Abstract
Many cellular processes rely on the ordered assembly of macromolecular structures. Here, we uncover an unexpected link between two such processes, endocytosis and transcription. Many endocytic proteins, including eps15, epsin1, the clathrin assembly lymphoid myeloid leukemia (CALM), and α-adaptin, accumulate in the nucleus when nuclear export is inhibited. Endocytosis and nucleocytoplasmic shuttling of endocytic proteins are apparently independent processes, since inhibition of endocytosis did not appreciably alter nuclear translocation of endocytic proteins, and blockade of nuclear export did not change the initial rate of endocytosis. In the nucleus, eps15 and CALM acted as positive modulators of transcription in a GAL4-based transactivation assay, thus raising the intriguing possibility that some endocytic proteins play a direct or indirect role in transcriptional regulation.
Keywords: endocytosis, nucleocytoplasmic shuttling, eps15, CRM1, transcription
Introduction
Bidirectional transport of proteins through the nuclear pore complex represents an important regulatory mechanism for a variety of cellular processes. Transported proteins display specialized sequences, nuclear localization signals or nuclear export signals (NESs), which bind to shuttling receptor molecules (importins and exportins, respectively), thus allowing their transport in and out of the nucleus (Nigg 1997).
The major nuclear export pathway relies on the interaction between NES-containing proteins and the chromosomal region maintenance protein (CRM)1/exportin1 receptor (Fornerod et al. 1997; Fukuda et al. 1997; Ossareh-Nazari et al. 1997; Stade et al. 1997). The small GTPase Ran in its active form (Ran-GTP) is absolutely required for this interaction. By cooperatively binding to CRM1 in the presence of NES-containing cargo molecules, Ran-GTP allows the formation of a trimeric Ran-GTP–CRM1–NES complex (Fornerod et al. 1997; Izaurralde et al. 1997). The antifungal antibiotic leptomycin B (LMB) specifically inhibits CRM1 (Kudo et al. 1998). By selectively modifying a single cysteine residue in CRM1 (Kudo et al. 1999), LMB interferes with the formation of RanGTP–CRM1–NES complexes, leading to nuclear accumulation of NES-containing proteins (Askjaer et al. 1998). Thus, LMB has represented a powerful tool to investigate nuclear shuttling of several proteins. More interestingly, LMB has allowed the unexpected detection of nuclear translocation of a variety of proteins with predominant extranuclear localization and function, such as the membrane-associated guanylate kinase CASK/LIN-2 (Hsueh et al. 2000), LIM-containing lipora-preferred partner (an actin cytoskeleton protein) (Petit et al. 2000), and actin (Wada et al. 1998). Therefore, it is predicted that LMB-aided analysis will unveil more nuclear translocation events of proteins whose export rate is too high to determine substantial nuclear accumulation at steady state.
Endocytic proteins might constitute one such class of molecules. Scattered evidence supports the idea that the molecular machinery of endocytosis might also play some currently undefined function in the nucleus. Box-dependent myc-interacting protein-1, which is related to amphiphysin, displays nuclear localization (Sakamuro et al. 1996). Eps15R, a bona fide endocytic protein, also shows a dual cytosolic/nuclear distribution (Coda et al. 1998). Epsin1, another endocytic protein, accumulates in the nucleus in the presence of LMB when overexpressed (Hyman et al. 2000). Functional evidence linking endocytic proteins to nucleocytosolic transport also exists as shown by findings that eps15 and eps15R play a role in the CRM1-based nuclear export pathway (Doria et al. 1999).
A major argument against a hypothetical function in the nucleus is that, by and large, endocytic proteins do not display nuclear localization. However, this might be due to a high rate of nuclear export. In this study, we undertook a systematic analysis of the subcellular distribution of several endocytic proteins under conditions of blockade of nuclear export as obtained by LMB treatment. Surprisingly, we found that several of the analyzed proteins accumulate in the nucleus under these conditions, thus indicating nucleocytoplasmic shuttling of endocytic machinery. In the nucleus, two endocytic proteins, eps15 and CALM, acted as transcriptional modulators in a GAL4-based transactivation assay. Our results uncover an unexpected property of several endocytic proteins and suggest their participation in nuclear events, possibly including regulation of transcription.
