Abstract
Angiogenesis depends on growth factors and vascular cell adhesion events. Integrins and growth factors are capable of activating the ras/MAP kinase pathway in vitro, yet how these signals influence endothelial cells during angiogenesis is unknown. Upon initiation of angiogenesis with basic fibroblast growth factor (bFGF) on the chick chorioallantoic membrane (CAM), endothelial cell mitogen-activated protein (MAP) kinase (ERK) activity was detected as early as 5 min yet was sustained for at least 20 h. The initial wave of ERK activity (5–120 min) was refractory to integrin antagonists, whereas the sustained activity (4–20 h) depended on integrin αvβ3, but not β1 integrins. Inhibition of MAP kinase kinase (MEK) during this sustained αvβ3-dependent ERK signal blocked the formation of new blood vessels while not influencing preexisting blood vessels on the CAM. Inhibition of MEK also blocked growth factor induced migration but not adhesion of endothelial cells in vitro. Therefore, angiogenesis depends on sustained ERK activity regulated by the ligation state of both a growth factor receptor and integrin αvβ3.
Angiogenesis is influenced by both growth factors and adhesion proteins associated with the extracellular matrix (ECM).1 The induction of angiogenesis is characterized by vascular cell proliferation, migration, and differentiation, which depends on contacts with the ECM. While it is assumed that ECM-derived signaling events are required for angiogenesis (Ingber and Folkman, 1989; Klagsburn and D'Amore, 1991; Folkman and Shing, 1992; Brooks et al., 1994a ,b), little is known regarding which signaling pathways initiate or maintain the angiogenic phenotype of vascular cells in vivo.
Recently, we demonstrated that antagonists of integrin αvβ3 could disrupt angiogenesis induced by bFGF in various animal models (Brooks et al., 1994a ; Friedlander et al., 1995). This was based on the finding that antagonists of αvβ3 induced p53-dependent endothelial cell apoptosis during angiogenesis (Stromblad et al., 1996). These findings suggest that during angiogenesis integrin αvβ3 and the receptor for bFGF cooperate to promote signaling events necessary for vascular cell survival, thereby facilitating the induction and/or maintenance of the angiogenic phenotype. Cell survival has been shown to be dependent on adhesion to the ECM requiring specific intracellular signaling events (Meredith et al., 1993; Brooks et al., 1994b ; Montgomery et al., 1994; Frisch et al., 1996). In fact, recent evidence has implicated various mitogen-activated protein (MAP) kinases (ERK) in cell survival in vivo. For example, activation of ERK and the suppression of p38 and JNK MAP kinases promotes increased cell survival (Xia et al., 1995). While growth factor receptors (Boulton et al., 1991; Cobb et al., 1991; Berrou et al., 1996; Molloy et al., 1996) as well as integrins can lead to the activation of the MAP kinase cascade in vitro (Chen et al., 1994; Morino et al., 1995; Schlaepfer et al., 1995; Miyamoto et al., 1995, 1996; Klemke et al., 1997), how these signaling events contribute to a complex biological process such as angiogenesis is poorly understood.
In vitro, both cell adhesion events and growth factors induce a rapid yet transient (5–60 min) activation of MAP kinase. However, little is known about the kinetics and regulation of MAP kinase activity in vivo, where the more complex extracellular matrix environment in a tissue may influence both the level and duration of this activity. Since both growth factor receptors and integrins activate MAP kinase in vitro, we examined the role of MAP kinase and the requirement for integrin ligation during angiogenesis in vivo. Specifically, we investigated the ability of integrin αvβ3 and bFGF to influence MAP kinase activity in angiogenic blood vessels within the chick chorioallantoic membrane (CAM). Evidence is provided that bFGF promotes two MAP kinase signals that are distinguished both by their kinetics of activation and their dependency on αvβ3 ligation. Initiation of angiogenesis is characterized by a rapid induction of MAP kinase that is αvβ3 independent, which is followed by a late acting sustained signal lasting for at least 20 h that is dependent on αvβ3 ligation.
Materials and Methods
Antibodies and Reagents
A rabbit polyclonal antibody, C-14, raised against rat ERK2 was used for immunoprecipitation and Western blotting (Santa Cruz Biotechnology, Santa Cruz, CA). An affinity-purified rabbit polyclonal antibody to the phosphorylated form of ERK2 was used for immunofluorescence to localize the phosphorylated ERK (New England Biolabs, Beverly, MA and Promega Corp., Madison, WI). The commercially available phospho-specific antibodies used in this study were affinity purified to recognize the phosphotyrosine in the T-E-Y recognition site in ERK2 and the phosphothreonine and phosphotyrosine in the T-E-Y sequence, respectively. The antiphosphotyrosine antibody, 4G10, was used for Western blotting (Upstate Biotechnology, Inc., Lake Placid, NY). Antiactin antibody was obtained from Sigma Chemical Co. (St. Louis, MO) for Western blotting control. mAb LM609 (anti-αvβ3) has been previously described (Cheresh and Spiro, 1987). The anti-β3 COOH-terminal antibody (8275) used for immunoblots was a gift from M. Hemler (Harvard University, Boston, MA). mAb CSAT (anti-β1) was obtained from Dr. C. Buck (Wistar Institute, Philadelphia, PA). FITC and rhodamine-labeled secondary antibodies were obtained from BioSource (Camarillo, CA). Cyclic peptides 66203 (cyclo-RGDfV; f = d-phenylalanine) directed to αvβ3 and control peptide 69601 (cyclo-RADfV) (Pfaff et al., 1994; Freidlander et al., 1995) were provided by Dr. A. Jonczyk (Merck KGaA, Darmstadt, FRG). PD98059 (2-[2′-amino-3′methoxyphenyl]-oxanphthalen-4-one) is a compound that specifically inhibits MEK by binding directly to MEK via a proline rich region on MEK, allosterically blocking MEK activity and its ability to phosphorylate and activate ERK (Alessi et al., 1995). PD98059 was the kind gift of Dr. A. Saltiel (Parke-Davis, Ann Arbor, MI). Recombinant bFGF was kindly supplied by Dr. J. Abraham (Scios Inc., Mountain View, CA). Myelin basic protein (MBP) was from Upstate Biotechnology, Inc.
