biol. 1997;136:1333–1347. doi: 10.1083/jcb.136.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L., Chavanas S., Schaapveld R.Q.J., Gagnoux-Palacios L., Calafat J., Meneguzzi G., Sonnenberg A. Role of the bullous pemphigoid antigen (BP180) in the assembly of hemidesmosomes and cell adhesion. Reexpression of BP180 in generalized atrophic benign epidermolysis bullosa. Exp. Cell Res. 1998;239:463–476. doi: 10.1006/excr.1997.3923. [DOI] [PubMed] [Google Scholar]
- Borradori L., Sonnenberg A. Structure and function of hemidesmosomesmore than simple adhesion complexes. J. Invest. Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Bretscher M.S. Circulating integrinsα5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R.E., Christiano A.M. The dermal-epidermal junction. Curr. Opin. Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Burridge K., Chrzanowska-Wodnicka M., Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- Carter W.G., Ryan M., Gahr P.J. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Calafat J., Janssen H., Stahle-Backdahl M., Zuurbier A.E., Knol E.F., Egesten A. Human monocytes and neutrophils store transforming growth factor-alpha in a subpopulation of cytoplasmic granules. Blood. 1997;90:1255–1266. [PubMed] [Google Scholar]
- Defilippi P., van Hinsbergh A., Bertolotto A., Rossino P., Silengo L., Tarone G. Differential distribution and modulation of expression of α1β1 on human endothelial cells. J. Cell Biol. 1991;114:855–863. doi: 10.1083/jcb.114.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel G.O., Hogervorst F., Kuikman I., Paulsson M., Timpl R., Sonnenberg A. Expression and function of the cytoplasmic variants of the integrin α6 subunit in transfected K562 cells. Activation-dependent adhesion and interaction with isoforms of laminin. J. Biol. Chem. 1993;268:25865–25875. [PubMed] [Google Scholar]
- Delwel G.O., Sonnenberg A. Laminin isoforms and their receptors. In: Horton M.A., editor. Adhesion Receptors as Therapeutic Targets. CRC Press; Boca Raton, FL: 1997. pp. 9–36. [Google Scholar]
- de Melker A.A., Sterk L.M.Th., Delwel G.O., Fles D.L.A., Daams H., Weening J.J., Sonnenberg A. The A and B variants of the α3 integrin subunittissue distribution and functional characterization. Lab. Invest. 1997;76:547–563. [PubMed] [Google Scholar]
- DiPersio C.M., Shah S., Hynes R.O. α3Aβ1 integrin localizes to focal contacts in response to diverse extracellular matrix proteins. J. Cell Sci. 1995;106:2321–2336. doi: 10.1242/jcs.108.6.2321. [DOI] [PubMed] [Google Scholar]
- DiPersio C.M., Hodivala-Dile K.M., Jaenisch R., Kreidberg J.A., Hynes R.O. α3β1 integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J., Yu Q.-C., Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter S., Sincock P.M., Jolliffe C.N., Ashman L.K. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with α3β1 and αIIbβ3 integrins in haemopoietic cell lines and modulates cell-cell adhesion. Biochem. J. 1999;338:61–70. [PMC free article] [PubMed] [Google Scholar]
- Fontao L., Dirrig S., Owaribe K., Kedinger M., Launay J.F. Polarized expression of HD1relationship with the cytoskeleton in cultured human colonic carcinoma cells. Exp. Cell Res. 1997;231:319–327. doi: 10.1006/excr.1996.3465. [DOI] [PubMed] [Google Scholar]
- Gaietta G., Redelmeier T.E., Jackson M.R., Tamura R.N., Quaranta V. Quantitative measurement of α6β1 and α6β4 integrin internalization under cross-linking conditionsa possible role for a6 cytoplasmic domains. J. Cell Sci. 1994;107:3339–3349. doi: 10.1242/jcs.107.12.3339. [DOI] [PubMed] [Google Scholar]