Materials and Methods
Confocal Microscopy Analysis
Cells grown on glass coverslips were treated overnight with 10 ng/ml LMB or mock-treated and subsequently subjected to immunofluorescence analysis. The following antibodies were used: monoclonals anti-eps15, anti–α-adaptin (AP.6; Affinity BioReagents, Inc.), anti–γ-adaptin and –β-adaptin (100/3 and 100/1, respectively; Sigma-Aldrich), anti–μ2-adaptin (AP50; Transduction Laboratories), anti-clathrin (X22; provided by T. Kirchhausen, Harvard Medical School, Boston, MA), polyclonals anti-CALM (C-18; Santa Cruz Biotechnology, Inc.), anti-dynamin II (C-18; Santa Cruz Biotechnology, Inc.), anti-epsin1 (R-20; Santa Cruz Biotechnology, Inc.), and anti-rab5 (provided by M. Zerial, Max-Planck Institute, Dresden, Germany). Alexa red and green secondary antibodies (Molecular Probes) were used. Images were acquired using a confocal 1024MRC Bio-Rad Laboratories microscope equipped with a krypton-argon laser.
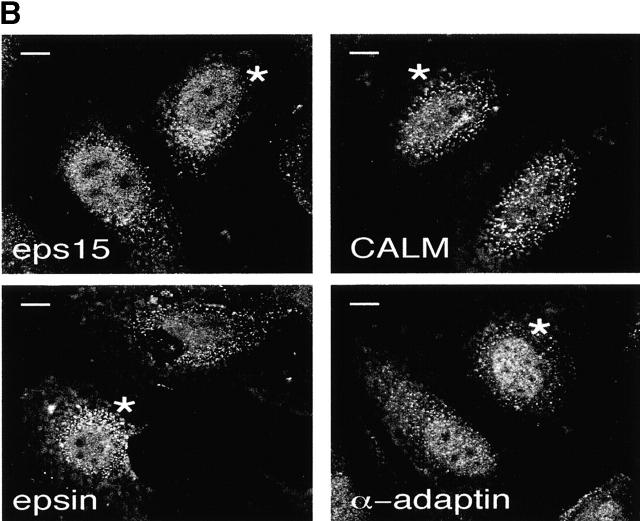
A semiquantitative analysis of the experiment shown in Fig. 3 B was performed with the NIH Image software by measuring the pixel density in the nucleus relative to the cytosol on central sections obtained by confocal microscopy. Values were obtained for five cells for each experimental condition. Results, expressed as the cytosol/nucleus ratio for each protein, were (the first value refers to untransfected cells, the second to rab5S34N-transfected cells): eps15, 3.4, 3.8; CALM, 5.6, 5.0; epsin, 4.7, 4.1; α-adaptin, 2.6, 3.0. SD was <20% of the mean in all cases.
Figure 3.
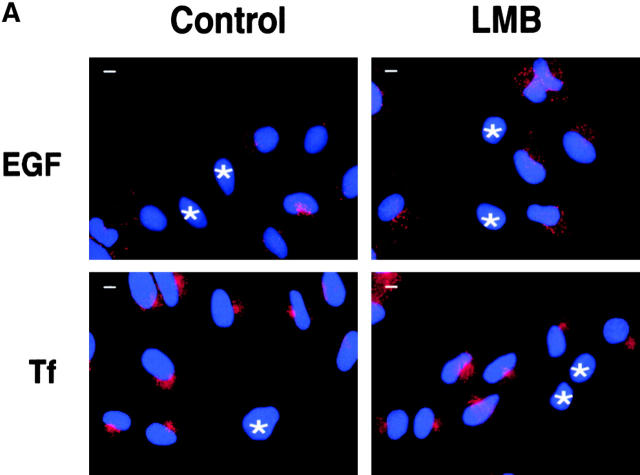
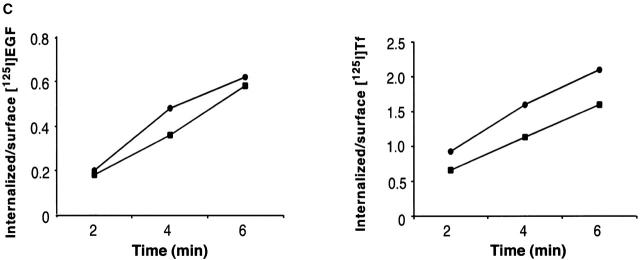
Endocytosis and nucleocytoplasmic shuttling of endocytic proteins are independent processes. (A) CV-1 cells were transiently transfected with a dominant negative rab5 mutant (rab5S34N) and either mock-treated (control) or treated with LMB for 16 h. Cells were then exposed to rhodamine-EGF (top) or rhodamine-Tf (bottom) for internalization assays. Transfected cells were identified by immunostaining with an anti-rab5 antibody and are indicated by asterisks. Red, rhodamine-EGF or -Tf; blue, DAPI nuclear counterstain. (B) Cells transiently transfected with rab5S34N were treated with LMB and stained with the indicated antibodies. Asterisks indicate rab5S34N-transfected cells. Central sections obtained by confocal microscopy are shown. (C) HeLa cells treated for 16 h with LMB (squares) or mock-treated (circles) were tested for internalization of 125I-EGF (2 ng/ml; left) or 125I-Tf (1 μg/ml; right). Bars, 10 μm.
