Abstract
The acyclic nucleoside phosphonates HPMPC (cidofovir), PMEA (adefovir), and PMPA (tenofovir) have proved to be effective in vitro (cell culture systems) and in vivo (animal models and clinical studies) against a wide variety of DNA virus and retrovirus infections: cidofovir against herpesvirus (herpes simplex virus types 1 and 2 varicella-zoster virus, cytomegalovirus [CMV], Epstein-Barr virus, and human herpesviruses 6, 7, and 8), polyomavirus, papillomavirus, adenovirus, and poxvirus (variola virus, cowpox virus, vaccinia virus, molluscum contagiosum virus, and orf virus) infections; adefovir against herpesvirus, hepadnavirus (human hepatitis B virus), and retrovirus (human immunodeficiency virus types 1 [HIV-1] and 2 [HIV-2], simian immunodeficiency virus, and feline immunodeficiency virus) infections; and tenofovir against both hepadnavirus and retrovirus infections. Cidofovir (Vistide) has been officially approved for the treatment of CMV retinitis in AIDS patients, tenofovir disoproxil fumarate (Viread) has been approved for the treatment of HIV infections (i.e., AIDS), and adefovir dipivoxil (Hepsera) has been approved for the treatment of chronic hepatitis B. Nephrotoxicity is the dose-limiting side effect for cidofovir (Vistide) when used intravenously (5 mg/kg); no toxic side effects have been described for adefovir dipivoxil and tenofovir disoproxil fumarate, at the approved doses (Hepsera at 10 mg orally daily and Viread at 300 mg orally daily).
INTRODUCTION
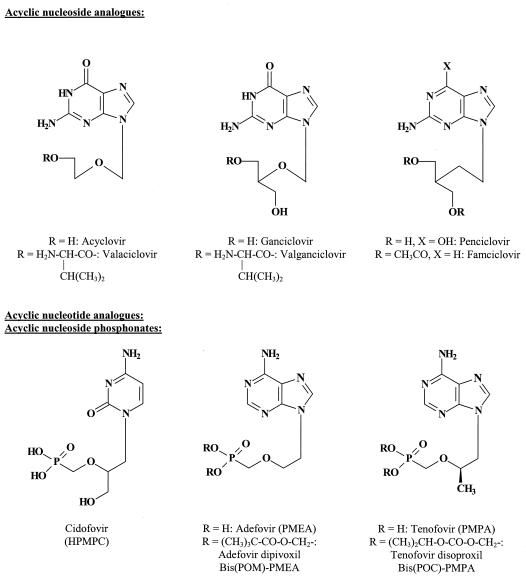
Most of the antiviral compounds that are currently used in the treatment of herpesvirus (herpes simplex virus (HSV), varicella-zoster virus (VZV), and cytomegalovirus (CMV)) infections (63) can be described as acyclic nucleoside analogues: acyclovir, penciclovir, and ganciclovir (Fig. 1). To increase their oral bioavailability, acyclovir, ganciclovir, and penciclovir have been converted to their oral prodrug forms (termed valaciclovir, valganciclovir, and famciclovir, respectively). Following their absorption from the gut, these compounds are reconverted to the parent compounds before reaching their target organ(s).
FIG. 1.
Acyclic nucleoside analogues and acyclic nucleotide analogues (acyclic nucleoside phosphonates).