Chicken Embryos and Treatments
Fertilized chick embryos (standard pathogen free grade; SPAFAS, Preston, CT) were incubated for 10 d at 37°C with 70% humidity. A small hole was made with a drill (Dremel, Emerson Electric, Racine, WI) directly over the air sac at the end of the egg. The embryos were candled to determine a location to drill a second hole directly over embryonic blood vessels. The CAM was separated from the egg shell by applying vacuum to the original hole. A 1.0 × 1.0–cm square window was cut in the egg shell over the dropped CAM with a grinding wheel, exposing the CAM to direct access for experimental manipulation. Cortisone acetate–treated filter disks were soaked with 250 ng of bFGF or 20 μl of 250 ng of bFGF in PBS or in serum and growth factor–free M199 medium (GIBCO BRL, Gaithersburg, MD) added directly to the CAM. The windows were sealed with tape and incubated at 37°C. Blocking antibodies (50 μg), peptides (100 μg), or the MEK inhibitor (50 μM) were added in a volume of 50 μl directly on the cytokine application point 1 h before harvest for ERK phosphorylation or kinase activity. The concentrations of the bFGF, blocking antibodies, and blocking peptides were chosen based on previously published experiments (Brooks et al., 1994b ). Doses of PD98059 were based on previously published results (Alessi et al., 1995; Klemke et al., 1997). We have not been able to wash out the inhibitor from the CAM tissue since the effects of the bFGF and PD98059 remain as they have thoroughly soaked into the CAM tissue during the course of the experiment. None of these inhibitors detectably influenced the preexisting blood vessels or the preexisting levels of endogenous ERK activity.
Cell Culture
Early passage primary human umbilical vein endothelial cells (HUVEC; Jaffe et al., 1973) were maintained in M199, 20% FBS, 10 mM Hepes, 9 mg/ml heparin, 3 μg/ml endothelial cell growth supplement (ECGS; H-Neurext; Upstate Biotechnology Inc.), and 50 μg/ml gentamicin. Cells were starved for 24 h by replacing serum-containing culture media with FBS and ECGS-free media.
Immunofluorescence and Microscopy
Cryosections of CAMs treated with bFGF were examined for the tissue distribution of phosphorylated ERK using a primary antibody directed to phosphorylated ERK peptide (New England Biolabs and Promega Corp.). CAM sections were removed from embryos treated with PD98059 or controls were washed, embedded in OCT (Sakura Finetek, Torrance, CA), and snap frozen in liquid nitrogen. Sections of CAM tissue 4 μm in thickness were cut, fixed in acetone for 30 s, and stored at −70°C until use. Tissue sections were prepared for immunostaining by a brief rinse in PBS, block in 2.5% BSA, incubation in a 1:50 dilution of the phospho-ERK rabbit polyclonal antibody, or 5 μg/ml of control primary antibodies for 2 h. After 20 min of PBS washes, FITC- or rhodamine-labeled secondary antibodies were incubated for 2 h at 1:200. Slides were mounted, and images were collected on a microscope (model Axiovert 100; Carl Zeiss, Inc., Thornwood, NY) with a 20× 0.7 NA lens with a cooled CCD camera (model CE200A;Photometrics, Tucson, AZ) as 12-bit, 512 × 384 pixel arrays using rhodamine and fluorescein filter sets from Chromatech (Brattleboro, VT). Fluorescence images were processed using ONCOR Image (Gaithersburg, MD), and Adobe Photoshop (Mountain View, CA). Quantitation of vessel staining intensity was performed in the BDS Image program (Gaithersburg, MD) on a Macintosh computer (Apple Computer Co., Cupertino, CA). The final specific values for the pixel intensity of immunostaining on the vessels was derived from the result of the raw pixel intensity values of regions on the vessel subtracted by the pixel intensity values of surrounding nonvascular tissue. Using the surrounding tissue from at least three specimens in each treatment as an internal control, we measured significant changes in pixel intensity values between different treatments. CCD images were contrast stretched and exposure matched between experimental treatments (Castleman, 1989). Data are expressed as the mean intensity ± SD.
Immunoprecipitation and Immunoblotting
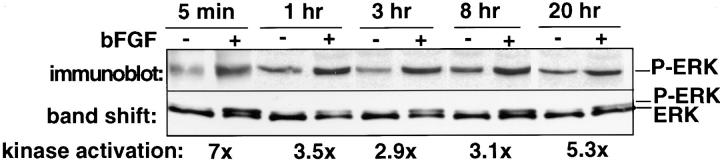
CAM tissue was harvested and snap frozen in liquid nitrogen before homogenization. Samples were homogenized with a motorized grinder in a modified RIPA buffer containing 100 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 2 mM PMSF, and the phosphatase inhibitors NaF (500 mM) and vanadate (1 mM). Two to three CAMs were homogenized in 1 ml of the RIPA buffer and then centrifuged for 15 min at 15,000 g. The total amount of protein was determined using the BCA protein assay reagent (Pierce, Rockford, IL). Equivalent amounts of protein were immunoprecipitated (400–700 μg) with anti-ERK antibody bound to protein A–Sepharose beads. Whole cell lysates or immunoprecipitates were washed in RIPA and PBS and then separated by 12% SDS-PAGE (SDS-PAGE), transferred to nitrocellulose with a semidry transfer apparatus, and blocked in 3% BSA in TBST. Antiphosphotyrosine, anti-ERK, anti–phospho-ERK, or anti-β3 antibodies used as primary antibodies were detected with horseradish peroxidase–conjugated goat anti–mouse or rabbit secondary antibodies and detected using the Enhanced Chemiluminescence (ECL; Amersham Corp., Arlington Heights, IL) system and Kodak x-ray film (Rochester, NY). The tyrosine phosphorylation of ERK in the Western blots was quantitated by laser scanning densitometry (Molecular Dynamics, Sunnyvale, CA) of ERK bands in the linear range of sensitivity of the x-ray film after ECL. The measurements of the bFGF-induced ERK phosphorylation with PD98059 or antiintegrin antagonists were assayed on at least three trials containing two to three CAMs from separate embryos. Gel shifts of the phosphorylated ERK were detected when using 12% SDS-PAGE (Fig. 1, top), but not by 10% SDS-PAGE (Fig. 1, bottom). The changes in ERK phosphorylation observed in chick CAM tissues were confirmed by in vitro kinase assays (below).
Figure 1.
Kinetics of bFGF-induced ERK phosphorylation in the CAM. bFGF (+) or PBS (−) saturated filter disks were placed on the CAM of 10-d-old chick embryos. At various times thereafter, CAMs were resected, lysed in detergent, and subjected to immunoprecipitation with an antibody to ERK. The immunoprecipitated material was subjected to SDS-PAGE and immunoblotting with an antiphosphotyrosine antibody (top). Band shifts of phosphorylated ERK in bFGF-treated CAMs was detected by immunoblotting with an anti-ERK antibody of parallel lysates (bottom). The relative increase in kinase activation of endogenous ERK after bFGF treatment compared with CAMs not treated with bFGF was measured by phosphorylation of myelin basic protein in an in vitro kinase assay using anti-ERK immunoprecipitates of CAM tissues as described in the Materials and Methods.
