Summary
Quorum sensing (QS) is the process through which bacteria communicate utilizing small diffusible molecules termed autoinducers. It has been demonstrated that QS controls a plethora of microbial processes including the expression of virulence factors. Here, we report an immunopharmacotherapeutic approach for the attenuation of QS in the Gram-positive human pathogen Staphylococcus aureus. An anti-autoinducer monoclonal antibody, AP4-24 H11, was elicited against a rationally-designed hapten, and efficiently inhibited QS in vitro through the sequestration of the autoinducing peptide (AIP)-4 produced by S. aureus RN4850. Importantly, AP4-24H11 suppressed S. aureus pathogenicity in an abscess formation mouse model in vivo and provided complete protection against a lethal S. aureus challenge. These findings provide a strong foundation for further investigations of using immunopharmacotherapy for the treatment of bacterial infections in which QS controls the expression of virulence factors.
The ability of micro-organisms to coordinate their gene expression in a population density-dependent manner has been coined “quorum sensing” (QS) [1]. This chemical exchange of information among single-cell organisms is mediated by secreted signaling molecules termed autoinducers (AI) [2]. Bacterial autoinducers can be classified into three major chemical groups: i) N-acyl homoserine lactones (AHLs) that have been shown to be produced by over 70 species of Gram-negative bacteria [3, 4], ii) oligopeptides, which are generally employed by Gram-positive bacteria [5, 6], and iii) the ribose-like S-4,5-dihydroxy-2,3-pentanedione (DPD)/autoinducer-2 (AI-2), which is utilized by both Gram-negative and -positive bacteria and, thus, can be regarded as an interspecies QS signaling molecule [7, 8].
Important biological and clinical aspects of QS include the regulation of bacterial virulence factors [9, 10], hence inhibition of QS signaling could provide a promising new strategy for the attenuation of bacterial infections [11-13]. Indeed, small molecule antagonists using AI analogs have been examined in a number of QS circuits as a means of signaling interference [14-18]. Alternatively, our laboratory pioneered an antibody-based strategy to inhibit AHL-mediated QS in Gram-negative bacteria [19]. Notably, following our initial report, an active immunization model in mice using an AHL-based vaccine was disclosed [20]. Ramifications arising from these studies have provided us further impetus to evaluate immunopharmacotherapeutic approaches targeting other bacterial QS systems.
Staphylococcus aureus is the most common cause of hospital-acquired infections [21] including various diseases raging from skin infections and food poisoning to life-threatening nosocomial infections. Increasing resistance of S. aureus isolates to glycopeptide antibiotics, most prominently vancomycin, is a major concern in today’s intensive care units, therefore, an alternative strategy to combat this pathogen is urgently required. Accessory gene regulator (agr) is the best-characterized QS circuit in S. aureus. The agr system utilizes cyclic oligopeptides, termed autoinducing peptide (AIP), and these contribute to bacterial pathogenesis by orchestrating the temporal cell density-dependent expression of virulence genes [22]. Genes regulated by agr encode cell surface proteins such as protein A, coagulase, fibronectin-binding proteins; secreted proteins including proteases, hemolysins, toxic shock syndrome toxin 1 (TSST-1), and enterotoxin B.
In addition, the agr QS system has also been linked to resistance with glycopeptide antibiotics in S. aureus [23]. Notably, Novick and co-workers have demonstrated that transient inactivation of the agr QS circuit might indeed be sufficient to prevent the deleterious effects of certain S. aureus infections [24]. Thus far, four different AIPs, with varying degrees of sequence similarities have been identified as agr QS molecules (Fig. 1) [25]. As a starting point for antibody-based interference with AIP-mediated QS, we focused on the AIP-4 QS system and its cognate strains RN4850 and NRS168 [16].
Figure 1. Structures of the AIPs used by S. aureus.
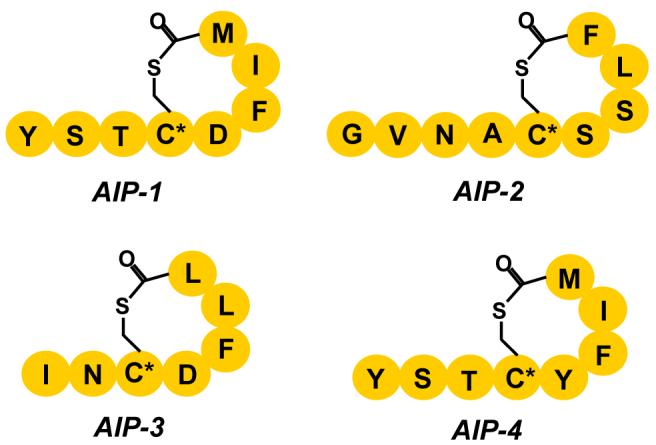
The oligopeptides are cyclized post-translationally via a thioester linkage between the thiol moiety of the conserved (*)Cys and the carboxyl group of the C-terminal residue.