Production of Recombinant Proteins and In Vitro Binding Assays
GST-eps15wt(1–897), GST-eps15(1–874), and GST-CALM were prepared by recombinant PCR. GST-epsin1(402–576) was a gift from P. De Camilli (Yale University School of Medicine, New Haven, CT). In vitro binding experiments were performed by incubating 15 μg of each GST fusion with 20 μl of in vitro–translated [35S]Met–labeled hCRM1 in binding buffer (50 mM Hepes/KOH, pH 7.5, 200 mM NaCl, 5 mM [CH3COO]2Mg, 1% boiled FBS, 1 mM PMSF, 10 μg/ml aprotinin, and 10 μg/ml leupeptin) in the presence or absence of 2 μM GTP-loaded RanQ69L. The incubation was performed at 4°C for 2 h. The beads were subsequently washed with binding buffer, and proteins were resolved by SDS-PAGE followed by autoradiography.
Internalization Assays
Internalization assays using fluorochrome-conjugated ligands have been described previously (Lanzetti et al. 2000). For internalization experiments using radiolabeled ligands, HeLa cells grown in 6-well dishes were serum starved for 3 h before the addition of 2 ng/ml of 125I-EGF or 1 μg/ml of 125I-transferrin (Tf) in binding medium (DME with 20 mM Hepes, pH 7.4, and 0.1% BSA) for 2, 4, and 6 min at 37°C. The amount of surface-bound and internalized radioactivity was determined as previously described (Sorkin et al. 1993). The rate of internalization is expressed as the ratio between internalized and surface 125I-ligand for each time point. A low concentration of 125I-EGF was used to avoid saturation of the internalization machinery. Specific binding is reported for all data points. Nonspecific binding was measured by adding a 100-fold excess of cold EGF.
Luciferase Reporter Assays
For transcriptional assays, epsin1, CALM, eps15, and its truncated versions were cloned into the PM2 vector (Sadowski et al. 1992) fused to the GAL4 DNA-binding domain (amino acids positions 1–147). COS-7 cells grown in 6-well dishes were transiently transfected in triplicate with 0.3 μg of the GAL4-TK-luciferase reporter (provided by K. Helin, European Institute of Oncology, Milan, Italy) and with 1.2 μg of the different GAL4 fusion constructs using lipofectamine (Life Technologies). Cells were lysed after 48 h and analyzed by immunoblotting with anti-GAL4 antibodies (Santa Cruz Biotechnology, Inc.) to verify the levels of expression of the various GAL4 fusion proteins. Transactivation assays were then performed only on sets of transfectants that showed comparable levels of expression of the various proteins. Luciferase activity was measured on identical amounts of total cellular lysates from the various transfectants using a commercial kit (Promega).
Results and Discussion
Endocytic Proteins Shuttle In and Out of the Nucleus
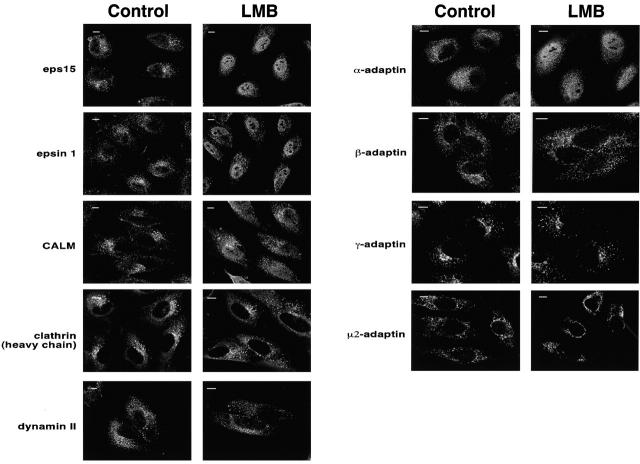
We undertook a systematic analysis of the subcellular distribution of several endocytic proteins under conditions of inhibition of nuclear export as obtained by LMB treatment. In HeLa or CV-1 cells, all of the tested endocytic proteins showed a typical punctate staining pattern (Fig. 1). However, upon LMB treatment we could readily detect nuclear accumulation of eps15, α-adaptin, epsin1, and CALM (Fig. 1), whereas the distribution of clathrin, dynamin II, β-adaptin, μ2-adaptin, and γ-adaptin remained unchanged (Fig. 1). We note that in all cases the size of the shuttling endocytic proteins is above the cut-off value for free diffusion into the nucleus, which is ∼50 kD.