- Geerts D., Fontao L., Nievers M.G., Schaapveld R.Q.J., Purkis P.E., Wheeler G.N., Lane E.B., Leigh I.M., Sonnenberg A. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzardits localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Georges-Labouesse E., Messadeq N., Yehia G., Cadalbert L., Dierich A., LeMeur M. Absence of the α6 integrin leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Goldfinger L., Stack M.S., Jones J.C.R. Processing of laminin-5 and its functional consequencesrole of plasmin and tissue-type plasminogen activator. J. Cell Biol. 1998;141:255–266. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger L.E., Hopkinson S.B., deHart G.W., Collawn S., Couchman J.R., Jones J.C. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J. Cell Sci. 1999;12:2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Hall D.E., Reichardt L.F., Crowley E., Holley B., Moezzi H., Sonnenberg A., Damsky C.H. The α1β1 and α6β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J. Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Utsunomiya T., Kishimoto K., Yanagisawa K., Fujita S. SFA-1, a novel cellular gene induced by human T-cell leukemia virus type 1, is a member of the transmembrane 4 superfamily. J. Virol. 1996;70:3258–3263. doi: 10.1128/jvi.70.5.3258-3263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa H., Nomura T., Kishimoto K., Yanagisawa K., Fujita S. SFA-1/PETA-3 (CD151), a member of the transmembrane 4 superfamily, associates preferentially with α5β1 integrin and regulates adhesion of human T cell leukemia virus type 1-infected T cells to fibronectin. J. Immunol. 1998;161:3087–3095. [PubMed] [Google Scholar]
- Hemler M.E. Integrin associated proteins. Curr. Opin. Cell Biol. 1998;5:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hessle H., Sakai L.Y., Hollister D.W., Burgeson R.E., Engvall E. Basement membrane diversity detected by monoclonal antibodies. Differentiation. 1984;26:49–54. doi: 10.1111/j.1432-0436.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., Noteboom E., Sonnenberg A. The role of phosphorylation in activation of the α6Aβ1 laminin receptor. J. Biol. Chem. 1993;268:18427–18430. [PubMed] [Google Scholar]
- Hopkinson S.B., Jones J.C.R. The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome. Mol. Biol. Cell. 2000;11:277–286. doi: 10.1091/mbc.11.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hořejší V., Vlček C. Novel structurally distinct family of leucocyte surface glycoproteins including CD9, CD37, CD53 and CD63. FEBS Lett. 1991;288:1–4. doi: 10.1016/0014-5793(91)80988-f. [DOI] [PubMed] [Google Scholar]
- Imai T., Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation in human T cell leukemia virus 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J. Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- Indig F.E., Diaz-Gonzalez F., Ginsberg M.H. Analysis of the tetraspanin CD9-integrin αIIbβ3 (GPIIb-IIIa) complex in platelet membranes and transfected cells. Biochem. J. 1997;327:291–298. doi: 10.1042/bj3270291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch B.M., Bubeck P., Giehl K., Kroemker M., Moschner J., Rothkegel M., Rüdiger M., Schluter K., Stanke G., Winkler J. The molecular architecture of focal adhesions. Annu. Rev. Cell Dev. Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Jones J.C.R., Hopkinson S.B., Goldfinger L.E. Structure and assembly of hemidesmosomes. Bioessays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kantor R.R.S., Mattes M.J., Lloyd K.O., Old L.J., Albino A.P. Biochemical analysis of two cell surface glycoprotein complexes, very common antigen 1 and very common antigen 2. J. Biol. Chem. 1987;262:15158–15165. [PubMed] [Google Scholar]
- Kennel S.J., Epler R.G., Lankford T.K., Foote L.J., Dickas V., Canamucio M., Cavalierie R., Cosimelli M., Venturo I., Falcioni R. Second generation monoclonal antibodies to the human integrin α6β4. Hybridoma. 1990;9:243–255. doi: 10.1089/hyb.1990.9.243. [DOI] [PubMed] [Google Scholar]
- Kinsella T.M., Nolan G.P. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gen. Ther. 1996;7:1405–1413. doi: 10.1089/hum.1996.7.12-1405. [DOI] [PubMed] [Google Scholar]