Results and Discussion
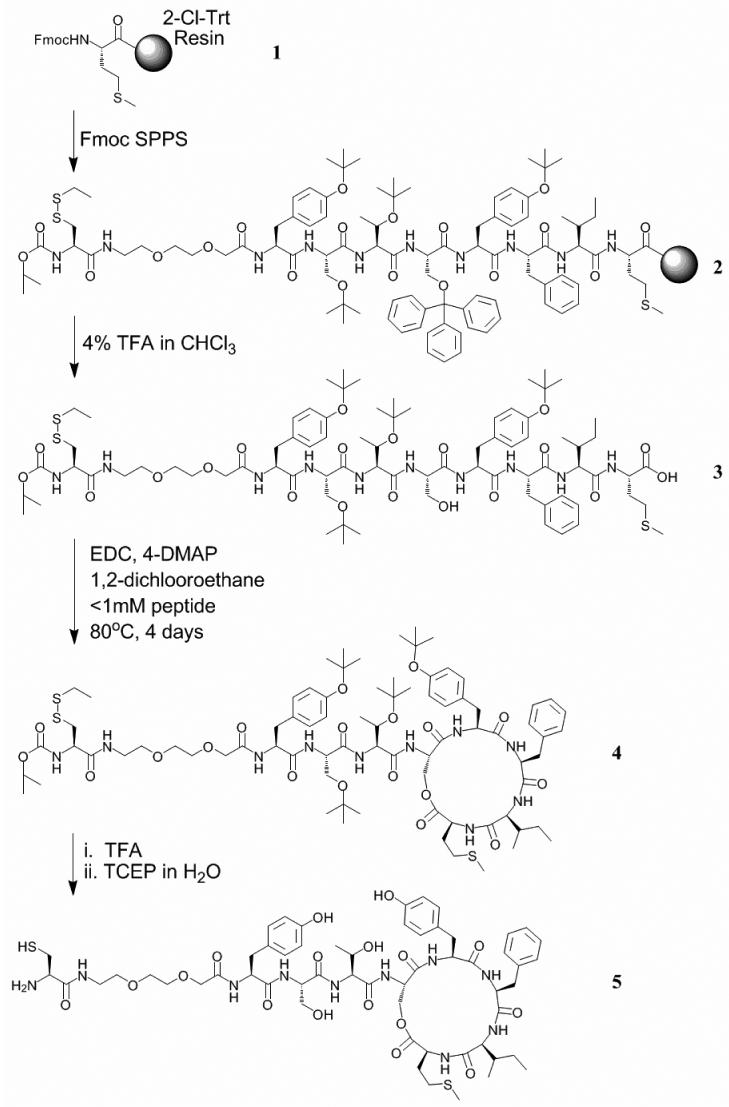
Design and Synthesis of AIP-4 Hapten
Based on the reported structural information of AIP-4 [26], we designed and synthesized the hapten AP4-5 to elicit an anti-AIP-4 antibody immune response in mice (Fig. 2). Our reasoning for the chemical switch from the native thiolactone to a lactone-containing hapten was based on a lactones greater aminolytic stability [27]. This strategy ensured that the hapten conjugates remained structurally intact during the immunization process and subsequent immune response; thus, avoiding the generation of degradation products with unknown chemical and biological properties as previously uncovered for other QS molecules by our laboratory [28]. Furthermore, this substitution was also intended to prevent a possible intramolecular thiol exchange between the conserved thiolactone and the pendant cysteine thiol. Therefore, Fmoc-Serine(Trt)-OH was incorporated at position 4 in place of the native cysteine residue.
Figure 2. Synthesis of the AP4 hapten 5.
The linear peptide was synthesized on 2-chlorotrityl resin preloaded with Fmoc-Methionine 1 using standard Fmoc chemistry employing DIC/HOBt as coupling reagents. The N-terminal pendant cysteine was incorporated for conjugation to a carrier protein and the short flexible linker was added between the hapten and the carrier protein as spacer. The protected linear peptide was released from the resin using 4% trifluoroacetic acid in chloroform, which also selectively removed the trityl protection group from the serine. Intramolecular lactonization under dilute conditions was performed using EDC/4-DMAP, and subsequent side chain deprotections afforded the AP4 hapten 5.
(For full details, see Experimental Procedures).
The hapten 5 was conjugated to the carrier proteins keyhole limpet hemocyanin (KLH) and bovine serum albumin (BSA) via a bifunctional linker (see Supplemental Fig. 1). Balb/c mice were immunized with the KLH conjugate using standard protocols [19]. Overall, the immunizations resulted in moderate titers (1600 - 3200), and based on ELISA analysis, 20 monoclonal antibodies (mAbs) were prepared. The affinities of the AP4-mAbs were determined against all four natural AIPs using competition ELISA methodology (see Supplemental Table 1). One of the mAbs, namely AP4-24H11, possessed strong binding affinity (Kd AIP-4 ≈ 90 nM) and high specificity to AIP-4 while displaying little cross reactivity for the other AIPs (Kd AIP-1 ≈ 5 μM, Kd AIP-2 = >25 μM, Kd AIP-3 = >25 μM). The ability of AP4-24H11 to discriminate between AIP1 and AIP4 is noteworthy as these two oligopeptides differ only at position 5 with an aspartic acid residue in AIP-1, and a tyrosine moiety in AIP-4. Thus, AP4-24H11 was selected for further biological evaluation.
AP4-24H11 alters expression of the virulent factors in S. aureus
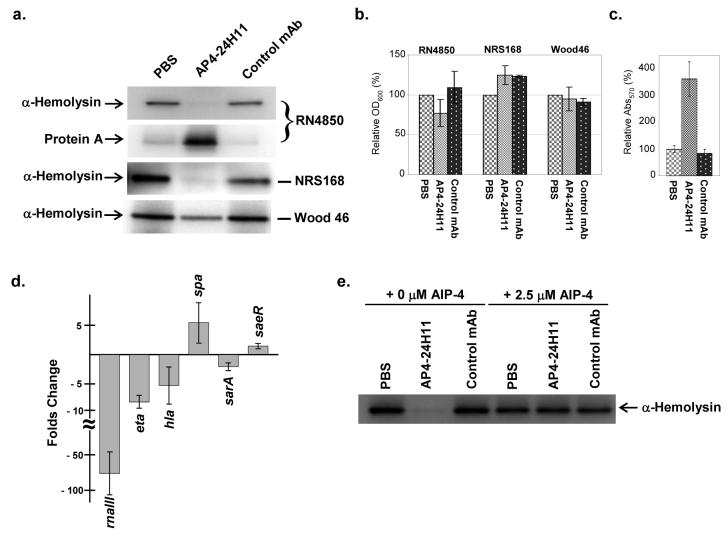
α-Hemolysin and protein A are two major virulence factors in S. aureus, and expression of these proteins is tightly regulated by S. aureus signaling networks including the AIP-based agr QS system. The agr QS system positively regulates expression of α-hemolysin while protein A production is down-regulated by QS signaling. In order to test our rationale that anti-AIP antibodies are able to interfere with QS signaling in S. aureus, we examined whether the anti-AIP-4 mAb AP4-24H11 could modulate the expression of α-hemolysin and protein A in agr group IV strains, RN4850 and NRS168. First, we observed that AP4-24H11 affects the expression and/or secretion of S. aureus exoproteins, some of which might also be regulated by the agr QS circuits (see Supplemental Fig. 2a). As seen in Figure 3a, mAb AP4-24H11 can successfully reduce the α-hemolysin expression in S. aureus, furthermore, no hemolytic activity was observed on blood agar plates with the AP4-24H11 treated supernatant (Supplemental Fig. 2b). In contrast, protein A expression was significantly increased by mAb AP4-24H11 in RN4850, which is also consistent with agr QS inhibition.
