Abstract
Studies of the cell invasion mechanism of the parasite Trypanosoma cruzi led to a series of novel findings, which revealed a previously unsuspected ability of conventional lysosomes to fuse with the plasma membrane. This regulated exocytic process, previously regarded mostly as a specialization of certain cell types, was recently shown to play an important role in the mechanism by which cells reseal their plasma membrane after injury.
Keywords: lysosome; Trypanosome cruzi; exocytosis; secretion
Lysosomes have a clearly defined identity in animal cells. They are dense, acidic compartments rich in hydrolases, in which endocytosed material destined for degradation accumulates. Although pathways of membrane recycling to the plasma membrane and/or the Golgi complex are known to exist along the endocytic pathway, lysosomes are generally believed to be much more limited in their communication skills. Membrane exchange in the endocytic pathway is evident only as far as late endosomes; the dense vesicles of defined size and shape known as lysosomes, which originate from late endosomes, have until recently been regarded as terminal compartments, “dead-end” organelles. Here we discuss recent evidence that significantly expands this view of lysosomes, suggesting that these organelles also function as regulated secretory compartments, capable of direct interaction with the plasma membrane.
The lysosome-mediated cell invasion mechanism of Trypanosoma cruzi
Unexpected findings were made during studies of the cell invasion mechanism of the protozoan parasite Trypanosoma cruzi. This process was originally assumed to be similar to the entry mechanism of many bacterial pathogens, which mobilize the actin cytoskeleton of host cells in a phagocytosis-like process (Galan and Bliska, 1996). Surprisingly, however, no polymerized actin was detected around recently formed T. cruzi–containing intracellular vacuoles, and invasion was actually significantly enhanced by disruption of host cell microfilaments (Tardieux et al., 1992). Images of the invasion process in fibroblasts revealed a gradual accumulation of host cell lysosomes at the parasite entry site, and progressive fusion of these lysosomes with the plasma membrane as invasion proceeded (Tardieux et al., 1992) (Fig. 1). These findings led to the suggestion that the cortical actin cytoskeleton, similar to what is observed in regulated exocytosis (Trifaró et al., 1992), acts as a barrier for lysosome recruitment and fusion, and trypomastigote entry. Subsequent studies showed that fusion of lysosomes with the plasma membrane is required for T. cruzi entry into several cell types, and that the process is triggered by elevations in intracellular free Ca2+ concentration ([Ca2+]i) induced by the parasite (Burleigh and Andrews, 1998).
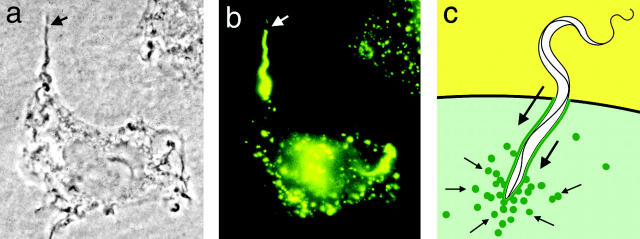
Figure 1.
The lysosome-mediated cell invasion mechanism of T. cruzi. (a) Phase-contrast image of a trypomastigote in the process of entering a HeLa cell. The arrow points to the extracellular portion of the parasite. (b) Immunofluorescence image of the same cell shown in panel a stained with antibodies against human Lamp-1. The arrow points to the extracellular portion of the parasite, not yet surrounded by Lamp-1–containing membranes. (c) Diagram of the process originating the trypomastigote-containing intracellular vacuole. The green line represents lysosomal membranes that are gradually incorporated into the vacuole, the small arrows indicate the direction of lysosome movement, and the large arrows indicate the direction of parasite movement.
In addition to [Ca2+]i transients, T. cruzi trypomastigotes also trigger cAMP elevation in host cells (Rodríguez et al., 1999). This event also appears to play a key role in the invasion mechanism, as trypomastigote entry is markedly reduced in cells treated with adenylyl cyclase inhibitors (Rodríguez et al., 1999). Interestingly, cAMP elevation enhances Ca2+-regulated exocytosis in several cell types (Morgan et al., 1993; Rodríguez et al., 1999). Although the mechanisms responsible for this effect are not completely understood, facilitated vesicular transport/docking at the plasma membrane and removal of the physical barrier posed by the actin cytoskeleton may be involved (Heuser, 1989; Morgan, 1995). These findings reinforce the view that rearrangements in the cortical actin cytoskeleton of host cells are essential for the recruitment and fusion of lysosomes required for T. cruzi invasion.
