Abstract
Matrix vesicles have a critical role in the initiation of mineral deposition in skeletal tissues, but the ways in which they exert this key function remain poorly understood. This issue is made even more intriguing by the fact that matrix vesicles are also present in nonmineralizing tissues. Thus, we tested the novel hypothesis that matrix vesicles produced and released by mineralizing cells are structurally and functionally different from those released by nonmineralizing cells. To test this hypothesis, we made use of cultures of chick embryonic hypertrophic chondrocytes in which mineralization was triggered by treatment with vitamin C and phosphate. Ultrastructural analysis revealed that both control nonmineralizing and vitamin C/phosphatetreated mineralizing chondrocytes produced and released matrix vesicles that exhibited similar round shape, smooth contour, and average size. However, unlike control vesicles, those produced by mineralizing chondrocytes had very strong alkaline phosphatase activity and contained annexin V, a membrane-associated protein known to mediate Ca2+ influx into matrix vesicles. Strikingly, these vesicles also formed numerous apatite-like crystals upon incubation with synthetic cartilage lymph, while control vesicles failed to do so. Northern blot and immunohistochemical analyses showed that the production and release of annexin V-rich matrix vesicles by mineralizing chondrocytes were accompanied by a marked increase in annexin V expression and, interestingly, were followed by increased expression of type I collagen. Studies on embryonic cartilages demonstrated a similar sequence of phenotypic changes during the mineralization process in vivo. Thus, chondrocytes located in the hypertrophic zone of chick embryo tibial growth plate were characterized by strong annexin V expression, and those located at the chondro–osseous mineralizing border exhibited expression of both annexin V and type I collagen. These findings reveal that hypertrophic chondrocytes can qualitatively modulate their production of matrix vesicles and only when induced to initiate mineralization, will release mineralization-competent matrix vesicles rich in annexin V and alkaline phosphatase. The occurrence of type I collagen in concert with cartilage matrix calcification suggests that the protein may facilitate crystal growth after rupture of the matrix vesicle membrane; it may also offer a smooth transition from mineralized type II/type X collagen-rich cartilage matrix to type I collagen-rich bone matrix.
Biomineralization has a key role in the normal replacement of the cartilaginous skeleton with definitive bone skeleton via endochondral ossification during prenatal and early postnatal life. In this complex process, mineralization is tightly controlled both temporally and spatially and is limited to a few layers of hypertrophic chondrocytes at the chondro–osseous border. Mineralization is also crucial for the development and function of other mineralized tissues, such as the intramembranous craniofacial bones and teeth. Changes in mineralization can have serious pathological ramifications. Excessive mineral deposition accompanies atherosclerosis and osteoarthritis, probably causing loss of normal tissue elasticity and resilience (2, 3, 65). Despite the fundamental and multiple roles of mineralization, the mechanisms regulating it remain poorly understood.
Much effort has been devoted to identifying and characterizing the structure and/or components that initiate mineralization, which is the nucleational site for calcification. Studies have suggested that focal accumulations of proteoglycans in hypertrophic cartilage may represent such nucleational sites (25, 26, 53). Because of their high negative charge density, the proteoglycans would bind large amounts of Ca2+ ions; inorganic phosphate would then displace the concentrated Ca2+, leading to salt precipitation and mineral deposition (25, 26, 53). Other studies have provided evidence that matrix vesicles may represent the nucleational site for mineralization (5, 6, 14). These vesicles are cell-derived, membrane-bound microstructures, averaging 30 to 100 nm in diameter, that are present in mineralizing tissues including hypertrophic cartilage, bone, and tendons.
Matrix vesicles contain several specific proteins, including alkaline phosphatase and annexins II, V, and VI (6, 21). Annexin V appears to play major roles in the function of the vesicles, particularly during the onset of calcification when the first mineral phase forms and grows inside the vesicle lumen. The protein mediates the influx of Ca2+ ions into the vesicles, which in turn permit mineral growth from a preexisting nucleational core complex (30, 34, 59). This core complex is Ca2+ and Pi rich and is thought to form intracellularly before the vesicles are released (30, 69, 71). In addition, annexin V binds directly to types II and X collagen, thereby anchoring the vesicles to the extracellular matrix (32, 34, 68). The second step of vesiclemediated mineralization is characterized by crystal growth from the vesicle lumen into the extracellular matrix. Once the crystals rupture the vesicle membrane and penetrate the extracellular matrix, additional proteins probably regulate apatite deposition and growth. For example, in turkey tendons the apatitic crystals emerging from matrix vesicles exhibit directed growth along type I collagen fibrils, suggesting a role of this collagen in crystal elongation, orientation, and propagation (9, 10, 13, 36).
If indeed matrix vesicles have the key role of initiating mineralization it would be reasonable to expect that the vesicles be present exclusively at the mineralization front of calcifying tissues and be absent in areas of the same tissues devoid of mineral and in tissues that do not mineralize. Unfortunately, this is not the case, since matrix vesicles are present in noncalcifying tissues such as normal articular cartilage (8, 15, 16, 67). One explanation for this puzzle is that nonmineralizing tissues may contain components that inhibit the function of matrix vesicles and block mineralization. Another equally interesting possibility is that matrix vesicles are not all equal and that tissues engaged in mineralization produce mineralization-competent vesicles, whereas nonmineralizing tissues produce vesicles unable to initiate mineralization. Support for this possibility can be found in studies demonstrating an increase of alkaline phosphatase activity in matrix vesicles as they approach the calcification front in growth plate cartilage and in articular cartilage matrix vesicles that lie close to the underlying bone (41, 55). We have tested the latter possibility by using mineralization systems that we have developed in recent years; they consist of maturing and hypertrophic chondrocytes isolated from chick embryos and maintained in monolayer culture in which mineralization can be triggered rapidly and efficiently by treatment with vitamin C or retinoid acid (28, 29, 37). Specifically, we have asked whether mineralizing hypertrophic chondrocytes produce vesicles that are mineralization competent and contain annexin V whereas nonmineralizing cells do not. We have also asked whether production of such mineralizationcompetent vesicles is followed by the initiation of type I collagen expression by the mineralizing chondrocytes, thus suggesting a role for this matrix component in the propagation of vesicle-mediated mineralization of cartilage matrix.
Materials and Methods
Cell Culture
Chondrocytes were isolated from the hypertrophic region of day 19 embryonic chick tibia growth plate cartilage by trypsin followed by collagenase digestion as described previously (49). Cells were plated at an initial density of 3 × 106 cells into 100-mm tissue culture dishes in Dulbecco's modified high glucose Eagle's medium (HG-DMEM; GIBCO BRL, Gaithersburg, MD) containing 10% defined fetal bovine serum (Hyclone, Logan, UT), 2 mM l-glutamine, and 50 U/ml penicillin and streptomycin (complete medium). Chondrocytes reached confluency in ∼7 d. Once confluent, cells were treated with 50 μg/ml of freshly prepared vitamin C and 2.5 mM phosphate. Medium was changed daily.
RNA Isolation and Analysis
Total RNAs were isolated from different zones of tibia growth plate cartilage from day 19-old chick embryos and from cultured cells by the method of Chomczynski and Sacchi (17). For Northern blot analysis, 10 μg of total RNA was denatured by glyoxalation, fractionated on 1% agarose gels, and transferred to Hybond-N membranes by capillary blotting as described previously (46, 50). Blots were stained with 0.04% methylene blue to verify that each sample has been transferred efficiently. Blots were hybridized in 6× SSC, 5× Denhardt's solution, 100 μg/ml sheared salmon sperm DNA, 2% SDS, and 50% formamide at 45°C overnight with 32P-labeled cDNA probes. The cDNA clones used to prepare probes for type X collagen, annexin V, and type I collagen mRNAs are pDLr10 (40), pACII.1 (52), and pCOL3 (72), respectively. Blots were washed at high stringency (0.1× SSC, 0.1% SDS at 60°C) and exposed to Kodak X-ray films at −70°C.
Immunofluorescence Staining and Alizarin Red S Staining
This procedure was carried out as previously described (33, 35). Briefly, longitudinal 8-μm-thick frozen sections from day 19 chick embryonic tibia growth plate cartilage were fixed for 10 min in ice-cold acetone. Before immunostaining, the fixed sections were decalcified with 0.1 M EDTA, pH 7.4, for 1 h and pretreated with sheep testicular hyaluronidase (2 mg/ml; Sigma Chemical Co., St. Louis, MO) for 30 min at 37°C. Cells were washed with PBS, pH 7.4, and fixed with 70% ethanol. The sections or fixed cells were incubated with a mixture of monoclonal mouse anti-chick type I collagen IgG and polyclonal rabbit anti–chick type X collagen IgG or a mixture of monoclonal mouse anti–chick type I collagen IgG and polyclonal rabbit anti–chick annexin V IgG for 3 h at room temperature, followed by incubation with a mixture of rhodamine-conjugated goat anti– mouse IgG and FITC-conjugated goat anti–rabbit IgG (Cappel, Durham, NC) for 1 h at room temperature.
To localize calcium deposits, sections were stained with 0.5% alizarin red S solution, pH 4.0, for 5 min at room temperature. To verify that alizarin staining was specific, sections were decalcified and then stained with alizarin red S. Stained sections were washed three times with water and ethanol. Sections and cells were examined under a Zeiss microscope and viewed in both epifluorescence and phase modes.
Isolation of Matrix Vesicles and Ca2+ Uptake Studies
Matrix vesicles were isolated from cultured chondrocytes by enzymatic digestion as described previously (20, 34). Briefly, adherent chondrocytes were washed with PBS and then incubated in PBS containing 0.1% trypsin (type III; Sigma Chemical Co.) at 37°C for 30 min. The trypsin was removed by washing with PBS, and the cells were then treated with crude collagenase (500 U/ml; type IA; Sigma Chemical Co.) at 37°C for 3 h. Matrix vesicles were harvested by differential ultracentrifugation as described previously (20). Three dishes (100 mm) of confluent chondrocyte cultures were used to isolate matrix vesicle fractions containing between 300 and 500 μg of total proteins. For Ca2+ uptake studies, matrix vesicles (50 μg of total proteins) were sedimented by centrifugation and resuspended in 500 μl of synthetic cartilage lymph; synthetic cartilage lymph, pH 7.4, contained 2 mM Ca2+ and 1.42 mM Pi, in addition to 104.5 mM Na+, 133.5 mM Cl−, 63.5 mM sucrose, 16.5 mM Tris, 12.70 mM K+, 5.55 mM d-glucose, 1.83 mM HCO3−, 0.57 mM Mg2+, and 0.57 mM SO4 2−. The matrix vesicle suspension was incubated at 37°C, centrifuged, and resuspended in 50 μl of 0.1 M HCl. Aliquots of the suspensions were analyzed for calcium using the microcolorimetric method of Baginski et al. (11).