Figure 1.
Endocytic proteins shuttle in and out of the nucleus. CV-1 cells were mock-treated (control) or treated for 16 h (LMB) with 10 ng/ml LMB and stained with the antibodies indicated on the left. Central sections obtained by confocal microscopy are shown. Bars, 10 μm.
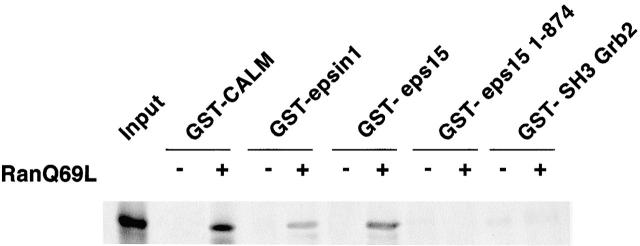
We next investigated whether endocytic proteins bind to CRM1. Immobilized GST-eps15, GST-CALM, or GST-epsin1 (Chen et al. 1998) were incubated with an in vitro–translated hCRM1 in the presence or absence of GTP-loaded RanQ69L, a constitutively active mutant of the Ran GTPase. We could detect RanQ69L-dependent binding of all three proteins to hCRM1 (Fig. 2).
Figure 2.
Eps15, Epsin, and CALM bind to hCRM1 in a Ran-dependent manner. Immobilized GST-CALM, GST-epsin1 (a fragment encompassing amino acid positions 402–576 and encompassing a putative NES), GST-eps15, GST-eps15 (amino acids 1–874), and GST-SH3 of Grb2 (as a negative control) were incubated with in vitro–translated hCRM1 (the lane input represents one-fourth of the amount of hCRM1 used in the assay) in the presence or absence of a GTP-loaded constitutively active mutant of the Ran GTPase (RanQ69L).
In the last 23 amino acids of eps15, an NES sequence is present whose deletion causes nuclear accumulation of the protein (our unpublished results). A truncated form of eps15 (amino acids 1–874), lacking the last 23 amino acids, was unable to bind to CRM1 (Fig. 2), supporting the idea that at least in the case of eps15 the formation of a complex with CRM1 is mediated by a CRM1–NES interaction. Finally, the nuclear accumulation of endocytic proteins appeared to be a saturable process. Only a fraction of their total pool (roughly ≥20–30%) shuttled in a CRM1-dependent manner as witnessed by the fact that we could never observe a dramatic depletion of the extranuclear pool (Fig. 1) even when higher doses and longer times of treatment with LMB were used (data not shown). The sum of our results indicates that endocytic proteins shuttle in and out of the nucleus and are exported through binding to CRM1.
Endocytosis and Nucleocytoplasmic Shuttling of Endocytic Proteins Are Independently Regulated Processes
We next investigated the reciprocal relationships between endocytosis and nucleocytoplasmic shuttling of endocytic proteins. Initially, we inhibited endocytosis and studied nuclear accumulation of endocytic proteins in the presence of LMB. A dominant negative mutant of rab5 (rab5S34N) (Lanzetti et al. 2000), which blocked endocytosis of both Tf and EGF receptors (Fig. 3 A), was transfected into CV-1 cells. Inhibition of endocytosis did not appreciably affect the nuclear accumulation of endocytic proteins (Fig. 3 B; a semiquantitative analysis is reported in Materials and Methods).
In a second set of experiments, we treated CV-1 cells with LMB for 16 h, a condition under which nuclear export is completely blocked, and then measured the uptake of radiolabeled ligands. As shown in Fig. 3 C, the initial rate of internalization of EGF and Tf was comparable in the presence or absence of LMB. Thus, endocytosis and nucleocytoplasmic shuttling of endocytic proteins are apparently independent processes.