This series of intriguing similarities between the T. cruzi cell invasion process and Ca2+-regulated exocytosis raised the possibility that the parasites might be taking advantage of a previously unsuspected property of conventional lysosomes, namely the capacity for mobilization to the cell periphery and fusion with the plasma membrane. As discussed below, a direct investigation of the ability of conventional lysosomes to exocytose in response to Ca2+ generated several lines of evidence in support of this view. A particularly intriguing finding, also further discussed below, is the involvement of Ca2+-regulated lysosomal exocytosis in the repair of plasma membrane lesions. T. cruzi trypomastigotes trigger [Ca2+]i elevation in host cells by an IP3-mediated pathway leading to Ca2+ mobilization from intracellular stores (Rodríguez et al., 1996). This signaling process requires a parasite cytosolic serine peptidase, oligopeptidase B (OpdB)* (Burleigh et al., 1997). Interestingly, OpdB-null trypomastigotes, although markedly deficient in both signaling and invasion, still show a residual capacity to elevate [Ca2+]i and to enter host cells (Caler et al., 2000). It is thus conceivable that the OpdB-independent signaling activity is a consequence of direct permeabilization of the host cell plasma membrane by the parasite. Indeed, earlier studies showed that trypomastigotes secrete a hemolytic protein with the capacity to form discrete channels on lipid bilayers (Andrews and Whitlow, 1989). It remains to be investigated if the Ca2+ influx triggered by the T. cruzi hemolysin plays a role in triggering a lysosome-mediated plasma membrane repair process, which would then be subverted by the parasite for gaining access to the intracellular environment.
Common features of conventional and “secretory” lysosomes
Although the concept of regulated exocytosis of conventional lysosomes is relatively new, secretory properties have long been associated with lysosome-related organelles. As reviewed extensively elsewhere (Marks and Seabra, 2001; Blott and Griffiths, 2002), the regulated secretory compartments of several specialized cells have many properties in common with lysosomes. The most widely recognized examples are found in hemopoietic cells: α-granules from platelets, azurophil granules from neutrophils, lytic granules from cytotoxic lymphocytes, and mast cell granules have acidified lumens and contain acidic hydrolases and lysosomal membrane markers. Several of these granules, referred to as “secretory lysosomes,” were also shown to be accessible to tracers trafficking through the endocytic pathway (Stinchcombe and Griffiths, 1999). Osteoclasts, which also belong to the hemopoietic lineage, show a dramatic reorganization of the lysosomal compartment, with translocation of lysosomal glycoproteins to the ruffled border membrane and secretion of lysosomal enzymes at the site of bone resorption (Mostov and Werb, 1997).
There are, however, several examples of cells with secretory lysosomes that do not belong to the hemopoietic lineage. Melanosomes, the melanin-containing granules that are transferred from melanocytes to keratinocytes, share several characteristics with conventional lysosomes, in spite of the existence of unique biogenetic steps (Marks and Seabra, 2001; Raposo et al., 2001). In pulmonary alveolar type II cells, the lysosome-related lamellar bodies are responsible for the Ca2+-regulated secretion of surfactant (Ashino et al., 2000). The acrosome of mammalian spermatozoa, another Ca2+-regulated exocytic compartment, has also been considered to be a modified lysosome, owing to its acidic lumen containing a full set of acidic hydrolases (Tulsiani et al., 1998). In addition, recent observations revealed an intriguing overlap between markers for lysosomes and Weibel-Palade bodies, the regulated secretory granules of endothelial cells (Tulsiani et al., 1998). In response to injury, endothelial cells are activated by inflammatory mediators, such as thrombin or histamine, triggering exocytosis of Weibel-Palade bodies and release of von Willebrand factor. Interestingly, von Willebrand factor, an adhesive protein involved in primary hemostasis, is also secreted by the lysosome-related α-granules of megakayocytes and platelets (Wagner, 1993). Therefore, it is clear that a capacity for Ca2+-regulated exocytosis is commonly associated with lysosomal properties, and that this occurs independently of cell lineage. The acidic lumenal pH of lysosomes and lysosome-related organelles may play a central role in the processing of specific secretory products, as suggested by the low pH requirement for the synthesis and polymerization of melanin in melanosomes (Marks and Seabra, 2001).
