Abstract
Background
We previously showed that infusion of TNF-α induces hypertension and vascular dysfunction in late pregnant but not virgin rats. In the present study we tested the hypothesis that levels of ovarian hormones to mimic pregnancy are required for TNF-α induced changes in vascular function and blood pressure in rats.
Methods
21 day release pellets containing 17β-estradiol, progesterone, or both were implanted in ovariectomized (OVX) rats. Sham OVX rats were used as controls. 12 days after implantation, TNF-α or vehicle was infused via osmotic minipumps (days 12-17). On day 18, mean arterial pressure was measured and animals were sacrificed to assess vascular function.
Results
Average estrogen and progesterone levels across all groups were 106±6 pg/ml and 88±5 ng/ml. TNF-α was 41±7 pg/ml compared to OVX rats infused with vehicle (4±1 pg/ml). The results show that TNF-α did not cause elevated mean arterial pressure in OVX rats with increased estrogen, progesterone, both. Vascular responses to the endothelium dependent and independent agonists, acetylcholine and sodium nitroprusside, were also not changed. Phenylephrine induced contraction was moderately but significantly increased at the highest concentrations (10-4 M) only in TNF-α infused rats.
Conclusion
These data suggest that increased ovarian hormones to levels observed during pregnancy are not sufficient to promote TNF-α induced increases in blood pressure or vascular dysfunction.
Keywords: Hypertension, preeclampsia, cytokine, estrogen, progesterone
Introduction
Preeclampsia is defined as new onset hypertension with proteinuria during pregnancy. Blood pressure typically increases during the third trimester and remits after delivery of the placenta. Increased arterial pressure during preeclampsia is accompanied by elevated circulating inflammatory cytokines such as TNF-α, IL-1 and IL-6 [1;2]. Previously, our laboratory demonstrated that a two-fold increase in circulating TNF-α in a normal pregnant rat is sufficient to cause hypertension and increased renal vascular resistance [3;4]. Importantly, this renal hemodynamic and blood pressure response occurs only in pregnant, but not virgin rats suggesting that placental factors or the hormonal environment of pregnancy may be required for cytokine mediated hypertension during pregnancy in the rat. Currently, the physiological mechanisms that facilitate enhanced blood pressure sensitivity to TNF-α during pregnancy are unknown. One possible mechanism may be related to the increased circulating levels of sex steroids. The role of sex steroids in promoting changes in vascular function and blood pressure regulation remains a controversial area of investigation. The physiological response to estrogenic hormones varies between animal models, vascular beds, and with concentration. While a large body of evidence points to a vascular protective and anti-hypertensive role for ovarian hormones, others suggest a potential pro-inflammatory role for both estrogens and progestans. For example, a recent report shows that 17β-estradiol facilitates TNF-α induced leukocyte adhesion to endothelial cells by increasing adhesion molecule expression [5]. Whether the increase in blood pressure caused by TNF-α in pregnant rats requires elevated estrogen and progesterone is not clear. In order to examine this, we asked whether administration of sex steroids (estrogen and/or progesterone) to mimic levels observed during pregnancy would be sufficient to promote TNF-α induced hypertension and vascular dysfunction in ovariectomized (OVX) rats.
Methods
Animals
All studies were performed in 15 week old OVX Sprague Dawley rats purchased from Harlan Inc. (Indianapolis IN). Rats were ovariectomized by Harlan Inc. at 12 weeks of age. Animals were housed in a temperature controlled room (23°C) with a 12:12 light:dark cycle. Experimental procedures were executed in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi Medical Center.
Experimental Design
Twenty one day release intradermal pellets of 17β-estradiol (0.5 mg/pellet) and/or progesterone (200 mg/pellet, Innovative Research of America) were implanted between the scapulae of OVX rats. The target level of estrogen and progesterone were based on evidence that pregnancy levels are greater than 80 pg/ml and 100 ng/ml respectively [6]. After twelve days of hormone administration, TNF-α (Biosource International, Camarillo CA) with heparin or saline with heparin (vehicle control) was infused intravenously at a rate of 50 ng/day for 5 days (day 12 to day 17) via mini-osmotic pumps (model 2001, Alzet Scientific Corporation, Palo Alto,Ca).