Nucleocytoplasmic Shuttling of eps15 Is Not Regulated by Several Signaling Pathways
Nucleocytoplasmic transport is regulated frequently by signaling pathways. For example, nuclear shuttling of signal transducer and activator of transcriptions, the Net transcriptional repressor, and actin are regulated by tyrosine phosphorylation, UV/heat shock, and heat shock, respectively (Wada et al. 1998; Ducret et al. 1999; Milocco et al. 1999). Eps15 is tyrosine phosphorylated by active EGF receptors (Fazioli et al. 1993); thus, we employed it as a model to study the possible regulation of shuttling of endocytic proteins by signaling pathways.
Initially, we compared the nuclear accumulation of a mutant eps15 (FLAG-Y850F) in which the tyrosine phosphorylation site was mutagenized to Phe (Confalonieri et al. 2000) with that of a comparable wild-type construct (FLAG-eps15). Both the wild-type and mutant eps15 were clearly detectable in the nucleus upon LMB treatment (Fig. 4 A). Notably, wild-type eps15 accumulated in the nucleus upon LMB treatment even under serum starvation conditions (Fig. 4 A). In addition, EGF, serum, and phorbol ester treatment did not cause a significant increase in nuclear accumulation of eps15 (Fig. 4 B). Similarly, various stress-inducing stimuli, including UV, H2O2, and heat shock, did not appreciably change the partitioning of eps15 between the cytosol and the nucleus (Fig. 4 B). Finally, no appreciable changes in the nuclear accumulation of eps15 were evident even when the various stimuli were applied in the presence of LMB when compared with treatment with the inhibitor alone (data not shown). Thus, at least in the case of eps15, nucleocytoplasmic shuttling appears to be a constitutive process, which is not regulated by major signaling pathways.
Figure 4.
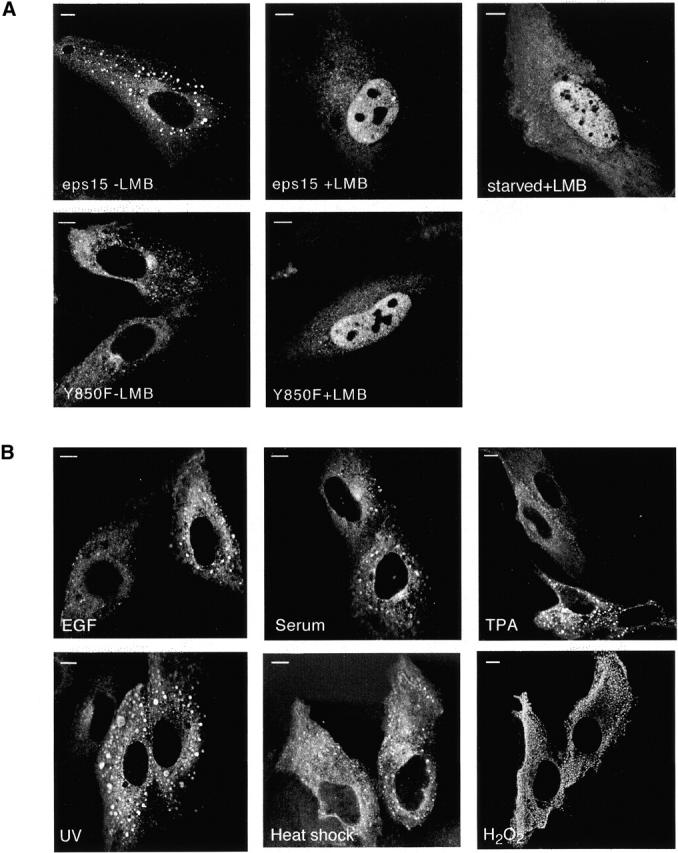
Nucleocytoplasmic shuttling of Eps15 is independent of several signaling pathways. (A) CV-1 cells were transiently transfected with FLAG epitope-tagged constructs encoding either wild-type eps15 (eps15) or the tyrosine phosphorylation-negative mutant eps15-Y850F (Y850F). Cells were then either mock-treated (−LMB) or treated for 2 h with LMB (+LMB) in complete medium. In a separate experiment, FLAG-eps15–transfected cells were also serum starved overnight before LMB addition (top, right; starved+LMB). Cells were fixed and stained with an anti-FLAG antibody (M2; Sigma-Aldrich). Central sections obtained by confocal microscopy are shown. (B) CV-1 cells were transiently transfected with FLAG-eps15 and then serum starved. Cells were subsequently individually treated with EGF (100 ng/ml), serum (10%), or phorbol ester (TPA) (100 ng/ml) for 30 min. Similar results were obtained for prolonged treatments, >90 min (not shown). Treatments with stress inducers were performed on logarithmically growing cells in the presence of serum as follows: UV (50 J/m2) followed by incubation at 37°C for 30 min; H2O2 (50 μM for 30 min); heat shock (42°C for 15 min). Subsequently, cells were fixed, stained with anti-FLAG, and analysed by confocal microscopy. Bars, 10 μm.
