Abstract
The high-risk human papillomavirus (HPV) E6 and E7 proteins act cooperatively to mediate multiple activities in viral pathogenesis. For instance, E7 acts to increase p53 levels while E6 accelerates its rate of turnover through the binding of the cellular ubiquitin ligase E6AP. Interferons are important antiviral agents that modulate both the initial and persistent phases of viral infection. The expression of HPV type 16 E7 was found to sensitize keratinocytes to the growth-inhibitory effects of interferon, while coexpression of E6 abrogates this inhibition. Treatment of E7-expressing cells with interferon ultimately resulted in cellular senescence through a process that is dependent upon acetylation of p53 by p300/CBP at lysine 382. Cells expressing mutant forms of E6 that are unable to bind p300/CBP or bind p53 failed to block acetylation of p53 at lysine 382 and were sensitive to growth arrest by interferon. In contrast, mutant forms of E6 that are unable to bind E6AP remain resistant to the effects of interferon, demonstrating that the absolute levels of p53 are not the major determinants of this activity. Finally, p53 acetylation at lysine 382 was found not to be an essential determinant of other types of senescence such as that induced by overexpression of Ras in human fibroblasts. This study identifies an important physiological role for E6 binding to p300/CBP in blocking growth arrest of human keratinocytes in the presence of interferon and so contributes to the persistence of HPV-infected cells.
The E6 and E7 oncoproteins of human papillomaviruses (HPVs) act cooperatively to immortalize and transform human keratinocytes, with one factor complementing the activity of the other. While E7 stabilizes p53, E6 acts to reduce levels by recruiting the cellular ubiquitin ligase E6AP to the p53 complex, resulting in increased rates of turnover (32). Similarly, E6 activates hTert expression, which, together with E7 inactivation of the RB pathway, acts to immortalize human primary cells (22). Additional pathways that may require the cooperative action of both viral proteins involve resistance to growth arrest and senescence induced by either high-level expression of cell cycle regulators such as Ras or exposure to extracellular agents such as interferon.
Interferon is a major signaling component of the innate immune response to viral infection. Type I interferons, which consist primarily of alpha and beta types, induce the transcription of multiple genes via the Jak-Stat signaling pathway, leading to immunomodulatory, as well as growth-inhibitory, effects (7, 34). Recent work has linked the tumor suppressor p53 as a regulator of the interferon response induced upon viral infection (35). While p53 is not directly activated by type I interferons, it can modulate the interferon response, thereby playing a central role in the cellular response to viral infection.
The p53 protein acts as a transcription factor that controls the expression of genes involved in cell cycle regulation, apoptosis, and senescence (23, 28). p53 activity can be further enhanced through complex formation with the transcriptional coactivators CBP/p300. The CBP/p300 proteins are histone acetyltransferases that modulate p53 transcription through the acetylation of histones at target genes (8, 16, 38). In addition, p53 itself can be acetylated by CBP/p300 at the C terminus, resulting in enhanced stability of the protein and augmented transcriptional activity (13, 40). Many DNA tumor viruses target p53 in order to block its effects on cell cycle progression, and abrogation of this activity may also contribute to the resistance of infected cells to interferon.
Inhibition of the cell cycle regulators p53 and Rb by the E6 and E7 proteins results in deregulated cellular proliferation (11, 27, 32, 39). The E7 protein binds to Rb family members, leading to constitutive activation of E2F-inducible genes, as well as increased levels of p53 (20). The E6 protein decreases the steady-state levels of p53 through the formation of a trimeric complex with the tumor suppressor and the cellular E3 ubiquitin ligase E6AP. This results in ubiquitination of p53 and increased protein turnover. E6 can also inhibit the transcriptional capabilities of p53 by binding the transcriptional coactivator CBP/p300 in a trimeric complex, resulting in inhibition of p53 acetylation (29, 37).
Many functions of E6 involve complex formation with p53 and E6AP, while only a limited number of activities have been described for its interaction with CBP/p300. Mutants of E6 that are unable to bind p53 or p300/CBP fail to block p53 acetylation (29, 41). Since p53 has been implicated in mediating the antiproliferative effects of interferon, we investigated how HPV proteins control these effects. Our studies indicate that expression of E7 sensitizes human keratinocytes to interferon and that E6 counteracts this in a p53-dependent manner. This activity of E6 is dependent upon its association with the transcriptional coactivators CBP/p300 but not upon the increased turnover of p53 mediated by the binding of E6AP. Our work identifies an important function for E6 association with CBP/p300 in modulating the response to components of the innate immune surveillance mechanism.
MATERIALS AND METHODS
Cell culture.
Normal human foreskin keratinocytes (NHKs) were isolated from neonatal human foreskin epithelium as described previously and were cultured in keratinocyte basal medium supplemented with hydrocortisone, bovine pituitary extract, insulin, and epidermal growth factor (Lonza, Chicago, IL). Retroviral transductants were cultured with mitomycin C-treated NIH 3T3 J2 fibroblast feeders in E medium plus mouse epidermal growth factor (5 ng/ml; Collaborative Biomedical Products, Bedford, MA). Prior to the harvesting of cells for Western analyses, J2 3T3 fibroblast feeders were removed via treatment with 0.5 mM EDTA in phosphate-buffered saline (PBS).
Creation and mutagenesis of retroviral constructs.
The LXSN, 16E6, 16E7, and 16E6E7 retroviral constructs have been described previously (17). For mutagenesis of LXSN plasmids, the Stratagene (IDT, Coralville, IA) QuikChange XL kit was used with appropriate primers in accordance with the manufacturer's specifications.
Creation of retroviral cell lines.