Measurement of arterial pressure
Mean arterial pressure was measured as previously described [3;4]. Briefly, rats were catheterized on day 17 with V-3 tubing (SCI, Lake Hayasu City, AZ) inserted into the carotid artery for blood sampling and blood pressure monitoring. The catheter was tunneled to the back of the neck and exteriorized after implantation. On day 18, rats were placed in individual restraining cages and allowed to acclimate for 1 hour. Arterial pressure was recorded with a pressure transducer (Cobe III Transducer CDX Sema, Birmingham, AL) continuously for 2 hours.
Vascular Ring Experiments
Rat carotid arteries were removed and prepared for vessel reactivity studies in organ chamber baths as previously published [7-9]. Carotid arteries were chosen in order to be consistent with our earlier work performed using conduit vessel strips [10]. Resting tension was adjusted step-wise to reach a final tension of 0.75 grams. For studies of vessel relaxation, carotid segments were pre-contracted with the thromboxane mimetic, U46619 (0.4 μg/ml). After the vessel reached a stable tension, concentration responses to acetylcholine and sodium nitroprusside (10-8 to 10-4 M) were performed to assess endothelial dependent and smooth muscle dependent relaxation, respectively. Contractile responses to phenylephrine (10-8 to 10-4 M) were also tested. For each animal, at least two vessel segments were studied with the averaged response equal to an n of 1.
Determination of serum TNF-α and Soluble ICAM levels
A rat TNF-α colorimetric sandwich ELISA (R&D Systems, Minneapolis, MN) was used for quantification of serum TNF-α levels between 12.5-800 pg/ml. This assay displayed a sensitivity level of 5 pg/ml and inter-assay variability of 10% and intra-assay of 5.1%. For measurement of soluble intercellular cell adhesion molecule as a marker of inflammation, a colorimetric sandwich ELISA (R&D Systems) was used. The assay has a sensitivity of 0.35-50 ng/ml and inter- and intra- assay variability of 10.1% and 4.8%, respectively.
Serum Estrogen and Progesterone levels
Diluted serum samples were evaluated using the Biosource International progesterone or 17β-estradiol kits. Minimum detectable levels were 0.08±0.03 ng/ml and 5±2 pg/ml for each assay, respectively. Progesterone and estrogen levels during pregnancy in the rat are typically greater than 100 ng/ml and 60 pg/ml, respectively [6].
Determination of Kidney Preproendothelin mRNA Levels
The cortex and medulla of the kidneys were separated immediately after harvesting and quickly frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the Totally RNA kit supplied by Ambion after the cortex and medulla were crushed in liquid nitrogen with a mortar and pestle. Isolation procedure was then performed as outlined in the instructions provided by the manufacturer.
Genomic DNA was digested with DNAse1 following instructions outlined by Ambion. RNA was quantified by spectrophotometry using an eppendorf BioPhotometer. cDNA was synthesized from 5 micrograms of RNA with Invitrogen’s Superscript II reverse transcriptase using the following primers: preproendothelin forward 1: CTAGGTCTAAGCGATCCTTG and preproendothelin reverse 1: TCTTTGTCTGCTTGGC, supplied by custom primers from Life technologies. Real time PCR was performed using the BioRad SYBR Green supermix and iCycler using a nested forward primer; preproendothelin forward2: CTAGGTCTAAGCGATCCTTG and the reverse primer outlined above. Invitrogen’s RT-PCR primer control kit was used to amplify β-actin transcripts as control. Levels of mRNA expression were calculated using the mathematical formulas for delta/delta CT recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997). Statistical analysis of real time PCR results was done using the mean normalized cycle threshold (delta/delta CT) values and standard deviations analyzed by One-Way ANOVA and Tukey-Kramer multiple comparison test.