In Vitro Kinase Assays for MAP Kinase
The kinase activity of endogenous MAP kinase was assessed by the ability of immunopurified ERK to phosphorylate MBP in an in vitro assay, as described previously (Boulton and Cobb, 1991). ERK was immunoprecipitated with anti-ERK antibodies conjugated to protein A–Sepharose from 500 μg of whole cell extract of bFGF and antagonist treated CAM tissue. Immunoprecipitates were washed with RIPA and then washed in 0.1 M NaCl with 50 mM Hepes, pH 8.0, and finally the beads were resuspended in reaction buffer containing 0.5 μCi [γ-32P]ATP, 10 mM MgCl2, 50 μM ATP, 1 mM DTT, 1 mM benzamidine, 0.3 mg/ml MBP, and 25 mM Hepes, pH 8.0, for 15 min at 30°C. Boiling SDS loading buffer was used to stop the reaction, and the samples were fractionated by 15% SDS-PAGE, stained with Coomassie blue, dried, and exposed to x-ray film overnight. For quantitation, radioactive gels were exposed to Phosphoimager screens (Molecular Dynamics) and analyzed with ImageQuant for the PC (Molecular Dynamics). The kinase data in Figs. 1 and 3 were normalized to mock-treated CAM tissue. These data were representative of at least three trials and significant as measured by the Student t test (P < 0.05).
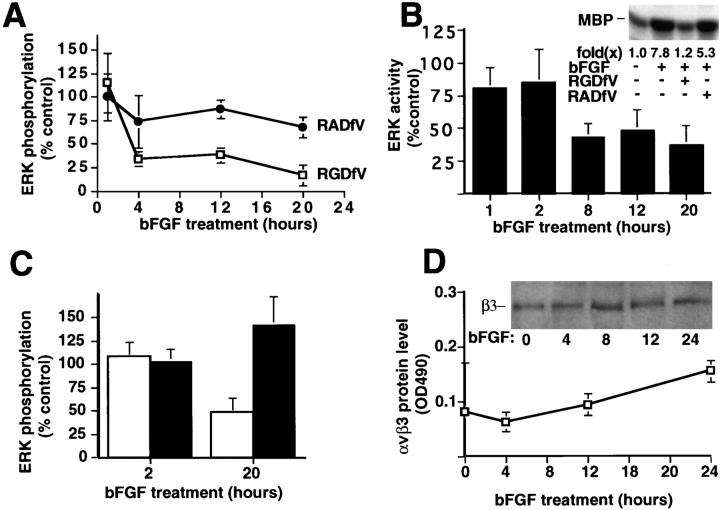
Figure 3.
The effect of αvβ3 antagonists on ERK activity during bFGF-induced angiogenesis. (A) ERK phosphorylation was measured in CAMs treated with bFGF-saturated filter disks as described in the Materials and Methods. The αvβ3 antagonist, cyclic peptide RGDfV directed to αvβ3, or a control peptide (RADfV) was added to the filter disks (100 μg/50 μl) 1 h before harvest at the indicated times, after bFGF stimulation. Each point depicts ERK phosphorylation on CAMs treated with bFGF and cyclic peptide compared with CAMs treated with bFGF alone. Data are expressed as the mean percent of control ERK phosphorylation ± SD (n = 3). (B) ERK activity was measured in bFGF-treated CAM lysates in the presence or absence of cyclic peptides (1 h) measured by an in vitro kinase assay (MBP) as described in Materials and Methods. Data are expressed as the mean percent of ERK activity of RGDfV-treated CAMs relative to CAMs treated with control peptide (RADfV). Each bar represents the mean ± SD (n = 3). (Inset) A representative kinase assay from lysates derived from CAMs treated with buffer or bFGF, for a total of 20 h, in the presence or absence of RGDfV or RADfV for 1 h before harvest as described in the Materials and Methods. (C) ERK phosphorylation was detected in bFGF-treated CAMs in the presence of either mAb CSAT (50 μg/50 μl) directed to chick β1 or LM609 directed to αvβ3. Data are expressed as the percentage of ERK phosphorylation relative to control (no antibody). Each bar is the mean ± SD (n = 3). Open bar, LM609; black bar, CSAT. (D) Expression of αvβ3 in CAM tissue after bFGF stimulation in whole tissue lysates was measured for the expression of αvβ3 at each time point indicated by ELISA. Expression of β3 protein was measured by Western blotting of whole tissue extracts (inset) using a primary antibody to the COOH terminus of β3 as described in Materials and Methods. For the ELISA assay, protein extracts from control or bFGF-treated CAM tissue were immobilized on ELISA plates and detected with an anti-αvβ3 primary antibody (LM609) and a horseradish peroxidase–conjugated anti–mouse secondary antibody. Data are expressed as the mean concentration of αvβ3 ± SD (n = 3) and represent arbitrary units of αvβ3 reactivity measured by OD 490.
Enzyme-linked Immunoadsorbent Assay for αvβ3
An ELISA was performed to measure the endogenous levels of αvβ3 in the CAM after bFGF treatment. Briefly, CAMs were treated with bFGF as described above and homogenized in PBS, and 2–4 μg of total protein as determined by BCA protein assay was coated in triplicate in each well of a 96-well plastic dish. The plates were dried at 37°C, blocked in PBS/5% BSA, incubated with anti-αvβ3 (LM609, 1 μg/ml), washed with ELISA wash buffer (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.2% Tween 20, 0.01% Thimerosal), incubated with HRP goat anti–mouse secondary (Bio-Rad Laboratories, Hercules, CA), and developed in o-phenylenediamide (Sigma Chemical Co.). Plates were read in an ELISA scanner at 492 nm. To assure that measurements were performed in the linear range, a standard curve with various amounts of CAM homogenates were analyzed in parallel. Whole tissue extracts of CAMs as prepared above for measurements of ERK phosphorylation and activity were used for immunoblotting to identify β3 protein.
bFGF-induced Angiogenesis Assay
Angiogenesis was induced with bFGF as described above with bFGF-soaked filter disks. After 20 h, 50 μM PD98059 was added to filter disk. The doses and time of addition of bFGF and PD98059 were based on previous observations (Brooks et al., 1994a ; Klemke et al., 1997). 48 h later, CAMs were removed, and the tissue underlying the filter disks was examined for the number of blood vessel branch points as previously described (Brooks et. al., 1994b; Friedlander et. al., 1995). Photographs were taken at 4× magnification. Each bar represents the mean branch points ± SD of triplicates as determined in a double blind manner. PD98059 treatment produced a significant reduction in the angiogenic index as measured by the Student's t test (P < 0.05).
Cell Migration Assays
The modified Boyden chamber (Costar Corp., Cambridge, MA) migration assays of the HUVEC were performed as previously described (Klemke et al., 1997). The serum-starved HUVECs were induced to migrate toward a gradient of bFGF (25 ng/ml) or vascular endothelial growth factor (VEGF) (25 ng/ml) placed in the bottom chamber and harvested after 2 hours. The top and bottom surface of the chamber membrane was coated with vitronectin (10 μg/ml). Cells that had migrated to bottom of the chamber were enumerated by counting random 10× fields of crystal violet–stained cells as previously described (Klemke et al., 1997).