PT67 cells (Clontech, Mountain View, CA) were transfected with previously described LXSN retroviral constructs by using FuGENE transfection reagent (Roche, Indianapolis, IN). Stable, virus-producing cells were selected by using 1,000 μg/ml G418 for 4 days, followed by an additional 4 days of selection with 500 μg/ml G418. Supernatants from cells were harvested, filtered, and used to infect NHKs for 6 h in the presence of 8 μg/ml Polybrene (Sigma, St. Louis, MO). Infected cells underwent antibiotic selection to generate stable cell lines as described previously (18).
Western blot analysis.
To isolate whole cell extracts, subconfluent cells were first treated with 0.5 mM EDTA in PBS to remove fibroblast feeders, trypsinized, and then pelleted by centrifugation at 1,200 rpm for 4 min. Pellets were washed with PBS, pelleted, and resuspended in 300 μl of RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 5 mM EDTA, 1% Triton X-100, protease inhibitors [Complete Mini; Roche], 100 μM phenylmethylsulfonyl fluoride). Samples were placed in ice for 15 min and then centrifuged at 16,100 × g for 5 min at 4°C. Supernatants were recovered and stored at −80°C. Protein was quantified against bovine serum albumin standards by using the Bradford method (Biotin Protein Assay Reagent; Bio-Rad, Hercules, CA) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described (18). Western analysis was conducted with anti-p53 (Oncogene, La Jolla, CA), anti-Rb (Santa Cruz, Santa Cruz, CA), anti-glyceraldehyde-3-phosphate dehydrogenase (Abcam, Cambridge, MA), and anti-acetylated p53 (K305 or K382) (Biovision, Mountain View, CA) antibodies.
Interferon sensitivity assay.
Prior to plating for the interferon sensitivity assay, cells were trypsinized and stained with trypan blue and viable cells were counted with a hemocytometer. Fifty thousand viable cells were plated on J2 fibroblast feeders in each well of a six-well plate in E medium with three wells designated as controls and three designated for interferon treatment. On days 0, 2, and 4, the medium was changed to fresh with or without 1,000 IU/ml human recombinant beta interferon (EMD Biosciences, San Diego, CA). On the second day after plating, fresh J2 fibroblast feeders were added to each well. On day 5, cells were trypsinized after treatment with 0.5 mM EDTA in PBS and 2 aliquots from each well were trypan blue stained and counted on a hemocytometer, for a total of six counts each of untreated and interferon-treated cells. The highest and lowest values for each group were excluded, and the average number of interferon-treated cells was determined. For experiments extending to 12 days, 100,000 cells were initially plated in 10-cm dishes, fresh J2 cells were added, and the medium was replaced every 3 days.
Ras-induced senescence of IMR90 human fibroblasts.
IMR90 human fibroblasts were first infected with retroviruses expressing wild-type or mutant E6 as described above to obtain stable cell lines. Ras-expressing retroviruses were obtained after transfection of PT67 cells with the vector control pBabe or H-rasV12 (pBabe-ras). Stable, virus-producing cells were selected, and supernatants were harvested after 72 h in 5 ml of medium. The virus-containing medium was filtered, supplemented with 8 μg/ml Polybrene, and used for infections. IMR90 cells expressing E6 and mutant forms of E6 were counted, plated at equal densities, and incubated overnight. For infections, the culture medium was replaced with the pBabe vector or Ras-containing supernatant and then incubated overnight at 37°C. Following infection, cells were expanded and selected for 2 days with 1 μg/ml puromycin. To screen for senescence-associated β-galactosidase activity at 5 days following selection, cells were washed three times with PBS, fixed with 3% formaldehyde, and then washed again with PBS. Cells were then incubated at 37°C with fresh SA-β-gal stain solution for 2 to 16 h. SA-β-gal solution (pH 6.0) contained 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal), 0.5 mM K3Fe(CN)6, 0.5 mM K4Fe(CN)6, 2 mM MgCl2, 150 mM NaCl, 40 mM citric acid, Na2HPO4 for pH, and PBS up to 20 ml (2, 33).
RESULTS
Cells expressing HPV type 16 (HPV-16) E7 exhibit increased sensitivity to interferon-induced growth inhibition.
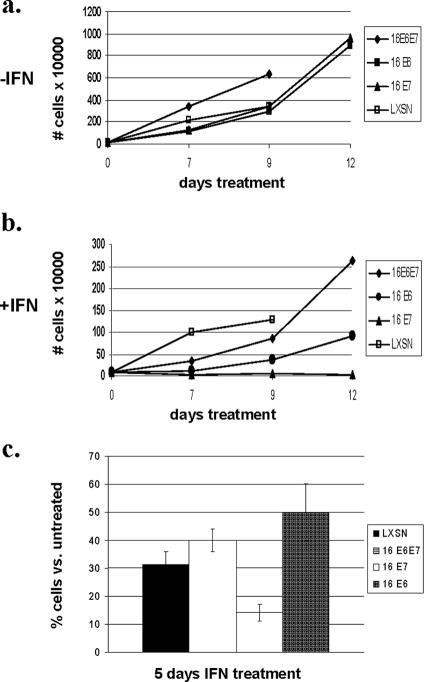
In order to investigate how HPV oncoproteins modulate the cellular response to interferon, we generated a series of five independently derived matched sets of keratinocyte lines in which the HPV-16 E6 and E7 proteins were expressed either individually or in combination. NHKs were isolated from neonatal foreskins and infected with retroviruses expressing HPV-16 E6, E7, or both E6 and E7. Following selection, colonies were expanded and pooled. Cells were then treated with beta interferon for periods of up to 12 days. At various times after initiation of treatment, cells were harvested and stained with trypan blue to identify viable cells. A representative analysis of one matched set of cells expressing HPV proteins is shown in Fig. 1A and B. Similar effects were seen in all five sets of cells treated with interferon. We observed differences in the number of viable cells as early as day 3 of treatment with beta interferon, and by 5 days significant reductions in cell numbers, as well as growth rates, were observed (Fig. 1C). After 5 days, cells expressing E7 alone and treated with interferon exhibited little to no proliferation compared to E6- and E6-E7-expressing cells, which continued to divide (Fig. 1C). Untreated, E7-expressing cells showed growth rates similar to those of untreated cells expressing E6 alone but slightly reduced from those of cells expressing the combination. Similar effects were seen with all five sets of keratinocytes expressing HPV proteins. After 12 days of interferon treatment, cells expressing E7 alone were completely growth arrested while cells expressing E6 alone or both E6 and E7 continued to proliferate (Fig. 1A and B). Identical effects were obtained with cells infected with retroviruses that contained both E6 and E7 but had a translation termination mutation in E6 (data not shown).