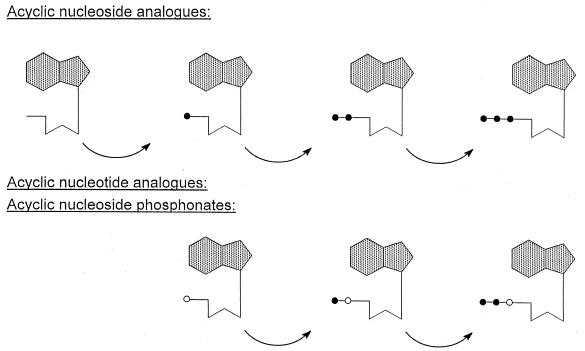
After the acyclic nucleoside analogues have been taken up by the cells, they have to be phosphorylated through three consecutive phosphorylation steps (Fig. 2) before they can interact, in their triphosphate form, with their target enzyme, the viral DNA polymerase. Of crucial importance in this phosphorylation process is the first phosphorylation step which is ensured by a specific virus-encoded thymidine kinase (TK) (for HSV and VZV) or a specific virus-encoded (UL97) protein kinase (PK) (for CMV). Once that the compounds have been phosphorylated to the monophosphate, cellular kinases (i.e., GMP kinase and NDP kinase) will afford their further phosphorylation to the di- and triphosphate stages. In their triphosphate form, the compounds then interact as competitive inhibitors or alternate substrates with the normal substrates [2′-deoxynucleoside 5′-triphosphates (dNTPs)], and if they are incorporated into the DNA chain, they may act as chain terminators, thus preventing further chain elongation. It should be noted that, as while acyclovir obligatorily acts as a chain terminator, ganciclovir and penciclovir may also be incorporated, via an internucleotide linkage, in the interior of the DNA chain.
FIG. 2.
Intracellular metabolism of acyclic nucleoside analogues and acyclic nucleotide analogues. The former need three phosphorylation steps whereas the latter only need two to be converted to their active metabolites (the dNTP analogues). Symbols: •, phosphate; ○, phosphonate.
The first phosphorylation step is crucial for the antiviral activity of the acyclic nucleoside analogues, since it confines the effectiveness of the compounds to viruses that do induce a specific kinase phosphorylating the compounds while making the compounds inactive against viruses that either do not induce a specific TK or PK or have developed resistance to the compounds through mutations in these enzymes (83). Thus, acyclovir, penciclovir, and ganciclovir are ineffective against TK− HSV, TK− VZV, PK− CMV, and any other DNA viruses (polyomavirus, papillomavirus, adenovirus, poxvirus) that fail to ensure phosphorylation of the nucleoside to the nucleoside monophosphate (nucleotide).
Acyclic nucleoside phosphonates (61) possess a phosphonate group attached to the acyclic nucleoside moiety through a stable P—C bond. In contrast to the phosphate group (which is attached through a P—O—C bond), a phosphonate group (P—C bound) cannot be cleaved off by cellular hydrolases (esterases). Foremost among the acyclic nucleoside phosphonates that have been pursued as antiviral agents are cidofovir (HPMPC) [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine], adefovir (PMEA) [9-(2-phosphonylmethoxyethyl)adenine], and tenofovir (PMPA) [(R)-9-(2-phosphonylmethoxypropyl)adenine]. Because of their limited oral bioavailability, the last two compounds have been converted to their oral prodrug forms, adefovir dipivoxil or bis(pivaloyloxymethyl)-PMEA [bis(POM)-PMEA] and tenofovir disoproxil or bis(isopropyloxycarbonyloxymethyl)-PMPA [bis(POC)-PMPA], respectively (Fig. 1).
MECHANISM OF ACTION
Since the acyclic nucleoside phosphonates already contain a phosphate-mimetic group, stably attached through a P—C bond, they need only two, instead of three, phosphorylation steps to reach the active metabolite stage (Fig. 2). Thus, acyclic nucleoside phosphonates do not depend on the virus-induced kinase to exert their antiviral action, and, in “bypassing” the nucleoside kinase step, the acyclic nucleoside phosphonates may be expected to act against a broad range of DNA viruses, including hepadnaviruses (hepatitis B virus [HBV]) and retroviruses (human immunodeficiency virus [HIV]), i.e., all the viruses that use for their replication a DNA polymerase via which the active metabolites of the acyclic nucleoside phosphonates could enter into competition with the normal substrates (dNTPs).