Statistical Analysis
Statistical analysis was performed with Statworks (Cricket Software, Philadelphia, PA), a program for the Macintosh computer, as previously described by Brooks et al. (1994b).
Results
Induction of MAP Kinase in Blood Vessels during Angiogenesis
To examine the role of MAP kinase during angiogenesis, we measured the kinetics of ERK activity in 10-d-old chick CAMs that were stimulated with bFGF. Detergent lysates of these CAMs were evaluated for ERK activity by immunoprecipitation with an anti-ERK antibody followed by phosphotyrosine immunoblotting. Increased phosphorylation of ERK was detected in these tissues within 5 min after their exposure to bFGF, and this was maintained for at least 20 h (Fig. 1, top). These findings were confirmed by immunoblotting with anti-ERK antibody, which identifies activated ERK as a band shift (Fig. 1 bottom). In fact, ERK activity was directly demonstrated in these lysates by an in vitro kinase assay using myelin basic protein as a substrate. As shown in Fig. 1, within 5 min after exposure to bFGF, ERK activity was sevenfold over nonactivated control tissue, and much of this activity remained detectable for at least 20 h. A single ERK species was observed in these tissues that comigrated with human ERK 2 (data not shown) consistent with that observed in chick embryo fibroblasts (Sanghera et al., 1992). Together, these findings demonstrate that bFGF activates an immediate ERK activity, which is sustained for at least 20 h in these tissues.
Phosphorylated ERK Immunolocalized to Blood Vessels
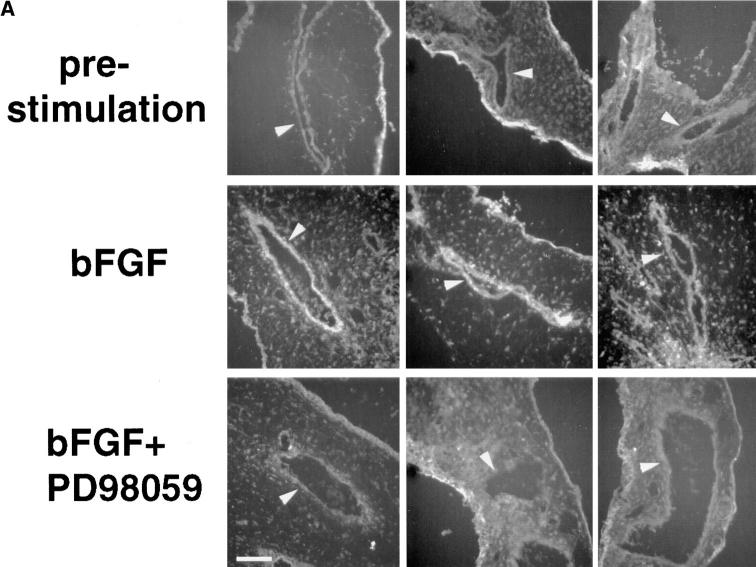
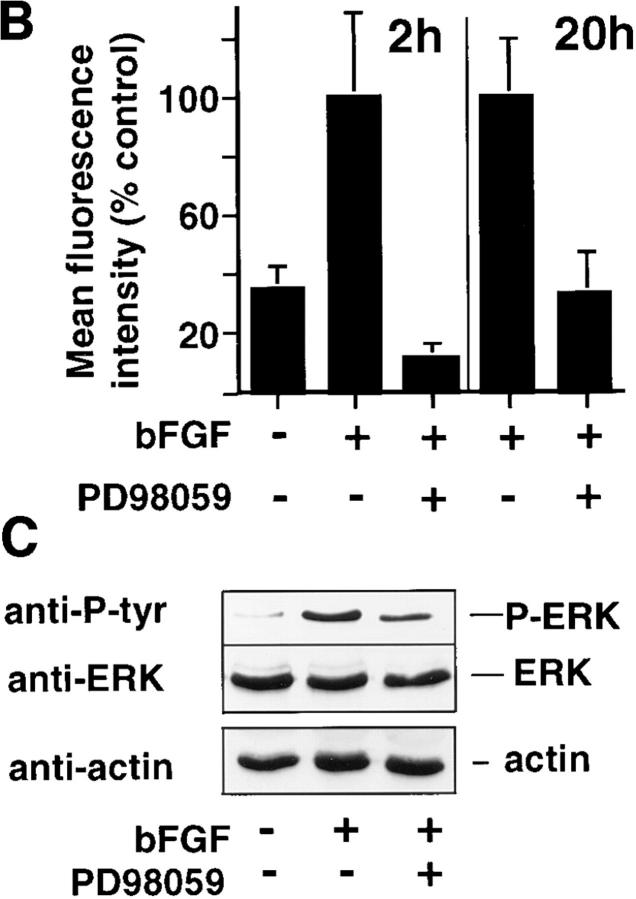
To identify the localization of active ERK in these tissues, CAMs were resected at 2 (early) or 20 h (late) after bFGF treatment, and cryostat sections were examined for immunoreactivity with an antibody directed to an ERK-derived phosphopeptide that reacts specifically with the phosphorylated (activated) form of ERK as described in the Materials and Methods. Activated ERK was localized to blood vessels (Fig. 2 A, arrowheads) in these tissues within 2 h of bFGF stimulation (Fig. 2 A, middle), compared with unstimulated CAMs (Fig. 2 A, top), and was blocked with a synthetic inhibitor (PD98059) of MEK (Fig. 2 A, bottom), which prevents phosphorylation and activation of ERK in vitro and in vivo (Alessi et al., 1995). The immunolocalization of the phosphorylated ERK after 20 h of bFGF treatment was identical to that observed in CAMs treated for 2 h with bFGF. The uniform immunostaining of the phosphorylated ERK observed in these tissues at time points between 2 and 20 h of bFGF treatment suggested that the activation of ERK in vascular cells within these vessels was a synchronized, uniform response to bFGF. Blocking MEK activity eliminated the bFGF-induced phosphorylation of ERK in these bFGF-treated blood vessels after both short (2 h) and long term (20 h) exposure to bFGF (Fig. 2), as measured by fluorescence image intensity analysis (Fig. 2 B). The ERK phosphorylation in parallel CAMs treated with bFGF for 20 h was blocked with a 1-h treatment with PD98059, as measured by an immunoprecipitation with an anti-ERK antibody followed by immunoblotting with an antiphosphotyrosine antibody. This immunoblot was stripped and reprobed with an anti-ERK antibody to demonstrate that there was not a detectable change in ERK protein levels. An immunoblot of whole CAM tissue lysates detected with an antiactin antibody suggests that changes in ERK phosphorylation were not due to a general change in protein levels since the quantity of both ERK and actin remained the same for at least 20 h after exposure to bFGF (Fig. 2 C).