Fourier Transform Infrared Spectroscopy
Matrix vesicle samples were freeze dried and stored desiccated. Each sample was analyzed by Fourier transform infrared spectroscopy (FT-IR; Magna IR 550 spectrometer; Nicolet Instrument Technologies, Madison, WI), operated in the diffuse reflectance mode. Vesicle preparations were milled in an agate mortar and layered on KBr (ratio of KBr to sample, 300:1, wt/ wt); routinely, 300 interferograms were collected at 4 cm−1 resolution; background due to organic matrix was subtracted using a chondrocyte membrane preparation. Spectra were coadded and the resultant interferograms Fourier transformed, and second derivative spectra (1,200–500 cm−1) were obtained using a software package (Omnic; Nicolet Instrument Technologies).
Electron Microscopy
Matrix vesicles were sedimented by centrifugation, and the resulting pellets were fixed in Karnovsky's fixative, postfixed in 2% osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in epoxy resin. Ultrathin sections were cut on an Ultratome (LKB Instruments, Inc., Stockholm, Sweden), picked up on formvar-coated grids, and contrasted with uranyl acetate and lead citrate. Specimens were evaluated in a transmission electron microscope (100CX II; JEOL USA, Peabody, MA) operated at 80 kV.
To analyze matrix vesicle preparations for calcium and sulfur, freezedried matrix vesicle pellets were mounted on SEM specimen stubs and coated with carbon. The samples were analyzed using a scanning electron microscope (T330A; JEOL USA) and EDS microanalysis system (Delta I; Kevex Corp., Foster City, CA).
Dot Blot Analysis
To analyze for cartilage proteoglycan (aggrecan) in matrix vesicle preparations, aliquots of matrix vesicle fractions (2 μg of total protein) were dotted onto nitrocellulose filters. After blocking with low fat milk powder the filters were immunostained with polyclonal antibodies against chicken aggrecan followed by secondary horseradish-conjugated anti–rabbit IgG (Rockland Inc., Gilbertsville, PA) and α-chloronaphthol as color substrate. The optical density of the color reaction was determined using a densitometer. To determine the amount of aggrecan in the matrix vesicle fractions, purified aggrecan in different concentrations (1–20 μg) was applied onto nitrocellulose filters and immunostained with the aggrecan antibodies.
Antibodies
The preparation and specificity of a polyclonal antibody against chicken annexin V were described elsewhere (42). The preparations of antibodies against chick aggrecan and types X, II, and I collagen were described elsewhere (1, 23, 38, 47). These antibodies have been shown to be specific and do not cross-react with other collagens or matrix proteins (1, 23, 38, 47).
SDS–polyacrylamide Gel Electrophoresis and Immunoblotting
Samples were dissolved in 3% SDS sample buffer with dithiothreitol, denatured at 100°C for 3 min, and analyzed by electrophoresis in 8 or 10% (wt/vol) polyacrylamide gels. Gels were stained with Coomassie blue, or proteins were electroblotted onto nitrocellulose filters (Schleicher & Schuell, Inc., Keene, NH). After blocking with low fat milk protein, blotted proteins were immunostained with the appropriate antibodies using peroxidase-conjugated secondary antibodies and α-chloronaphthol as a color substrate.
Other Methods
To measure the protein content and alkaline phosphatase activity in the cell layer, cells were washed in PBS and then suspended in 10 mM Tris/ HCl, pH 7.5, 0.1% Triton X-100, 0.5 mM MgCl2. Protein content was analyzed by the BCA assay from Pierce (Rockford, IL). Alkaline phosphatase activity was determined using p-nitrophenyl phosphate as substrate (63). To measure calcium and phosphate levels, the suspension was centrifuged, and the pellet was resuspended in 0.1 M HCl. Inorganic phosphate was determined by a modified method of Ames (4).
Results
Calcification and Production of Matrix Vesicles in Chondrocyte Cultures
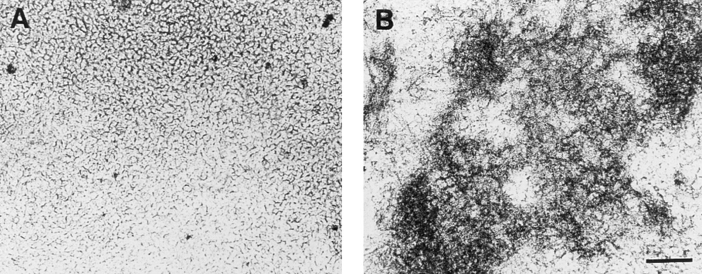
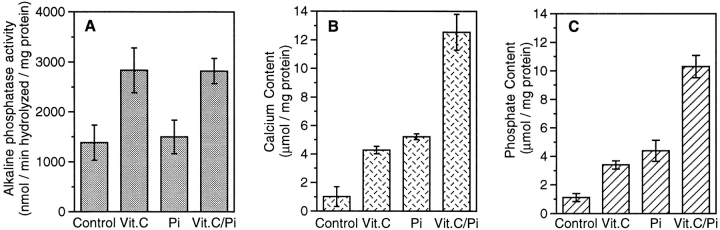
In a first series of experiments we investigated whether mineralization in chondrocyte cultures and the formation of matrix vesicles were affected by vitamin C and phosphate treatment. Chondrocytes isolated from the hypertrophic zone of day 19 embryonic chick tibia growth plate cartilage were cultured in control medium or medium containing 50 μg/ml vitamin C and 2.5 mM phosphate. After 14 d, mineralization in vitamin C/phosphate-treated chondrocyte cultures was observed (Fig. 1 B); bright field microscopy failed to show mineral in the matrix of untreated chondrocytes (Fig. 1 A). Vitamin C increased the activity of alkaline phosphatase; in contrast, phosphate did not stimulate alkaline phosphatase activity (Fig. 2 A). Cultures supplemented with vitamin C or phosphate led to a 4-fold elevation in the calcium and phosphate content of the cell layer; together both reagents led to a more than 12-fold increase in ion accumulation (Fig. 2, B and C). After 7 d, little calcification was evident in vitamin C and phosphatesupplemented cultures (data not shown).
Figure 1.
Phase contrast micrographs of cultures of hypertrophic chondrocytes treated with vitamin C and phosphate. Tibia chondrocytes were grown to confluency and then maintained in culture for a further 14 d in the absence (A) or presence of vitamin C (50 μg/ml) and 2.5 mM phosphate (B). Note the presence of abundant opaque mineral deposits in vitamin C/phosphate-treated cultures (B).
Figure 2.
Histograms showing alkaline phosphatase activity and calcium and phosphate content of chondrocyte cultures supplemented with or without vitamin C and/or phosphate. Confluent cultures of day 19 chick embryonic growth plate chondrocytes were treated for 14 d with vitamin C (50 μg/ml) and 2.5 mM phosphate. Control cultures were maintained for 14 d in the absence of either of these supplements. Cultures were harvested and alkaline phosphatase, Ca2+, and Pi content determined. Data were obtained from three different cultures; values are mean ± SE.
Since the vitamin C and phosphate treatment significantly increased the rate and extent of calcification of chondrocyte cultures, we determined next whether this treatment affected the composition of matrix vesicles. Matrix vesicles were isolated from the cell layers of day 14 control nonmineralizing and vitamin C/phosphate-treated mineralizing cultures by mild trypsin and collagenase digestion followed by ultracentrifugation. Table I summarizes the general composition of the vesicles. The total protein content of vesicles from control cultures was about half that of vesicles from mineralizing cultures. Most important was the finding that the alkaline phosphatase activity and the Ca2+ and Pi content of the vesicles from mineralizing cultures were over 300- and 10-fold higher than those in vesicles from nonmineralizing control cells, respectively (Table I).
Table I.
Characterization of Matrix Vesicles Isolated from Control Chondrocyte Cultures or from Cultures Treated with Vitamin C and Phosphate
| MV-fraction from control cells | MV-fraction from Vit.C/Pi-treated cells | |||
|---|---|---|---|---|
| Protein content | 56 ± 7 | 92‡ ± 5 | ||
| (μg/mg cell protein) | ||||
| Alkaline phosphatase activity | 125 ± 32 | 45,112‡ ± 3,795 | ||
| (nmol/min/mg protein)* | ||||
| Calcium content | 0.037 ± 0.013 | 0.462‡ ± 0.086 | ||
| (μmol/mg protein) | ||||
| Phosphate content | 0.034 ± 0.009 | 0.381‡ ± 0.126 | ||
| (μmol/mg protein) | ||||
| Calcium uptake after 24 h | 2.3 ± 0.9 | 29.3‡ ± 4.1 | ||
| (percent of total Ca2+ in 1 ml | ||||
| of reaction mixture) |
Alkaline phosphatase activity is expressed as nmol of p-nitrophenyl phosphate hydrolyzed per min/mg of protein.
Significantly different from levels in control matrix vesicles (P < 0.001). Four different samples of matrix vesicles isolated from control or vitamin C/phosphate-treated cultures were analyzed; values shown are means ± S.E. (n = 4). The protein content of the vesicles was normalized to 1 mg of cell protein.
Given the quite different characteristics of vesicles from control and mineralizing cultures, we asked whether the vesicles would also differ in their ability to take up Ca2+. Vesicles isolated from control cultures showed no significant Ca2+ uptake after 24 h incubation in synthetic cartilage lymph (2% of the total amount of Ca2+ present in the reaction mixture), while vesicles isolated from mineralizing cultures accumulated ∼30% of the total amount of Ca2+ in the reaction mixture (Table I). Similar rates of ∼30% Ca2+ uptake over 24 h test period were also found in matrix vesicles isolated from calcified cartilage (30, 34).