- Lagaudrière-Gesbert C., Le Naour F., Lebel-Binay S., Billard M., Lemichez E., Boquet P., Boucheix C., Conjeaud H., Rubinstein E. Functional analysis of four tetraspans, CD9, CD53, CD81, and CD82, suggests a common role in costimulation, cell adhesion, and migrationonly CD9 upregulates HB-EGF activity. Cell Immunol. 1997;182:105–112. doi: 10.1006/cimm.1997.1223. [DOI] [PubMed] [Google Scholar]
- Liou W., Geuze H.J., Slot J.W. Improving structural integrity of cryosections for immunogold labeling. Histochem. Cell Biol. 1996;106:41–58. doi: 10.1007/BF02473201. [DOI] [PubMed] [Google Scholar]
- Maecker H.T., Todd S.C., Levy S. The tetraspanin superfamilymolecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Mannion B.A., Berditchevski F., Kraeft S.K., Chen L.B., Hemler M.E. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin α4β1 (CD49d/CD29) J. Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- Marinkovich M.P., Lundstrum G.P., Burgeson R.E. The anchorage filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J. Biol. Chem. 1992;119:695–703. [PubMed] [Google Scholar]
- Nakamura K., Iwamoto R.I., Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell-cell contact sites. J. Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen C.M., Hogervorst F., Jaspars L.H., de Melker A.A., Delwel G.O., Hulsman E.H.M., Kuikman I., Sonnenberg A. The α6β4 integrin is a receptor for both laminin and kalinin. Exp. Cell Res. 1994;211:360–367. doi: 10.1006/excr.1994.1099. [DOI] [PubMed] [Google Scholar]
- Niessen C.M., Hulsman E.H.M., Rots E.S., Sánchez-Aparicio P., Sonnenberg A. Integrin α6β4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol. Biol. Cell. 1997;8:555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievers M.G., Schaapveld R.Q.J., Oomen L.C.J.M., Fontao L., Geerts D., Sonnenberg A. Ligand-independent role of the β4 integrin subunit in the formation of hemidesmosomes. J. Cell Sci. 1998;111:1659–1672. doi: 10.1242/jcs.111.12.1659. [DOI] [PubMed] [Google Scholar]
- Nievers M.G., Schaapveld R.Q.J., Sonnenberg A. Biology and function of hemidesmosomes. Matrix Biol. 1999;18:5–17. doi: 10.1016/s0945-053x(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Nievers M.G., Kuikman I., Geerts D., Sonnenberg A. Formation of hemidesmosome-like structures in the absence of ligand binding by the α6β4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the β4 integrin subunit. J. Cell Sci. 2000;113:963–973. doi: 10.1242/jcs.113.6.963. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y., Uematsu J., Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major glycoprotein of the hemidesmosome. J. Biochem. 1993;113:493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- Okochi H., Kato M., Nashiro K., Yoshie O., Miyazono K., Furue M. Expression of tetra-spans transmembrane family (CD9, CD37, CD53, CD63, CD81, CD82) in normal and neoplastic human keratinocytesan association of CD9 with α3β1 integrin. Brit. J. Dermatol. 1997;136:857–863. [PubMed] [Google Scholar]
- Okumura M., Uematsu J., Hirako Y., Nishizawa Y., Shimizu H., Kido N., Owaribe K. Identification of the hemidesmosomal 500 kDa protein (HD1) as plectin. J. Biochem. 1999;126:1144–1150. doi: 10.1093/oxfordjournals.jbchem.a022560. [DOI] [PubMed] [Google Scholar]
- Rezniczek G.A., de Pereda J.M., Reipert S., Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeletondirect interaction between the β4 subunit and plectin at multiple molecular sites. J. Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddelle K.S., Green K.J., Jones J.C.R. Formation of hemidesmosomes in vitro by a transformed rat bladder cell line. J. Cell Biol. 1991;112:159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouselle P., Lundstrum G.P., Keene D.R., Burgeson R.E. Kalininan epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzzi L., Gagnoux-Palacios L., Pinola M., Belli S., Meneguzzi G., D'Alessio M., Zambruno G. A homozygous mutation in the integrin α6 gene in junctional epidermolysis bullosa with pyloric atresia. J. Clin. Invest. 