Endocytic Proteins Act as Transcriptional Regulators in a GAL4-based Transactivation Assay
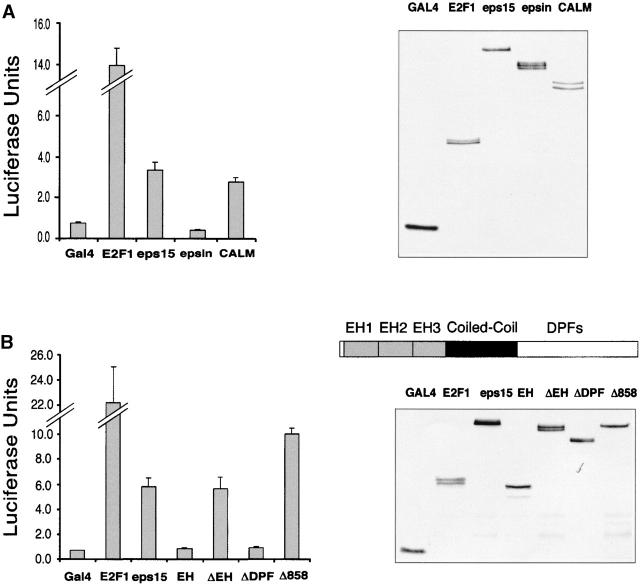
To investigate possible functions of endocytic proteins in the nucleus, we employed a GAL4-based transactivation assay. Expression constructs encoding the GAL4 DNA-binding domain fused to full-length eps15, CALM, or epsin1 were cotransfected together with a reporter plasmid encoding the luciferase gene under the transcriptional control of a GAL4-responsive promoter. We observed a four- to fivefold transactivation over the basal value obtained in the presence of GAL4 alone with both the GAL4-CALM and GAL4-eps15 fusions but not with the GAL4-epsin1 construct (Fig. 5 A). The extent of the transactivation detected was lower but in the same order of magnitude than that obtained in the presence of the bona fide transactivator E2F1 under identical conditions (Fig. 5 A). To determine the structural basis for the eps15-mediated transcriptional activity, we constructed various eps15 truncation mutants fused to the GAL4 DNA-binding domain and assessed their ability to activate the GAL4-responsive promoter. The full-length molecule (GAL4-eps15) and an eps15 fragment bearing the DPF and the coiled-coil domains (GAL4-ΔEH) were equally active, whereas a construct harboring the eps15 homology (EH) domains alone (GAL4-EH) or a mutant lacking the entire DPF region (GAL4-EH+coil) displayed no activity (Fig. 5 B). Thus, determinants contained in the COOH-terminal region of eps15 appear necessary for its effect on transcriptional regulation. Finally, deletion of the eps15 NES sequence (GAL4-Δ858) resulted in a partial nuclear accumulation of this fusion protein at steady-state (data not shown) and in supertransactivation of the GAL4 promoter when compared with wild-type eps15 (Fig. 5 B).
Figure 5.
Eps15 and CALM act as transcriptional regulators in GAL4-based assays. COS-7 cells were co-transfected with a GAL4-regulated luciferase reporter construct, and chimeric constructs encompassing the GAL4 DNA-binding domain fused to endocytic proteins. Luciferase activity (left) was measured 48 h after transfection on equal amounts of total cellular lysates that expressed comparable levels of the various GAL4 fusion proteins as assessed by anti-GAL4 immunoblot (right). Results are typical and representative of three independent experiments performed in triplicate. (A) Transcriptional activity of GAL4-fused endocytic proteins. (B) Transcriptional activity of GAL4-fused mutants of eps15 (a schematic of the various domains of eps15 is also shown). The following eps15 mutants are used: EH (containing the EH domains and encompassing amino acids 1–277); ΔEH (containing the coiled-coil plus the DPF domain and encompassing amino acids 313–897); ΔDPF (containing the EH domain plus the coiled-coil and encompassing amino acids 1–645); Δ858 (bearing a COOH-terminal truncation removing the putative NES and encompassing amino acids 1–858).
A Moonlighting Function for Endocytic Proteins?