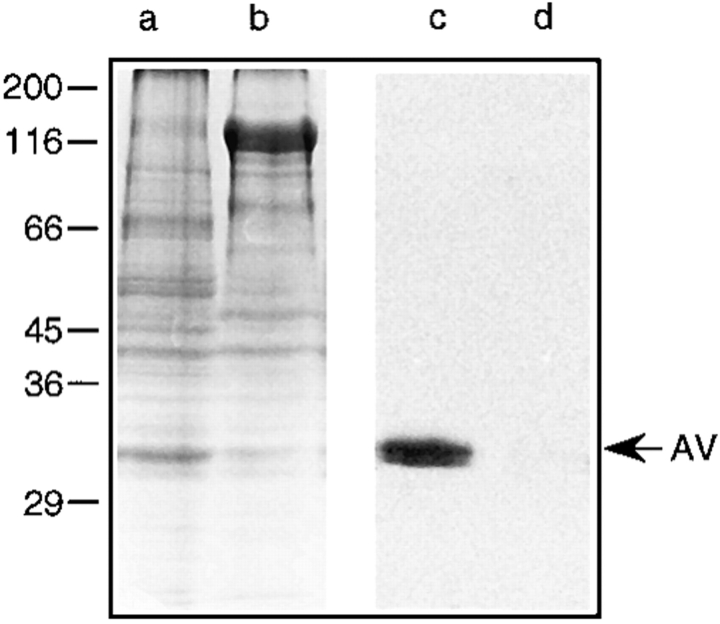
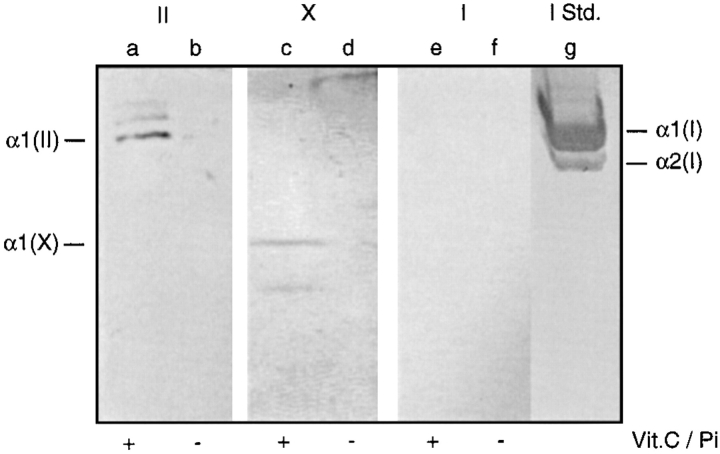
Ca2+ uptake by matrix vesicles is probably mediated by annexin V (30, 34, 59); in addition, annexin V mediates binding of types II and X collagen to matrix vesicles (32, 34, 68). Binding of these collagens to matrix vesicles stimulates Ca2+ uptake by these particles (30, 34). Thus, we asked whether the differences in Ca2+ uptake seen above reflected differences in annexin V and types II and X collagen content in the vesicles. Vesicle samples were subjected to SDS-PAGE and immunoblotting analyses. Fig. 3 (lanes b and d, control matrix vesicles; lanes a and c, mineralizing matrix vesicles) shows that there were several major differences in the protein profile of vesicles from control (Fig. 3, lane b) versus mineralizing cultures (Fig. 3, lane a). For example, a major band with an apparent molecular mass of 120 kD was present in control vesicles (Fig. 3, lane b) but absent in mineralizing vesicles (lane a). Immunoblot analysis using antibodies against annexin V revealed that indeed only matrix vesicles from mineralizing chondrocytes contained annexin V (Fig. 3, lane c), while vesicles from control cultures showed no appreciable amounts of this protein (Fig. 3, lane d). Immunoblot analyses using antibodies against types I, II, and X collagen revealed that only mineralizing matrix vesicles contained types II (Fig. 4, lane a) and X collagen (Fig. 4, lane c), while no immunostaining was obtained in control matrix vesicles (Fig. 4, lane b and d). No appreciable amounts of type I collagen were detected in both mineralizing (Fig. 4, lane e) and control matrix vesicles (Fig. 4, lane f).
Figure 3.
SDS-PAGE and immunoblot analysis of matrix vesicles isolated from vitamin C/phosphate-treated and control chondrocyte cultures. Pellets of matrix vesicles which were isolated from the cell layer of vitamin C/ phosphate-treated (lanes a and c) or control chondrocyte cultures (lanes b and d) were boiled in SDS–sample buffer, analyzed by SDS-PAGE on 10% (wt/vol) polyacrylamide gels, and stained with Coomassie blue to visualize the protein bands (lanes a and b), or the proteins were transferred electrophoretically to nitrocellulose filters for immunoblot analysis of annexin V (lanes c and d). Note the difference in the protein profile of both matrix vesicle preparations. Also note the immunostaining for annexin V (AV) in vesicles isolated from vitamin C/phosphate-treated cultures (lane c), whereas there was no staining in vesicles prepared from control cultures (lane d).
Figure 4.
Immunoblot analyses of matrix vesicles from vitamin C/phosphate-treated and control chondrocyte cultures using antibodies against types I, II, and X collagen. Pellets of matrix vesicles isolated from vitamin C/phosphate-treated (lanes a, c, and e) or control chondrocyte cultures (lanes b, d, and f) were analyzed by SDS-PAGE on 8% (wt/vol) polyacrylamide gels and transferred electrophoretically to nitrocellulose filters for immunoblot analysis of type II collagen (II, lanes a and b), type X collagen (X, lanes c and d) and type I collagen (I, lanes e and f). Note the immunostaining for type II collagen (lane a) and type X collagen (lane c) in vesicles isolated from vitamin C/phosphate-treated cultures, whereas there was no staining in vesicles prepared from control cultures (lanes b and d). No immunostaining for type I collagen was obtained in both vesicle preparations (lanes e and f). (Lane g [I Std.]) Purified type I collagen immunostained with antibodies against type I collagen.
Ultrastructural analysis revealed that matrix vesicles freshly isolated from vitamin C/phosphate-treated cultures varied in size, shape, and content. In general, they were round to oval with diameters ranging from 30 to 100 nm. A few preformed crystallites were observed in these freshly isolated matrix vesicles (Fig. 5 A). After 24 h incubation in synthetic cartilage lymph at 37°C, numerous needle-like crystals were formed in association with matrix vesicles (Fig. 5 B); by 48 h, these vesicle preparations were heavily calcified (Fig. 5 C). Strikingly, no needle-like crystals were formed in association with matrix vesicles isolated from control cultures (Fig. 5 D).
Figure 5.
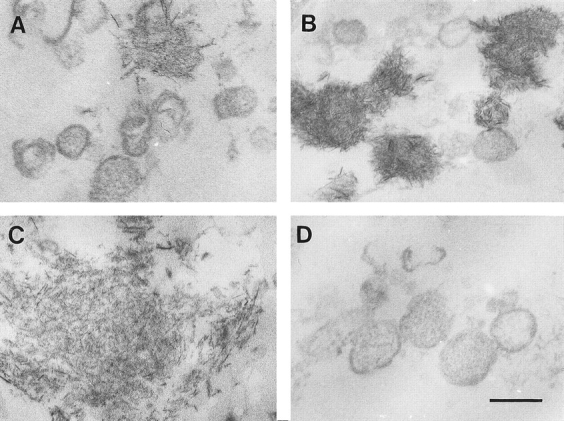
Electron micrographs of matrix vesicles isolated from vitamin C/phosphate-treated chondrocyte (A–C) and control cultures (D) and incubated in synthetic cartilage lymph for 24 and 48 h. Matrix vesicles were isolated from control and vitamin C/phosphate-treated chondrocyte cultures as described in Materials and Methods and then incubated in synthetic cartilage lymph for 24 and 48 h at 37°C. (A) Freshly isolated matrix vesicles from vitamin C/phosphate-treated chondrocyte cultures without incubation in synthetic cartilage lymph. (B and C) Matrix vesicles isolated from vitamin C/ phosphate-treated cultures and incubated for 24 (B) and 48 h (C) in synthetic cartilage lymph. (D) Matrix vesicles isolated from control cultures and incubated for 48 h in synthetic cartilage lymph. Note numerous needle-like crystals associated with matrix vesicles isolated from vitamin C/phosphate-treated cultures, while vesicles from control cultures showed no evidence of calcification. Bar, 100 nm.
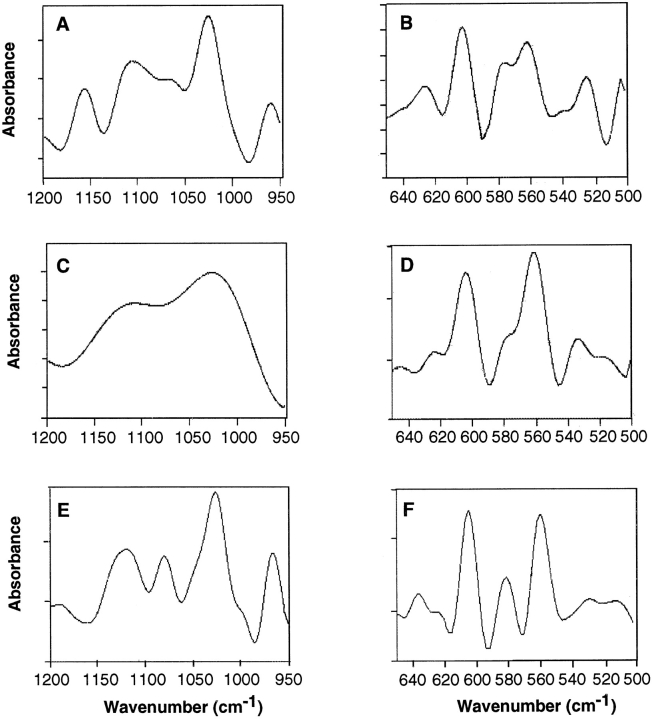
To determine the nature of the crystals, vesicle samples incubated in synthetic lymph for 24 h were analyzed by Fourier transform infrared spectroscopy. Fig. 6 A shows the second derivative spectra of the ν1ν3 phosphate region (1,200–950 cm−1) of matrix vesicle preparations obtained from mineralizing cultures. Peaks can be seen at 960, 1,025, 1,102, 1,065, and 1,157 cm−1, consistent with apatite structure. In contrast, the spectra of the ν1ν3 region of control vesicles exhibited no such peaks but rather a nondescript pattern consistent with lack of a mineral phase (Fig. 6 C). Fig. 6, B and D shows the second derivative structure in the ν4 domains (640–500 cm−1). Peaks at 626 and 539 cm−1, also characteristic of HPO4 apatites, were clearly evident in mineralizing vesicle samples (Fig. 6 B) but were markedly reduced in control samples (Fig. 6 D). Both samples contained prominent bands at 561, 576, and 602 cm−1 which are representative of phosphate ions (Fig. 6, B and D).