FIG. 1.
HPV-16 E7 sensitizes human keratinocytes to the growth-inhibitory effects of interferon. (a and b) NHKs were stably infected with E6-, E7-, or E6-E7-expressing, as well as LXSN control, retroviruses and treated with 1,000 U/ml beta interferon for periods of up to 12 days. Cells were stained with trypan blue, and the viable cells were counted at each time point. A representative set of experiments is shown. (c) Histogram showing the percentage of viable cells following interferon treatment for 5 days compared to that of untreated cells. Cells expressing E6, E7, or E6-E7, along with LXSN vector-only controls, were treated with 1,000 U/ml beta interferon for 5 days. Results shown are the averages of five separate experiments with five individually derived sets of cells expressing various combinations of HPV-16 E6 and E7.
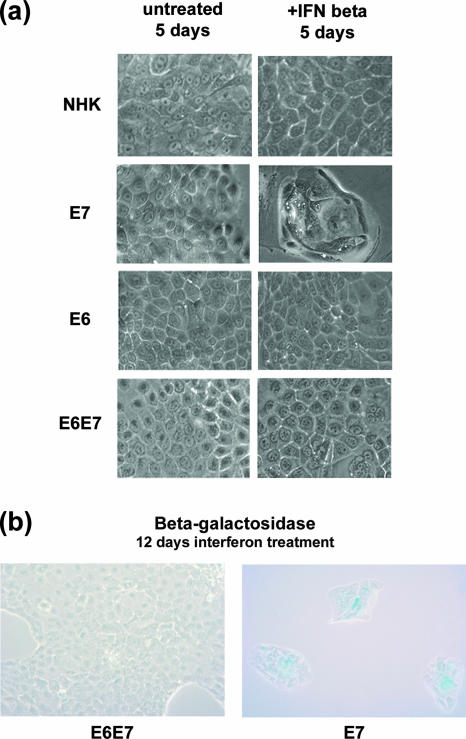
The morphology of the cells expressing E7 alone changed following treatment with interferon, while no significant alterations were seen in LXSN vector-infected control cells or cells expressing E6 alone or in combination with E7 (Fig. 2A). By phase-contrast microscopy, we observed that vector control cells treated with interferon resembled untreated cells. Similarly, no dramatic changes in cell morphology were observed in interferon-treated cells expressing E6 alone or E6 together with E7. In contrast, cells expressing E7 alone formed colonies of large flat cells whose morphology resembled that observed in cells undergoing senescence. By 12 days of interferon treatment, cells expressing E7, but not E6 and E7, became positive for acidic β-galactosidase (SA-β-gal), indicating that the cells were undergoing senescence (Fig. 2B). No blebbing of cytoplasmic membranes or nuclear condensation was seen, indicating that the cells were not undergoing apoptosis (data not shown). Taken together, these results suggest that the presence of E7 leads to increased sensitivity to interferon-induced growth arrest and senescence. Importantly, the coexpression of E6 together with E7 abrogates these effects.
FIG. 2.
Morphological changes in keratinocytes expressing HPV-16 E7 following 5 days of interferon treatment. (a) Phase-contrast microscopy of keratinocytes that stably express control retroviruses, E6, E7, or E6-E7 following 5 days of treatment with 1,000 U/ml beta interferon. (b) Light microscopy of cells expressing both E6 and E7 or E7 alone following 12 days of interferon treatment and staining for senescence-associated β-galactosidase expression. SA-β-gal staining is observable in E7-expressing cells but not in E6-E7-expressing cells.
Inhibition of p53 pathway engagement correlates with sustained proliferation in interferon-treated cells.