Cidofovir (HPMPC) is phosphorylated by pyrimidine nucleoside monophosphate (PNMP) kinase to cidofovir monophosphate (HPMPCp), which is then further phosphorylated by nucleoside diphosphate (NDP) kinase, pyruvate kinase, or creatine kinase to cidofovir diphosphate (HPMPCpp) (50). Both phosphorylation steps can occur in both uninfected and virus-infected cells. For adefovir (PMEA), and presumably tenofovir (PMPA) as well, phosphorylation to the diphosphate form (PMEApp and PMPApp) can be achieved in one or two steps through the aid of 5-phosphoribosyl-1-pyrophosphate synthetase (14) or AMP (dAMP) kinase (139), respectively.
Cidofovir inhibits CMV replication at a 50% antivirally effective concentration of 0.1 μg/ml, which is 1,000-fold lower than the 50% cell-inhibitory concentration (IC50) required to inhibit normal cell proliferation (199). This antiviral selectivity is also reflected at the viral DNA synthesis level, since cidofovir inhibits CMV DNA synthesis at a concentration (IC50, 0.1 μg/ml) that is 1,000-fold lower than the concentration (IC50, 100 μg/ml) required to inhibit cellular DNA synthesis (155). In contrast to ganciclovir, which provides only a weak and transient inhibition of viral DNA synthesis and viral replication, cidofovir was found to confer a pronounced and prolonged inhibition of viral DNA synthesis and viral replication, lasting for at least 7 days after an exposure time as short as 6 h postinfection (156).
The long-lasting antiviral action of cidofovir allows infrequent dosing with the drug (i.e., only once a week or every other week), which clearly distinguishes cidofovir from other antiviral drugs (acyclovir, penciclovir, and ganciclovir), which have to be administered several times daily to sustain an antiviral response. The long-lasting antiviral action of cidofovir can be attributed to the long half-life of the HPMPC metabolites (HPMPCp, HPMPCpp, and HPMPCp-choline) that are formed intracellularly following uptake of HPMPC by the cells (presumably by endocytosis) (53). In particular, HPMPCp-choline may serve as the intracellular depot form of HPMPC, since its intracellular half-life as extremely long (48 h) (49, 100).
The cellular uptake of HPMPC is rather slow, due to the presence of the negatively charged phosphonate group. This uptake can be greatly enhanced (2) if the phosphonate group is esterified, as in 1-O-hexadecyloxypropyl-cidofovir (HDP-CDV); compared to cidofovir itself, HDP-CDV demonstrated a multiple-log enhancement in antiviral activity against both poxviruses (vaccinia virus and cowpox virus) (110) and herpesviruses (HSV and CMV) (18).
The acyclic nucleoside phosphonates owe their selective antiviral activity to the fact that in their diphosphorylated form, they have a higher affinity (lower Ki value) for the viral DNA polymerases (HSV-1 DNA polymerase, CMV DNA polymerase, and HIV-1 reverse transcriptase [RT]) than for cellular DNA polymerases α, β, γ, δ, and ɛ (references 116 and 147 and references therein). HPMPCpp, PMEApp, and PMPApp interact as competitive inhibitors or alternate substrates with the normal substrates (i.e., HPMPCpp with dCTP and PMEApp and PMPApp with dATP) for the viral DNA polymerases. The incorporation of one molecule of PMEA or PMPA at the 3′ end of the growing DNA chain suffices to terminate further chain elongation (147). PMPA is more faithful a chain terminator of the HIV-1 RT reaction than PMEA, since it is incorporated to a lower extent by the cellular DNA polymerases α, β, and γ (51). The 3′ → 5′ exonuclease activity of DNA polymerases δ and ɛ have proved to be able to excise PMEA from the 3′-OH end of DNA, albeit at a rate 1 order of magnitude lower than that of the dAMP residue (24). PMPApp was found to be an exceptionally poor substrate (1,000-fold less efficient than dATP) and a weak inhibitor of cellular DNA polymerases α, δ, and ɛ (25). Thus, PMPApp may minimally interfere with nuclear DNA synthesis, and this may at least partially explain its low cytotoxicity and favorable safety profile in the treatment of HIV infections.