Figure 2.
Localization of bFGF-induced ERK phosphorylation in the CAM. (A) Cryosections (4 μm) of bFGF-treated (2 h) or control CAMs treated without growth factors incubated in the presence or absence of the MEK inhibitor, PD98059 (50 μM), were examined for phosphorylated ERK, by indirect immunofluorescence using a primary antibody directed to the phosphorylated ERK peptide (New England Biolabs) as described in the Materials and Methods. Arrowheads indicate blood vessels. The edge staining of the tissues reflects nonspecific signal, which is routinely observed with secondary antibody alone in these cryopreserved CAM tissues. (B) Quantitation of immunofluorescence staining intensity with antiphosphorylated ERK antibody after bFGF treatment for 2 or 20 h, in the presence or absence of PD98059 by digital image analysis, as described in the Materials and Methods. (C) Phosphorylated ERK (top) and total ERK (middle) in bFGF-treated (20 h) CAM tissue lysates, in the presence or absence of PD98059, was detected by immunoprecipitation with an anti-ERK antibody followed by immunoblotting with either antiphosphotyrosine antibody to detect phosphorylated ERK (top) or anti-ERK antibody (middle) to show levels of ERK protein in the same lysate. Actin content of these CAM tissue extracts was detected by immunoblotting with an antiactin antibody (bottom). Bar, 50 μm.
Requirement of Integrin αvβ3 Ligation for Sustained ERK Activity
Since both peptide and antibody antagonists of αvβ3 block bFGF-mediated angiogenesis (Brooks et al., 1994a ,b), we examined whether αvβ3 ligation might contribute to the ERK activity induced by bFGF. CAMs were treated with bFGF (1–20 h) and either a cyclic peptide antagonist of αvβ3 (RGDfV) or a control peptide (RADfV) for 1 h before harvest. The levels of ERK phosphorylation were measured by immunoprecipitation with anti-ERK antibody followed by antiphosphotyrosine immunoblotting. Administration of RGDfV to CAMs treated with bFGF between 4 and 20 h specifically blocked this late and sustained ERK phosphorylation (Fig. 3 A), yet it had no effect on the early ERK phosphorylation measured at 1 h (Fig. 3 A). ERK activity was then measured in lysates of bFGF-treated CAMs exposed to RGDfV or RADfV using an in vitro kinase assay with MBP as a substrate (Fig. 3 B). We also tested the effects of the RGDfV and RADfV antagonists in the absence of bFGF on ERK activity in the CAM after a 1-h treatment and observed no significant difference or inhibition of ERK activity (data not shown). Consistent with the effect of RGDfV on ERK phosphorylation, this peptide specifically blocked bFGF-induced sustained ERK activity (8, 12, and 20 h; inset, 20 h) but had little effect on ERK activity measured within 1 or 2 h after bFGF stimulation (Fig. 3 B). These data suggest that αvβ3 ligation selectively influences the late/sustained ERK activity (>4 h) induced by bFGF during angiogenesis.
To evaluate the specificity of αvβ3 in the activation of ERK in these tissues, CAMs exposed to bFGF for either 2 or 20 h were treated with function-blocking monoclonal antibodies to either αvβ3 (LM609) or chick integrin β1 (CSAT; Buck et al., 1986). 2 h after exposure of cells to bFGF, neither integrin antibody impacted the ERK phosphorylation in these tissues (Fig. 3 C) as measured by immunoprecipitation of ERK followed by immunoblotting with an antiphosphotyrosine antibody. However, LM609 caused a significant decrease in the sustained ERK phosphorylation (20 h), while CSAT had no effect (Fig. 3 C). These data were supported by an in vitro kinase assay using MBP as a substrate (data not shown). Although the MEK inhibitor PD98059 blocked ERK phosphorylation and activity, other kinase inhibitors such as calphostin C, which disrupts protein kinase C, or LY294002, which blocks phosphatidylinositol-3 kinase, did not measurably impact the sustained ERK activity (data not shown). The failure of CSAT to influence MAP kinase was not due to its lack of reactivity with these tissues as this antibody readily localized to both preexisting and angiogenic blood vessels in the chick CAM (Brooks et al., 1994a ). These findings are consistent with previous studies demonstrating that both LM609 and RGDfV, but neither CSAT nor RADfV, blocked bFGF-induced angiogenesis on these CAMs when added up to 20 h after addition of bFGF (Brooks et al., 1994b ). Importantly, these αvβ3 antagonists did not influence preexisting blood vessels on these CAMs nor were they toxic to the embryo (Brooks et al., 1994a ,b, 1995; Friedlander et al., 1995). Together, these data suggest that both αvβ3 antagonists, LM609 or RGDfV, can block the late-acting and sustained ERK activity that is critical for angiogenesis.
Kinetics of αvβ3 Expression during Angiogenesis
While bFGF initiates angiogenesis, it also leads to increased expression of integrin αvβ3 on vascular cells (Cheng and Kramer, 1989; Brooks et al., 1994a ,b) based on its ability to first induce expression of the Hox D3 homeobox gene (Boudreau et al., 1997). Blood vessels from the CAMs of 10-d-old embryos expressed detectable αvβ3, which was increased fourfold after 72 h of bFGF exposure (Brooks et al., 1994a ). Thus, a simple increase in αvβ3 expression on vascular cells could account for this late/sustained ERK signal. To address this possibility, 10-d-old CAMs were exposed to bFGF for various times, and lysates prepared from these tissues were examined for αvβ3 expression. The αvβ3 level remained unchanged in these tissues for at least 12 h after bFGF treatment as determined by ELISA (Fig. 3 D). In addition, we observed no increase in the level of β3 protein in these tissues as measured by Western blot analysis (Fig 3 D, inset). That antagonists of αvβ3 blocked the level of ERK phosphorylation 4 h after addition of bFGF (Fig. 3 A), before de novo expression of αvβ3, demonstrates that preexisting levels of αvβ3 could potentiate the late-acting ERK signal in these blood vessels. Thus, the sustained level of ERK activity in these tissues was not simply the result of increased αvβ3 expression.