Figure 6.
FT-IR spectra of matrix vesicles isolated from vitamin C/phosphate-treated or control chondrocyte cultures. (A, C, and E) Second derivative spectra of the ν1ν3 phosphate region of matrix vesicles isolated from vitamin C/phosphate-treated chondrocyte cultures and incubated in synthetic cartilage lymph for 24 h at 37°C (A), matrix vesicles isolated from control cultures and incubated in synthetic cartilage lymph for 24 h at 37°C (C), and hydroxyapatite (E). Note that in A, peaks can be seen at 960, 1,025, 1,065, 1,102 and 1,157 cm−1, while these bands are absent in matrix vesicles from control cultures (C). Also note the similarity of the spectra of mineralizing matrix vesicles (A) and hydroxyapatite (E). (B, D, and F) Second derivative structure in the ν4 domains (640–500 cm−1) of matrix vesicles isolated from vitamin C/ phosphate-treated cultures (B), matrix vesicles isolated from control cultures (D), and hydroxyapatite (F). Note that peaks at 626 and 539 cm−1, characteristic of HPO4 apatites, were clearly evident in mineralizing vesicle samples (B) but were markedly reduced in control samples (D). Both samples contained peaks at 561, 576, and 602 cm−1, which are representative of phosphate ions. Also note the similarity of the spectra of mineralizing matrix vesicles (B) and hydroxyapatite (F).
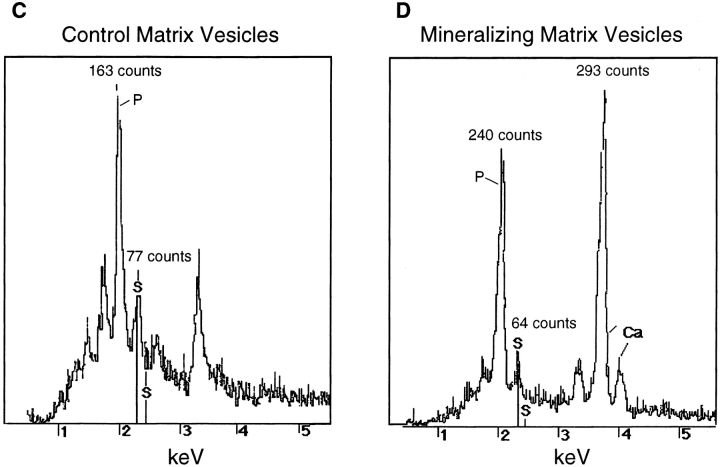
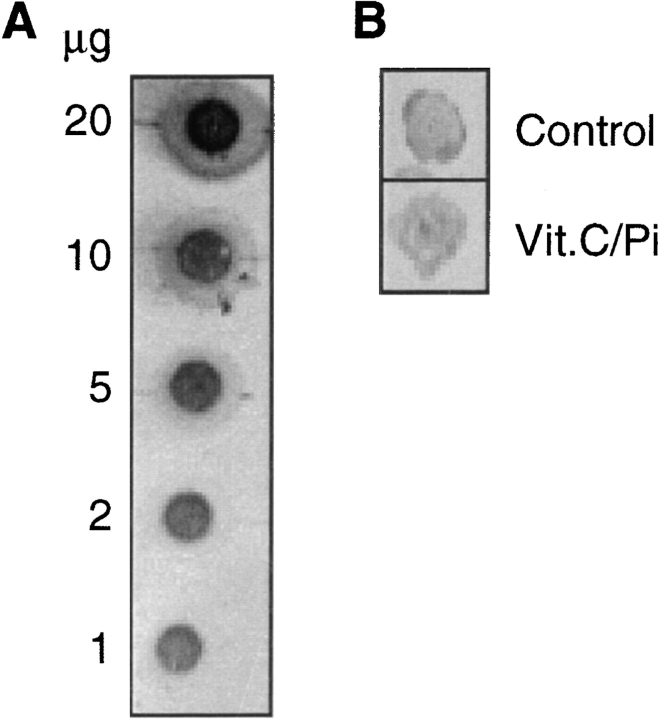
Previous studies have suggested that proteoglycans are involved in the nucleation of cartilage mineralization (25, 26, 53). Thus, it is possible that the different calcification properties of matrix vesicles isolated from control and mineralizing chondrocyte cultures may be due to differences in vesicle-associated proteoglycan content. To analyze this possibility we carried out two quantitative analyses of proteoglycan content. In the first set of experiments, aliquots of the matrix vesicle preparations (2 μg of total protein) were dotted onto nitrocellulose filters and immunostained with antibodies against the cartilage proteoglycan aggrecan (Fig. 7 B); as a control, purified aggrecan at concentrations from 1 to 20 μg/dot was also applied to the filters (Fig. 7 A). Vesicle preparations from both control and vitamin C/phosphate-treated cultures exhibited immunostaining for aggrecan (Fig. 7 B). However, densitometric analysis revealed that similar amounts of aggrecan were present in both preparations (192 μg/mg total protein in vesicles from control cultures; 141 μg/mg total protein in vesicles from mineralizing cultures). In a second set of experiments we analyzed the sulfur and calcium content in the vesicle preparations using scanning electron microscopy and EDS microanalysis. A similar sulfur content was observed in vesicles from control cultures (Fig. 7 C, peak labeled with S) and mineralizing cultures (Fig. 7 D, peak labeled with S). Interestingly, strong calcium peaks were only detected in vesicles from mineralizing cultures (Fig. 7 D, peaks labeled with Ca) but were absent in vesicles from control cultures (Fig. 7 C). In addition, mineralizing matrix vesicles contained more phosphate than control vesicles (Fig. 7, C and D, peaks labeled with P). These findings indicate that differences in proteoglycan content cannot account for the differences in mineralizing properties of vesicles produced by control and mineralizing chondrocytes.
Figure 7.
Proteoglycan content in control and mineralizing matrix vesicle preparations. Aliquots of matrix vesicle preparations isolated from control cultures (B, Control) or vitamin C/phosphate-treated chondrocyte cultures (B, Vit.C/Pi) were dotted onto nitrocellulose filters and immunostained with antibodies against aggrecan as described in Materials and Methods. (A) Different concentrations of purified aggrecan were dotted onto nitrocellulose filters and immunostained with antibodies against aggrecan. Densitometry was used to calculate the amount of aggrecan in the vesicle preparations using the staining intensities of the aggrecan dots as a standard curve. (C and D) EDS analysis of control matrix vesicles (C) and mineralizing matrix vesicles (D). Note that both vesicle preparations contain similar amounts of sulfur (peaks labeled with S; 77 counts in control matrix vesicles; 64 counts in mineralizing matrix vesicles). Also note that Ca2+ peaks are only present in mineralizing matrix vesicles (D, peaks labeled with Ca); in addition, mineralizing matrix vesicles contain more phosphate (peaks labeled with P) than control matrix vesicles.
Annexin V and Type I Collagen Expression
We asked next whether the presence of annexin V-rich, mineralization-competent matrix vesicles in mineralizing cultures reflected increased gene expression of this protein. We also asked whether possible changes in annexin V expression may be accompanied by changes in type I collagen expression, given the possible prominent role of this matrix protein in mineral propagation (9, 10, 13, 36).
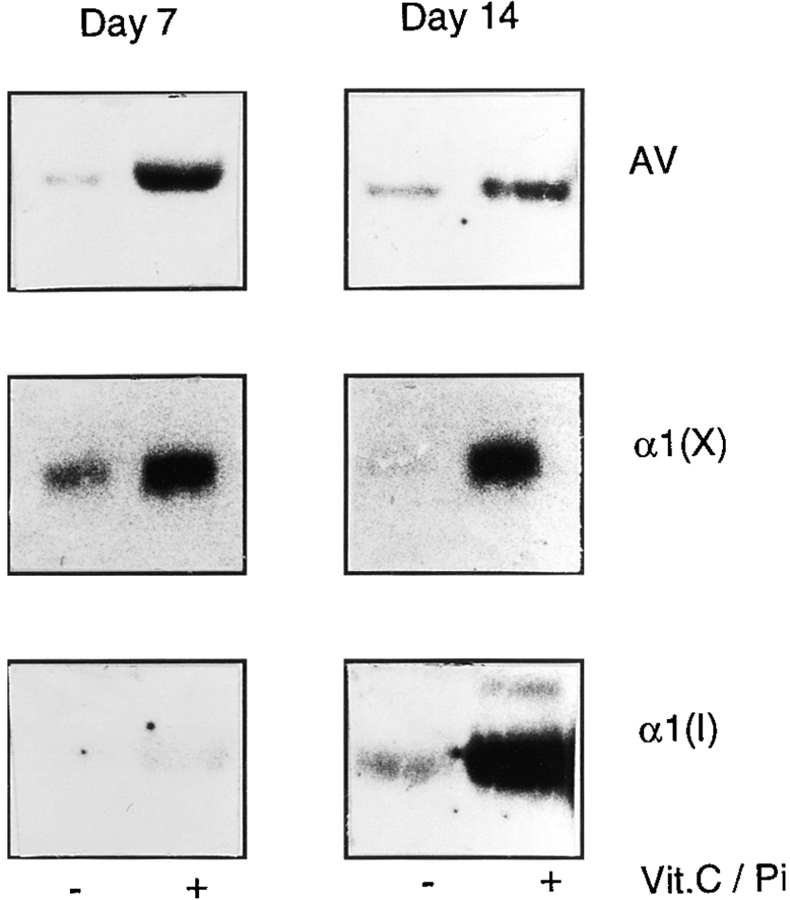
Chondrocytes were cultured for 7 and 14 d in the absence or presence of vitamin C and phosphate, and total cellular RNAs were then isolated and examined by Northern blot analysis. Indeed, annexin V gene expression increased several fold in the vitamin C/phosphate-treated cultures already by day 7 (Fig. 8, Day 7, AV) and remained high by day 14 (Fig. 8, Day 14, AV). Likewise, gene expression of type X collagen, a marker of hypertrophic chondrocytes, was also markedly stimulated (Fig. 8, α1(X)). Interestingly at day 14, vitamin C/phosphate-treated cultures exhibited high levels of type I collagen expression compared to control cultures (Fig. 8, Day 14, α1(I)), while only low levels of type I collagen expression were detected after 7 d of treatment (Fig. 8, Day 7, α1(I)). Thus, during induction of mineralization in chondrocyte cultures, expression of annexin V and type X collagen genes increases first and is followed by increased expression of type I collagen.