For HPMPC, two consecutive incorporations (at the 3′ end of the DNA chain) are required to efficiently shut off CMV DNA elongation (226). HPMPC is incorporated in the DNA product with the correct complementation to dGMP in the template, and the incorporated HPMPC is not excised by CMV DNA polymerase. Incorporation of one HPMPC molecule causes a marked decrease in the rate of DNA elongation; incorporation of two consecutive HPMPC molecules causes a virtual stop in DNA synthesis, so that incorporation of a third (consecutive) HPMPC molecule cannot be detected. Incorporation of two HPMPC molecules separated by a dNMP (dAMP, dGMP, or dTMP) also drastically decreases the rate of DNA chain elongation by CMV DNA polymerase (226).
While the antiviral effects of cidofovir can be attributed to the interaction of HPMPCpp with the viral DNA polymerase and incorporation of HPMPC into the viral DNA chain, its specific inhibitory activity against the proliferation of human papillomavirus (HPV)-infected cells (6) must imply additional or alternative mechanisms of action. The antiproliferative effect of cidofovir on the growth of HPV-infected cells, akin to its inhibitory effect on the growth of nasopharyngeal carcinoma (142, 154), may be ascribed to the induction of apoptosis (4, 7). This, in turn, may be related to the ability of cidofovir to restore the function of the tumor suppressor proteins p53 and pRb (which are neutralized by the oncoproteins E6 and E7, respectively) in HPV-infected cells (1).
Andrei et al. (4) have reported that in HPV-infected, but not uninfected, human keratinocytes, cidofovir caused cell death by apoptosis, as evidenced by several parameters of apoptosis: (i) induction of caspase protease activity, (ii) translocation of phosphatidylserine from the inner part of the plasma membrane to the outer layer, (iii) disintegration of the nuclear matrix protein, (iv) DNA fragmentation, and (v) number of cells in apoptotic phase following cell cycle analysis. Induction of apoptosis in HPV-positive cells by cidofovir was associated with accumulation of the tumor suppressor protein p53 and the cyclin-dependent kinase inhibitor p21/WAF-1 (4).
Polyomaviruses are genomically and functionally related to papillomaviruses; like papillomaviruses, polyomaviruses are able to induce tumors, e.g., hemangiomas in rats (127). In fact, cidofovir can successfully suppress polyomavirus-induced tumor formation in rats (127). It is therefore tempting to speculate that in its action against polyomavirus- associated lesions, cidofovir follows the same strategy as against HPV-associated lesions, namely, induction of apoptosis.
ANTIVIRAL ACTIVITY SPECTRUM
The acyclic nucleoside phosphonates possess an antiviral activity spectrum that is quite different from that of the “classical” nucleoside analogues acyclovir, ganciclovir, and penciclovir. Whereas the activity spectrum of acyclovir, ganciclovir, and penciclovir is restricted to the main herpesviruses HSV, VZV, CMV, and Epstein-Barr virus (EBV) and only strains that are either TK+ (HSV, VZV, and EBV) or PK+ (CMV), cidofovir (62, 64) is active against all herpesviruses (including EBV and its murine counterpart, murine herpesvirus 68 [MHV-68] [151], human herpesvirus 6 [HHV-6], HHV-7, and HHV8, the putative cause of Kaposi's sarcoma [kS] [109, 138], and the TK− HSV, TK− VZV, and PK− CMV strains); furthermore, cidofovir is also active against adenovirus, polyomavirus, papillomavirus, and poxvirus (Table 1). In particular, the activity of cidofovir against poxviruses (including vaccinia virus, cowpox virus, camelpox virus, monkeypox virus, and variola virus [192]) and parapoxviruses (such as orf virus [148]) has recently received considerable attention (66-68). The activity of cidofovir against molluscum contagiosum virus (MCV) is surmised from recent observations that cidofovir in HIV-infected patients effected a complete resolution of recalcitrant molluscum contagiosum lesions following either topical or systemic administration (59, 103, 137, 212, 231). The comparative activities of cidofovir and various other compounds against HHV-6, HHV-7, HHV-8, and (murine and primate) polyomaviruses (8, 150, 173, 205) have been reviewed recently (70).
