Abstract
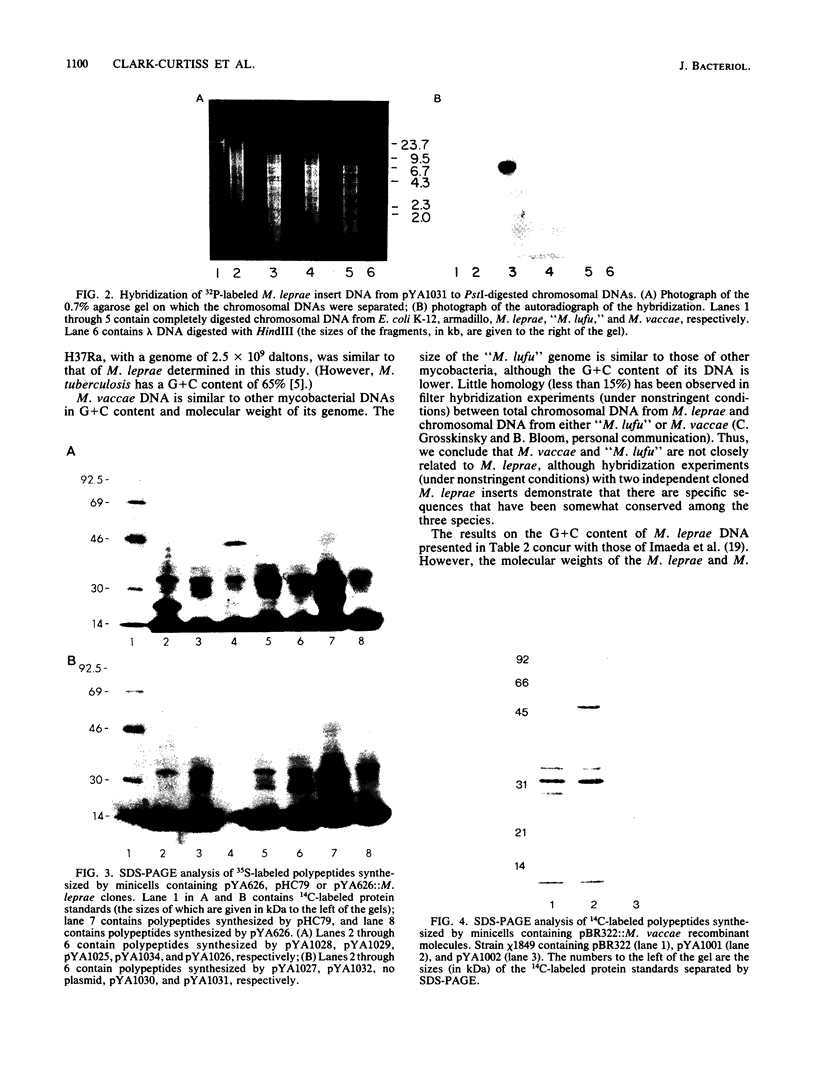
Molecular analysis of DNA from Mycobacterium leprae, "Mycobacterium lufu," and Mycobacterium vaccae has demonstrated that the G + C (guanine plus cytosine) contents of the DNAs are 56, 61, and 65%, respectively, and that the genome sizes are 2.2 X 10(9), 3.1 X 10(9), and 3.1 X 10(9) daltons, respectively. Because of the significant differences in both G + C content and genome size among M. leprae, "M. lufu," and M. vaccae DNAs, these species are not related, although hybridization experiments under nonstringent conditions, with two separate cloned M. leprae DNA inserts as probes, indicate that there are some conserved sequences among the DNAs. The G + C content of Dasypus novemcinctus (armadillo, the animal of choice for cultivating M. leprae) DNA was determined to be 36%. Genomic libraries potentially representing more than 99.99% of each genome were prepared by cloning into the cosmid vector, pHC79, in Escherichia coli K-12. A genomic library representing approximately 95% of the genome of M. vaccae was prepared in pBR322. M. leprae DNA was subcloned from the pHC79::M. leprae library into an expression vector, pYA626. This vector is a 3.8-kilobase derivative of pBR322 in which the promoter region of the asd (aspartate semialdehyde dehydrogenase) gene from Streptococcus mutans has been inserted in place of the EcoRI-to-PstI fragment of pBR322. Several (44% of those tested) pYA626::M. leprae recombinants and one pBR322::M. vaccae recombinant synthesized new polypeptides in minicells of E. coli, indicating that mycobacterial DNA can be expressed in E. coli K-12, although expression is probably dependent upon use of nonmycobacterial promoters recognized by the E. coli transcription-translation apparatus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradley S. G. Relationships among mycobacteria and nocardiae based upon deoxyribonucleic acid reassociation. J Bacteriol. 1973 Feb;113(2):645–651. doi: 10.1128/jb.113.2.645-651.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Curtiss R., 3rd Analysis of recombinant DNA using Escherichia coli minicells. Methods Enzymol. 1983;101:347–362. doi: 10.1016/0076-6879(83)01026-5. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper P. Cell walls of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):95–98. [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gillis T. P., Abe M., Bullock W. E., Rojas-Espinosa O., Garcia-Ortigoza E., Draper P., Kirchheimer W., Buchanan T. M. Comparison of 22 species of Mycobacteria by immunodiffusion against an absorbed reference leprosy serum. Int J Lepr Other Mycobact Dis. 1981 Sep;49(3):287–293. [PubMed] [Google Scholar]