The pathology of a group of autosomal recessive diseases of humans and mice that includes the Hermansky-Pudlak (HPS) and Chediak-Higashi (CHS) syndromes is usually attributed to the dysfunction of specialized lysosome-related organelles, mainly because of the common symptoms of oculocutaneous albinism (caused by the defective transfer of melanosomes to keratinocytes) and prolonged bleeding (caused by impaired secretion of platelet clotting mediators). However, it is important to note that there is ample evidence indicating that conventional lysosomes are also affected in these diseases. The most striking example is CHS, caused by mutations in Lyst in the mouse (beige) and CHS1 in humans. Although the function of the very large (>400 kD) cytosolic protein encoded by Lyst/CHS1 remains elusive, it is clear that all cells in beige mice and CHS human patients show an abnormal enlargement of lysosomes (Ward et al., 2000). Severe immunodeficiency, which is usually responsible for early death in humans, has been attributed to defects in the exocytosis of cytotoxic T lymphocyte lytic granules (Baetz et al., 1995), and to delayed major histocompatibility complex (MHC) class II–dependent antigen presentation (Faigle et al., 1998). Due to the severity of CHS in humans, not much information is available on additional lysosome-related disorders in these patients. In this respect, analysis of the symptoms associated with HPS has been more informative and revealed many intriguing features.
Analysis of the various mouse models of HPS has led to the identification of a series of genes clearly involved in organelle biogenesis and membrane traffic events (Marks and Seabra, 2001). Because this syndrome is less severe than CHS and more heterogeneous in clinical presentation, a number of intriguing symptoms have been reported. In addition to hypopigmentation and blood clotting defects, human HPS patients (and the mice, in several instances) show a series of additional abnormalities, not all obviously related to specialized secretory lysosomes. Importantly, accumulation of partially degraded proteolipids (ceroid/lipofuscin) is observed in the lysosomes of many different cell types, and secretion of lysosomal enzymes into the urine, a phenomenon normally observed in male mice, is greatly reduced. Massive amounts of ceroid accumulate in the epithelium of kidney proximal tubule cells and other cell types of HPS patients, a process proposed to be a consequence of defective secretion of lysosomal contents (Swank et al., 1998). HPS patients also develop a serious restrictive lung fibrosis, and occasional granulomatous hemorragic colitis, granulomatous gengivitis, and cardiomyopathy (Swank et al., 1998). The pulmonary fibrosis has been attributed to the intracellular ceroid deposition in the lung, although it is conceivable that defective secretion of surfactant by the lysosome-related lamellar bodies is also involved. No obvious explanation, however, has yet been offered for the colitis, gengivitis, and cardiomyopathy observed in HPS patients. In light of the recent evidence discussed below, it is tempting to speculate that these symptoms might be, at least in part, related to defects in lysosome-mediated repair of plasma membrane lesions.
Regulated exocytosis of conventional lysosomes
Regulated secretion has generally been considered to be a specialization of only a few cell types. It therefore came as a surprise when a large component of Ca2+-regulated exocytosis was detected in a variety of cells previously believed to only be capable of constitutive secretion, such as fibroblasts and epithelial cells (Chavez et al., 1996; Coorsen et al., 1996; Ninomiya et al., 1996). Membrane capacitance measurements revealed a 20–30% increase in the surface area of CHO and 3T3 fibroblasts after elevation in [Ca2+]i (Coorsen et al., 1996). In CHO cells, the diameter of the Ca2+-regulated exocytic vesicles detected by capacitance measurements was estimated to be between 0.4 and 1.5 μm in diameter (Ninomiya et al., 1996), a size consistent with the dimensions of lysosomes in these cell types. Interestingly, even in cells such as PC-12 and adrenal chromaffin cells, which contain “classical” Ca2+-regulated secretory granules, an additional population of exocytic vesicles with distinct properties was detected by electrophysiological methods (Xu et al., 1998; Kasai et al., 1999). Such studies reinforced the growing perception that most cell types contain a population of vesicles that can be mobilized for fusion with the plasma membrane upon elevation in [Ca2+]i. Detailed studies performed with normal rat kidney (NRK) cells in our laboratory identified these vesicles as conventional lysosomes (Rodríguez et al., 1997). Upon stimulation with 1 μM Ca2+, the lumenal domain of lysosomal membrane glycoproteins is exposed on the plasma membrane, and lysosomal contents are released extracellularly. In contrast, no significant increase in the traffic of early endosomal markers to the cell surface is observed under the same conditions. Furthermore, only the lysosomally processed form of cathepsin D is secreted in response to Ca2+, reinforcing the conclusion that mature lysosomes, and not biosynthetic carrier vesicles, are involved in this exocytic process (Rodríguez et al., 1997).