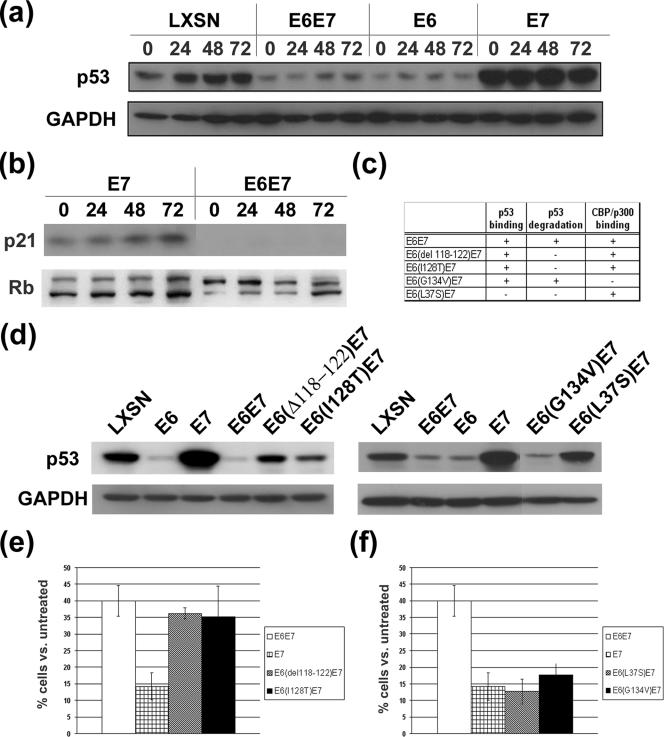
Expression of high-risk E7 proteins in the absence of E6 results in increased levels of p53, as well as enhanced expression of downstream targets such as p21. As p53 has been implicated as a regulator of the type I interferon response (35), we next wanted to investigate whether it contributed to the response of HPV-positive cells to interferon. We therefore examined p53 protein levels in cells expressing E6, E7, or E6-E7 following treatment with beta interferon. As shown in Fig. 3A, cells expressing E7 alone contained levels of p53 higher than those observed in E6- or E6-E7-expressing cells or in vector controls. Following treatment with interferon, little or no change in p53 levels was observed in E6-, E7-, or E6-E7-expressing cells or in vector controls. Consistent with the known downstream targets of p53, the levels of the p53-regulated protein p21 were increased in E7-expressing cells following treatment with interferon but not when E6 was also present (Fig. 3B). Both hypo- and hyperphosphorylated forms of Rb were detected in both E7- and E6-E7-expressing cells following interferon treatment. The ratio of hyper- to hypophosphorylated forms changed slightly in E6-E7-positive cells following interferon treatment, while no such changes were seen in cells expressing E7 alone. Similar effects were seen in three independently derived cell lines. These observations are consistent with the hypothesis that high levels of p53 in E7-expressing cells may be important for the growth-repressive effects of beta interferon.
FIG. 3.
Western analysis of p53 and Rb levels in keratinocytes expressing E6 and E7 following treatment with interferon. (a) Western analysis of p53 levels in keratinocytes expressing vector controls, E6, E7, or E6-E7 following interferon treatment for up to 72 h. LXSN designates cells expressing empty vector control retroviruses. (b) Western analysis of p21 and Rb levels in E7- and E6-E7-expressing cells following interferon treatment for up to 72 h. Hyper- and hypophosphorylated forms of Rb are shown. (c) Characteristics of mutant forms of E6 used in subsequent analyses of p53 binding and degradation and p300/CBP binding. (d) Steady-state p53 levels in keratinocytes infected with E6, E7, or mutant forms of E6 together with E7. (e and f) Proliferation of cells expressing mutant forms of E6 in combination with E7 following 5 days of interferon treatment. Results shown are the average of three independent experiments with three individually derived sets of keratinocytes expressing mutant forms of E6 in combination with E7. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
E6 binding of p53 and CBP/p300, but not p53 degradation, are essential to overcome interferon-mediated growth inhibition.
The studies described above suggested a correlation between increased levels of p53 and sensitivity of E7-expressing cells to interferon. This would be consistent with the increase in proliferation that was observed when E6 was coexpressed with E7 as it targets p53. We next investigated which activities of E6 were responsible for overcoming the growth inhibition of E7-expressing cells. For these studies, we analyzed a series of retrovirally transduced NHKs that expressed several previously characterized mutant E6 proteins in combination with wild-type E7 (Fig. 3C). We first analyzed two mutations that inhibit the ability of E6 to degrade p53. The Δ118-122 and I128T mutant E6 proteins block the increased turnover of p53 mediated through E6 binding to E6AP, resulting in higher steady-state levels of p53. NHKs were infected with retroviruses expressing these two mutant E6 proteins in combination with wild-type E7, and cell lines were isolated following drug selection. Surprisingly, no significant differences in growth were seen following interferon treatment in cells expressing either of the two mutant E6 proteins in combination with E7 compared to cells expressing wild-type E6-E7 (Fig. 3E). After 5 days of treatment, the number of wild-type E6-E7-positive cells was similar to that of cells expressing either the Δ118-122 or I128T mutant form of E6 in combination with E7. Examination of the p53 levels by Western blot analysis demonstrated elevated amounts in cells that expressed mutant E6 proteins compared to those seen in cells expressing wild-type E6 or E6-E7, although they did not reach the levels seen in cells expressing E7 alone (Fig. 3D). These results suggest that the absolute levels of p53 are not a major determinant of sensitivity to interferon.
A second activity of E6 that is directed against p53 function is the inhibition of acetylation through the binding of the histone acetyltransferases CBP/p300 (29, 37). We therefore examined the effects of E6-mediated interaction with these proteins in modulating the interferon-induced growth inhibition of E7-expressing cells (Fig. 3C and D). E6 forms a trimeric complex with p53 and p300/CBP. The abrogation of the binding of E6 to p300 or loss of E6 binding to p53 allows the acetylation of p53. The G134V mutation in HPV-16 E6 abrogates the ability to bind CBP/p300, thereby allowing p53 to be acetylated, but does not alter its ability to bind and degrade p53 (29, 37). Furthermore, the L37S mutation abolishes the ability of E6 to bind p53, thus blocking its increased rate of degradation, as well as the inhibition of acetylation (24). Cell lines were generated following infection with retroviruses expressing these mutant E6 proteins in combination with wild-type E7 and monitored for growth in the presence of interferon. Interestingly, neither the L37S nor the G134V mutant E6 protein was able to overcome growth arrest following treatment with interferon (Fig. 3F). After 5 days of treatment, the number of viable cells infected with either mutant was comparable to that of cells expressing E7 alone, suggesting that the ability of E6 to bind both p53 and CBP/p300 is necessary to overcome interferon-mediated growth inhibition. As expected, the levels of p53 in the G134V mutant were similar to those of E6-expressing cells while in the L37S cells the levels were similar to those seen in LXSN-infected control cells (Fig. 3D).