The unexpected finding of nucleocytoplasmic shuttling of some endocytic proteins poses the question of the physiological meaning of this event. One obvious possibility is that endocytic proteins need a “nuclear phase” to be rendered somehow competent for their action at the plasma membrane. A similar situation is described in yeast where the scaffold protein Ste5 requires shuttling through the nucleus to localize at the plasma membrane and activate the mitogen-activated protein kinase cascade (Mahanty et al. 1999).
However, two lines of evidence suggest additional hypotheses. First, no immediate relationship between endocytosis and nucleocytoplasmic shuttling could be experimentally evidenced. Second, nuclear translocation of endocytic proteins was not influenced by several signaling stimuli, suggesting participation of these proteins in “basic” nuclear functions. Such a function could be transcription as witnessed by stimulation of transcription originating from a model promoter for two of the three endocytic proteins tested. We note that endocytic proteins contain multiple protein–protein interaction surfaces and are endowed with self-assembling properties (Marsh and McMahon 1999). Both endocytosis and transcription rely on the assembly of macromolecular scaffolds (Marsh and McMahon 1999; Lemon and Tjian 2000). Thus, it is tempting to speculate that the endocytic machinery functions in the nucleus as a whole, possibly by providing scaffold structures, which modify nuclear localization of specific transcription factors thereby regulating their function.
Our results might have important implications for the process of leukemogenesis. In acute myeloid leukemias, chromosomal translocations led to the formation of fusion proteins, frequently involving transcriptional regulators (Tenen et al. 1997). The emerging paradigm is that fusion proteins alter the subnuclear distribution of the transcriptional regulators involved, leading to subversion of the ordered series of transcriptional events required in the normal control of cell proliferation (Tenen et al. 1997). Endocytic proteins (eps15, CALM, and EEN) are also partners of fusion proteins in acute myeloid leukemia (Floyd and De Camilli 1998): a finding so far that could not be rationalized, given current theories. On the basis of our results, a unifying scenario can be proposed in which endocytic proteins participate to leukemogenesis by mislocalizing transcription factors and thus lead to transcriptional deregulation.
Acknowledgments
We thank P. De Camilli for GST-epsin1(402–576), M. Zerial for rab5S34N and rab5 antibodies, T. Kirchhausen for the anti-clathrin antibody, S.K. Bohlander for the pQE-31 CALM vector, J. Mattaj for the pQE-31 RANQ69L plasmid, and M. Yoshida for generously providing LMB and the T7-hCRM1 plasmid. We also thank B. Canciani and P. Transidico for assistance with confocal microscopy.
This work was supported by grants from Associazione Italiana Ricerca sul Cancro, Telethon Italy (D-90), Istituto Superiore di Sanitá (AIDS 1999) and Centro Nazionale Ricerche (target project Biotechnology) to P.P. Di Fiore, and the Association pour la Recherche contre le Cancer (5807) to A. Benmerah.
M. Vecchi, J.W. van de Loo, and V. Poupon were supported by fellowships from Fondazione Italiana Ricerca sul Cancro, European Community (Marie Curie fellowship), and the Ligue Nationale contre le Cancer (Claude Bernard grant), respectively.
Footnotes
M. Vecchi and S. Polo contributed equally to this work.
Abbreviations used in this paper: CALM, clathrin assembly lymphoid myeloid leukemia; CRM, chromosomal region maintenance; EH, eps15 homology; LMB, leptomycin B; NES, nuclear export signal; Tf, transferrin.