TABLE 1.
Antiviral activity spectrum of the acyclic nucleoside phosphonates
| Virus | Activity
|
||
|---|---|---|---|
| Cidofovir | Adefovir | Tenofovir | |
| DNA viruses | |||
| Papovaviridae | |||
| Polyomavirus | • | ||
| Papillomavirus | • | ||
| Adenoviridae | |||
| Adenovirus | • | ||
| Herpesviridae | |||
| HSV-1 | • | • | |
| HSV-2 | • | • | |
| VZV | • | • | |
| CMV | • | • | |
| EBV | • | • | |
| HHV-6 | • | • | |
| HHV-7 | • | • | |
| HHV-8 | • | • | |
| Poxviridae | |||
| Variola virus | • | ||
| Cowpox virus | • | ||
| Monkeypox virus | • | ||
| Camelpox virus | • | ||
| Vaccinia virus | • | ||
| MCV | • | ||
| Orf virus | • | ||
| Hepadnaviridae | |||
| HBV | • | • | |
| RNA virus infections | |||
| Retroviridae | |||
| HIV-1 | • | • | |
| HIV-2 | • | • | |
| SIV | • | • | |
| FIV | • | • | |
The antiviral activity spectrum of adefovir is unique in that it encompasses retroviruses and hepadnaviruses as well as herpesviruses (147). This means that adefovir could be used for the treatment of HIV and HBV infections and for the prophylaxis (or therapy) of herpesvirus (including CMV) infections that may occur as opportunistic pathogens during the course of the HIV disease. In contrast to adefovir, tenofovir does not afford activity against herpesviruses. Its activity spectrum is confined to retroviruses and hepadnaviruses (Table 1). Both adefovir and tenofovir have demonstrated activity against a wide range of retroviruses, i.e. simian immunodeficiency virus (SIV), feline immunodeficiency virus (FIV), visna-maedi virus, and murine leukemia/sarcoma viruses (147).
ANIMAL MODEL INFECTIONS
Cidofovir
The antiviral efficacy of cidofovir has been demonstrated in a number of experimental animal model infections that are reminiscent of all major DNA virus infections in humans (62, 64): herpetic encephalitis, herpetic keratitis, adenovirus keratoconjunctivitis, herpetic retinitis, genital herpes, herpes labialis, mucocutaneous HSV infections in immunocompromised patients, varicella, herpes zoster, CMV infections in immunocompromised patients, CMV pneumonitis in bone marrow transplant recipients, EBV-associated diseases, poxvirus infections (molluscum contagiosum, monkeypox, smallpox, and progressive vaccinia), polyomavirus infections (progressive multifocal leukoencephalopathy [PML]), and papillomavirus infections (warts and other HPV-associated diseases) (64).
In particular, the efficacy of topical cidofovir against adenovirus infections has been demonstrated in the rabbit ocular model (79, 175, 177). In the rabbit model, topical cidofovir proved significantly more efficacious against HSV-1 keratitis than did topical acyclovir or trifluridine (176). Cidofovir and trifluridine were both highly effective in preventing the development of experimental HSV-1 stromal disease in rabbits (108).
Wherever cidofovir was directly compared for its antiviral efficacy with other antiviral drugs (acyclovir or ganciclovir), it proved clearly superior; e.g., to acyclovir, in the treatment of mice infected intracerebrally with HSV (69, 227) and to ganciclovir in the treatment of both immunocompetent and immunodeficient mice infected with murine CMV (152, 153, 187). In the treatment of acute murine CMV-induced myocarditis in mice, with treatment beginning 24 h postinfection, cidofovir was also clearly more efficacious than ganciclovir (125).