Growth inhibition corresponds to p53 acetylation at lysine 382.
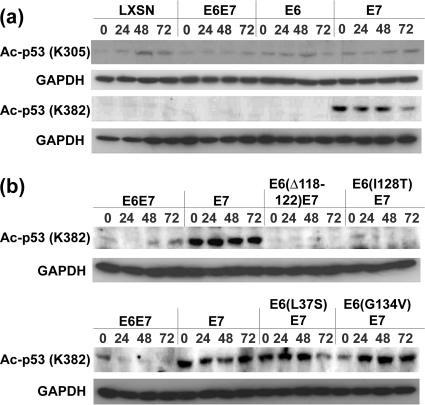
We next wanted to investigate the acetylation status of p53 in cells expressing the various HPV oncoproteins. We first examined keratinocytes expressing wild-type E6, E7, and E6-E7 for the levels of p53 acetylated at two sites targeted by CBP/p300. For these analyses, we used antibodies specific for acetylation of p53 at lysine 305 or lysine 382 (Fig. 4A). No differences in the levels of p53 acetylated at lysine 305 were found in cells expressing any of combination of the viral proteins compared to LXSN-infected controls. Furthermore, levels of p53 acetylated at lysine 305 were not altered by treatment with interferon in either control or HPV-expressing cells. In contrast, high levels of acetylation of p53 at lysine 382 were observed in cells expressing E7 alone but not in control cells or cells expressing E6 or E6-E7. This acetylated form of p53 was seen in E7-expressing cells in the absence of interferon, and the levels were not altered following addition of interferon.
FIG. 4.
Acetylation of p53 at lysine 382 correlates with growth inhibition induced by interferon. (a) Western blot analysis of p53 acetylated at lysine 305 or lysine 382 in cells containing vector controls (LXSN), E6, E7, or E6-E7. Antibodies specific to p53 acetylated at lysine 305 or lysine 382 were used. (b) Levels of p53 acetylated at lysine 382 in keratinocytes expressing E6 mutant proteins along with E7 following interferon treatment for periods of up to 72 h. Results shown are from three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Similar analyses of the levels of p53 acetylation at lysine 382 were next performed by using extracts of cells expressing mutant forms of E6 in the presence of wild-type E7 (Fig. 4B). In cells expressing either the Δ118-122 deletion or the I128T mutant form, the levels of p53 acetylation at lysine 382 were similar to those seen in cells expressing wild-type E6 or E6-E7. In contrast, cells containing the L37S or the G134V mutant form of E6 exhibited levels of p53 acetylation at lysine 382 significantly higher than those seen in wild-type E6-E7-expressing cells and nearly identical to levels observed in cells expressing E7 alone. Our results indicate that inhibition of p53 acetylation at lysine 382 closely correlates with the ability of E6 to overcome interferon-mediated growth inhibition in the presence of the E7 protein.
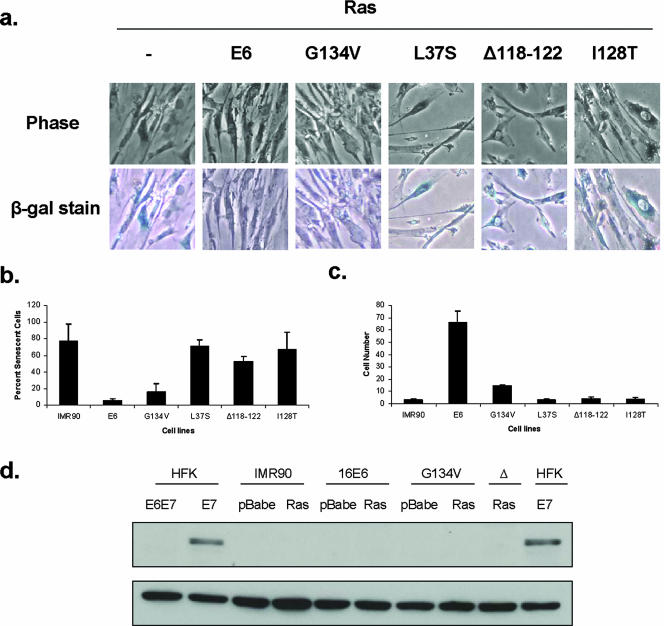
To investigate if acetylation of p53 at lysine 382 was necessary for the induction of other forms of growth arrest leading to senescence, we used a well-characterized method by which growth arrest, and eventually senescence, is induced through the overexpression of Ras in human fibroblasts (2, 33). The ability of E6 to overcome Ras-mediated senescence of human fibroblasts has previously been reported, but the mechanisms responsible have not been characterized (25, 26). To determine which activities of E6 were important in this system, we first infected IMR90 human embryonic lung fibroblasts with control retroviruses or E6-expressing retroviruses and selected stable cell lines. These cell lines were then superinfected with Ras-expressing retroviruses and after 5 days, screened for the number of viable cells. In addition, we examined the cells for expression of SA-β-gal to assay for senescence. Consistent with previous studies, we observed that high-level expression of Ras in IMR90 cells inhibited cell growth, caused flattening of cells, and induced expression of SA-β-gal (Fig. 5A). In contrast, cells expressing both wild-type E6 and Ras continued to proliferate, exhibited a small-cell phenotype, and were not positive for β-galactosidase (Fig. 5A). We next repeated these experiments with the panel of mutant forms of E6 described above and observed that cells expressing the mutant forms of E6 that were unable to degrade p53 were not able to overcome Ras-induced growth arrest (Fig. 5B). In addition, the mutant forms of E6 that failed to block acetylation of p53 (G134V) in keratinocytes were still able to overcome Ras-mediated growth arrest, although at a reduced rate compared to wild-type E6 (Fig. 5B). The L37S mutant that does not bind p53 was also not able to overcome Ras-mediated arrest. We confirmed the presence of E6 in these cells by the ability to reduce p53 levels (data not shown). Our data suggest that blocking the ability to bind p53 or inhibiting its rapid turnover is important for overcoming Ras-induced growth arrest and senescence in fibroblasts. In contrast, E6-mediated effects on p53 acetylation were not essential for blocking senescence induced in this system. Consistent with this observation, we failed to observe acetylation of p53 in Ras-expressing IMR90 cells with or without the presence of E6 or mutant forms of E6 (Fig. 5D). We conclude that the ability of E6 to block p53 acetylation through p300/CBP binding is important in overcoming interferon-induced senescence but not other types of senescence.