Recent findings from our laboratory revealed that synaptotagmin (Syt) VII, a ubiquitously expressed member of the Syt family of Ca2+-binding proteins, is a powerful tool for modulating Ca2+-dependent exocytosis of lysosomes. Syts are transmembrane proteins with a short amino terminus ectodomain, a single transmembrane region, and two highly conserved, independently folding Ca2+-binding C2 domains (C2A and C2B) homologous to the C2 regulatory region of protein kinase C (Sudhof and Rizo, 1996). Syt I, the most extensively studied isoform, is present on the membrane of synaptic vesicles in neurons, where it was proposed to function as a Ca2+ sensor for rapid exocytosis. Genetic studies in mice, Drosophila, and Caenorhabditis elegans demonstrated that Syt I ablation or mutation strongly decreases the Ca2+ dependency of neurotransmitter release (Nonet et al., 1993; DiAntonio and Schwarz, 1994; Geppert et al., 1994). Several Syt isoforms have been described to date, some of which appear to be more abundantly expressed in brain (Li et al., 1995). Syt VII, however, was shown by hybridization studies to be expressed at significant levels on most mouse tissues (Ullrich and Sudhof, 1995). Consistent with this ubiquitous pattern of expression, recent work from our laboratory showed that Syt VII is localized on the membrane of lysosomes in NRK (Martinez et al., 2000) and other cell types (Caler et al., 2001).
Several lines of evidence indicate that the C2A domain plays a central role in the mechanism by which Syts regulate Ca2+-triggered exocytosis. Antibodies generated against the Syt I C2A domain, and recombinant peptides containing the Syt I C2A domain, were reported to inhibit Ca2+-triggered exocytosis when introduced into neuronal cells (Schiavo et al., 1998). Similarly, the C2A domain of Syt VII or antibodies against this domain block Ca2+-triggered exocytosis of lysosomes in permeabilized NRK cells (Martinez et al., 2000). Importantly, inhibition of lysosomal exocytosis was only observed in the presence of the Syt VII C2A domain, and not the C2A domain of the exclusively neuronal isoform Syt I. Consistent with our previous evidence indicating that common molecular mechanisms regulate lysosomal exocytosis and cell entry by T. cruzi, the recombinant Syt VII C2A domain and anti–Syt VII C2A antibodies also specifically inhibited host cell invasion by trypomastigotes (Caler et al., 2001).
Lysosomal exocytosis and plasma membrane repair
The existence of a ubiquitous pathway for Ca2+-regulated lysosomal exocytosis raised the question of what could be its physiological role in mammalian cells. Interestingly, a series of studies in the last decade concluded that the repair of lesions on the plasma membrane of animal cells involves the Ca2+-regulated delivery of intracellular membrane to wound sites. Ca2+ influx through plasma membrane disruptions triggers a high rate of vesicular exocytosis at the wound site, an event that is required for membrane resealing (McNeil and Steinhardt, 1997). The mechanism by which exocytosis promotes resealing is still unclear, but it may involve the reduction in plasma membrane tension known to result from intracellular membrane delivery (Togo et al., 2000).
Although this series of studies strongly suggested that a ubiquitous form of Ca2+-regulated exocytosis was a necessary and rate-limiting step in plasma membrane repair (McNeil and Steinhardt, 1997), the exact nature of the intracellular vesicles involved was not clear. In sea urchin eggs, resealing of plasma membrane disruptions was initially proposed to be mediated by cortical granules (Bi et al., 1995), but in subsequent studies, yolk granules were implicated (McNeil, 2002). Interestingly, yolk granules from eggs of several species contain acidic hydrolases, in addition to being accessible to tracers chased through the endocytic pathway (Wall and Meleka, 1985). Labeling experiments using the dye FM-143 also implicated the endosomal/lysosomal pathway in the repair of injured endothelial cells and fibroblasts, although the vesicular population involved was not directly identified (Miyake and McNeil, 1995).