In a lethal immunosuppression model in BALB/c mice, cidofovir (at 5 mg/kg/day) provided significant protection (i.e., 13-day delay in death and 3,000-fold reduction in virus titers in the lungs) against MHV-68, a rodent virus related to EBV (191); it was concluded that cidofovir may be an excellent candidate for treating EBV infections in humans, particularly in immunocompromised patients.
In the cottontail rabbit papillomavirus model, complete cures were obtained with topical 1% cidofovir at dosing schedules of twice daily for 8 weeks beginning at 4 weeks after infection, when papillomas were clearly visible (44, 81). If cidofovir treatment was combined with viral DNA vaccination, recurrence rates could be further reduced (45).
Cidofovir has proven to be efficacious in the treatment of lethal (systemic) vaccinia virus infections in mice with severe combined immune deficiency (SCID mice) (149), as well as in the treatment of lethal vaccinia virus respiratory infections in BALB/c mice (188, 190), where a single dose of the compound (given subcutaneously 1 day postinfection) sufficed to reduce mortality from 100 to 0%.
A single subcutaneous or intranasal (aerosolized) dose of cidofovir, administered 1 day before or after an intranasal (aerosolized) cowpox virus infection, resulted in 100% survival of the infected mice (33, 34, 189), which means that aerosolized cidofovir could be effective in the prophylaxis or early postexposure therapy of human smallpox or monkeypox virus infection. Given the enhanced oral bioavailability of HDP-CDV compared to cidofovir itself (J. W. Huggins, R. O. Baker, J. R. Beadle, and K. Y. Hostetler, Abstr. 15th Int. Conf. Antiviral Res., abstr. 104, 2002; K. L. Winegarden, S. L. Ciesla, K. A. Aldern, J. R. Beadle, and K. Y. Hostetler, Abstr. 15th Int. Conf. Antiviral Res., abstr. 105, 2002), oral dosing of HDP-CDV may be a viable alternative to intravenous or aerosol delivery of cidofovir to human lungs in patients with a respiratory poxvirus infection or DNA virus infections in general.
Cidofovir has demonstrated remarkable activity as an antitumor agent in a number of animal models of tumor growth: (i) nasopharyngeal carcinoma xenografts in athymic nude mice (142, 154), (ii) human cervical carcinoma xenografts in athymic nude mice (7), (iii) polyomavirus-induced hemangiomas in rats (127), (iv) hemangiosarcoma development in nude mice (126), (v) fibroblast growth factor 2-induced vascular tumor formation in nude mice and SCID mice (128), and (vi) murine melanoma B16 in C57B16/J mice (172). While the mechanism of action of cidofovir against melanoma cell growth was not resolved (172), its inhibitory effect on the growth of hemangiosarcoma (124), endothelium-derived tumors (128), and nasopharyngeal carcinoma (142, 154) could be unequivocally attributed to the induction of apoptosis.
Adefovir
Adefovir has demonstrated remarkable efficacy in the therapy and prophylaxis of a number of retrovirus infections in animal models, as reviewed previously (147). The bis(pivaloyloxymethyl) ester of adefovir, adefovir dipivoxil, was shown to effect a significant reduction in serum viral DNA load in woodchucks chronically infected with woodchuck hepatitis virus (WHV), i.e., >1.6 log10 units after 2 weeks and >2.5 log10 units after 12 weeks of treatment with oral adefovir dipivoxil at 15 mg/kg daily (55).
Oral adefovir dipivoxil was also shown to significantly reduce the viral load in the liver as well as in the serum of transgenic mice expressing HBV: the minimum effective dose was less than 0.1 mg/kg/day for the serum HBV load (106). When evaluated under the same conditions, oral lamivudine at doses up to 500 mg/kg/day only marginally reduced the level of HBV DNA in serum and did not significantly reduce the HBV DNA level in the liver (106).
Adefovir has also proven to be more effective than lamivudine in suppressing viremia and intrahepatic viral DNA in ducklings experimentally infected with duck hepatitis B virus. Although adefovir effected a potent reduction of viremia, it was unable to prevent the initial formation of covalently closed circular DNA (cccDNA). Nor was lamivudine able to do so (74).
