Abstract
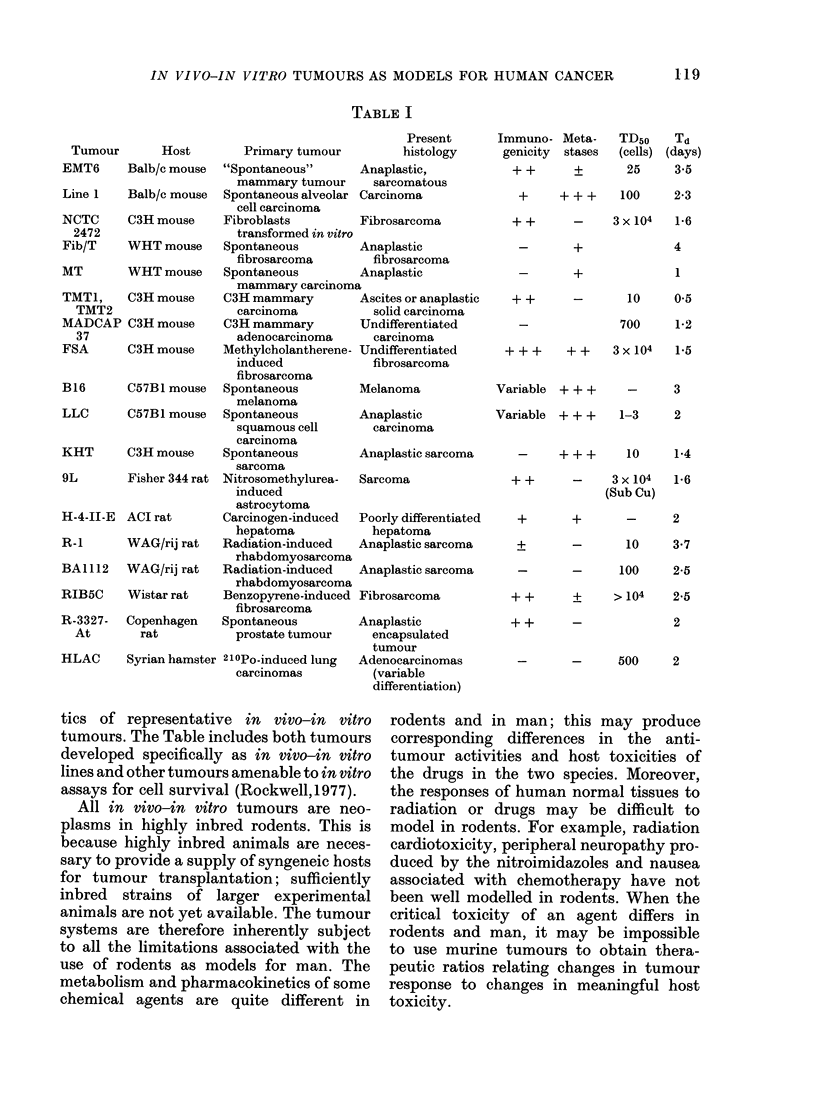
In vivo-in vitro tumour cell lines are widely used to study the biology of cancer and to examine the factors influencing the response of tumours to therapeutic agents and regimens. The existing in vivo-in vitro tumours form a uniform and artificial population of experimental neoplasms, with biological characteristics which limit the acceptability of any of these tumours or panel of these tumours as an accurate model for human cancer. All are rapidly growing, transplanted tumours in highly inbred rodents. All have growth rates, cell proliferation patterns and tumour-host interactions different from those of primary tumours in animals or man. Most are immunogenic. Most are anaplastic sarcomas: few are carcinomas; none are well differentiated. The biological differences between in vivo-in vitro tumours and human neoplasms must be considered when the experimental systems are used as models for human cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Kovacs C. J. Properties of the H-4-II-E tumor cell system. I. Growth and cell proliferation kinetics of an experimental hepatoma. Cell Tissue Kinet. 1977 May;10(3):233–243. [PubMed] [Google Scholar]
- Hewitt H. B. The choice of animal tumors for experimental studies of cancer therapy. Adv Cancer Res. 1978;27:149–200. doi: 10.1016/s0065-230x(08)60932-x. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Moulder J. E., Fischer J. J. Tumour response endpoints in the BA1112 rat sarcoma. Br J Cancer Suppl. 1980 Apr;4:271–274. [PMC free article] [PubMed] [Google Scholar]
- McNally N. J., Denekamp J., Sheldon P., Flockhart I. R., Stewart F. A. Radiosensitization by misonidazole (Ro 07-0582). The importance of timing and tumor concentration of sensitizer. Radiat Res. 1978 Mar;73(3):568–580. [PubMed] [Google Scholar]
- Meyer K. R., Hopwood L. E., Gillette E. L. Radiation response and characteristics of a cell line derived from a mouse mammary adenocarcinoma. Radiat Res. 1978 Feb;73(2):315–329. [PubMed] [Google Scholar]
- Rao B. R., Nakeff A., Eaton C., Heston W. D. Establishment and characterization of an in vitro clonogenic cell assay for the R-3327-At Copenhagen rat prostatic tumor. Cancer Res. 1978 Dec;38(12):4431–4439. [PubMed] [Google Scholar]
- Rockwell S. In vivo-in vitro tumor systems: new models for studing the response of tumours to therapy. Lab Anim Sci. 1977 Oct;27(5 Pt 2):831–851. [PubMed] [Google Scholar]
- Rockwell S., Kallman R. F. Growth and cell population kinetics of single and multiple KHT sarcomas. Cell Tissue Kinet. 1972 Nov;5(6):449–457. doi: 10.1111/j.1365-2184.1972.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Rosenblum M. K., Knebel K. D., Vasquez D. A., Wilson C. B. Brain-tumor therapy. Quantitative analysis using a model system. J Neurosurg. 1977 Feb;46(2):145–154. doi: 10.3171/jns.1977.46.2.0145. [DOI] [PubMed] [Google Scholar]
- Thomson J. E., Rauth A. M. An in vitro assay to measure the viability of KHT tumor cells not previously exposed to culture conditions. Radiat Res. 1974 May;58(2):262–276. [PubMed] [Google Scholar]
- Ullrich R. L., Adams L. M. Combined use of local irradiation and Corynebacterium parvum in the treatment of the murine line 1 lung carcinoma. Radiat Res. 1978 Feb;73(2):267–273. [PubMed] [Google Scholar]