Figure 3. Inhibition of quorum sensing signaling in S. aureus by AP4-24H11.
(a) Western blot analyses of α-hemolysin and Protein A expression in S. aureus (RN4850 and Wood 46). S. aureus culture supernatants were prepared as described in Experimental Procedures. (b) Relative OD600 (%) of RN4850, NRS168 and Wood 46 after 20-24 h incubation in the presence/absence of AP4-24H11. (c) Analysis of static biofilm formation in RN4850. (d) Real-Time PCR analysis. The amounts of the selected mRNAs were measured in RN4850 grown in the presence or absence of AP4-24H11. Relative quantification was performed using gyrA as a calibrator. At least two independent experiments were carried out for each experiment in duplicate. Actual numbers of fold-change; rnaIII (- 77±48), eta (- 8.1 ±1), hla (- 5.2±3.1), spa (+ 5.7±3.6), sarA (- 2.1±0.6) and saeR (+ 1.4±0.4). (e) Suppression of AP4-24H11-mediated QS inhibition in S. aureus by AIP-4. AP4-24H11 (≈ 1.3 μM) was incubated with the native AIP-4 (2.5 μM) in CYPG medium for 20 min at room temperature. Overnight cultured S. aureus cells were diluted into the above medium (OD600 ≈ 0.03) and grown for 20 to 24 h at 37 °C under the static condition. The supernatants were prepared and analyzed. (For full details, see Experimental Procedures)
The only structural difference between AIP-1 and AIP-4 is position 5, and our data suggest that AP4-24H11 is able to bind to AIP-1 with moderate affinity (≈ 5 μM). Therefore, we investigated whether AP4-24H11 could affect QS signaling in an agr group I strain, namely Wood 46. Clearly, AP4-24H11 was not able to block α-hemolysin expression in Wood 46 as effectively as in RN4850; however, a notable decrease in α-hemolysin production in Wood 46 grown in the presence of AP4-24H11 was evident (Fig. 3a). These data suggest that it might be possible to generate cross-reactive mAbs that suppress S. aureus QS signaling of two or more different agr groups.
While it could be argued that the decrease in toxin production and overall protein secretion is caused by an antibody-mediated growth defect, it is important to note that no significant growth changes of S. aureus were observed over a 24-hour growth period in the presence of AP4-24H11 (Fig. 3b). In addition, no discernable growth effects were observed with mAb SP2-6E11, an unrelated isotype control (κγ2a) for AP4-24H11.
One of the important bacterial virulent factors regulated by QS is biofilm formation. In S. aureus, biofilm formation is known to be negatively regulated by agr QS signaling [29], which is indeed one of the problems in controlling S. aureus virulence through agr QS inhibition [30]. Consistent with previous studies, AP4-24H11-mediated QS inhibition led to increased biofilm formation in RN4850 (Fig. 3c). Although the increase of biofilm formation poses a significant problem in chronic infection of S. aureus, it represent a lesser predicament in acute infections and thus, mAb AP4-24H11 might be still an effective way to control such S. aureus infections.
Real time PCR analysis
To further examine agr QS inhibition by AP4-24H11, we performed real time-polymerase chain reaction (RT-PCR) analysis to evaluate if the observed changes in virulent factor expression were indeed caused by interference with the agr QS system, i.e. whether the presence of AP4-24H11 affects the transcription of rnaIII, the immediate product of agr autoinduction and the main QS effector in S. aureus [31]. As expected, the rnaIII transcriptional level in RN4850 during stationary growth phase was reduced significantly (> 50 fold), by AP4-24H11; this finding advocates that the alteration of α-hemolysin and protein A expression is a direct result of the interference of AIP-4-mediated QS signaling by AP4-24H11 (Fig. 3d). Yet, the subtle changes in overall exoprotein expression (see Supplemental Fig. 2) might be misconstrued to mean that AP4-24H11 does not block the QS signaling efficiently, however, our RT-PCR analysis provides evidence that AP4-24H11 significantly inhibits AIP4-based QS in S. aureus RN4850.
To gain greater appreciation of the specificity and potential limitations of antibody-based QS interference in S. aureus, we investigated the transcriptional level of two additional virulence regulators, namely sarA (staphylococcal accessory regulator) and saeR (staphylococcal accessory protein effector) [6, 32, 33], which control the response to environmental stresses as well as virulence factor expression in S. aureus. Importantly, no significant changes (≤ 2-fold) were observed in either sarA or saeR transcription, supporting that AP4-24H11 only affects agr QS system (Fig. 3d).
The transcription of α-hemolysin and protein A was analyzed by RT-PCR. As stated, (vide supra), significant changes were seen in protein expression level. In terms of transcription, the hla and spa genes were suppressed and elevated respectively ≈ 3 to 5 fold, again confirming that rnaIII affects not only transcription but also translation of these proteins as reported previously [31]. Finally, exofoliatin A (eta) transcription was investigated, which is another agr QS regulated toxin exclusively produced by AIP-4 utilizing S. aureus strains [34]. Gratifyingly, our data indicated that AP4-24H11 also decreased eta transcription by ≈ 10 fold (Fig. 3d).