Statistical Analysis
Blood pressure data was analyzed using a One Way ANOVA with Student Newman Keuls Post Hoc test. Concentration dependent relaxation and contraction was analyzed using a Repeated Measures ANOVA with all pair wise comparison and a Student Newman Keuls Post Hoc test. Data were considered statistical different at p values < 0.05.
Results
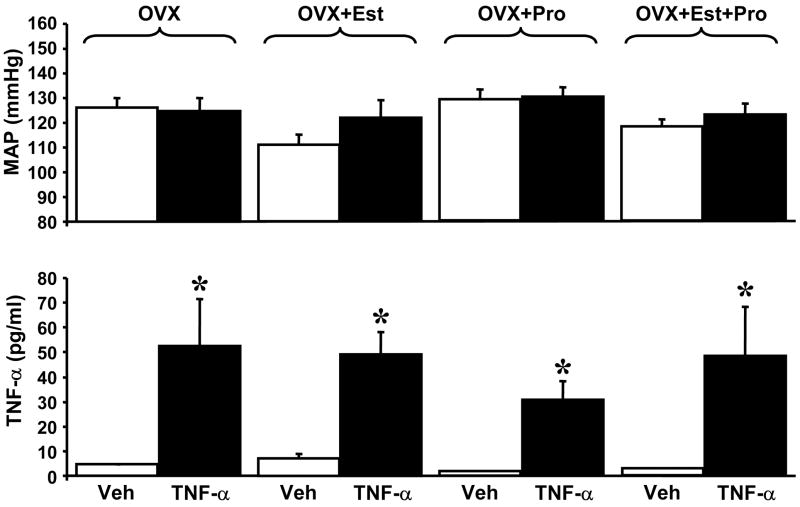
We have previously reported that infusion of TNF-α in late pregnant rats causes an increase in blood pressure in pregnant but not virgin rats [3;4;11]. The results showed a 27 mmHg increase in blood pressure caused by a two-fold increase in serum TNF-α levels [4]. In the present study, OVX rats were subjected to a 5 day infusion of TNF-α that increased serum levels of TNF-α from 4.5 ± 0.3 pg/ml to 52.5 ± 18.5 pg/ml. The increased TNF-α did not result in elevated MAP (126± 4 vs. 125± 5 mmHg) (Figure 1). Serum levels of estrogen in OVX and OVX rats with TNF-α were 62±12 and 49±16 pg/ml, respectively. Serum progesterone levels were 37±6 and 41±6 ng/ml, in OVX rats or OVX rats with TNF-α.
Figure 1.
TNF-α infusion significantly increased serum TNF-α in ovariectomized (OVX), ovariectomized with estrogen (OVX+Est), ovariectomized with progesterone (OVX+Pro), and ovariectomized with both (OVX+Pro+Est), but did not alter blood pressure.
We next asked whether the presence of increased estrogen is necessary to mediate TNF-α induced hypertension. 17β–estradiol was administered to OVX rats for a period of seventeen days to mimic levels observed during pregnancy in the rat. TNF-α or vehicle was administered for five days beginning on day 12. Plasma estrogen levels were significantly elevated in 17β-estradiol OVX rats treated with either vehicle (89 ± 16 pg/ml) or TNF-α (146 ± 10 pg/ml). In rats administered 17β-estradiol, progesterone levels were not different from control (20±7 and 56±10 ng/ml compared to OVX and OVX + TNF-α, respectively) OVX rats infused with TNF-α and treated with 17β-estradiol had elevated serum TNF-α levels (49 ± 9 pg/ml compared to vehicle 7.2 ± 2 pg/ml) but this did not significantly increase mean arterial pressure (122 ± 7 mmHg vs. 111 ± 4 mmHg) (Figure 1).