The capacity of conventional lysosomes to respond to [Ca2+]i by fusing with the plasma membrane suggested that lysosomes might correspond to the exocytic vesicles involved in cell resealing. A recent study generated several lines of evidence in support of this view (Reddy et al., 2001). Lumenal epitopes of the lysosomal glycoprotein Lamp-1 readily appear on the cell surface of wounded cells (Fig. 2), in a process strictly dependent on the presence of extracellular Ca2+ (Reddy et al., 2001). Wounding in the presence of the inhibitory Syt VII C2A peptides or antibodies inhibits both the surface appearance of Lamp-1 and resealing of the plasma membrane. Furthermore, microinjection of agglutinating antibodies directed against the cytosolic tail of Lamp-1 (Bakker et al., 1997) also impairs resealing, directly implicating lysosomes in the plasma membrane repair process (Reddy et al., 2001).
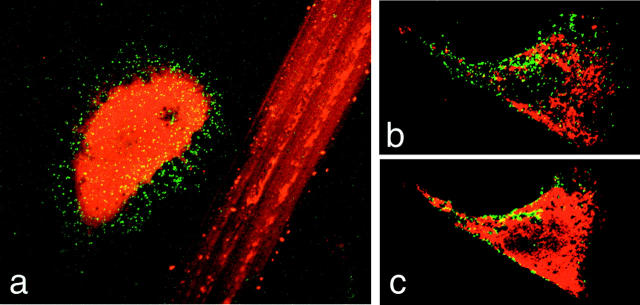
Figure 2.
Exposure of the lumenal domain of Lamp-1 on the surface of wounded cells. (a) A wounded NRK cell, containing cytosolic Texas red–dextran, is shown next to the track left on the coverslip by scratching the monolayer. (b and c) Sequential confocal Z-sections through the bottom (b) and middle (c) of a wounded 3T3 fibroblast. In all images, the green/yellow punctate staining corresponds to the lumenal epitope of Lamp-1, recognized by a monoclonal antibody on the surface of nonpermeabilized cells.
Plasma membrane disruption appears to be a frequent event in mechanically active tissues (McNeil, 2002). Previous studies in rodents showed that cells from the skin, gastrointestinal tract, and muscle are frequently injured, as indicated by the incorporation of membrane-impermeant molecules into their cytosol (McNeil, 2002). Interestingly, the frequency of wounding in rat skeletal muscle cells was reported to increase proportionally with exercise (McNeil and Khakee, 1992). Primary skin fibroblasts, when moving in culture, also suffer frequent and rapidly reversible plasma membrane disruptions, during retraction of the trailing edge (Chen, 1981). Such lesions, believed to be caused by rupture of the focal adhesions formed between fibroblasts and the substrate, also occur extensively in the fibroblast-collagen-matrix model of wound contraction (Lin et al., 1997). This is a model developed to study the regulation of wound contraction, a critical step in the healing of cutaneous lesions (Grinnell, 2000). In this system, skin fibroblasts embedded in a three-dimensional matrix of polymerized collagen attached to a substrate (an environment that mimics the granulation tissue secreted by fibroblasts in cutaneous wounds) develop extensive stress fibers and strong focal adhesions. The tension generated by the fibroblasts under these conditions is believed to represent the force responsible for the contraction and closure of cutaneous wounds in vivo. This contraction event can be reproduced in an accelerated time scale when the collagen-embedded fibroblast matrices are mechanically detached from the substrate. After release, the floating matrix condenses rapidly, a result of isometric tension generated by the fibroblasts. During this contraction event, the majority of the fibroblasts contained in the matrix suffer synchronous plasma membrane disruptions, followed by rapid, Ca2+-dependent resealing (Lin et al., 1997). This system provided a very sensitive and quantitative assay for investigating the role of lysosomes in the repair of plasma membrane injuries suffered by primary cells. When human foreskin fibroblast-collagen-matrices were released from the substrate in the presence of agents that inhibit lysosomal exocytosis, release of the lysosomal enzyme β-hexosaminidase was inhibited while leakage of cytosolic enzyme lactate dehydrogenase was enhanced. Sustained inhibition of lysosomal exocytosis in this system caused significant loss in cell viability, strongly suggesting that this Ca2+-regulated process has a physiological role in the maintenance of plasma membrane integrity.