References
- Askjaer P., Jensen T.H., Nilsson J., Englmeier L., Kjems J. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 1998;273:33414–33422. doi: 10.1074/jbc.273.50.33414. [DOI] [PubMed] [Google Scholar]
- Chen H., Fre S., Slepnev V.I., Capua M.R., Takei K., Butler M.H., Di Fiore P.P., De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- Coda L., Salcini A.E., Confalonieri S., Pelicci G., Sorkina T., Sorkin A., Pelicci P.G., Di Fiore P.P. Eps15R is a tyrosine kinase substrate with characteristics of a docking protein possibly involved in coated pits-mediated internalization. J. Biol. Chem. 1998;273:3003–3012. doi: 10.1074/jbc.273.5.3003. [DOI] [PubMed] [Google Scholar]
- Confalonieri S., Salcini A.E., Puri C., Tacchetti C., Di Fiore P.P. Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 2000;150:905–912. doi: 10.1083/jcb.150.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria M., Salcini A.E., Colombo E., Parslow T.G., Pelicci P.G., Di Fiore P.P. The eps15 homology (EH) domain–based interaction between eps15 and hrb connects the molecular machinery of endocytosis to that of nucleocytosolic transport. J. Cell Biol. 1999;147:1379–1384. doi: 10.1083/jcb.147.7.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret C., Maira S.M., Dierich A., Wasylyk B. The net repressor is regulated by nuclear export in response to anisomycin, UV, and heat shock. Mol. Cell. Biol. 1999;19:7076–7087. doi: 10.1128/mcb.19.10.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazioli F., Minichiello L., Matoskova B., Wong W.T., Di Fiore P.P. eps15, a novel tyrosine kinase substrate, exhibits transforming activity. Mol. Cell. Biol. 1993;13:5814–5828. doi: 10.1128/mcb.13.9.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd S., De Camilli P. Endocytosis proteins and cancera potential link? Trends Cell Biol. 1998;8:299–301. doi: 10.1016/s0962-8924(98)01316-6. [DOI] [PubMed] [Google Scholar]
- Fornerod M., Ohno M., Yoshida M., Mattaj I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Asano S., Nakamura T., Adachi M., Yoshida M., Yanagida M., Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Hsueh Y.P., Wang T.F., Yang F.C., Sheng M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature. 2000;404:298–302. doi: 10.1038/35005118. [DOI] [PubMed] [Google Scholar]
- Hyman J., Chen H., Di Fiore P.P., De Camilli P., Brunger A.T. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH(2)-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn(2)+ finger protein (PLZF) J. Cell Biol. 2000;149:537–546. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E., Kutay U., von Kobbe C., Mattaj I.W., Gorlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N., Wolff B., Sekimoto T., Schreiner E.P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E.P., Wolff B., Yoshida M., Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetti L., Rybin V., Malabarba M.G., Christoforidis S., Scita G., Zerial M., Di Fiore P.P. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- Lemon B., Tjian R. Orchestrated responsea symphony of transcription factors for gene control. Genes Dev. 2000;14:2551–2569. doi: 10.1101/gad.831000. [DOI] [PubMed] [Google Scholar]
- Mahanty S.K., Wang Y., Farley F.W., Elion E.A. Nuclear shuttling of yeast scaffold Ste5 is required for its recruitment to the plasma membrane and activation of the mating MAPK cascade. Cell. 1999;98:501–512. doi: 10.1016/s0092-8674(00)81978-9. [DOI] [PubMed] [Google Scholar]
- Marsh M., McMahon H.T. The structural era of endocytosis. Science. 1999;285:215–220. doi: 10.1126/science.285.5425.215. [DOI] [PubMed] [Google Scholar]
- Milocco L.H., Haslam J.A., Rosen J., Seidel H.M. Design of conditionally active STATsinsights into STAT activation and gene regulatory function. Mol. Cell. Biol. 1999;19:2913–2920. doi: 10.1128/mcb.19.4.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. Nucleocytoplasmic transportsignals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Ossareh-Nazari B., Bachelerie F., Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- Petit M.M., Fradelizi J., Golsteyn R.M., Ayoubi T.A., Menichi B., Louvard D., Van de Ven W.J., Friederich E. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol. Biol. Cell. 2000;11:117–129. doi: 10.1091/mbc.11.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski I., Bell B., Broad P., Hollis M. GAL4 fusion vectors for expression in yeast or mammalian cells. Gene. 1992;118:137–141. doi: 10.1016/0378-1119(92)90261-m. [DOI] [PubMed] [Google Scholar]
- Sakamuro D., Elliott K.J., Wechsler-Reya R., Prendergast G.C. BIN1 is a novel MYC-interacting protein with features of a tumour suppressor. Nat. Genet. 1996;14:69–77. doi: 10.1038/ng0996-69. [DOI] [PubMed] [Google Scholar]
- Sorkin A., Di Fiore P.P., Carpenter G. The carboxyl terminus of epidermal growth factor receptor/erbB-2 chimerae is internalization impaired. Oncogene. 1993;8:3021–3028. [PubMed] [Google Scholar]
- Stade K., Ford C.S., Guthrie C., Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Tenen D.G., Hromas R., Licht J.D., Zhang D.E. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489–519. [PubMed] [Google Scholar]
- Wada A., Fukuda M., Mishima M., Nishida E. Nuclear export of actina novel mechanism regulating the subcellular localization of a major cytoskeletal protein. EMBO J. 1998;17:1635–1641. doi: 10.1093/emboj/17.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]