Figure 8.
Expression of annexin V (AV), type X collagen (α1(X)), and type I collagen (α1(I)) genes in control and vitamin C/phosphatetreated chondrocyte cultures. Cultures of day 19 chick embryonic hypertrophic tibia chondrocytes were maintained in culture in DMEM containing 10% FCS. When the cells had reached confluency, half of the cultures received 50 μg/ml of freshly prepared vitamin C and 2.5 mM phosphate; the medium of both the treated ( + Vit.C/Pi) and control (− Vit.C/Pi) cultures was changed daily. After 7 or 14 d, total RNA was isolated from the control cultures or supplemented cultures and subjected to Northern blot analysis using annexin V, type X, and type I collagen 32P-labeled cDNA probes.
To ascertain whether the above changes in gene expression were accompanied by corresponding changes at the protein level, cultures were immunostained with antibodies against annexin V, type X collagen, or type I collagen. Control cultures were immunopositive for type X collagen but exhibited no staining for type I collagen on day 7 (not shown) and day 14 (Fig. 9, B and C), even though low levels of α1(I) mRNA were detectable on day 14 (Fig. 8, Day 14, α1(I)); the control cells were also immunopositive for annexin V (Fig. 9 A). Cultures treated with vitamin C and phosphate were strongly immunopositive for annexin V and type X collagen on day 7, while only a few cells were positive for type I collagen (Fig. 9, D–F). After 14 d of treatment, the cultures not only exhibited strong annexin V and type X collagen immunoreactivity (Fig. 9, G and H) but also strong type I collagen immunoreactivity, resulting in >90% of the cells being surrounded by a type I collagen-rich matrix (Fig. 9 I).
Figure 9.
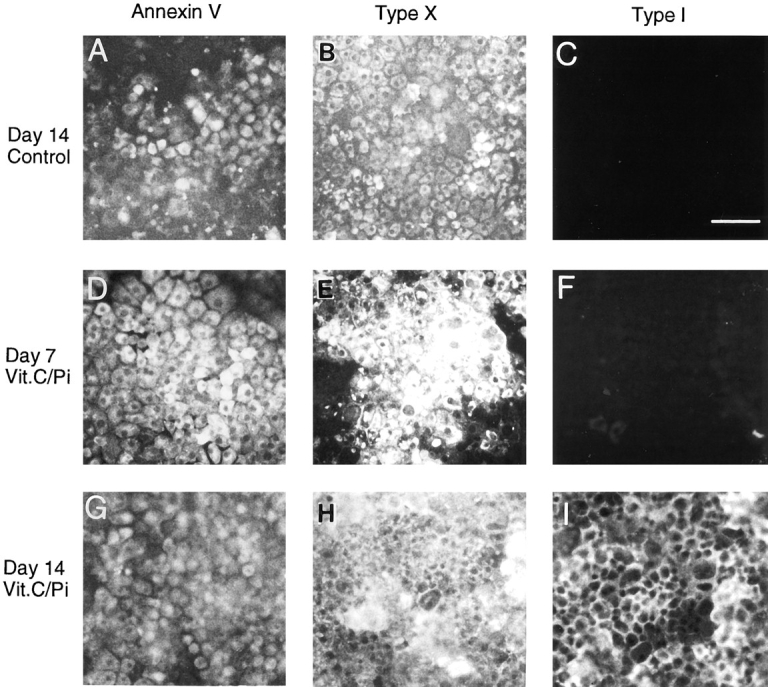
Immunofluorescence staining of chondrocyte cultures with antibodies against annexin V (Annexin V), type X (Type X), and type I collagen (Type I). Chondrocytes isolated from day 19 chick embryonic tibia cartilage were maintained in culture until confluent. They were then grown for additional 7 or 14 d in the absence (Control) or presence of vitamin C (50 μg/ml) and phosphate (2.5 mM; Vit.C/Pi) and then immunostained for annexin V, type X, and type I collagen. Note that after 14 d, control cultures were positive for annexin V (A) and type X collagen (B) but showed no staining for type I collagen (C). After 7 d of treatment with vitamin C and phosphate, chondrocytes were positive for annexin V (D) and type X collagen (E) and exhibited no staining for type I collagen (F). After 14 d of treatment, chondrocytes showed strong staining for annexin V (G), type X collagen (H), and type I collagen (I). Bar, 100 μm.
Annexin V and Type I Collagen Expression in Tibia Growth Plate Cartilage
Our in vitro studies above show that chondrocytes undergoing mineralization first upregulate annexin V gene expression followed by onset of type I collagen expression and matrix calcification. We determined next whether a similar sequence of events occurs in vivo. To address this question we examined annexin V and type I collagen gene expression in the developing chick tibia growth plate cartilage in vivo by Northern blots and immunohistochemistry.
The proliferative, upper and lower hypertrophic zones of tibia growth plate cartilage were microsurgically isolated from day 19 chick embryos; the resulting tissue fragments were processed for RNA isolation and Northern blot analyses. We found that the upper hypertrophic zone contained large amounts of annexin V transcripts while minimal levels were present in the proliferative and lower hypertrophic zones (Fig. 10, AV). In contrast, mRNA encoding type I collagen was undetectable in the proliferative and upper hypertrophic zones but was extremely prominent in the lower hypertrophic zone (Fig. 10, α1(I)). To ascertain that the different growth plate zones had been microdissected accurately, blots were hybridized with a type X collagen cDNA probe. The upper hypertrophic cartilage exhibited large amounts of type X collagen transcripts, while no detectable type X collagen transcripts were found in the proliferative zone, and minimal levels were present in the lower hypertrophic zone (Fig. 10, α1(X)).
Figure 10.
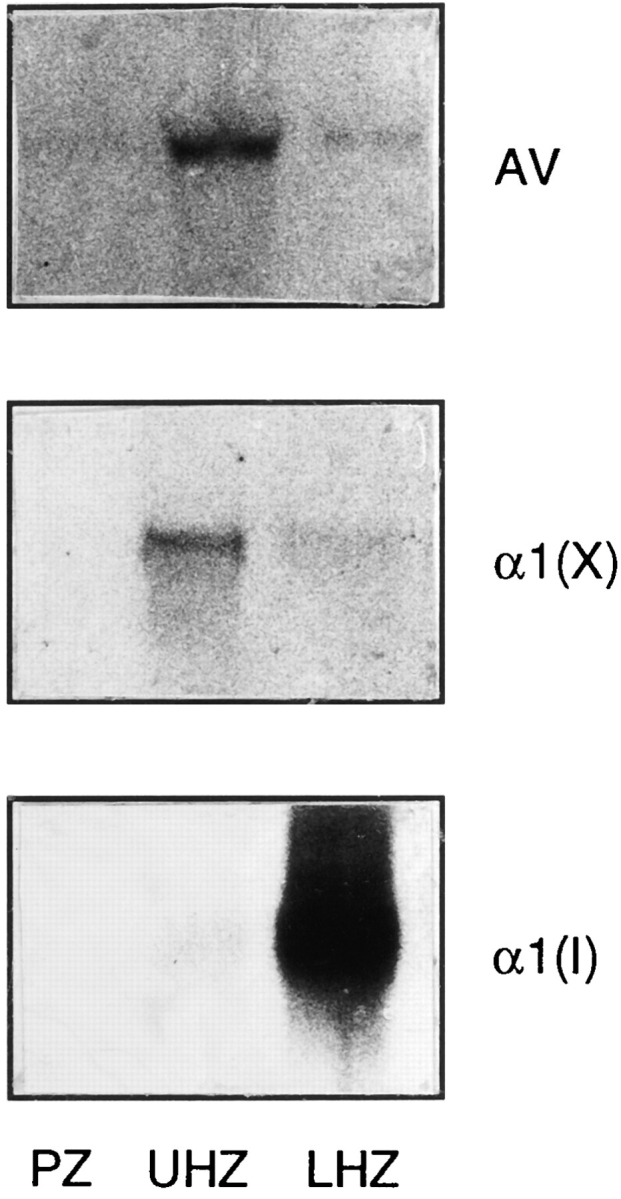
Northern blot analysis showing annexin V (AV), type X collagen (α1(X)), and type I collagen (α1(I)) in different zones of growth plate cartilage. RNA (10 μg/lane) isolated from the proliferative zone (PZ), upper hypertrophic zone (UHZ), and lower hypertrophic, calcifying zone (LHZ) of day 19 chick embryonic tibia were separated on agarose gels, blotted onto nylon membranes, and hybridized to 32P-labeled annexin V (AV), type X collagen (α1(X)), and type I collagen (α1(I)) cDNA probes. Note the presence of high amounts of annexin V and type X collagen message in the upper hypertrophic zone; in contrast, α1(I) is confined to the lower hypertrophic, calcifying zone.
To ascertain if type I collagen mRNA is expressed by chondrocytes, we used immunostaining to localize type X collagen, type I collagen, and annexin V in embryonic tibia growth plate cartilage. Double immunostaining using antibodies against types X and I collagen revealed that type I collagen was restricted to a narrow zone of chondrocytes that bordered the chondro–osseous junction, which also showed strong immunostaining for type X collagen. In contrast, hypertrophic chondrocytes away from the border were only type X collagen positive, while adjacent osteoid tissue was immunopositive for type I collagen but not type X collagen (Fig. 11, A and B). Immunostaining with antibodies against annexin V showed strong staining of the matrix only in a few cell layers close to the chondro–osseous junction, while in other areas of the hypertrophic zone staining for annexin V was restricted to the cell surface (Fig. 11 C). Immunostaining for annexin V was also observed in the osteoid matrix (Fig. 11 C). Staining of sections derived from similar areas with alizarin red S revealed a strong staining of the narrow type I collagen, type X collagen, and annexin V–positive zone, demonstrating the codistribution of type I collagen and annexin V in the matrix of calcified cartilage (Fig. 11 D).
Figure 11.