Inactivation of AP4-24H11 by the synthetic AIP-4
Balaban and co-workers have proposed that the RNAIII activating protein (RAP) regulates agr QS signaling in S. aureus in an AIP-independent manner [35]. In addition, the same group has reported that a linear peptide, termed RNAIII-inhibiting peptide (RIP), could blunt RAP-mediated QS signaling resulting in a decrease of RNAIII transcription [36, 37]. Although we have presented evidence that AP4-24H11 inhibited agr QS through binding to AIP-4 and sequestering it from the cell growing medium, there was still the possibility that AP4-24H11 might affect other signaling systems in S. aureus including RAP, which in turn could affect agr QS network. As such, we investigated whether external addition of AIP-4 could restore the agr QS signaling network in S. aureus RN4850 in the presence of AP4-24H11. Thus, we treated AP4-24H11 with an equimolar amount of synthetic AIP-4 before addition to the S. aureus growth medium; this then would assure saturation of the antibody binding sites with the AIP-4 peptide. As seen in Figure 3e, the addition of synthetic AIP-4 efficiently reduced the quorum quenching effect of AP4-24H11, and as a result fully restored expression of α-hemolysin in S. aureus RN4850. As expected, this finding provides additional confirmation that AP4-24H11 indeed sequesters AIP-4 in S. aureus growth medium and inhibits AIP-dependent QS signaling in S. aureus in a strictly AIP-4-dependent manner.
AP4-24H11 inhibits S. aureus-induced apoptosis in mammalian cells
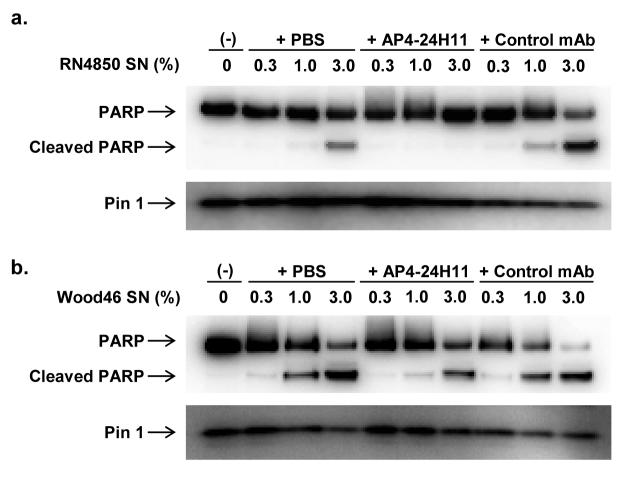
Recent studies have shown that incubation of Jurkat T cells with supernatant of S. aureus culture results in induction of apoptosis [38]. We treated Jurkat cells with the supernatants of S. aureus (RN4850 and Wood 46) cultures grown in the presence or absence of AP4-24H11. After incubation for 4 hours with the supernatant, the cleavage of poly(ADP-ribose) polymerase (PARP), a biochemical marker indicative of apoptosis induction, was evaluated in Jurkat cell protein extracts. As shown in Figure 4, AP4-24H11 prevented RN4850 supernatant (1 %) to induce PARP cleavage in Jurkat cells, and also partially inhibited the effect of Wood 46 supernatant. In fact, previous mechanistic studies proposed that one of the major factors in S. aureus supernatant responsible for inducing apoptosis might be α-hemolysin; our findings, (Fig. 3a and Fig. 4), support this positive correlation between expression of α-hemolysin and S. aureus-induced apoptosis.
Figure 4. Inhibition of S. aureus-induced PARP cleavage by AP4-24H11.
PARP cleavage in Jurkat cells after treating with S. aureus (A) RN4850, (B) Wood 46 supernatants. Human Jurkat leukemic T cells were maintained in RPMI 1640 supplemented with 10 % heat-inactivated fatal bovine serum, 10 mM (L)-glutamine, and 50 mg/mL of streptomycin and penicillin (GIBCO, Invitrogen Corp.). S. aureus supernatants were prepared as described in Experimental Procedures, and the supernatants of RN4850 were further concentrated to 1/3 of original volume using Amicon Ultra-4 (5,000 NMWL) centrifugal filter devices (MILLIPORE, Billerica MA) Confluent cells were distributed to 24-well plate in fresh medium (0.5 mL) and incubated for 6 hours before adding the S. aureus supernatants. After 4 hours incubation with the indicated amount of S. aureus supernatants, cell extracts were prepared and analyzed by Western blotting using anti-PARP antibody.
AP4-24H11 blocks S. aureus-induced dermal injury in mice
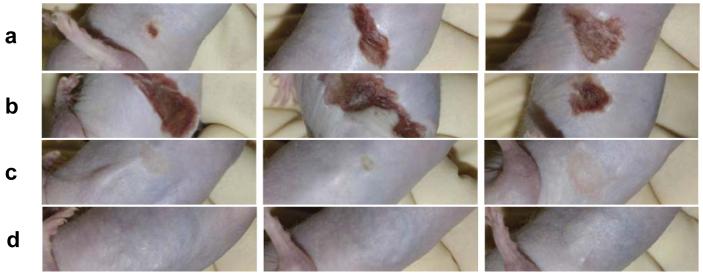
Next, we investigated the potential of mAb AP4-24H11 to mitigate S. aureus induced injury in vivo by employing a murine subcutaneous infection model. Freshly grown log phase S. aureus RN4850 were suspended in PBS containing Cytodex beads and where indicated AP4-24H11 or control IgG. Subcutaneous injections of bacterial suspension or vehicle control were made in the flank of SKH1 hairless mice followed by close monitoring over seven days. Doses administered were 107 or 108 bacteria (colony forming units; cfu) and 0.6 or 0.06 mg AP4-24H11 or control IgG. Mice receiving 107 cfu developed minimal hyperemia/edema followed by limited induration over 7 days (see Supplemental Fig. 3a). However as early as six hours after injection, mice receiving 108 cfu suspended in saline or control IgG showed early-stage hyperemia / redness at the injection site and extending 3-5 mm horizontally and 5-10 mm vertically in a diagonal pattern along the flank (Fig. 5a). Upon reexamination at 18 hours, the same areas surrounding the injection site were devitalized, and the skin was transformed to a brittle, reddish-brown scab. Over the 7-day observation period, the hardened scab began to detach from the surrounding relatively normal appearing skin, and small amounts of purulent exudate were observed at the normal/necrotic junction. In contrast, skin injury was abrogated in mice that received 108 bacteria with 0.6 mg AP4-24H11 (Fig. 5c). As anticipated, the lower dose of AP4-24H11 (0.06 mg) was not protective (Fig. 5b), and control mice receiving 108 cfu with 0.6 mg control IgG were not protected (see Supplemental Fig. 3b). Mice that received an injection of PBS/Cytodex alone or containing 0.6 mg AP4-24H11 remained normal over the observation period with the exception of occasional local induration (Fig. 5d). Excitingly, animals that had received the protective dose of 0.6 mg AP4-24H11 in combination with S. aureus RN4850 did not develop any significant lesions over the 7 day observation period.