Cells from mechanically challenged tissues probably suffer repeated cycles of plasma membrane wounding and repair. Recent studies examining sequentially wounded cells found that brefeldin A, although not affecting repair of the initial wound, inhibits resealing of the second wound (Togo et al., 1999). These results were interpreted as reflecting the involvement of distinct types of intracellular vesicles in the initial and subsequent waves of exocytosis–repair. Although this possibility cannot be ruled out, these findings are also consistent with the involvement of lysosomes located at distinct cellular sites. It is conceivable that the initial wound could trigger an exocytic response from lysosomes located in the close proximity of the plasma membrane, while mobilizing an additional population from inside the cell to replace it. Because brefeldin A also affects the morphology and functional properties of lysosomes (Lippincott-Schwartz et al., 1991), it might inhibit replenishing of the peripheral lysosomal population, and subsequent cycles of exocytosis–repair. Future studies using cells impaired in lysosomal exocytosis should be useful in clarifying this interesting issue.
Concluding remarks
The findings discussed above illustrate well the enormous potential that the study of host–pathogen interactions has for uncovering novel, surprising aspects of the physiology of animal cells. The investigation of a highly unusual form of host cell invasion by a trypanosome led to the discovery of Ca2+-regulated lysosomal exocytosis, a widespread process with a central role in the mechanism of plasma membrane repair. These fundamental findings, in turn, significantly advanced our understanding of the strategy used by T. cruzi to invade host cells. It now appears that this parasite subverts a very basic housekeeping process for its own advantage: contact with the parasite triggers a “repair” response by host cell lysosomes, which are then hijacked for formation of the intracellular vacuole. Exocytosis-mediated membrane repair is believed to represent a primitive form of secretion (McNeil and Steinhardt, 1997); it is an intriguing idea that encounters with microbes may have played a key role in the selection of lysosomes for this role (Reddy et al., 2001).
Acknowledgments
The author thanks all present and past members of her laboratory for their essential contributions to the work discussed, and H. Tan for the artwork shown in Fig. 1 c.
Research in the author's laboratory is supported by grants from the National Institutes of Health, Human Frontier Science Program, and a Burroughs Wellcome Molecular Parasitology Scholar award.
Footnotes
Abbreviations used in this paper: CHS, Chediak-Higashi syndrome; HPS, Hermansky-Pudlak syndrome; NRK, normal rat kidney; OpdB, oligopeptidase B; Syt, synaptotagmin.
References
- Andrews, N.W., and M.B. Whitlow. 1989. Secretion by Trypanosoma cruzi of a hemolysin active at low pH. Mol. Biochem. Parasitol. 33:249–256. [DOI] [PubMed] [Google Scholar]
- Ashino, Y., X. Ying, L.G. Dobbs, and J. Bhattacharya. 2000. [Ca(2+)](i) oscillations regulate type II cell exocytosis in the pulmonary alveolus. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L5–L13. [DOI] [PubMed] [Google Scholar]
- Baetz, K., S. Isaaz, and G.M. Griffiths. 1995. Loss of cytotoxic T lymphocyte function in Chediak-Higashi syndrome arises from a secretory defect that prevents lytic granule exocytosis. J. Immunol. 154:6122–6131. [PubMed] [Google Scholar]
- Bakker, A.C., P. Webster, W.A. Jacob, and N.W. Andrews. 1997. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J. Cell Sci. 110:2227–2238. [DOI] [PubMed] [Google Scholar]
- Bi, G.Q., J.M. Alderton, and R.A. Steinhardt. 1995. Calcium-regulated exocytosis is required for cell membrane resealing. J. Cell Biol. 131:1747–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blott, E.J., and G.M. Griffiths. 2002. Secretory lysosomes. Nat. Rev. Mol. Cell Biol. 3:122–131. [DOI] [PubMed] [Google Scholar]