In a similar study OVX rats were administered progesterone over the seventeen days at levels to mimic pregnancy. Progesterone administration increased serum levels in OVX rats (75±9 ng/ml) and in OVX rats infused with TNF-α (99±11 ng/ml). 17β-estradiol levels were not different from OVX (44±8 pg/ml) or OVX with TNF-α (53±10 pg/ml). TNF-α infused animals had higher serum TNF-α levels compared to vehicle treated controls (30.8 ± 7.1 pg/ml vs. 1.8 ± 0.2 pg/ml); however, MAP was not significantly altered (129 ± 4 mmHg OVX + progesterone vs. 130 ± 4 mmHg OVX + progesterone + TNF-α) (Figure 1).
Finally, we tested whether the presence of both progesterone and estrogen at levels to mimic pregnancy were required to cause hypertension during the infusion of TNF-α. OVX rats administered both 17β-estradiol and progesterone had significantly increased serum levels (99±8 pg/ml and 78±4 ng/ml, respectively). Similarly, TNF-α infused OVX rats receiving both 17β-estradiol and progesterone had significantly increased serum levels (114±11 pg/ml and 108±13 ng/ml, respectively). Although serum TNF-α levels were increased following infusion (3.0±0.04 pg/ml vs. 48.5 ± 19.4 pg/ml), again blood pressure was not increased by TNF-α (118 ± 3 mmHg vs.123 ± 4 mmHg) (Figure 1).
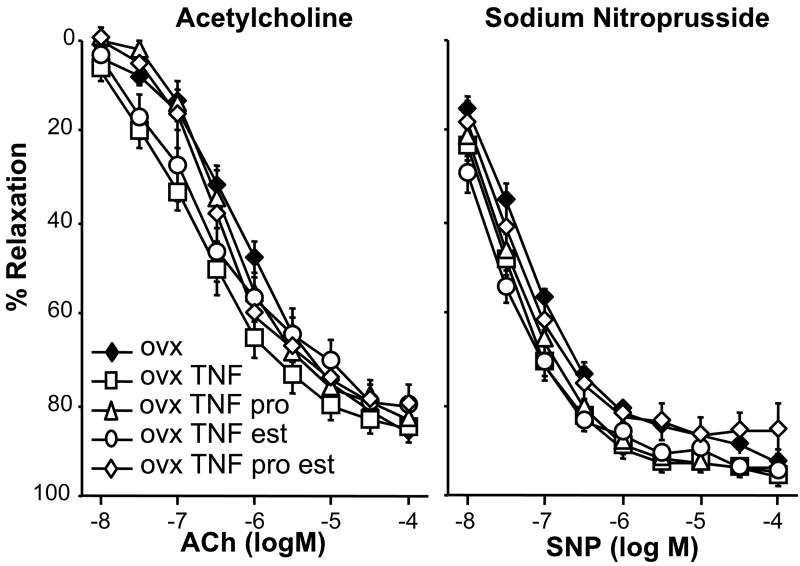
In addition to examining the interaction of sex steroids with TNF-α on blood pressure, we also determined whether there were any changes in vascular function. Increased TNF-α has been shown in numerous studies to alter endothelial dependent relaxation as well as vascular contraction. Concentration dependent relaxation to acetylcholine was not different between any of the experimental groups (Figure 2) suggesting that the five day infusion of TNF-α in OVX rats did not cause endothelial dysfunction. Similarly, relaxation responses to sodium nitroprusside were not impaired with infusion of TNF-α and sex steroid administration did not affect this response (Figure 2).
Figure 2.
(A) Endothelium dependent relaxation is not impaired by TNF-α infusion in OVX rats, or OVX rats treated with estrogen and/or progesterone. Carotid arteries were pre-contracted with U46619 and endothelium dependent relaxation was tested with increasing concentrations of acetylcholine. (B) Nitric oxide dependent relaxation is not impaired by TNF-α infusion in OVX rats, or OVX rats treated with estrogen and/or progesterone. Carotid arteries were pre-contracted with U46619 and smooth muscle dependent relaxation was tested with increasing concentrations of sodium nitroprusside.