1997;99:2826–2831. doi: 10.1172/JCI119474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Aparicio P., Martínez de Velasco A.M., Niessen C.M., Borradori L., Kuikman I., Hulsman E.H., Fässler R., Owaribe K., Sonnenberg A. The subcellular distribution of the high molecular mass protein, HD1, is determined by the cytoplasmic domain of the integrin β4 subunit. J. Cell Sci. 1997;110:169–178. doi: 10.1242/jcs.110.2.169. [DOI] [PubMed] [Google Scholar]
- Schaapveld R.Q.J., Borradori L., Geerts D., van Leusden M.R., Kuikman I., Nievers M.G., Niessen C.M., Steenbergen R.D., Snijders P.J., Sonnenberg A. Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin, and involves a direct interaction between β4 and the bullous pemphigoid antigen 180. J. Cell Biol. 1998;142:271–284. doi: 10.1083/jcb.142.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serru V., Le Naour F., Billard M., Azorsa D.O., Lanza F., Boucheix C., Rubinstein E. Selective tetraspan-integrin complexes (CD81/α4β1, CD151/α3β1, CD151/α6β1) under conditions disrupting tetraspan interactions. Biochem. J. 1999;340:103–111. [PMC free article] [PubMed] [Google Scholar]
- Sincock P.M., Mayrhofer G., Ashman L.K. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissuescomparison with CD9, CD63, and α5β1 integrin. J. Histochem. Cytochem. 1997;45:515–525. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Sincock P.M., Fitter S., Parton R.G., Berndt M.C., Gamble J.R., Ashman L.K. PETA-3/CD151, a member of the transmembrane 4 superfamily, is localised to the plasma membrane and endocytic system of endothelial cells, associates with multiple integrins and modulates cell function. J. Cell Sci. 1999;112:833–844. doi: 10.1242/jcs.112.6.833. [DOI] [PubMed] [Google Scholar]
- Sugiura T., Berditchevski F. Function of α3β1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J. Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Korman N.J., Shimizu H., Eady R.A.J., Klaus-Kovtun V., Cehrs K., Stanley J.R. Production of rabbit antibodies against carboxy-terminal epitopes encoded by bullous pemphigoid cDNA. J. Invest. Dermatol. 1990;94:617–623. doi: 10.1111/1523-1747.ep12876200. [DOI] [PubMed] [Google Scholar]
- Uematsu J., Nishizawa Y., Sonnenberg A., Owaribe K. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J. Biochem. 1994;115:469–476. doi: 10.1093/oxfordjournals.jbchem.a124361. [DOI] [PubMed] [Google Scholar]
- van der Neut R., Krimpenfort P., Calafat J., Niessen C.M., Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F.N., Kain H.E., Kammen R.A., Michiels F., Kranenburg O.W., Collard J.G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J. Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal F., Aberdam D., Miquel C., Christiano A.M., Pulkkinen L., Uitto J., Ortonne J.-P., Meneguzzi G. Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- Watt F.M., Hertle M.D. Keratinocyte integrins. In: Leigh I.M., Lane E.B., Watt F.M., editors. The Keratinocyte Handbook. Cambridge University Press; Cambridge: 1994. pp. 153–164. [Google Scholar]
- Wright M.D., Tomlinson M.G. The ins and outs of the transmembrane 4 superfamily. Immunol. Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M., Alfranca A., Cabanas C., Marazuela M., Tejedor R., Ursa M.A., Ashman L.K., de Landazuri M.O., Sanchez-Madrid F. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with α3β1 integrin localized at endothelial lateral junctions. J. Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch R.L., Berditchevski F., Harler M.B., Reichner J., Hemler M.E. Highly stoichiometric, stable, and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4- kinase, and may regulate cell migration. Mol. Biol. Cell. 1998;9:2751–2765. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]