- Burleigh, B., E. Caler, P. Webster, and N.W. Andrews. 1997. A cytosolic serine endopeptidase from Trypanosoma cruzi is required for the generation of Ca2+ signaling in mammalian cells. J. Cell Biol. 136:609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh, B.A., and N.W. Andrews. 1998. Signaling and host cell invasion by Trypanosoma cruzi. Curr. Opin. Microbiol. 1:461–465. [DOI] [PubMed] [Google Scholar]
- Caler, E.V., R. Morty, B. Burleigh, and N.W. Andrews. 2000. Dual role for localized intracellular Ca2+ elevation and cAMP in cell invasion by Trypanosoma cruzi. Infect. Immun. 68:6602–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caler, E.V., S. Chakrabarti, K.T. Fowler, S. Rao, and N.W. Andrews. 2001. The exocytosis-regulatory protein synaptotagmin VII mediates cell invasion by Trypanosoma cruzi. J. Exp. Med. 193:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez, R.A., S.G. Miller, and H.P. Moore. 1996. A biosynthetic regulated secretory pathway in constitutive secretory cells. J. Cell Biol. 133:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W.T. 1981. Surface changes during retraction-induced spreading of fibroblasts. J. Cell Sci. 49:1–13. [DOI] [PubMed] [Google Scholar]
- Coorsen, J.R., H. Schmitt, and W. Almers. 1996. Ca2+ triggers massive exocytosis in Chinese hamster ovary cells. EMBO J. 15:3787–3791. [PMC free article] [PubMed] [Google Scholar]
- DiAntonio, A., and T.L. Schwarz. 1994. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 12:909–920. [DOI] [PubMed] [Google Scholar]
- Faigle, W., G. Raposo, D. Tenza, V. Pinet, A.B. Vogt, H. Kropshofer, A. Fischer, G. de Saint Basile, and S. Amigorena. 1998. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency disease: the Chediak-Higashi syndrome. J. Cell Biol. 141:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan, J.E., and J.B. Bliska. 1996. Cross-talk between bacterial pathogens and their host cells. Annu. Rev. Cell Dev. Biol. 12:221–255. [DOI] [PubMed] [Google Scholar]
- Geppert, M., Y. Goda, R.E. Hammer, C. Li, T.W. Rosahl, C.F. Stevens, and T.C. Sudhof. 1994. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 79:717–727. [DOI] [PubMed] [Google Scholar]
- Grinnell, F. 2000. Fibroblast-collagen-matrix contraction: growth-factor signalling and mechanical loading. Trends Cell Biol. 10:362–365. [DOI] [PubMed] [Google Scholar]
- Heuser, J.E. 1989. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 108:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, H., T. Kishimoto, T.-T. Liu, Y. Miyashita, P. Podini, F. Grohovaz, and J. Meldolesi. 1999. Multiple and diverse forms of regulated exocytosis in wild type and defective PC12 cells. Proc. Natl. Acad. Sci. USA. 96:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., B. Ullrich, J.Z. Zhang, R.G.W. Anderson, N. Brose, and T.C. Sudhof. 1995. Ca2+-dependent and independent activities of neural and non-neural synaptotagmins. Nature. 375:594–599. [DOI] [PubMed] [Google Scholar]
- Lin, Y.C., C.H. Ho, and F. Grinnell. 1997. Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca2+. Mol. Biol. Cell. 8:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., L. Yuan, C. Tipper, M. Amherdt, L. Orci, and R.D. Klausner. 1991. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 67:601–616. [DOI] [PubMed] [Google Scholar]
- Marks, M.S., and M.C. Seabra. 2001. The melanosome: membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2:738–748. [DOI] [PubMed] [Google Scholar]
- Martinez, I., S. Chakrabarti, T. Hellevik, J. Morehead, K. Fowler, and N.W. Andrews. 2000. Synaptotagmin VII regulates Ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 148:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, P.L. 2002. Repairing a torn cell surface: make way, lysosomes to the rescue. J. Cell Sci. 115:873–879. [DOI] [PubMed] [Google Scholar]
- McNeil, P.L., and R. Khakee. 1992. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- McNeil, P.L., and R.A. Steinhardt. 1997. Loss, restoration and maintenance of plasma membrane integrity. J. Cell Biol. 137:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake, K., and P.L. McNeil. 1995. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J. Cell Biol. 131:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, A. 1995. Exocytosis. Essays Biochem. 30:77–95. [PubMed] [Google Scholar]