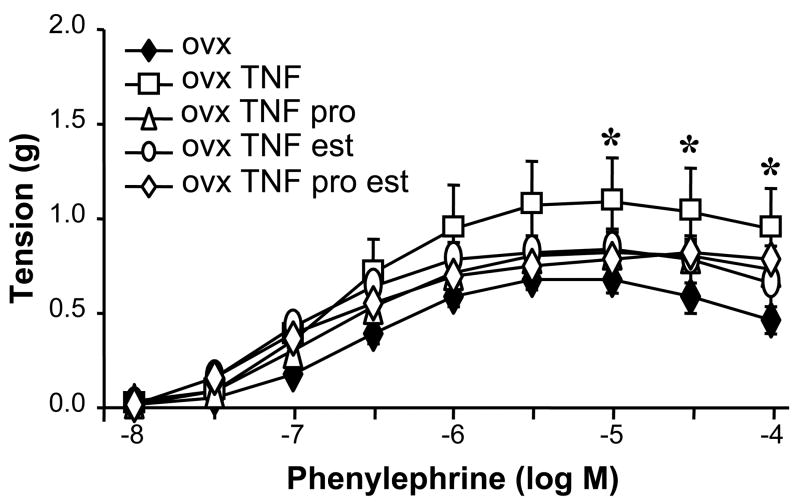
Because our earlier data showed that TNF-α infusion increased vascular contraction in pregnant but not virgin rats, we examined whether vascular contractility was altered by TNF-α or sex steroids. Our data show that there is a modest but significant increase in the contractile response to maximal concentrations of phenylephrine in the OVX rats infused with TNF-α when compared to OVX rats (Figure 3). No other differences in vascular function were detected among the other experimental groups.
Figure 3.
Carotid artery contractile responses to phenylephrine were significantly increased in OVX rats infused with TNF-α. The response was not enhanced in the presence of estrogen and/or progesterone. * p<0.05 vs. OVX.
Although there was no change in endothelial function after the five day infusion of TNF-α, it is possible that TNF-α infusion could increase cell adhesion molecule expression as an early marker of endothelial changes that could progress to vascular damage. Therefore, we tested whether soluble ICAM was increased in control and TNF-α infused rats and whether the presence of either estrogen or progesterone would alter levels of soluble ICAM. OVX rats infused with TNF-α had significantly increased soluble ICAM levels compared to control OVX rats (0.9±0.2 ng/ml, n=26 vs. 10391±536 ng/ml, n=9). Treatment with TNF-α and progesterone (10144±1549 ng/ml, n=4), estrogen (9474±1229 ng/ml, n=6), or both (11394±1267 ng/ml, n=8) did not significantly alter levels of soluble ICAM in this study when compared to OVX rats infused with TNF-α alone.
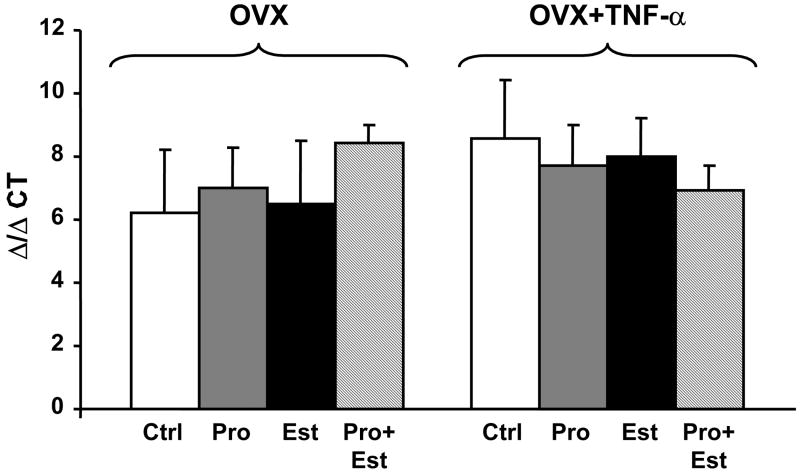
In a previous study we demonstrated that TNF-α induced hypertension in pregnant rats is due to a TNF-α mediated increase in endothelin-1. Therefore, we measured the expression of renal preproendothelin using quantitative real time PCR. The results show that there is no difference in preproendothelin expression among any of the experimental groups (Figure 4).