Figure 5. Inhibition of S. aureus-induced abscess formation by AP4-24H11 in mice models.
SKH1 euthymic hairless mice (6-8 weeks old) received 200 μl intradermal flank injections containing S. aureus (1 × 108 bacteria), 4 μl packed volume Cytodex beads, DPBS, mAb AP4-24H11 or control IgG (0.06 mg or 0.6 mg). Additional control animals received 200 μl intradermal injections containing Cytodex beads or beads plus antibody. After injections were made the mice were monitored at least three times each day over a period of 4-7 days. At the conclusion of the monitoring period the mice were euthanized and tissues harvested for bacteriologic and histologic analysis. (a) S. aureus + PBS; (b) S. aureus + AP4-24H11 (0.06 mg); (c) S. aureus + AP4-24H11 (0.6 mg); (d) Cytodex + AP4-24H11 (0.6 mg).
Passive immunization with AP4-24H11 protected mice from S. aureus-induced fatality
To evaluate the effectiveness of a passive immunization approach using AP4-24H11 against a lethal challenge with S. aureus, SKH1 hairless mice received a 1 ml i.p. injection of AP4-24H11, control IgG or vehicle (DPBS) followed 2 hours later by 0.5 ml DPBS- containing 3 × 108 S. aureus RN4850. As shown in Figure 6, all of the mice receiving AP4-24H11 (6/6) survived through the 8-day observation period. In contrast, only one of the DPBS treated control mice (1/6) and none of the control IgG treated mice (0/6) survived longer than 24 hours. These data further validated our immunopharmcaothereutic approach for combating acute S. aureus infections.
Figure 6. Passive immunization of mice with AP4-24H11 against S. aureus infection.
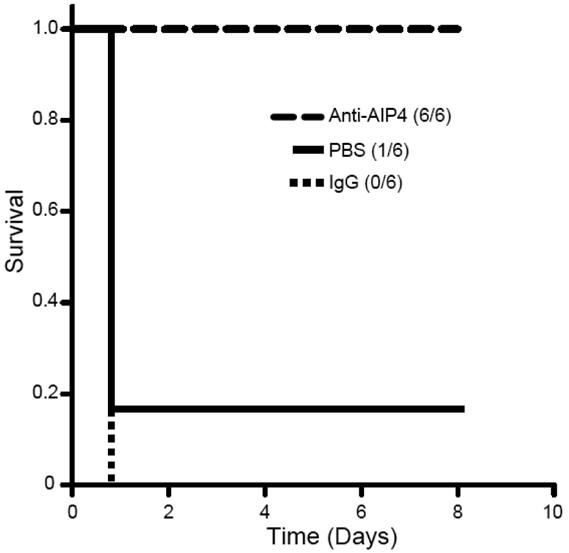
Survival in mice that were pretreated with mAb AP4-24H11 or control IgG followed two hours later by S. aureus injection (3 × 108 i.p.). The numbers in parenthesis show number of survivors/number per group. The log rank statistic, p = 0.001; n = 6 for each group.
Significance
We have sought to elucidate the consequences of quorum sensing signaling using highly specific anti-autoinducer antibodies elicited against a rationally-designed synthetic hapten. Our data indicate that the scavenging of an AIP by a monoclonal antibody is sufficient to suppress QS-controlled expression of virulence factors in S. aureus, which establishes this as the first report of AIP removal from bacterial culture without genetic manipulations of the organism. Antibodies now comprise over one third of the molecules undergoing clinical evaluation, mostly for the cancer immunotherapy [39]. However, antibody-based therapies for the treatment of bacterial infections using QS as a molecular target are still in their infancy [39-41]. Conventional QS interference strategies utilizing small molecule antagonists are based on a competition between the bacterial autoinducer and the inhibitor for the bacterial AI receptor protein. In contrast, quorum quenching antibodies engage in competition with the AI receptor for the autoinducer. In this context, an immunopharmacotherapeutic strategy is highly appealing as the antibody acts as a “decoy-receptor” while possessing well documented pharmacokinetic behavior and pharmacodynamic response.
In total, antibodies generated against bacterial autoinducers represent a new and valuable set of immunological tools for both the study of QS-controlled processes and potentially an alternative strategy engaging immunopharmacotherapy for the prevention or treatment of infections in which QS signaling contributes to bacterial pathogenesis.
Experimental Procedures
Synthesis of the linear protected peptide (3)
All N-α-Fmoc protected amino acids, coupling reagents and the resins for peptide synthesis were purchased from EMD Biosciences, Inc. (San Diego, CA). All other chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO). ESI-MS analyses were performed with API150EX (PESCIEX, Foster City, CA), and HITACHI L-7300 and SHIMADZU SCL-10A were used for analytical and preparative HPLC experiments, respectively.
The peptide was synthesized by Fmoc SPPS on 2-chlorotrityl resin preloaded with the Fmoc-Met 1. An Fmoc-Ser(Trt)-OH was incorporated at the position of lactonization. All other residues were chosen with side chain protecting groups stable to dilute TFA and labile in 95% TFA. A short flexible linker was incorporated penultimate to the N-terminus by coupling Fmoc-8-amino-3,6-dioxaoctanoic acid. The N-terminal residue was Boc-Cys(SEt)-OH for eventual use in conjugation to carrier proteins. Specific Conditions: Batch synthesis was carried out on 1 mmol of resin swollen in DMF for at least 1 h. A solution of the protected amino acid, DIC, and HOBt (4 eq each) in 5 mL DMF was prepared and allowed to sit for 5 min for pre-activation, followed by the addition of 0.5 mL symcollidine. The cocktail was added to the resin for coupling, which was generally complete in 1 h. The resin was then washed with DMF and subjected to Fmoc deprotection with 20 % (v/v) piperidine in DMF (2 × 7 min). The resin was then washed with DMF and the next coupling reaction was carried out. When synthesis was complete, the resin was washed with DMF, then CH2Cl2, and finally with ether before it was placed in the desiccator. Cleavage (and Trityl Deprotection): The resin was added to a cocktail of 4 % TFA, 4 % triisopropylsilane (TIS) and 0.5 % H2O in chloroform, and shaken for 6 h. The mixture was filtered, allowing the filtrate to drip into cold ether to precipitate the peptide. The ether mixture was centrifuged and the supernatant was decanted. The peptide was then washed (2x) with ether by re-suspending the solid in ether, centrifugation, and decanting the supernatant. The resulting solid was placed in a desiccator. Purification: The fully protected peptide 3 was dissolved in methylene chloride and purified by normal phase silica gel chromatography eluted with 5 % methanol in methylene chloride.