- Hansen J. B., Abiko Y., Curtiss R., 3rd Characterization of the Streptococcus mutans plasmid pva318 cloned into Escherichia coli. Infect Immun. 1981 Mar;31(3):1034–1043. doi: 10.1128/iai.31.3.1034-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Imaeda T., Kirchheimer W. F., Barksdale L. DNA isolated from Mycobacterium leprae: genome size, base ratio, and homology with other related bacteria as determined by optical DNA-DNA reassociation. J Bacteriol. 1982 Apr;150(1):414–417. doi: 10.1128/jb.150.1.414-417.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagusztyn-Krynicka E. K., Smorawinska M., Curtiss R., 3rd Expression of Streptococcus mutans aspartate-semialdehyde dehydrogenase gene cloned into plasmid pBR322. J Gen Microbiol. 1982 May;128(5):1135–1145. doi: 10.1099/00221287-128-5-1135. [DOI] [PubMed] [Google Scholar]
- Kirchheimer W. F., Storrs E. E. Attempts to establish the armadillo (Dasypus novemcinctus Linn.) as a model for the study of leprosy. I. Report of lepromatoid leprosy in an experimentally infected armadillo. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):693–702. [PubMed] [Google Scholar]
- Kvach J. T., Veras J. R. A fluorescent staining procedure for determining the viability of mycobacterial cells. Int J Lepr Other Mycobact Dis. 1982 Jun;50(2):183–192. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Mandel M., Igambi L., Bergendahl J., Dodson M. L., Jr, Scheltgen E. Correlation of melting temperature and cesium chloride buoyant density of bacterial deoxyribonucleic acid. J Bacteriol. 1970 Feb;101(2):333–338. doi: 10.1128/jb.101.2.333-338.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaels F. Unclassified mycobacterial strains susceptible to dapsone isolated from the environment in Central Africa. Int J Lepr Other Mycobact Dis. 1980 Sep;48(3):330–330. [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seydel J. K., Wempe E. G. Bacterial growth kinetics of "M. lufu" in the presence and absence of various drugs alone and in combination. A model for the development of combined chemotherapy against M. leprae? Int J Lepr Other Mycobact Dis. 1982 Mar;50(1):20–30. [PubMed] [Google Scholar]
- Shepard C. C., Draper P., Rees R. J., Lowe C. Effect of purification steps on the immunogenicity of Mycobacterium leprae. Br J Exp Pathol. 1980 Aug;61(4):376–379. [PMC free article] [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A. Taxonomic studies on the leprosy bacillus. Int J Lepr Other Mycobact Dis. 1976 Jan-Jun;44(1-2):216–221. [PubMed] [Google Scholar]
- Storrs E. E. The nine-banded armadillo: a model for leprosy and other biomedical research. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):703–714. [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]