FIG. 5.
Effects of mutant forms of E6 on the ability to rescue Ras-induced growth arrest in IMR90 human fibroblasts. (a, top row) Phase-contrast microscopy of IMR90 cells with and without HPV-16 E6 and mutant forms of E6 at 5 days following infection with Ras. The IMR90 cells alone with Ras appear spindly, larger, and fewer in number compared to E6 cells plus Ras. (a, bottom row) IMR90 cells with E6 and mutant forms of E6 that are also expressing Ras were stained for senescence-associated β-galactosidase expression 5 days after infection and selection with Ras retroviruses. (b) Graph depicting the percentage of senescent cells in cell lines after Ras infection as assayed by counting the blue cells after staining with SA-β-gal solution. Upon Ras infection, IMR90 cells show a high number of senescent cells compared to cells expressing wild-type E6 and Ras. The E6G134V mutation partially rescues Ras-induced senescence, while the other mutants do not. (c) Graph depicting relative cell survival in all cell lines at 5 days postinfection and selection with Ras. Cells were counted prior to being stained with SA-β-gal solution. Following infection with retroviruses expressing Ras, the IMR90, L37S, Δ118-122, and I128T cell lines exhibited low levels of survival compared to wild-type E6-expressing cells, while an intermediate phenotype was observed with G134V E6 mutant-expressing cells. (d, top row) Western blot analysis of p53 acetylated at lysine 382 in IMR90 cells infected with E6 and mutant forms of E6, as well as the vector alone or Ras. NHKs expressing HPV-16 E7 and E6-E7 were included as positive and negative controls, respectively. (d, bottom row) Western blot analysis of glyceraldehyde-3-phosphate dehydrogenase as a loading control for lysates in the top row.
DISCUSSION
In this study, we demonstrate that expression of HPV-16 E7 sensitizes keratinocytes to the growth-inhibitory effects of interferon and that coexpression of E6 abrogates this inhibition. The growth arrest of E7-expressing keratinocytes induced by interferon ultimately results in senescence in a process that is dependent upon the acetylation of p53 mediated by p300/CBP. In the presence of E6, acetylation of p53 by p300/CBP has been shown to be blocked through the formation of a trimeric complex of E6, p53, and p300/CBP (29, 37, 41). In our study, this complex formation is necessary for the continued proliferation of cells in the presence of interferon, as mutant forms of E6 that cannot bind p300/CBP or p53 were unable to overcome E7-induced sensitivity to interferon. In contrast, mutant forms of E6 that abrogate binding to E6AP, which increases the rate of degradation of p53, had little or no effect in mediating resistance to interferon. This identifies an important in vivo activity for E6 binding to p300/CBP in human keratinocytes. Previous studies have shown that p300/CBP-mediated acetylation of p53 enhances its transcriptional activation ability, as well as its sequence-specific DNA-binding activity (29, 40). Furthermore, in vitro studies demonstrated that the ability of E6 to bind to p300/CBP is important for repression of p53-dependent gene activation. Our studies identify an important new in vivo physiological role for E6 binding to p300/CBP in mediating resistance to growth arrest induced by interferon.
Interferons are important antiviral agents that modulate both the initial phase of viral infection and persistence (1). Persistent infection with high-risk HPVs is a risk factor for the development of cancer and requires escape from immune surveillance. Our findings suggest that binding of E6 to p300/CBP may play an important role in counteracting surveillance by the innate immune system. Stratified keratinocytes constitutively express beta interferon so as to prime cells to inhibit viral infections (5). The interaction of E6 and p300/CBP may thus block the growth-suppressing effects of interferon and allow HPV infections to persist for extended periods of time. Interferon therapy has also been suggested as a treatment for high-grade HPV infections, but variable results have been observed in clinical trails (6, 14, 15). Previously, we reported that keratinocytes that maintain HPV-31 episomes are highly sensitive to interferon treatment but that resistant populations quickly appear (9). These resistant populations contain integrated copies of HPV-31 with expression limited to the E6-E7 oncoproteins. This effect was recently confirmed by using HPV-16-positive W12 cells, indicating that it is a property that is common to high-risk HPV types (19). These observations suggest that the failure of interferon therapy to clear HPV-positive lesions may have been the result of mixed populations of cells containing integrated and extrachromosomal genomes. Furthermore, interferon treatment may select for expansion of populations of cells that contain integrated HPV genomes while eliminating cells with episomes. Our present studies suggest the possibility that keratinocytes can be sensitized to interferon treatment if p53 can be acetylated at lysine 382 and this could potentially be achieved through the use of chemical inhibitors of histone deacetylases (HDACs). HDACs deacetylate histones and other proteins such as p53, and treatment with HDAC inhibitors induces p53 acetylation (21, 30, 31). We hypothesize that the use of these inhibitors in combination with interferon may be effective in clearing HPV lesions that contain cells with both integrated and episomal genomes.