Lactonization of (3)
The protected linear peptide 3 was dissolved in 1,2-dichloroethane (previously dried over anhydrous MgSO4) to give a final concentration of no greater than 1.0 mM. The solution was stirred and heated to 80 °C and 3 eq each of EDC and 4-DMAP were added; another equivalent each of EDC and 4-DMAP were added at both 24 h and 48 h into the reaction. The reaction was monitored by HPLC. After 4 days, the reaction mixture was cooled to room temperature, washed with 2 × 200 mL of 0.2 M KHSO4 (aq), dried over anhydrous Na2SO4, and evaporated to dryness. The cyclized peptide 4 was purified by prep-HPLC. Yields range from 30-60 % as determined by analytical HPLC integration.
Global Deprotection and Disulphide Deprotection of (4)
The solid, purified peptide was dissolved in TFA containing 2 % TIS and stirred for 1 h. The mixture was then evaporated to dryness. Water was added and the mixture was frozen and lyophilized. The lyophilized solid was then dissolved in H2O with tris(2-carboxyethyl)phosphine hydrochloride (TCEP). The mixture was stirred for 1 h and injected directly into the prep-HPLC for purification yielding AP4 hapten 5. The collected pure fractions were pooled, frozen, and lyophilized. ESI-MS: m / z calcd for C57H80N10O17S2 (M + H), 1241.5; found, 1242.2.
Conjugation of (5) to KLH/BSA
Attachment of Sulpho-SMCC
5 mg of the carrier protein were resuspended in 0.9 mL PBS, pH 7.4. To this solution was added 1 mg of the linker sulpho-SMCC (sulphosuccinimidyl 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate). The solution was stirred for 6-8 h and the protein-linker conjugate was purified by dialysis in PBS at 4 °C. Conjugation of the hapten 5. To the protein-linker conjugate in PBS were added 100 μL of DMF containing 2 mg of the hapten 5. The solution was shaken overnight and the protein-hapten conjugate was purified by dialysis. MALDI-TOF analysis confirmed the attachment on average of ≈ 6 haptens per BSA molecule.
Analysis of exoprotein secretion in S. aureus
After overnight growth on an agar plate at 37 °C, a single colony of S. aureus (RN4850 or Wood 46) was inoculated into 3 mL CYGP medium and grown for overnight (18 h) [42]. The overnight cultured cells were diluted to OD600 ≈ 0.03 in fresh CYGP medium, and distributed to 5 mL polystyrene cell-culturing tube, where each tube contained 0.5 mL of the diluted cells and the appropriate antibody (0.2 mg/mL). After growth for 20-24 h at 37 °C in a humid incubator without agitation, the samples were transferred to the microcentrifuge tubes (1.5 mL) and centrifuged at 13,000 rpm for 5 min. The supernatants were sterilized by filtration through a Millex®-GV filter unit (0.22 μm; Millipore, Ireland), and analyzed by SDS-PAGE (10 % Bis-Tris gel, Invitrogen, Carlsbad CA). To confirm α-hemolysin and protein A expression, Western blot analyses were performed using the HRP conjugated sheep polyclonal α-hemolysin antibody (abcam Inc., Cambridge MA) and anti-Protein A mouse monoclonal antibody (Sigma-Aldrich, St. Louis MO) and murine mAb SP2-6E11 (Park and Janda, unpublished data) was used as a control antibody. To test hemolytic activity, the S. aureus supernatants (75 μL × 3) were applied onto the sheep blood agar plate, and the plates were incubated at 37 °C for 18 hours and at room temperature for another 24 hours.
Static Biofim Analysis
The biofilm assay was conducted by following a literature procedure with a few modifications [43]. After S. aureus cells (200 μL) were grown in tryptic soy broth (TSB) medium containing 0.2 % glucose with or without the antibody (0.2 mg/mL) in the polystyrene 96-well plate for 20-24 h without agitation, the plate was washed by submersion in water and dried. A crystal violet solution (200 μL, aq. 0.1 %) was added to stain the biofilm, and then the plate was washed vigorously with water followed by adding acetic acid (250 μL, aq. 30 %) to solubilize the remaining crystal violet. Absorbance was measured at 570 nm with Spectramax 250 (Molecular Devices, Sunnyvale CA).
Real Time-PCR Analysis
Overnight cultured S. aureus RN4850 cells were diluted to OD600 ≈ 0.03 in fresh CYGP medium (1 mL) containing the antibody and grown for 20-24 h (OD600 ≈ 2) at 37 °C without shaking. RNA from the cells was isolated using RNeasy® Mini Kit (QIAGEN Inc., Valencia CA) according to the manufacturer’s instructions. Isolated RNA was further purified by treating with RNase-Free DNase (QIAGENE Inc.) for 30 min at room temperature. The first-strand DNA was synthesized using SuperScriptTM First-Strand Synthesis System for RT-PCR (Invitrogen) using ≈ 300 ng of purified RNA. RT-PCR experiments were performed with at least two independent samples, and each experiment was set up in duplicate using LightCycler® FastStart DNA MasterPLUS SYBR Green I (Roche Applied Science, Indianapolis, IN). Generic SYBR Green Protocol (Roche) was used for the PCR conditions, and relative quantification analyses were performed with LightCycler® 2.0 system (Roche Applied Science) using the housekeeping GyrA gene was a reference. The sequence information of the primers used in our experiments is listed in the Supplemental Data [44].