Requirement of ERK Activity for αvβ3-mediated Endothelial Cell Migration In Vitro
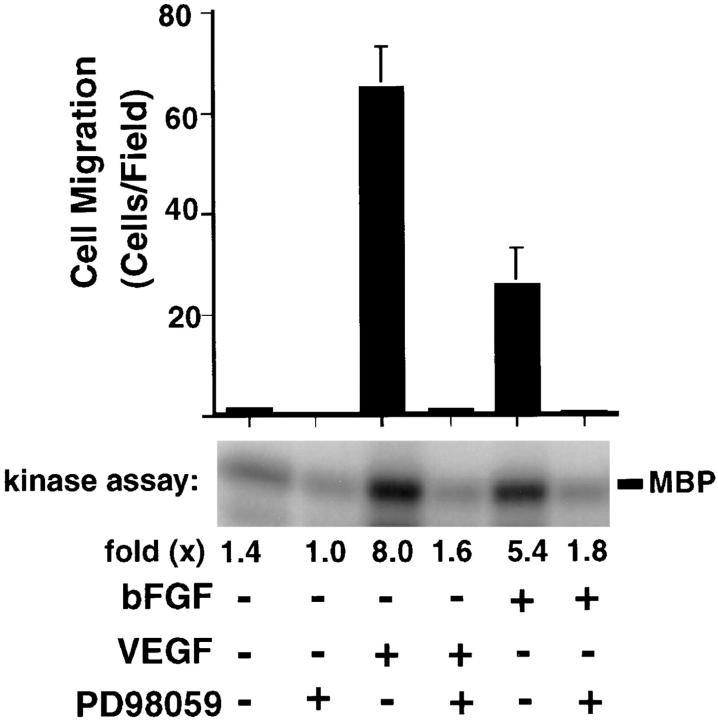
Angiogenesis is highly dependent on endothelial cell invasion and motility. Therefore, we tested the possibility that activation of MAP kinase may be involved in endothelial cell migration in response to angiogenic growth factors. Endothelial cells were serum starved, treated with either bFGF or VEGF, and then allowed to migrate on a vitronectin-coated substrate in the presence or absence of the MEK inhibitor, PD98059. As shown in Fig. 4, bFGF and VEGF stimulated endothelial cell MAP kinase activity and promoted their migration on vitronectin, and this was blocked with the MEK inhibitor even though it had no effect on the ability of these cells to attach and spread on a vitronectin substrate (data not shown). These results suggest that during angiogenesis, sustained MAP kinase activity may be required for endothelial cell migration, an event critical for the formation of new blood vessels.
Figure 4.
The role of ERK activity on endothelial cell migration. Endothelial cell migration on vitronectin in modified Boyden chambers of serum-starved HUVECs was induced with bFGF (25 ng/ml) or VEGF (25 ng/ml) in the presence or absence of 50 μM PD98059 for 2 h as described in Materials and Methods. ERK activity as detected by phosphorylation of MBP in an in vitro kinase assay was measured in parallel HUVEC cultures after treatment with bFGF, VEGF, or PD98059 for 30 min as described in the Materials and Methods. Migration data are expressed as the mean number of cells quantitated in at least three 10× microscope fields ± SD (n = 3). The kinase data are from a representative experiment.
Requirement of Sustained ERK Activity for Angiogenesis In Vivo
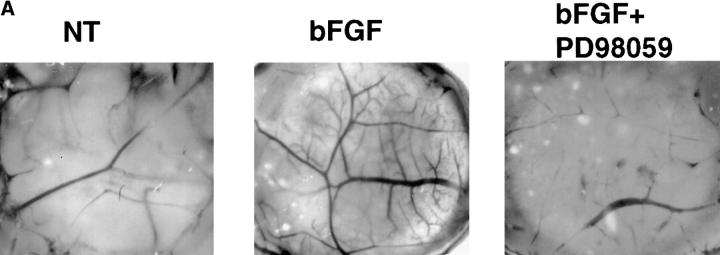
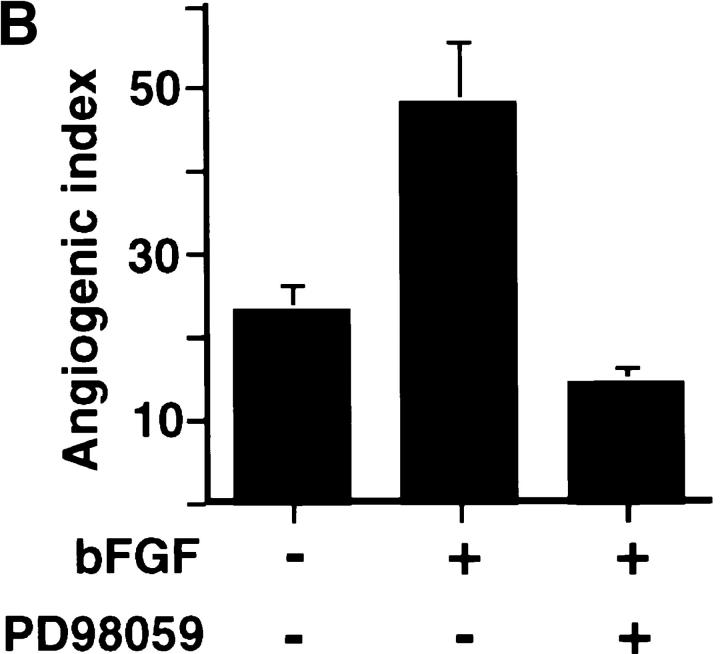
To establish whether the αvβ3-dependent sustained ERK activity in blood vessels was required for angiogenesis in vivo, we applied PD98059 to CAMs that had been pretreated with bFGF for 18 h. 48 h later, CAMs were resected and photographed. bFGF induced extensive neovascularization, and this was blocked by addition of PD98059 (Fig. 5), which inhibited the ERK phosphorylation in endothelial cells associated with these tissues (Figs. 1 and 2). While local administration of the MEK inhibitor blocked bFGF-induced angiogenesis, it had no measurable impact on preexisting blood vessels or embryo viability (data not shown). In fact, embryos treated in this manner appeared to develop normally as determined by a gross morphological examination 48 h after administration of the inhibitor (data not shown). Therefore, blocking the late acting sustained ERK signal during angiogenesis by inhibiting MEK directly or by antagonizing αvβ3 can disrupt the angiogenic cascade.
Figure 5.
The effects of PD98059 on bFGF-induced angiogenesis. (A) 10-d-old chick CAMs were exposed to filter paper disks saturated with bFGF (20 h) or buffer for 20 h and then incubated for an additional 48 h with or without PD98059 (50 μl of 50 μM). Photomicrographs were taken at 4× with a stereomicroscope and are representative of each group of treated CAMs. (B) The level of angiogenesis in bFGF-treated CAMs in the absence or presence of PD98059 was quantified by counting blood vessel branch points double blind as previously described (Friedlander et al., 1995).