- Morgan, A., M. Wilkinson, and R.D. Burgoyne. 1993. Identification of Exo2 as the catalytic subunit of protein kinase A reveals a role for cyclic AMP in Ca(2+)-dependent exocytosis in chromaffin cells. EMBO J. 12:3747–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov, K., and Z. Werb. 1997. Journey across the osteoclast. Science. 276:219–220. [DOI] [PubMed] [Google Scholar]
- Ninomiya, Y., T. Kishimoto, Y. Miyashita, and H. Kasai. 1996. Ca2+-dependent exocytic pathways in Chinese hamster ovary fibroblasts revealed by a caged-Ca2+ compound. J. Biol. Chem. 271:17751–17754. [DOI] [PubMed] [Google Scholar]
- Nonet, M.L., K. Grundahl, B.J. Meyer, and J.B. Rand. 1993. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 73:1291–1305. [DOI] [PubMed] [Google Scholar]
- Raposo, G., D. Tenza, D.M. Murphy, J.F. Berson, and M.S. Marks. 2001. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 152:809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A., E. Caler, and N. Andrews. 2001. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 106:157–169. [DOI] [PubMed] [Google Scholar]
- Rodríguez, A., E. Samoff, M. Rioult, A. Chung, and N.W. Andrews. 1996. Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. J. Cell Biol. 134:349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A., P. Webster, J. Ortego, and N.W. Andrews. 1997. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J. Cell Biol. 137:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A., I. Martinez, A. Chung, C.H. Berlot, and N.W. Andrews. 1999. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J. Biol. Chem. 274:16754–16759. [DOI] [PubMed] [Google Scholar]
- Schiavo, G., S.L. Osborne, and J.G. Sgouros. 1998. Synaptotagmins: more isoforms than functions? Biochem. Biophys. Res. Commun. 248:1–8. [DOI] [PubMed] [Google Scholar]
- Stinchcombe, J.C., and G.M. Griffiths. 1999. Regulated secretion from hemopoietic cells. J. Cell Biol. 147:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof, T.C., and J. Rizo. 1996. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron. 17:379–388. [DOI] [PubMed] [Google Scholar]
- Swank, R.T., E.K. Novak, M.P. McGarry, M.E. Rusiniak, and L. Feng. 1998. Mouse models of Hermansky Pudlak syndrome: a review. Pigment Cell Res. 11:60–80. [DOI] [PubMed] [Google Scholar]
- Tardieux, I., P. Webster, J. Ravesloot, W. Boron, J.A. Lunn, J.E. Heuser, and N.W. Andrews. 1992. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 71:1117–1130. [DOI] [PubMed] [Google Scholar]
- Togo, T., J.M. Alderton, G.Q. Bi, and R.A. Steinhardt. 1999. The mechanism of facilitated cell membrane resealing. J. Cell Sci. 112:719–731. [DOI] [PubMed] [Google Scholar]
- Togo, T., T.B. Krasieva, and R.A. Steinhardt. 2000. A decrease in membrane tension precedes successful cell-membrane repair. Mol. Biol. Cell. 11:4339–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaró, J.M., A. Rodriguez de Castillo, and M.L. Vitale. 1992. Dynamic changes in chromafin cell cytoskeleton as a prelude to exocytosis. Mol. Neurobiol. 6:339–358. [DOI] [PubMed] [Google Scholar]
- Tulsiani, D.R., A. Abou-Haila, C.R. Loeser, and B.M. Pereira. 1998. The biological and functional significance of the sperm acrosome and acrosomal enzymes in mammalian fertilization. Exp. Cell Res. 240:151–164. [DOI] [PubMed] [Google Scholar]
- Ullrich, B., and T.C. Sudhof. 1995. Differential distributions of novel synaptotagmins: comparison to synapsins. Neuropharmacology. 34:1371–1377. [DOI] [PubMed] [Google Scholar]
- Wagner, D.D. 1993. The Weibel-Palade body: the storage granule for von Willebrand factor and P-selectin. Thromb. Haemost. 70:105–110. [PubMed] [Google Scholar]
- Wall, D.A., and I. Meleka. 1985. An unusual lysosome compartment involved in vitellogenin endocytosis by Xenopus oocytes. J. Cell Biol. 101:1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, D.M., G.M. Griffiths, J.C. Stinchcombe, and J. Kaplan. 2000. Analysis of the lysosomal storage disease Chediak-Higashi syndrome. Traffic. 1:816–822. [DOI] [PubMed] [Google Scholar]
- Xu, T., T. Binz, H. Niemann, and E. Neher. 1998. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci. 1:192–200. [DOI] [PubMed] [Google Scholar]