Figure 4.
Renal expression of preproendothelin is not different in OVX rats (+/- TNF-α) treated with estrogen, progesterone, or both.
Discussion
Preeclampsia affects 5-8% of all pregnancies in the United States and is the most common cause for maternal and fetal mortality. While it is generally accepted that preeclampsia results from improper remodeling of the uterine spiral arteries leading to a reduction in uterine perfusion pressure, the mechanisms that account for this are not well understood. As a result, to date, the only effective treatment for preeclampsia is delivery of the fetus after which hypertension and proteinuria remit.
We have recently begun to uncover some of the pathophysiological mechanisms that are involved in preeclampsia using the reduced uterine perfusion pressure model in the rat (RUPP). In addition to elucidating a role for the renin angiotensin system [12] and endothelin [11;13] in pregnancy induced hypertension, we and others have recently demonstrated an important role for inflammatory cytokines [1-4;14-18]. During preeclampsia, the placenta has increased immune infiltrates [19] and levels of circulating TNF-α are elevated greater than two-fold. Similar to humans, the RUPP model also has a two-fold increase in TNF-α [1;20-22] suggesting it as a putative mechanism promoting hypertension during pregnancy. Furthermore, we have shown that if TNF-α is infused at a rate to mimic the increases reported during preeclampsia, pregnant rats, but not virgins, become hypertensive and have increased vascular contractility [3;4].
Based on these earlier studies, we asked whether ovarian sex steroids are required to cause hypertension in TNF-α infused female rats. The major new finding of this study is that ovarian hormones (17β-estradiol and/or progesterone) at levels to mimic pregnancy are not sufficient in combination with TNF-α to cause hypertension. These data do not completely exclude the possibility that the combination of ovarian hormones and TNF-α induce hypertension, but rather suggest at minimum, that some other factor, perhaps placental in origin, is required to cause the hypertension. This may explain why in pregnant rats, infusion of TNF-α results in increased pressure while infusion of TNF-α in the present study does not. One possible candidate promoting TNF-α induced hypertension in pregnant rats is endothelin. Previously published work from our laboratory shows that TNF-α induced hypertension in pregnant rats is prevented by blockade of the endothelin system [11;13]. However, in the present study there was no difference in renal expression of preproendothelin despite increased serum TNF-α concentrations. These data further support the likelihood that factors in addition to TNF-α and sex steroids are required to cause hypertension during pregnancy.
In addition to blood pressure, we examined vascular responsiveness in TNF-α infused OVX rats. Our data shows no change in relaxation responses to acetylcholine or sodium nitroprusside and only a moderate increase in the contractile response to phenylephrine. The latter finding varies slightly from our earlier report showing that only pregnant rats infused with TNF-α have increased contractile responses [23]. One possible explanation for this modest discrepancy is that the circulating levels of TNF-α achieved in the present study are higher than the earlier experiments (33.9±4.6 pg/ml vs. 13.5±1.8 pg/ml). However, we believe this is unlikely since more recent experiments from our laboratory demonstrated that serum TNF-α levels as high as 73.6±11 pg/ml still caused hypertension in pregnant rats [24]. In all of these studies, the levels of TNF-α achieved are substantially lower than what is expected for an acute inflammatory reaction [25-27]. Perhaps a more likely possibility is that the experimental set up to examine vascular function between experiments differs slightly. For example, the current study examined carotid vascular rings instead of aortic strips and tested a higher maximal concentration of phenylephrine (10-4 M versus 10-5 M). This increased phenylephrine mediated contraction was only evident at the highest concentrations. Although we recognize that conduit vessels may not play a major role in blood pressure regulation, it should be noted that there is a direct correlation between carotid artery hemodynamics and blood pressure in humans [28].