Dermal infection model in mice
All experiments on mice were performed in accordance with TSRI guidelines and regulations. SKH1 euthymic hairless mice, 6-8 weeks old were obtained from Charles River Laboratories and housed in the biocontainment vivarium for one week before use in experiments. Brain heart infusion agar was from BBL (#211065) and CYGP broth contained 1% casamino acids (Fisher BP1424) 1% yeast extract (EMD 1.03753) 0.59% sodium chloride, 0.5% dextrose and 60 mM β-glycerol phosphate disodium salt (Fluka 50020) as described by Novick [42]. Cytodex 1 beads (GE Healthcare 17-0448-01) were suspended (1 gram in 50 ml) in Dulbecco’s Phosphate Buffered Saline without calcium/magnesium (Gibco) overnight at 20°C. The supernatant was decanted and the beads washed three times by suspension in DPBS and 1G sedimentation followed by autoclaving (121°C, 15 psi, 15 minutes). Staphylococcus aureus RN4850 (AIP4) was grown from frozen stock (BHI + 20% glycerol) on brain heart infusion agar plates 35°C overnight. Three representative colonies were combined to inoculate 2 ml CYGP broth, and after overnight incubation without shaking, 0.25 ml of the culture was used to inoculate 5 ml of CYGP followed by incubation at 35°C, 200 rpm for 3 hours. The culture was centrifuged 1,300 × G at 4°C for 20 minutes, the supernatant poured off, and the bacterial pellet was suspended in 1 ml DPBS without calcium/magnesium. The SKH1 received 200 μl intradermal flank injections containing S. aureus (1 × 107 or 1 × 108 bacteria), 4 μl packed volume Cytodex beads, DPBS, anti-AIP4 antibody or control IgG (0.6 or 0.06 mg). Additional control animals received 200 μl intradermal injections containing Cytodex beads or beads plus antibody. After injections were made the mice were monitored at least three times each day over a period of 4-7 days. At the conclusion of the monitoring period the mice were euthanized and tissues harvested for bacteriologic and histologic analysis.
Passive immunization of mice with AP4-24H11
S. aureus RN4850 were stored at -80°C in 20% glycerol/BHI medium, thawed and grown on BHI-agar plates overnight, and three separate colonies sampled to inoculate 2 ml CYGP medium. The inoculum culture was maintained 1 hour at 35°C without shaking, followed by shaking at 200 rpm for 3 hours. Aliquots of the freshly grown inoculum culture were transferred to 5 ml CYGP medium in 50 ml conical polypropylene tubes (1/20 dilution) followed by shaking at 200 rpm, 35°C for 3 hours. The bacteria were pelleted by centrifugation at 3,000 rpm (1300 × G) for 10 minutes, 4°C. The bacterial pellets were resuspended in Dulbecco’s phosphate buffered saline without calcium or magnesium (DPBS-), and enumerated using a Petroff-Hausser counting chamber. Final dilutions were made in DPBS- so that 3 × 108 bacteria were administered i.p. in 0.5 ml. To maintain viability bacteria were administered within two hours of harvest.
MAb AP4-24H11, isotype-matched control IgG (1 mg each) or DPBS was administered i.p. in DPBS to SKH1 mice (6-9 weeks old; 6 animals per treatment group) followed two hours later by 0.5 ml DPBS- i.p. containing 3 × 108 S. aureus. The mice were monitored several times on the day of injection and twice each day on subsequent days, observing ambulation, alertness, response to handling and skin temperature measured by infrared thermometry (Raytek MiniTemp MT4) using a 1 cm diameter infrasternal skin site. Animals showing surface temperature consistently below 30°C and also diminished response to handling and weakened righting reflex were considered moribund and were euthanized.
Other Experimental Procedures
See Supplemental Data for details of syntheses AIPs (also see Supplemental Fig. 4), competition ELISA, and sequence information of the primers for RT-PCR.
Supplementary Material
Acknowledgements
We are grateful to Dr. Richard P. Novick (Skirball Institute, New York University Medical Center) for the generous gift of RN4850. We also thank the TSRI Antibody Production Core Facility for providing the purified monoclonal antibodies. A clinical isolate, NRS168 was obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) Program supported by NIAID/NIH (N01-AI-95359). This work was supported by the National Institutes of Health (AI055778 to K.D.J.) and The Skaggs Institute for Chemical Biology.
The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fuqua C, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 2.Nealson KH, Platt T, Hastings JW. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970;104:313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lazazzera BA, Grossman AD. The ins and outs of peptide signaling. Trends Microbiol. 1998;6:288–294. doi: 10.1016/s0966-842x(98)01313-4. [DOI] [PubMed] [Google Scholar]
- 6.Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 7.Meijler MM, Hom LG, Kaufmann GF, McKenzie KM, Sun C, Moss JA, Matsushita M, Janda KD. Synthesis and biological validation of a ubiquitous quorum-sensing molecule. Angew Chem Int Ed Engl. 2004;43:2106–2108. doi: 10.1002/anie.200353150. [DOI] [PubMed] [Google Scholar]
- 8.Schauder S, Shokat K, Surette MG, Bassler BL. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol. 2001;41:463–476. doi: 10.1046/j.1365-2958.2001.02532.x. [DOI] [PubMed] [Google Scholar]
- 9.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 10.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 11.Lyon GJ, Muir TW. Chemical signaling among bacteria and its inhibition. Chem Biol. 2003;10:1007–1021. doi: 10.1016/j.chembiol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen TB, Givskov M. Quorum sensing inhibitors: a bargain of effects. Microbiology. 2006;152:895–904. doi: 10.1099/mic.0.28601-0. [DOI] [PubMed] [Google Scholar]
- 13.Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. doi: 10.1016/s1369-5274(03)00008-0. [DOI] [PubMed] [Google Scholar]
- 14.Chan WC, Coyle BJ, Williams P. Virulence regulation and quorum sensing in staphylococcal infections: competitive AgrC antagonists as quorum sensing inhibitors. J Med Chem. 2004;47:4633–4641. doi: 10.1021/jm0400754. [DOI] [PubMed] [Google Scholar]