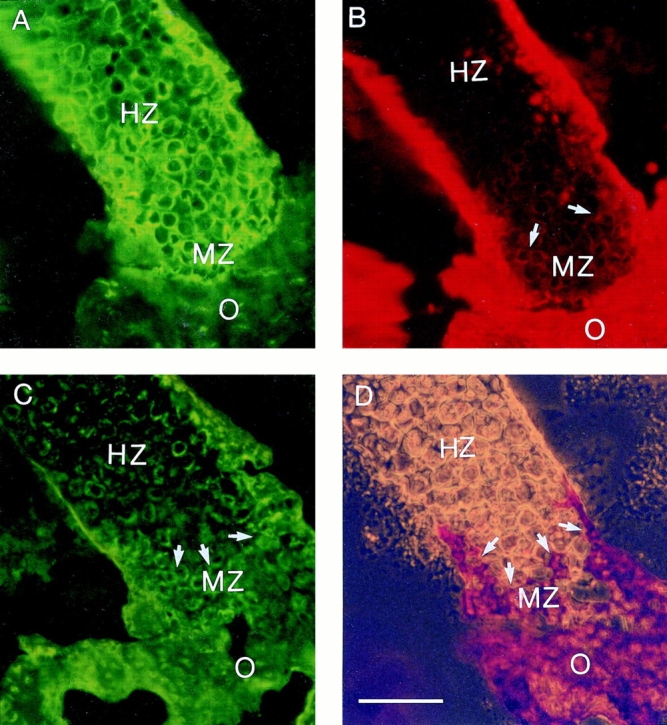
Localization of type X collagen, type I collagen, annexin V, and calcium deposits in growth plate cartilage. Longitudinal sections of day 19 chick embryonic tibia growth plate cartilage were double stained with antibodies against type X (A) and I collagen (B) or antibodies against annexin V (C). Sections derived from similar areas were stained with alizarin red S (D). Double staining demonstrated a colocalization of type X (A) and type I collagen (B) in the lower hypertrophic cartilage zone. Type I collagen staining was detected in 3–5 cell layers close to the chondro–osseous junction (arrows). Alizarin red S staining confirmed that these type I collagen-positive cartilage regions were calcified (D, arrow). Immunostaining with antibodies against annexin V revealed strong staining of the matrix only in the lower hypertrophic (calcified) cartilage zone, while staining for annexin V in hypertrophic cartilage was restricted to the cell surface (C). HZ, hypertrophic zone; MZ, mineralized zone; O, osteoid. Bar, 100 μm.
In summary, annexin V is expressed more widely than, and before, type I collagen in hypertrophic cartilage. However, both proteins are abundant in the restricted area of hypertrophic cartilage engaged in calcification.
Discussion
The results of this study demonstrate that matrix vesicle production can be qualitatively modulated in hypertrophic chondrocytes. Once the cells become engaged in mineralization under the stimulating activity of vitamin C, they release vesicles that are annexin V and alkaline phosphatase rich and that are able to undergo rapid and efficient mineralization. The mineral associated with these vesicles is apatitic and is thus quite similar, if not identical, to mineral that is normally deposited in vivo at the chondro–osseous border during endochondral ossification. Our results offer an answer to the puzzle posed above, namely that not all matrix vesicles are created equal, but cells, such as hypertrophic chondrocytes, have the ability to regulate the structural composition and functional properties of the vesicles. It is reasonable to postulate that matrix vesicles present in tissues not engaged in mineralization, such as articular cartilage (8, 15, 16, 67), have inadequate structural composition and are unable to initiate mineralization. What role mineralization-incompetent vesicles may have, if any, remains to be established.
The vesicles produced by the mineralizing chondrocytes have all the characteristics needed for the induction of calcification. First, by being extremely rich in alkaline phosphatase activity, the vesicles have the capacity to hydrolyze a variety of organic phosphate substrates, to increase substantially the local availability of free phosphate ions, and to permit their use for apatite crystallization. Such enzymatic activity may also remove putative inhibitors of mineralization, including pyrophosphate (2, 7, 56). Second, the vesicles contain a large amount of annexin V. As summarized above, this membrane-associated protein mediates the influx of Ca2+ into matrix vesicles, enabling intralumenal crystal growth from the preexisting nucleational core complex (30, 35, 59, 69). Recent molecular modeling studies have indicated that the protein forms an hexameric structure that contains a hydrophilic pore selective for the passage of cations (39). We have confirmed that annexin V is assembled as a multimeric structure in the vesicles from mineralizing chondrocytes (31). In addition, our previous work has shown that annexin V binds directly to types II and X collagen (32, 34). These interactions may be important for anchoring the vesicles to the fibrous components of the matrix and appear to stimulate Ca2+ influx into the vesicles (30, 34). Clearly, by lacking annexin V, the vesicles produced by nonmineralizing chondrocytes are not only unable to take up Ca2+, which favors intralumenal crystal growth (Table I and Figs. 3 and 6); they may also “fall off” the matrix and fail to produce functional extracellular matrix–vesicle interactions. Third, freshly isolated vesicles contain a relatively high Ca2+ and Pi content, suggesting that they already contain the preformed nucleational core complex rich in Ca2+ and Pi. By having a very low initial Ca2+/Pi content, the vesicles from nonmineralizing cells may not contain a nucleational core complex and thus are probably unable to carry out crystal assembly or do so at unphysiologically slow rates. Fourth, when incubated in synthetic cartilage lymph, the vesicles form needle-like crystals (Fig. 5), while vesicles from nonmineralizing cells are unable to form crystals. FT-IR analysis of the ν1ν3 and ν4 phosphate domains indicates that the vesicles from mineralizing cells generate well crystallized apatite (Fig. 6).
The assembly of mineralization-competent vesicles thus requires a coordinated effort on the part of the chondrocytes such that multiple specific components all appear in these vesicles. Matrix vesicle assembly must require sequestering mechanisms reminiscent of those involved in assembly of exo- and endocytotic vesicles. For instance, our data show that vitamin C stimulates alkaline phosphatase activity about 2-fold in total cell homogenates; in striking contrast, such activity is stimulated over 300-fold in matrix vesicles. Thus, the chondrocytes must possess mechanisms to concentrate the enzyme, which is phosphatidylinositol linked (24, 61, 66), in the nascent matrix vesicles before budding off the cell surface. Similar concentration mechanisms must also be involved in selecting the lipid composition of the matrix vesicles, which is markedly different from the lipid composition of the chondrocyte membrane and, in particular, is phosphatidylserine rich (51, 70). We should point out that hypertrophic chondrocytes exhibit elevated levels of intracellular calcium (22, 27, 35); interestingly, calcium is required for binding and association of annexin V with the membrane (19). Thus, elevated levels of intracellular calcium could facilitate the association of annexin V with the membrane inside the chondrocytes; assembly processes such as those above may then concentrate annexin V in nascent matrix vesicles. In addition, the high intracellular calcium concentration might also be required for the formation of the nucleational core complex inside the cells before the release of the vesicles into extracellular matrix (69). In this regard, it will be interesting to determine whether vitamin C treatment increases the intracellular calcium concentration in chondrocytes, thus favoring annexin V/matrix vesicle assembly. Because matrix vesicles are produced by nonmineralizing chondrocytes but are alkaline phosphatase, annexin V, and Ca2+/Pi poor, it seems, however, that these three components may not have a major direct role in vesicle assembly and budding per se.
Matrix vesicles produced by mineralizing chondrocytes also contain types II and X collagen, which are not present in vesicles produced by nonmineralizing chondrocytes, confirming previous studies showing that binding of types II and X collagen to matrix vesicles is mediated by annexin V (32, 35, 68). In addition, our previous studies have provided evidence that surface-attached collagens (types II and X) play an important role in stimulating influx of Ca2+ into matrix vesicles that already contain a preformed nucleational core complex inside their lumen (30, 34); however, these collagens are not able to initiate mineralization by themselves in the absence of matrix vesicles (30, 34). Thus, by having annexin V, surface-attached collagens, and a preformed nucleational core complex, matrix vesicles produced by mineralizing chondrocytes can actively take up Ca2+ and induce mineralization. Matrix vesicles produced by nonmineralizing chondrocytes lack annexin V, surface-attached collagens, and a nucleational core and thus are unable to accumulate mineral ions.
As summarized above, it has been proposed that focal accumulations of proteoglycans in the hypertrophic zone may serve as the nucleational site for calcification (25, 26, 53). However, our results show that matrix vesicles isolated from both control and mineralizing cultures contain similar amounts of proteoglycans, indicating that proteoglycans do not play a major role in the initial phase of mineralization mediated by matrix vesicles, when the first crystal phase grows inside the vesicle lumen (6). These findings are in agreement with a previous study showing that removal of proteoglycans from matrix vesicle preparations from hypertrophic cartilage does not change their ability to take up Ca2+ (69). Our data do not exclude the possibility that once the crystals rupture the vesicle membrane and penetrate the extracellular matrix, proteoglycans might still be involved in regulating apatite deposition and growth.
It is not surprising that hypertrophic chondrocytes depend on the stimulating activity of vitamin C to assemble mineralization-competent vesicles. It is quite likely that mineralization in vivo depends on a variety of exogenous cues and factors as well. We have shown previously that retinoic acid is also a powerful and efficient inducer of mineralization in chondrocytes (28, 29). Indeed, both lack of vitamin A or excessive amounts of it cause severe skeletal defects and produce abnormal cartilage mineralization (43, 60). Vitamin C and retinoic acid do not function as “master switches” such that the entire mineralization program is turned on at once. Rather, they appear to act as triggers that initiate a subsequent sequence of temporally separable events. This is exemplified by our findings that vitamin C treatment first leads to stimulation of alkaline phosphatase activity and annexin V gene expression and then to induction of type I collagen expression and matrix mineralization. Thus, cues such as vitamin C seem to trigger or stimulate the expression of the chondrocyte-endogenous developmental program, which then proceeds according to an endogenously controlled sequence. We demonstrate here that a similar sequence of phenotypic events characterizes the hypertrophic zone of growth plate in vivo in which we observe the presence of annexin V first and the codistribution of annexin V and type I collagen later at the mineralizing border. How this sequence of events is controlled in vivo and in vitro and what cellular and molecular mechanisms are involved remains to be worked out. For instance, both vitamin C and phosphate have been found to change the thiol status of chondrocytes undergoing terminal maturation (62); since thiol levels can regulate the expression of a number of matrix genes, it is plausible that thiol levels could affect the expression of annexin V, type I collagen, as well as type X collagen in maturing and mineralizing chondrocytes.
Previous studies have shown that in turkey tendons the apatitic crystals emanating from matrix vesicles grow along type I collagen fibrils (9, 10, 13, 36). Based on the highly oriented nature and parallel alignment of type I collagen fibrils, these fibrils probably provide an ideal environment for further crystal growth. Our data now demonstrate that hypertrophic chondrocytes engaged in mineralization in culture produce and deposit extracellularly large amounts of type I collagen; we also show that the mineralizing chondrocytes at the chondro–osseous border in vivo display type I collagen in their matrix. Since matrix vesicles isolated from mineralizing chondrocyte cultures do not contain type I collagen and are able to initiate calcification when incubated in synthetic cartilage lymph (Table I and Figs. 5 and 6), it is likely that this collagen is not involved in the initiation and nucleation of the first crystal phase. However, as in the case of tendons, this collagen may offer a structure onto which crystals can grow and propagate in appropriate spatial directions.