The role of ovarian hormones in the development of cardiovascular disease has been somewhat controversial. A large majority of studies show that estrogens have vascular protective effects and contribute to lower blood pressures in pre-menopausal females. However, there is accumulating evidence to suggest that estrogens also have pro-inflammatory properties. For example, increased serum levels of the acute phase reactant C-reactive protein have been reported by numerous studies in women taking estrogen [29]. Proinflammatory effects of ovarian hormones are also supported by in vitro studies [5]. Experiments in human umbilical vein endothelial cells show that estrogen and progesterone promote increased leukocyte binding to TNF stimulated cells by increasing the expression of cell adhesion molecules including E-selectin, intercellular adhesion molecule type 1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1). Our results show that TNF-α increases serum levels of soluble ICAM; however, serum levels were not affected by estrogen and/or progesterone.
The current study has some potential limitations. For example, in order to be consistent with our earlier work, pressure was measured on the final day of the protocol rather than monitored temporally. Therefore, the possibility for undetected changes in pressure over the course of the study exists. However, this is unlikely since both TNF-α and sex steroid levels were increased at the time of sacrifice when pressure was measured. A second limitation is the variability in estrogen and progesterone levels achieved in the OVX rats. The reason for the difficulty in consistently regulating these levels was not apparent; however, hormone levels may have been affected by the infusion of TNF-α. Despite this difficulty, it is important to note that the levels of estrogen and progesterone achieved are consistent with those reported during pregnancy in the rat [6]. Therefore this experimental design adequately tests the hypothesis.
In conclusion, the data from this study suggest that sex steroid levels mimicking levels observed during pregnancy, by themselves, are not sufficient to play a role in TNF-α induced hypertension. Therefore it is likely that some other factor or factors, possibly produced by the placenta during pregnancy, is required to cause increases in blood pressure or is necessary to interact with sex steroids to cause hypertension. Future experiments will be important to determine these factors.
Acknowledgments
We would like to gratefully acknowledge Kathy Cockrell for her patience and technical expertise. This work was supported in part by an individual National Research Service Award (HL38499, B.D.L. and HL51971 D.L.C.), an American Heart Association Scientist Development Award (0630089N, M.J.R.), and the National Institutes of Health (HL076145, PO078147, J.P.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 2.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Alexander BT, Cockrell KL, Massey MB, Bennett WA, Granger JP. Tumor necrosis factor-alpha-induced hypertension in pregnant rats results in decreased renal neuronal nitric oxide synthase expression. Am J Hypertens. 2002;15:170–5. doi: 10.1016/s0895-7061(01)02255-5. [DOI] [PubMed] [Google Scholar]
- 4.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–5. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 5.Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994;93:17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges RS. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–40. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 7.Ryan MJ, Didion SP, Davis DR, Faraci FM, Sigmund CD. Endothelial dysfunction and blood pressure variability in selected inbred mouse strains. Arterioscler Thromb Vasc Biol. 2002;22:42–8. doi: 10.1161/hq0102.101098. [DOI] [PubMed] [Google Scholar]
- 8.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. PPAR(gamma) agonist rosiglitazone improves vascular function and lowers blood pressure in hypertensive transgenic mice. Hypertension. 2004;43:661–6. doi: 10.1161/01.HYP.0000116303.71408.c2. [DOI] [PubMed] [Google Scholar]