Treatment of E7-expressing cells for 12 days with interferon was found to result in cellular senescence by a process that was dependent upon acetylation of p53 at lysine 382. A similar role for acetylation of p53 has been reported in human fibroblasts induced to undergo senescence following high-level expression of Ras, although other studies have not shown this effect (26). We also observed that E6 was able to overcome Ras-mediated senescence in IMR90 fibroblasts, and this was lost with mutants unable to bind p53 or degrade it through the action of E6AP. Mutants of E6 that were deficient in binding to p300/CBP were still able to overcome Ras-induced arrest in fibroblasts, although not as efficiently as wild-type E6. It therefore appears that the acetylation of p53 at lysine 382 by p300/CBP is not critical in all cell types for p53-dependent senescence and that other functions are also important. This is consistent with our failure to observe significant levels of p53 acetylation in Ras-expressing IMR90 cells. In the course of our studies, it was reported that interferon treatment of normal human fibroblasts induces growth arrest and senescence (26). This observation contrasts with our work on NHKs, which continue to proliferate in the presence of interferon, although at a slightly reduced rate. Furthermore, our work indicates that expression of E7 is required to sensitize human keratinocytes to the effects of interferon. While senescence induced by interferon in human fibroblasts was shown to be dependent upon p53, the same mechanism does not appear to play a role as differential acetylation of p53 at lysine 382 was not observed in these analyses (26). It thus appears that significant cell type differences exist in the response to interferon, and we believe our studies with human keratinocytes more accurately reflect what happens in natural infections.
While the acetylation of p53 at lysine 382 appears to be central to mediating sensitivity to interferon in keratinocytes, the mechanism by which E7 induces this modification is not clear. E7 has also been reported to bind p300/CBP, and it is possible that this may result in acetylation of p53 (4). However, E7 was found to abolish p300/CBP-mediated transactivation, suggesting that it acts to inhibit its function rather than activate it. Alternatively, E7 binding to the Rb family of proteins could indirectly lead to acetylation of p53. A third possibility is that p53 acetylation could result from E7 binding to HDACs that inhibit deacetylation. Finally, E7 expression induces genomic instability, which results in a DNA damage response that could activate p53 acetylation (10). Additional detailed mutational analyses are required to elucidate the exact mechanisms by which p53 acetylation is induced. In our studies, E7 acted to increase the levels of p53 and its downstream target, p21. Our previous study indicated that p53 is transcriptionally active in E7-expressing keratinocytes (36), but this was not seen in E7-positive fibroblasts (12) and may reflect cell type differences. Cell growth arrest and senescence have been shown to be the result of a convergence of signaling from both the Rb and p53 pathways (3). In our studies, the ratio of hypo- to hyperphosphorylated forms of Rb increased moderately with interferon treatment, suggesting that these may contribute to p53-mediated effects. This contrasts with the significant reductions seen in fibroblasts treated with interferon, further underscoring the cell type-specific effects of interferons. Overall, our studies demonstrate that the binding of E6 to p53 and CBP/p300 is crucial to the ability to overcome interferon-mediated growth arrest in human keratinocytes and identifies an important physiological activity for these interactions.
Acknowledgments
This work was supported by grants from the NCI (R37CA74202) and NIAID (UO1 AI31494) to L.A.L. M.B. was supported by carcinogenesis training grant T32 CA009560-20.
Footnotes
Published ahead of print on 26 September 2007.
REFERENCES
- 1.Barton, E. S., M. L. Lutzke, R. Rochford, and H. W. Virgin IV. 2005. Alpha/beta interferons regulate murine gammaherpesvirus latent gene expression and reactivation from latency. J. Virol. 79:14149-14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benanti, J. A., and D. A. Galloway. 2004. Normal human fibroblasts are resistant to RAS-induced senescence. Mol. Cell. Biol. 24:2842-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Porath, I., and R. A. Weinberg. 2005. The signals and pathways activating cellular senescence. Int. J. Biochem. Cell Biol. 37:961-976. [DOI] [PubMed] [Google Scholar]
- 4.Bernat, A., N. Avvakumov, J. S. Mymryk, and L. Banks. 2003. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene 22:7871-7881. [DOI] [PubMed] [Google Scholar]
- 5.Bielenberg, D. R., M. F. McCarty, C. D. Bucana, S. H. Yuspa, D. Morgan, J. M. Arbeit, L. M. Ellis, K. R. Cleary, and I. J. Fidler. 1999. Expression of interferon-beta is associated with growth arrest of murine and human epidermal cells. J. Investig. Dermatol. 112:802-809. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein, J., B. Pascal, D. Zarfati, N. Goldshmid, and H. Abramovici. 1997. Recombinant human interferon-beta for condylomata acuminata: a randomized, double-blind, placebo-controlled study of intralesional therapy. Int. J. STD AIDS 8:614-621. [DOI] [PubMed] [Google Scholar]
- 7.Brierley, M. M., and E. N. Fish. 2002. Review: IFN-α/β receptor interactions to biologic outcomes: understanding the circuitry. J. Interferon Cytokine Res. 22:835-845. [DOI] [PubMed] [Google Scholar]
- 8.Chan, H. M., and N. B. La Thangue. 2001. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114:2363-2373. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y. E., and L. A. Laimins. 2001. Interferon-inducible genes are major targets of human papillomavirus type 31: insights from microarray analysis. Dis. Markers 17:139-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duensing, S., A. Duensing, C. P. Crum, and K. Munger. 2001. Human papillomavirus type 16 E7 oncoprotein-induced abnormal centrosome synthesis is an early event in the evolving malignant phenotype. Cancer Res. 61:2356-2360. [PubMed] [Google Scholar]