Discussion
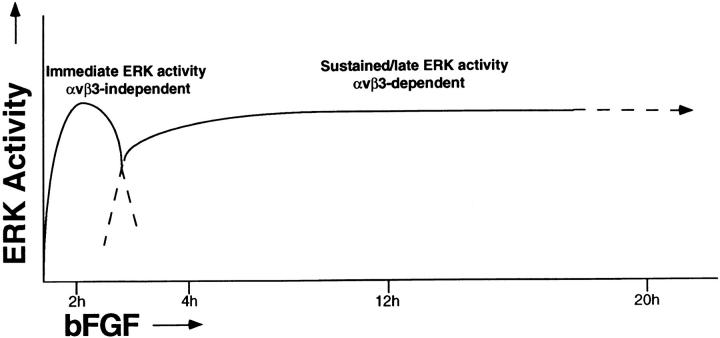
In this report, we focused on the role of ras/MAP kinase in angiogenesis since integrins such as αvβ3, which have been linked to angiogenesis (Brooks et al., 1994a ,b), are known to regulate MAP kinase activity in vitro (Chen et al., 1994; Schlaepfer et al., 1994; Miyamoto et al., 1995; Morino et al., 1995; Zhu and Assoian, 1995). Several lines of evidence suggest that two kinetically distinct MAP kinase–mediated signaling events are required for angiogenesis. Exposure of preexisting blood vessels to bFGF resulted in an immediate relatively short-term activation of MAP kinase that was resistant to integrin antagonists. This was followed by a sustained MAP kinase activity, maintained for at least 20 h, that was selectively blocked by antagonists of integrin αvβ3. Antibody or cyclic peptide antagonists of αvβ3 added during this late-acting sustained ERK signal blocked angiogenesis (Brooks et al., 1994b ; Friedlander et al., 1995). In contrast, CSAT, a function-blocking antibody to chick β1 integrins, failed to influence angiogenesis in the CAM even though it readily reacted with both preexisting and angiogenic blood vessels in these tissues (Brooks et al., 1994b ). Using an in situ marker of activated ERK (anti-phospho MAP kinase antibody), we determined that most if not all of the activated MAP kinase in these tissues was associated with bFGF-stimulated angiogenic blood vessels. The MEK inhibitor, PD98059, used to disrupt angiogenesis, also blocked the phosphorylation of MAP kinase detected in blood vessels in situ. Importantly, local administration of PD98059 or αvβ3 antagonists had no effect on blood vessels in other areas of the CAM and did not cause toxicity to the embryo. Taken together, these findings support a model in which αvβ3 ligation on the surface of angiogenic vascular cells promotes a late-acting sustained ERK signal that is critical for angiogenesis (Fig. 6).
Figure 6.
Model depicting kinetics of MAP kinase activity during angiogenesis and the distinct requirements for ligation of integrin αvβ3 for immediate versus sustained MAP kinase activity. A rapid increase in bFGF-induced MAP kinase activity that is independent of integrin αvβ3 is followed by a sustained MAP kinase activity which requires αvβ3 ligation.
Expression of integrin αvβ3 is increased on endothelial cells after exposure to bFGF in vitro (Cheng and Kramer, 1989; Senger et al., 1996; Boudreau et al., 1997) and angiogenic blood vessels in vivo (Brooks et al., 1994a ,b, 1995). In addition, integrin αvβ3 expression on chick CAM angiogenic blood vessels has been linked to the ability of bFGF to promote expression of the Hox D3 homeobox gene in these tissues (Boudreau et al., 1997). While αvβ3 was detectable on preexisting blood vessels in 10-d-old chick CAMs, αvβ3 levels were not significantly increased above this baseline level for at least 12 h after bFGF treatment. This suggests that the preexisting levels of αvβ3 are sufficient to initiate this sustained phase of MAP kinase activity in these blood vessels and that the requirement of αvβ3 ligation for the sustained MAP kinase activity in blood vessels within 4 h was independent of an increase in the total expression of αvβ3 protein.
To support the model that integrin-mediated MAP kinase activity contributes to biological events associated with angiogenesis, endothelial cells stimulated to migrate on vitronectin with bFGF or VEGF were treated with the MEK inhibitor, PD98059. This treatment blocked growth factor–induced cell migration but not αvβ3-dependent adhesion or spreading on vitronectin. Moreover, expression of a mutationally active form of MEK promotes cell migration on vitronectin in the absence of growth factors, and this is also sensitive to PD98059 (Klemke et al., 1997). These findings suggest that the sustained MAP kinase activity is dependent on αvβ3 ligation during angiogenesis, and this may contribute to endothelial cell migration or other processes critical for angiogenesis.
Previous studies have suggested that the duration of MAP kinase signaling profoundly influences the biological responses of cells (Marshall, 1995). For example, exposure of PC12 cells to epidermal growth factor induces a rapid and transient MAP kinase activity leading to cell proliferation, while nerve growth factor induces cell differentiation because of a sustained MAP kinase activation (Heasley and Johnson, 1992; Traverse et al., 1992; Nguyen et al., 1993). Therefore, it is likely that both immediate and late-acting ERK signals contribute to distinct biological events necessary for angiogenesis such as cell proliferation, migration, and differentiation (Traverse et al., 1994; Marshall, 1995). These results may clarify the role of integrin αvβ3 in angiogenesis. For example, signals potentiated by this integrin, through downstream signaling molecules such as focal adhesion kinase (FAK) (Schlaepfer et al., 1994; Chen et al., 1994; Clark and Hynes, 1996) and Shc (Buday and Downward, 1993; Obermeier et al., 1994; Stephens et al., 1994; Wary et al., 1996), cooperate with growth factor receptor signals to mediate the sustained MAP kinase activity during angiogenesis. However, αvβ3 antagonists used did not appear to influence FAK activity in these tissues. This is presumably due to other integrin-mediated adhesion events supporting endothelial cell interaction with the extracellular matrix (data not shown).
At present it is not clear how αvβ3 and growth factor receptors function together to promote a sustained signaling event. Recent studies suggest that the clustering of integrins, with growth factor receptors on the surface of cells (Ruoslahti and Engvall, 1997), appears to contribute to intracellular signal transduction events, including the activation of MAP kinases (Miyamoto et al., 1995, 1996). The kinetics of MAP kinase activation may also be regulated by MAP kinase phosphatases, which have been shown to suppress MAP kinase activity in vascular smooth muscle cells (Duff et al., 1995). In such a case, the sustained MAP kinase activity may be associated with a decrease in MAP kinase phosphatase activity which, in turn, is influenced by the ligation state of αvβ3.
We propose that during angiogenesis, ligation of αvβ3 through appropriate matrix contacts may provide the positional or molecular cues necessary for sustained ERK activity. This would allow prolonged cell survival as well as migration and differentiation of only those cells that are in the appropriate matrix microenvironment. The fact that antagonists of αvβ3 caused increased p53 activity and apoptosis of angiogenic endothelial cells (Brooks et al., 1994b ; Stromblad et al., 1996) suggests that the αvβ3-mediated MAP kinase activity may influence p53 activation and cell survival. In such a case, αvβ3-mediated sustained MAP kinase activity may suppress p53 activity long enough to ensure vascular cell survival leading to the invasion and maturation of newly sprouting blood vessels.