- 9.Ryan MJ, Didion SP, Mathur S, Faraci FM, Sigmund CD. Angiotensin II-induced vascular dysfunction is mediated by the AT1A receptor in mice. Hypertension. 2004;43:1074–9. doi: 10.1161/01.HYP.0000123074.89717.3d. [DOI] [PubMed] [Google Scholar]
- 10.Giardina JB, Green GM, Cockrell KL, Granger JP, Khalil RA. TNF-alpha enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R130–R143. doi: 10.1152/ajpregu.00704.2001. [DOI] [PubMed] [Google Scholar]
- 11.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–6. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 12.Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38:742–5. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- 13.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–8. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 14.Beckmann I, Efraim SB, Vervoort M, Visser W, Wallenburg HC. Tumor necrosis factor-alpha in whole blood cultures of preeclamptic patients and healthy pregnant and nonpregnant women. Hypertens Pregnancy. 2004;23:319–29. doi: 10.1081/PRG-200030334. [DOI] [PubMed] [Google Scholar]
- 15.Muzammil S, Singhal U, Gulati R, Bano I. Serum tumor necrosis factor-alpha in pre eclampsia. Indian J Physiol Pharmacol. 2005;49:236–40. [PubMed] [Google Scholar]
- 16.Schipper EJ, Bolte AC, Schalkwijk CG, Van Geijn HP, Dekker GA. TNF-receptor levels in preeclampsia--results of a longitudinal study in high-risk women. J Matern Fetal Neonatal Med. 2005;18:283–7. doi: 10.1080/14767050500246466. [DOI] [PubMed] [Google Scholar]
- 17.Gulati R. Raised serum TNF-alpha, blood sugar and uric acid in preeclampsia in third trimester of pregnancy. JNMA J Nepal Med Assoc. 2005;44:36–8. [PubMed] [Google Scholar]
- 18.Dong M, He J, Wang Z, Xie X, Wang H. Placental imbalance of Th1- and Th2-type cytokines in preeclampsia. Acta Obstet Gynecol Scand. 2005;84:788–93. doi: 10.1111/j.0001-6349.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 19.De WF, Brosens I, Robertson WB. Ultrastructure of uteroplacental arteries. Contrib Gynecol Obstet. 1982;9:86–99. [PubMed] [Google Scholar]
- 20.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–12. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 21.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–7. [PubMed] [Google Scholar]
- 22.Visser W, Beckmann I, Bremer HA, Lim HL, Wallenburg HC. Bioactive tumour necrosis factor alpha in pre-eclamptic patients with and without the HELLP syndrome. Br J Obstet Gynaecol. 1994;101:1081–2. doi: 10.1111/j.1471-0528.1994.tb13587.x. [DOI] [PubMed] [Google Scholar]
- 23.Davis JR, Giardina JB, Green GM, Alexander BT, Granger JP, Khalil RA. Reduced endothelial NO-cGMP vascular relaxation pathway during TNF-alpha-induced hypertension in pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R390–R399. doi: 10.1152/ajpregu.00270.2001. [DOI] [PubMed] [Google Scholar]
- 24.LaMarca BB, Speed J, Fournier L, Cockrell K, Chandler D, Granger JP. The role of angiotensin II type I receptor activation in mediating TNF alpha-induced hypertension in the pregnant rat. FASEB J. 2007;21(5):A592. [Google Scholar]
- 25.Adanin S, Yalovetskiy IV, Nardulli BA, Sam AD, Jonjev ZS, Law WR. Inhibiting adenosine deaminase modulates the systemic inflammatory response syndrome in endotoxemia and sepsis. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1324–R1332. doi: 10.1152/ajpregu.00373.2001. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Zolty E, Falk S, Basava V, Reznikov L, Schrier R. Pentoxifylline protects against endotoxin-induced acute renal failure in mice. Am J Physiol Renal Physiol. 2006;291:F1090–F1095. doi: 10.1152/ajprenal.00517.2005. [DOI] [PubMed] [Google Scholar]
- 27.Peschon JJ, Torrance DS, Stocking KL, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–52. [PubMed] [Google Scholar]
- 28.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–22. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 29.Stork S, van der Schouw YT, Grobbee DE, Bots ML. Estrogen, inflammation and cardiovascular risk in women: a critical appraisal. Trends Endocrinol Metab. 2004;15:66–72. doi: 10.1016/j.tem.2004.01.005. [DOI] [PubMed] [Google Scholar]