- 11.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 12.Eichten, A., M. Westfall, J. A. Pietenpol, and K. Munger. 2002. Stabilization and functional impairment of the tumor suppressor p53 by the human papillomavirus type 16 E7 oncoprotein. Virology 295:74-85. [DOI] [PubMed] [Google Scholar]
- 13.Fu, M., C. Wang, X. Zhang, and R. G. Pestell. 2004. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem. Pharmacol. 68:1199-1208. [DOI] [PubMed] [Google Scholar]
- 14.Garciá-Millián, R., A. Santos, S. E. Perea, R. Gonzalez-Cabanas, C. Valenzuela, and M. Arana. 1999. Molecular analysis of resistance to interferon in patients with laryngeal papillomatosis. Cytokines Cell. Mol. Ther. 5:79-85. [PubMed] [Google Scholar]
- 15.Gonzalez-Sanchez, J. L., J. C. Martinez-Chequer, M. E. Hernandez-Celaya, E. Barahona-Bustillos, and A. F. Andrade-Manzano. 2001. Randomized placebo-controlled evaluation of intramuscular interferon beta treatment of recurrent human papillomavirus. Obstet. Gynecol. 97:621-624. [DOI] [PubMed] [Google Scholar]
- 16.Grossman, S. R. 2001. p300/CBP/p53 interaction and regulation of the p53 response. Eur. J. Biochem. 268:2773-2778. [DOI] [PubMed] [Google Scholar]
- 17.Halbert, C. L., G. W. Demers, and D. A. Galloway. 1991. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J. Virol. 65:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hebner, C. M., R. Wilson, J. Rader, M. Bidder, and L. A. Laimins. 2006. Human papillomaviruses target the double-stranded RNA protein kinase pathway. J. Gen. Virol. 87:3183-3193. [DOI] [PubMed] [Google Scholar]
- 19.Herdman, M. T., M. R. Pett, I. Roberts, W. O. Alazawi, A. E. Teschendorff, X. Y. Zhang, M. A. Stanley, and N. Coleman. 2006. Interferon-beta treatment of cervical keratinocytes naturally infected with human papillomavirus 16 episomes promotes rapid reduction in episome numbers and emergence of latent integrants. Carcinogenesis 27:2341-2353. [DOI] [PubMed] [Google Scholar]
- 20.Iaquinta, P. J., A. Aslanian, and J. A. Lees. 2005. Regulation of the Arf/p53 tumor surveillance network by E2F. Cold Spring Harbor Symp. Quant. Biol. 70:309-316. [DOI] [PubMed] [Google Scholar]
- 21.Ito, A., C. H. Lai, X. Zhao, S. Saito, M. H. Hamilton, E. Appella, and T. P. Yao. 2001. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20:1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiyono, T., S. A. Foster, J. I. Koop, J. K. McDougall, D. A. Galloway, and A. J. Klingelhutz. 1998. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 396:84-88. [DOI] [PubMed] [Google Scholar]
- 23.Liu, G., and X. Chen. 2006. Regulation of the p53 transcriptional activity. J. Cell. Biochem. 97:448-458. [DOI] [PubMed] [Google Scholar]
- 24.Liu, Y., J. J. Chen, Q. Gao, S. Dalal, Y. Hong, C. P. Mansur, V. Band, and E. J. Androphy. 1999. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J. Virol. 73:7297-7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, Z., J. Ghai, R. S. Ostrow, R. C. McGlennen, and A. J. Faras. 1994. The E6 gene of human papillomavirus type 16 is sufficient for transformation of baby rat kidney cells in cotransfection with activated Ha-ras. Virology 201:388-396. [DOI] [PubMed] [Google Scholar]
- 26.Moiseeva, O., F. A. Mallette, U. K. Mukhopadhyay, A. Moores, and G. Ferbeyre. 2006. DNA damage signaling and p53-dependent senescence after prolonged beta-interferon stimulation. Mol. Biol. Cell 17:1583-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Münger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oren, M. 2003. Decision making by p53: life, death and cancer. Cell Death Differ. 10:431-442. [DOI] [PubMed] [Google Scholar]
- 29.Patel, D., S. M. Huang, L. A. Baglia, and D. J. McCance. 1999. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 18:5061-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy, S., K. Packman, R. Jeffrey, and M. Tenniswood. 2005. Histone deacetylase inhibitors differentially stabilize acetylated p53 and induce cell cycle arrest or apoptosis in prostate cancer cells. Cell Death Differ. 12:482-491. [DOI] [PubMed] [Google Scholar]
- 31.Roy, S., and M. Tenniswood. 2007. Site-specific acetylation of p53 directs selective transcription complex assembly. J. Biol. Chem. 282:4765-4771. [DOI] [PubMed] [Google Scholar]
- 32.Scheffner, M., B. A. Werness, J. M. Huibregtse, A. J. Levine, and P. M. Howley. 1990. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129-1136. [DOI] [PubMed] [Google Scholar]
- 33.Serrano, M., A. W. Lin, M. E. McCurrach, D. Beach, and S. W. Lowe. 1997. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88:593-602. [DOI] [PubMed] [Google Scholar]
- 34.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 35.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 424:516-523. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, J. T., and L. A. Laimins. 1998. Human papillomavirus oncoproteins E6 and E7 independently abrogate the mitotic spindle checkpoint. J. Virol. 72:1131-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas, M. C., and C. M. Chiang. 2005. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol. Cell 17:251-264. [DOI] [PubMed] [Google Scholar]
- 38.Vo, N., and R. H. Goodman. 2001. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 276:13505-13508. [DOI] [PubMed] [Google Scholar]
- 39.Werness, B. A., A. J. Levine, and P. M. Howley. 1990. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248:76-79. [DOI] [PubMed] [Google Scholar]
- 40.Yang, X. J. 2004. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays 26:1076-1087. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann, H., R. Degenkolbe, H. U. Bernard, and M. J. O'Connor. 1999. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 73:6209-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]