Acknowledgments
We thank A. Jonczyk, B. Diefenbach, and S. Goodman (Merck, KGaA, Darmstadt) for peptides (RGDfV [l-arginine, l-glycine, l-aspartic acid, d-phenylalanine, l-valine] and RADfV [l-arginine, l-glycine, l-aspartic acid, d-phenylalanine, l-valine]; J. Abraham (Scios, Mountain View, CA) for bFGF; C. Buck for CSAT antibody, K. Hahn for assistance with the cooled CCD camera imaging analysis, and C. Andrews for expert technical support.
Abbreviations used in this paper
- bFGF
basic fibroblast growth factor
- CAM
chorioallantoic membrane
- ECM
extracellular matrix
- ERK
endothelial cell MAP kinase
- HUVEC
human umbilical vein endothelial cell(s)
- MAP
mitogen-activated protein
- MEK
MAP kinase kinase
- MBP
myelin basic protein
Footnotes
B.P. Eliceiri (1F32 HL09435) and R. Klemke (1F32CA67442) were supported by NRSA postdoctoral fellowships, and D.A. Cheresh was supported by grants CA50286, CA45726, and HL54444 from the National Institutes of Health. This is manuscript 10715-IMM from The Scripps Research Institute.
Address all correpondence to D.A. Cheresh, Departments of Immunology and Vascular Biology, The Scripps Research Institute, (IMM-24), 10550 N. Torrey Pines Rd., La Jolla, CA 92037. Tel.: (619) 784-8281. Fax: (619) 784-8926. E-mail: cheresh@scripps.edu
References
- Alessi D, Cuenda A, Cohen P, Dudley D, Saltiel A. PD98059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Berrou E, Fontenay-Roupie M, Quarck R, McKenzie F, Levy-Toledano S, Tobelem G, Bryckaert M. Transforming growth factor β1 inhibits mitogen-activated protein kinase induced by basic fibroblast growth factor in smooth muscle cells. Biochem J. 1996;316:167–173. doi: 10.1042/bj3160167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton TG, Cobb MH. Identification of multiple extracellular signaling kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991;2:357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton T, Nye S, Robbins D, Ip N, Radziejewska E, Morgenbesser S, DePinho R, Panayotatos N, Cobb M, Yancopoulos G. ERKs: a family of protein serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark R AF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck C, Duggan S, Horwitz A. Integrin (the CSAT antigen): functionality requires oligomeric integrity. J Cell Biol. 1986;103:2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Castleman, K. 1989. Digital image processing. Vol. 11. Prentice Hall, NY. 667 pp.
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Cheng Y-F, Kramer RH. Human microvascular endothelial cells express integrin-related complexes that mediate adhesion to the extracellular matrix. J Cell Physiol. 1989;139:275–286. doi: 10.1002/jcp.1041390209. [DOI] [PubMed] [Google Scholar]
- Cheresh DA, Spiro RC. Biosynthetic and functional properties of Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen and von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- Clark RF, Tonnesen MG, Gailit J, Cheresh DA. Transient functional expression of αvβ3 on vascular cells during repair. Am J Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- Cobb M, Boulton T, Robbins D. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991;2:965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff JL, Monia BP, Berk BC. Mitogen-activated protein (MAP) kinase is regulated by the MAP kinase phosphatase (MKP-1) in vascular smooth muscle cells. J Biol Chem. 1995;270:7161–7166. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two distinct angiogenic pathways by distinct αv integrins. Science. 1995;270:1500–1502. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- Frisch S, Vuori K, Ruoslahti E, Chan-Hui P. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L, Johnson G. The beta PDGF receptor induces neuronal differentiation of PC12 cells. Mol Cell Biol. 1992;3:545–553. doi: 10.1091/mbc.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D, Folkman J. How does extracellular matrix control capillary morphogenesis? . Cell. 1989;58:803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Jaffe E, Hoyer L, Nachman R. Synthesis of anti-hemophilic factor antigen by cultured endothelial cells. J Clin Invest. 1973;52:2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagsburn M, D'Amore P. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–221. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, De Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr, Fazeli B, Schwartz MA. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama S, Yamada K. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto JS, Gutkind JS, Yamada K. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy C, Pawlowski J, Taylor D, Turner C, Weber H, Peluso M. Thrombin receptor activation elicits rapid protein tyrosine phosphorylation and stimulation of the raf-1/MAP kinase pathway preceding delayed mitogenesis in cultured rat aortic smooth muscle cells: evidence for an obligate autocrine mechanism promoting cell proliferation induced by G-protein–coupled receptor agonist. J Clin Invest. 1996;97:1173–1183. doi: 10.1172/JCI118531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery AMP, Reisfeld RA, Cheresh DA. Integrin αvβ3 rescues melanoma cells from apoptosis in three-dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowaki T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk1 and p42erk2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Scimeca J, Filloux C, Peraldi P, Carpentier J, van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1 and the 90 kD ribosomal S6 kinase in PC12 cells. J Biol Chem. 1993;268:9803–9810. [PubMed] [Google Scholar]
- Obermeier A, Bradshaw R, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO (Eur Mol Biol Organ) J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff M, Tangemann K, Muller B, Gurrath M, Muller G, Kessler H, Timpl R, Engel J. Selective recognition of cyclic RGD peptides of NMR defines conformation by αIIbβ3, αvβ3, and α5β1 integrins. J Biol Chem. 1994;269:20233–20238. [PubMed] [Google Scholar]
- Ruoslahti E, Engvall E. Integrins and vascular extracellular matrix assembly. J Clin Invest. 1997;99:1149–1152. doi: 10.1172/JCI119269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanghera J S, Peter M, Nigg EA, Pelech SL. Immunological characterization of avian MAP kinases: evidence for nuclear localization. Mol Biol Cell. 1992;3:775–787. doi: 10.1091/mbc.3.7.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D, Hanks D, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by Grb2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Perruzzi C, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the αvβ3 integrin, osteopontin and thrombin. Am J Path. 1996;149:293–305. [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Loeb D, Copeland T, Pawson T, Greene L, Kaplan D. Trk receptors use redundant signal transduction pathways involving SHC and PLC-γ 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21 WAF1/CIP1. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Seedorf K, Paterson H, Marshall C, Cohen P, Ullrich A. EGF receptor triggers a neuronal differentiation of PC12 cells that overexpress the EGF receptor. Curr Biol. 1994;4:694–701. doi: 10.1016/s0960-9822(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Wary K, Maineiro F, Isakoff S, Marcantonio E, Giancotti F. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis R, Greenberg M. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Zhu X, Assoian RK. Integrin-dependent activation of MAP kinase: a link to shape-dependent cell proliferation. Mol Cell Biol. 1995;6:273–282. doi: 10.1091/mbc.6.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]