Type I collagen may also play an additional, important function. It may provide for a smooth transition from the type II/X collagen-rich cartilage matrix to the type I collagen-rich bone matrix. It may also reinforce the mechanical properties of the chondro–osseous border, helping this heterogeneous and highly dynamic structure maintain structural integrity.
The onset of synthesis of type I collagen in chondrocytes should be discussed in relation to previous studies on a related issue. Chondrocytes have long been known to have the capacity to initiate type I collagen synthesis once placed in culture (12, 44, 54, 64). This finding has been traditionally interpreted to signify that chondrocytes are phenotypically unstable and can rapidly dedifferentiate into fibroblast-like cells (12, 44, 54, 64). In more recent studies, type I collagen synthesis by chondrocytes has been given an alternative interpretation, namely that it indicates that the chondrocytes “transdifferentiate” into osteoblast-like cells (18, 57, 58). However, our findings demonstrate that hypertrophic chondrocytes do not necessarily have to undergo “transdifferentiation” to osteoblastic cells to express type I collagen. Instead, morphologically identifiable, type X collagen-positive chondrocytes express type I collagen in concert with matrix calcification. Thus, mineralizing hypertrophic chondrocytes acquire and express most of the phenotypic characteristics of osteoblasts, including high alkaline phosphatase activity and expression of osteonectin and type I collagen (28, 29, 35, 37, 45, 48), while maintaining unique properties such as hypertrophy or type X collagen synthesis. In line with our conclusions above, it may be that these phenotypic traits shared with osteoblasts are needed not only to bring about the final phase of endochondral ossification, that is, the replacement of mineralized cartilage with bone; they may also permit mineralized cartilage to act as bone-like structure and tissue and allow for a seamless structural–functional transition from cartilage to bone.
Acknowledgments
We would like to thank Drs. Klaus von der Mark (University of Erlangen, Nuremberg, Germany) and Thomas Linsenmayer (Tufts University, Boston, MA) for providing us antibodies against annexin V and type I collagen. We would also like to thank Dr. Peter Berthold, Ms. Sylvia Decker, and Mr. Gerald Harrison (all of the School of Dental Medicine, University of Pennsylvania, Philadelphia, PA) for assistance with transmission and scanning electron microscopy, and Ms. Yooson Kim for technical assistance.
This work was supported by National Institutes of Health grants (AR 43732 to T. Kirsch, AR 40833 and DE 10354 to M. Pacifici, and DE 10875 and AR 41525 to I.M. Shapiro).
Footnotes
Please address all correspondence to Dr. Thorsten Kirsch, Department of Anatomy and Histology, School of Dental Medicine, University of Pennsylvania, 4001 Spruce Street, Philadelphia, PA 19104-6003. Tel.: (215) 5733502; Fax: (215) 573-2324.
References
- 1.Adams SL, Pallante KM, Niu Z, Leboy PS, Golden EB, Pacifici M. Rapid induction of type X collagen gene expression in cultured chick vertebral chondrocytes. Exp Cell Res. 1991;193:190–197. doi: 10.1016/0014-4827(91)90555-9. [DOI] [PubMed] [Google Scholar]
- 2.Ali SY. Analysis of matrix vesicles and their role in the calcification of epiphyseal cartilage. Fed Proc. 1976;35:135–142. [PubMed] [Google Scholar]
- 3.Ali SY. New knowledge of osteoarthritis. J Clin Pathol (Lond) 1992;178:205–213. [Google Scholar]
- 4.Ames BN. Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 5.Anderson HC. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969;41:59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson, H.C. 1990. Biology of disease—mechanism of mineral formation in bone. In Pathology Reviews. E. Rubin and I. Damjanov, editors. The Humana Press Inc., Clifton, NJ. 13–23.
- 7.Anderson HC, Reynolds JJ. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973;34:211–227. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]
- 8.Anderson HC, Stechschulte DJ, Jr, Collins DE, Jacobs DH, Morris DC, Redford PA, Zeiger S. Matrix vesicle biogenesis in vitro by rachitic and normal rat chondrocytes. Am J Path. 1990;136:391–398. [PMC free article] [PubMed] [Google Scholar]
- 9.Arsenault AL. Crystal-collagen relationships in calcified turkey leg tendons visualized by selected-area dark field electron microscopy. Calcif Tissue Int. 1988;43:202–212. doi: 10.1007/BF02555136. [DOI] [PubMed] [Google Scholar]
- 10.Arsenault AL, Frankland BW, Ottensmeyer FP. Vectorial sequence of mineralization in the turkey leg tendon determined by electron microscopic imaging. Calcif Tissue Int. 1991;48:46–55. doi: 10.1007/BF02555795. [DOI] [PubMed] [Google Scholar]
- 11.Baginsky ES, Marie SS, Clark WL, Zak B. Direct microdetermination of serum calcium. Clin Chim Acta. 1973;46:49–54. doi: 10.1016/0009-8981(73)90101-0. [DOI] [PubMed] [Google Scholar]
- 12.Benya PD, Padilla SR, Nimni ME. Independent regulation of collagen types by chondrocytes during the loss of differentiated function in culture. Cell. 1978;15:1313–1321. doi: 10.1016/0092-8674(78)90056-9. [DOI] [PubMed] [Google Scholar]
- 13.Berthet-Colominas C, Miller A, White SW. Structural study of the calcifying collagen in turkey leg tendons. J Mol Biol. 1979;134:431–445. doi: 10.1016/0022-2836(79)90362-0. [DOI] [PubMed] [Google Scholar]
- 14.Bonucci E. Fine structure and histochemistry of calcifying globules in epiphyseal cartilage. Z Zellforsch Mikrosk Anat. 1970;103:192–217. doi: 10.1007/BF00337312. [DOI] [PubMed] [Google Scholar]
- 15.Borg TK, Runyan R, Wuthier RE. A freeze-fracture study of avian epiphyseal cartilage differentiation. Anat Rec. 1981;199:449–457. doi: 10.1002/ar.1091990402. [DOI] [PubMed] [Google Scholar]
- 16.Buckwalter JA, Mower D, Schaeffer J. Differences in matrix vesicle concentration among growth plate zones. J Orthop Res. 1987;5:157–163. doi: 10.1002/jor.1100050202. [DOI] [PubMed] [Google Scholar]
- 17.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 18.Descalzi-Cancedda F, Gentili C, Manduca P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation in culture. J Cell Biol. 1992;117:427–435. doi: 10.1083/jcb.117.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisow, M.J., J.H. Walker, C. Boustead, and W. Taylor. 1988. Annexins—a new family of Ca2+ regulated phospholipid-binding proteins. In Molecular Mechanisms in Secretion. N.A. Thorn, M. Traiman, and O.H. Peterson, editors. Munksgaard, Copenhagen, Denmark. 598–608.
- 20.Genge BR, Sauer GR, Wu LN, McLean FM, Wuthier RE. Correlation between loss of alkaline phosphatase activity and accumulation of calcium during matrix vesicle-mediated mineralization. J Biol Chem. 1988;263:18513–18519. [PubMed] [Google Scholar]
- 21.Genge BR, Wu LNY, Wuthier RE. Identification of phospholipid-dependent calcium-binding proteins as constituents of matrix vesicles. J Biol Chem. 1989;264:10917–10921. [PubMed] [Google Scholar]
- 22.Gunter TE, Zuscik MJ, Puzas JE, Gunter KK, Rosier RN. Cytosolic free calcium concentrations in avian growth plate chondrocytes. Cell Calcium. 1990;11:445–457. doi: 10.1016/0143-4160(90)90077-8. [DOI] [PubMed] [Google Scholar]
- 23.Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collageninduced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- 24.Hsu HH, Morris DC, Davis L, Moylan P, Anderson CH. In vitro Ca deposition by rat matrix vesicles: is the membrane association of alkaline phosphatase essential for matrix vesicle-mediated calcium deposition? . Int J Biochem. 1993;25:1737–1742. doi: 10.1016/0020-711x(88)90301-1. [DOI] [PubMed] [Google Scholar]
- 25.Hunter GK. An ion-exchange mechanism of cartilage calcification. Connect Tissue Res. 1987;16:111–120. doi: 10.3109/03008208709001999. [DOI] [PubMed] [Google Scholar]
- 26.Hunter, G.K. 1991. Role of proteoglycan in the provisional calcification of cartilage. A review and reinterpretation. Clin. Orth. Relat. Res. 256–280. [PubMed]
- 27.Iannotti JP, Brighton CT. Cytosolic ionized calcium concentration in isolated chondrocytes from each zone of the growth plate. J Orthop Res. 1989;7:511–518. doi: 10.1002/jor.1100070408. [DOI] [PubMed] [Google Scholar]
- 28.Iwamoto M, Shapiro IM, Yagami K, Boskey AL, Leboy PS, Adams SL, Pacifici M. Retinoic acid induces rapid mineralization and expression of mineralization-related genes in chondrocytes. Exp Cell Res. 1993;207:413–420. doi: 10.1006/excr.1993.1209. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto M, Yagami K, Shapiro IM, Leboy PS, Adams SL, Pacifici M. Retinoic acid is a major regulator of chondrocyte maturation and matrix mineralization. Microsc Res Tech. 1994;28:483–491. doi: 10.1002/jemt.1070280604. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch T, Ishikawa Y, Mwale F, Wuthier RE. Roles of the nucleational core complex and collagens (types II and X) in calcification of growth plate cartilage matrix vesicles. J Biol Chem. 1994;269:20103–20109. [PubMed] [Google Scholar]
- 31.Kirsch T, Nah H-D, Demuth DR, Harrison G, Golub EE, Adams SL, Pacifici M. Annexin V-mediated calcium flux across membranes is dependent on the lipid composition. Implications for cartilage mineralization. Biochemistry. 1997;36:3359–3367. doi: 10.1021/bi9626867. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch T, Pfaeffle M. Selective binding of anchorin CII (annexin V) to type II and X collagen and to chondrocalcin (C-propeptide of type II collagen) FEBS (Fed Eur Biochem Soc) Lett. 1992;310:143–147. doi: 10.1016/0014-5793(92)81316-e. [DOI] [PubMed] [Google Scholar]