- 15.Geske GD, Wezeman RJ, Siegel AP, Blackwell HE. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J Am Chem Soc. 2005;127:12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- 16.Lyon GJ, Mayville P, Muir TW, Novick RP. Rational design of a global inhibitor of the virulence response in Staphylococcus aureus, based in part on localization of the site of inhibition to the receptor-histidine kinase, AgrC. Proc Natl Acad Sci U S A. 2000;97:13330–13335. doi: 10.1073/pnas.97.24.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muh U, Hare BJ, Duerkop BA, Schuster M, Hanzelka BL, Heim R, Olson ER, Greenberg EP. A structurally unrelated mimic of a Pseudomonas aeruginosa acyl-homoserine lactone quorum-sensing signal. Proc Natl Acad Sci U S A. 2006;103:16948–16952. doi: 10.1073/pnas.0608348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith KM, Bu Y, Suga H. Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem Biol. 2003;10:563–571. doi: 10.1016/s1074-5521(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann GF, Sartorio R, Lee SH, Mee JM, Altobell LJ, 3rd, Kujawa DP, Jeffries E, Clapham B, Meijler MM, Janda KD. Antibody interference with N-acyl homoserine lactone-mediated bacterial quorum sensing. J Am Chem Soc. 2006;128:2802–2803. doi: 10.1021/ja0578698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyairi S, Tateda K, Fuse ET, Ueda C, Saito H, Takabatake T, Ishii Y, Horikawa M, Ishiguro M, Standiford TJ, Yamaguchi K. Immunization with 3-oxododecanoyl-L-homoserine lactone-protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J Med Microbiol. 2006;55:1381–1387. doi: 10.1099/jmm.0.46658-0. [DOI] [PubMed] [Google Scholar]
- 21.Massey RC, Horsburgh MJ, Lina G, Hook M, Recker M. The evolution and maintenance of virulence in Staphylococcus aureus: a role for host-to-host transmission? Nat Rev Microbiol. 2006;4:953–958. doi: 10.1038/nrmicro1551. [DOI] [PubMed] [Google Scholar]
- 22.George EA, Muir TW. Molecular mechanisms of agr quorum sensing in virulent staphylococci. Chembiochem. 2007;8:847–855. doi: 10.1002/cbic.200700023. [DOI] [PubMed] [Google Scholar]
- 23.Sakoulas G, Eliopoulos GM, Moellering RC, Jr., Novick RP, Venkataraman L, Wennersten C, DeGirolami PC, Schwaber MJ, Gold HS. Staphylococcus aureus accessory gene regulator (agr) group II: is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis. 2003;187:929–938. doi: 10.1086/368128. [DOI] [PubMed] [Google Scholar]
- 24.Wright JS, 3rd, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc Natl Acad Sci U S A. 2005;102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji G, Beavis R, Novick RP. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 26.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick RP, Muir TW. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci U S A. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shigenaga A, Moss JA, Ashley FT, Kaufmann GF, Janda KD. Solid-phase synthesis and cyclative cleavage of quorum sensing depsipeptide analogues by acylphenyldiazene activation. SYNLETT. 2006;4:551–554. [Google Scholar]
- 28.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci U S A. 2005;102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J Infect Dis. 2000;182:1688–1693. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- 30.Harraghy N, Kerdudou S, Herrmann M. Quorum-sensing systems in staphylococci as therapeutic targets. Anal Bioanal Chem. 2007;387:437–444. doi: 10.1007/s00216-006-0860-0. [DOI] [PubMed] [Google Scholar]
- 31.Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. Embo J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penades JR, Lasa I. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol. 2003;48:1075–1087. doi: 10.1046/j.1365-2958.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- 33.Xiong YQ, Willard J, Yeaman MR, Cheung AL, Bayer AS. Regulation of Staphylococcus aureus alpha-toxin gene (hla) expression by agr, sarA, and sae in vitro and in experimental infective endocarditis. J Infect Dis. 2006;194:1267–1275. doi: 10.1086/508210. [DOI] [PubMed] [Google Scholar]
- 34.Jarraud S, Lyon GJ, Figueiredo AM, Gerard L, Vandenesch F, Etienne J, Muir TW, Novick RP. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J Bacteriol. 2000;182:6517–6522. doi: 10.1128/jb.182.22.6517-6522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaban N, Goldkorn T, Nhan RT, Dang LB, Scott S, Ridgley RM, Rasooly A, Wright SC, Larrick JW, Rasooly R, Carlson JR. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–440. doi: 10.1126/science.280.5362.438. [DOI] [PubMed] [Google Scholar]
- 36.Shaw LN, Jonnson IM, Singh VK, Tarkowski A, Stewart GC. Inactivation of traP has no effect on the Agr quorum sensing system or virulence of Staphylococcus aureus. Infect Immun. 2007 doi: 10.1128/IAI.00491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsang LH, Daily ST, Weiss EC, Smeltzer MS. Mutation of traP in Staphylococcus aureus has no impact on expression of agr or biofilm formation. Infect Immun. 2007 doi: 10.1128/IAI.00603-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bantel H, Sinha B, Domschke W, Peters G, Schulze-Osthoff K, Janicke RU. alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J Cell Biol. 2001;155:637–648. doi: 10.1083/jcb.200105081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 40.Yang G, Gao Y, Dong J, Liu C, Xue Y, Fan M, Shen B, Shao N. A novel peptide screened by phage display can mimic TRAP antigen epitope against Staphylococcus aureus infections. J Biol Chem. 2005;280:27431–27435. doi: 10.1074/jbc.M501127200. [DOI] [PubMed] [Google Scholar]
- 41.Yang G, Gao Y, Dong J, Xue Y, Fan M, Shen B, Liu C, Shao N. A novel peptide isolated from phage library to substitute a complex system for a vaccine against staphylococci infection. Vaccine. 2006;24:1117–1123. doi: 10.1016/j.vaccine.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Novick RP. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 43.O’Toole GA, Pratt LA, Watnick PI, Newman DK, Weaver VB, Kolter R. Genetic approaches to study of biofilms. Methods Enzymol. 1999;310:91–109. doi: 10.1016/s0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 44.Eleaume H, Jabbouri S. Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods. 2004;59:363–370. doi: 10.1016/j.mimet.2004.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.