- 33.Kirsch T, von der Mark K. Remodelling of collagen types I, II and X and calcification of human fetal cartilage. Bone Miner. 1992;18:107–117. doi: 10.1016/0169-6009(92)90851-4. [DOI] [PubMed] [Google Scholar]
- 34.Kirsch T, Wuthier RE. Stimulation of calcification of growth plate cartilage matrix vesicles by binding to type II and X collagens. J Biol Chem. 1994;269:11462–11469. [PubMed] [Google Scholar]
- 35.Kirsch T, Swoboda B, von der Mark K. Ascorbate independent differentiation of human chondrocytes in vitro: simultaneous expression of types I and X collagen and matrix mineralization. Differentiation. 1992;52:89–100. doi: 10.1111/j.1432-0436.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 36.Landis WJ. A study of calcification in the leg tendons from the domestic turkey. J Ultrastruct Mol Struct Res. 1986;94:217–238. doi: 10.1016/0889-1605(86)90069-8. [DOI] [PubMed] [Google Scholar]
- 37.Leboy PS, Vaias L, Uschmann B, Golub E, Adams SL, Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem. 1989;264:17281–17286. [PubMed] [Google Scholar]
- 38.Linsenmayer TF, Hendrix MJ, Little CD. Production and characterization of a monoclonal antibody to chicken type I collagen. Proc Natl Acad Sci USA. 1979;76:3703–3707. doi: 10.1073/pnas.76.8.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luecke H, Chang BT, Mailliard WS, Schlaepfer DD, Haigler HT. Crystal structure of the annexin XII hexamer and implications for bilayer insertion. Nature (Lond) 1995;378:512–515. doi: 10.1038/378512a0. [DOI] [PubMed] [Google Scholar]
- 40.LuValle PA, Ninomiya Y, Rosenblum ND, Olsen BR. The type X collagen gene. J Biol Chem. 1988;263:18378–18385. [PubMed] [Google Scholar]
- 41.Matsuzawa T, Anderson HC. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem. 1971;19:801–808. doi: 10.1177/19.12.801. [DOI] [PubMed] [Google Scholar]
- 42.Mollenhauer J, Bee JA, Lizarbe MA, von der Mark K. Role of anchorin CII a 31000 molecular weight membrane protein in interaction of chondrocytes with type II collagen. J Cell Biol. 1984;98:1572–1578. doi: 10.1083/jcb.98.4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore T, Sharman IM. Hypervitaminosis A combined with calcium deficiency in rats. Int J Vitam Nutr Res. 1979;49:14–20. [PubMed] [Google Scholar]
- 44.Mueller PK, Lemmen C, Gay S, Gauss V, Kuehn K. Immunochemical and biochemical study of collagen synthesis by chondrocytes in culture. Exp Cell Res. 1977;108:47–55. [PubMed] [Google Scholar]
- 45.Nah H-D, Bennett VD, Niu ZL, Adams SL. Alternative transcript of the chick alpha-2(I) collagen gene is transiently expressed during endochondral bone formation and during development of the central nervous system. Dev Dyn. 1996;206:146–158. doi: 10.1002/(SICI)1097-0177(199606)206:2<146::AID-AJA4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 46.Oettinger HF, Pacifici M. Type X collagen gene expression is transiently up-regulated by retinoic acid treatment in chick chondrocyte cultures. Exp Cell Res. 1990;191:292–298. doi: 10.1016/0014-4827(90)90017-5. [DOI] [PubMed] [Google Scholar]
- 47.Pacifici M, Soltesz R, Thal G, Shanley DJ, Boettiger D, Holtzer H. Immunological characterization of the major chick cartilage proteoglycan and its intracellular localization in cultured chondroblasts: a comparison with type II procollagen. J Cell Biol. 1983;97:1724–1736. doi: 10.1083/jcb.97.6.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pacifici M, Oshima O, Fisher LW, Young MF, Shapiro IM, Leboy PS. Changes in osteonectin distribution and levels are associated with mineralization of the chicken tibial growth cartilage. Calcif Tissue Int. 1990;47:51–61. doi: 10.1007/BF02555866. [DOI] [PubMed] [Google Scholar]
- 49.Pacifici M, Golden EB, Adams SL, Shapiro IM. Cell hypertrophy and type X collagen synthesis in cultured articular chondrocytes. Exp Cell Res. 1991a;192:266–270. doi: 10.1016/0014-4827(91)90185-w. [DOI] [PubMed] [Google Scholar]
- 50.Pacifici M, Golden EB, Iwamoto M, Adams SL. Retinoic acid treatment induces type X collagen gene expression in cultured chick chondrocytes. Exp Cell Res. 1991b;195:38–46. doi: 10.1016/0014-4827(91)90497-i. [DOI] [PubMed] [Google Scholar]
- 51.Peress NS, Anderson HC, Sajdera SW. The lipids of matrix vesicles from bovine fetal epiphyseal cartilage. Calcif Tissue Int. 1974;14:275–281. doi: 10.1007/BF02060301. [DOI] [PubMed] [Google Scholar]
- 52.Pfannmueller E, Turnay J, Bertling W, von der Mark K. Organisation of the chicken annexin V gene and its correlation with the tertiary structure of the protein. FEBS (Fed Eur Biochem Soc) Lett. 1993;336:467–471. doi: 10.1016/0014-5793(93)80857-q. [DOI] [PubMed] [Google Scholar]
- 53.Poole, A.R. 1991. The growth plate: cellular physiology, cartilage assembly and mineralization. In Cartilage: Molecular Aspects. B. Hall and S. Newman, editors. CRC Press, Boca Raton, FL. 179–211.
- 54.Quarto R, Dozin B, Tacchetti C, Campanile G, Malfatto C, Cancedda R. In vitro development of hypertrophic chondrocytes starting from selected clones of dedifferentiated cells. J Cell Biol. 1990;110:1379–1386. doi: 10.1083/jcb.110.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rees JA, Ali SY. Ultrastructural localisation of alkaline phosphatase activity in osteoarthritic human articular cartilage. Ann Rheum Dis. 1988;47:747–753. doi: 10.1136/ard.47.9.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Register TC, Wuthier RE. Effect of pyrophosphate and two diphosphonates on 45Ca and 32Pi uptake and mineralization by matrix vesicle-enriched fractions and by hydroxyapatite. Bone (NY) 1985;6:307–312. doi: 10.1016/8756-3282(85)90320-5. [DOI] [PubMed] [Google Scholar]
- 57.Roach HI, Shearer JR. Cartilage resorption and endochondral bone formation during the development of long bones in chick embryos. Bone Miner. 1989;6:289–309. doi: 10.1016/0169-6009(89)90035-4. [DOI] [PubMed] [Google Scholar]
- 58.Roach HI, Erenpreisa J, Aigner T. Osteogenic differentiation of hypertrophic chondrocytes involves asymmetric cell divisions and apoptosis. J Cell Biol. 1995;131:483–494. doi: 10.1083/jcb.131.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojas E, Arispe N, Haigler HT, Burns HL, Pollard HB. Identification of annexins as calcium channels in biological membranes. Bone Miner. 1992;13:214–218. doi: 10.1016/0169-6009(92)90739-z. [DOI] [PubMed] [Google Scholar]
- 60.Shepard, T.H. 1976. Catalog of Teratogenic Agents. Johns Hopkins University Press, Baltimore, MD. 239 pp.
- 61.Takami N, Ogata S, Oda K, Misumi Y, Ikehara Y. Biosynthesis of placental alkaline phosphatase and its post-translational modification by glycophospholipid for membrane-anchoring. J Biol Chem. 1988;263:3016–3021. [PubMed] [Google Scholar]
- 62.Teixeira CC, Shapiro IM, Hatori M, Rajpurohit R, Koch C. Retinoic acid modulation of glutathione and cysteine metabolism in chondrocytes. Biochem J. 1996;314:21–26. doi: 10.1042/bj3140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerd CJ, Rinker AD, Shney D, Zygowicz ER. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. 1983;29:751–761. [PubMed] [Google Scholar]
- 64.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature (Lond) 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 65.von der Mark, K., T. Kirsch, A. Nerlich, A. Kuss, G. Weseloh, K. Glueckert, and H. Stoess. 1992. Type X collagen synthesis in human osteoarthritic cartilage. Arthritis Rheum. 3 5:806–811. [DOI] [PubMed]
- 66.Wu LN, Valhmu WB, Lloyd GC, Genge BR, Wuthier RE. Isolation of two glycosylated forms of membrane-bound alkaline phosphatase from avian growth plate cartilage matrix vesicle-enriched microsomes. Bone Miner. 1989;7:113–125. doi: 10.1016/0169-6009(89)90069-x. [DOI] [PubMed] [Google Scholar]
- 67.Wu LNY, Sauer GR, Genge BR, Wuthier RE. Induction of mineral deposition by primary cultures of chicken growth plate chondrocytes in ascorbate-containing medium. J Biol Chem. 1989;264:21346–21355. [PubMed] [Google Scholar]
- 68.Wu LNY, Genge BR, Lloyd GC, Wuthier RE. Collagenbinding proteins in collagenase-released matrix vesicles from cartilageinteraction between matrix vesicle proteins and different types of collagen. J Biol Chem. 1991;266:1195–1203. [PubMed] [Google Scholar]
- 69.Wu LNY, Yoshimori T, Genge BR, Sauer GR, Kirsch T, Ishikawa Y, Wuthier RE. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J Biol Chem. 1993;268:25084–25094. [PubMed] [Google Scholar]
- 70.Wuthier RE. Lipid composition of isolated epiphyseal cartilage cells, membranes and matrix vesicles. Biochem Biophys Acta. 1975;409:128–143. doi: 10.1016/0005-2760(75)90087-9. [DOI] [PubMed] [Google Scholar]
- 71.Wuthier RE. Involvement of cellular metabolism of calcium and phosphate in calcification of avian growth plate cartilage. J Nutr. 1993;123:301–309. doi: 10.1093/jn/123.suppl_2.301. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto T, Sobel ME, Adams SL, Avvedimento VE, DiLauro R, Pastan I, Showalter A, Pesciotta D, Fietzek P, Olsen B. Construction of a recombinant bacterial plasmid containing pro-alpha 1(I) collagen DNA sequences. J Biol Chem. 1980;255:2612–2615. [PubMed] [Google Scholar]