Tenofovir
The three characteristic routes of HIV transmission are parenteral (i.e., intravenous, subcutaneous, and intramuscular), perinatal (i.e., from mother to child) and sexual (e.g., intravaginal). Tenofovir has proven effective in the pre- and postexposure prophylaxis of all these routes of SIV infection in macaques. Particularly striking are the effects that have been recorded for tenofovir in the prevention of SIV infection in adult macaques, even when tenofovir therapy was started 24 h after intravenous virus inoculation (213), as well as the therapy of chronic SIV infections in infant rhesus macaques (217) and adult cynomolgus macaques (214). In these macaques, tenofovir at a dose of 30 mg/kg, administered once daily beginning either 2 days before or 4 or 24 h after virus inoculation and continued for 4 weeks, completely prevented SIV infection in all macaques without toxicity, whereas all control animals became infected (213). When tenofovir was administered at the same dosage regimen in macaques with an established SIV infection, it caused a >99% reduction (in some macaques below the lower quantitation limit) of SIV levels in plasma and peripheral blood mononuclear cells, without side effects, while causing a meaningful rise in the mean CD4+ cell counts (214).
Follow-up studies ascertained that postexposure prophylaxis with tenofovir, as well as adefovir, can provide long-term protection against subsequent heterologous SIV challenge, and this was attributed to activation of the antiviral immune response (215). In another study, tenofovir treatment, started 7 days after inoculation of rhesus macaques with SHIV, reduced plasma viral RNA levels to undetectable, with parallel decreases in the infectivity of plasma and infectious cells in peripheral blood mononuclear cells and cerebrospinal fluid (CSF) and stabilization of CD4+ T-cell numbers; following cessation of treatment after 12 weeks, the CD4+ T-cell counts normalized and stabilized in the normal range, despite persistent low-level infection (194).
In newborn rhesus macaques inoculated orally with the highly virulent SIVmac251 strain within 3 days of birth, tenofovir treatment from 5 days old for either 14 or 60 days caused reduced virus levels and enhanced antiviral immune responses (220). If the newborn macaques, inoculated orally with SIVmac251 at the age of 3 days, received a subcutaneous injection of tenofovir (4 mg/kg) either 4 h before or 20 h after, or 1 and 25 h after infection, or a single dose of tenofovir (30 mg/kg) at 1 h after SIV inoculation, they remained SIV negative and seronegative (218).
The use of tenofovir has also been investigated in the postexposure prophylaxis of intravaginal infection of pig-tailed macaques with HIV-2 (159). Tenofovir was administered subcutaneously at 30 mg/kg for 28 days, starting at 12, 36, or 72 h after viral inoculation: early intervention (i.e., treatment started at either 12 or 36 h after virus inoculation) completely abrogated HIV infection via intravaginal exposure (159).
CLINICAL USEFULNESS
Cidofovir
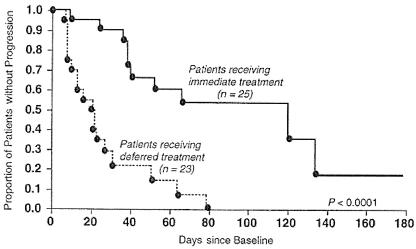
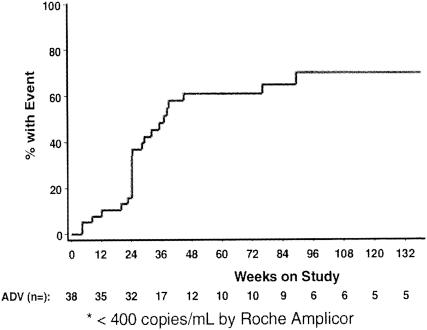
The acyclic nucleoside phosphonates offer great promise for the treatment of a large variety of DNA virus and retrovirus infections (Table 2). Three of these indications have received regulatory approval: cidofovir for the treatment of CMV retinitis in AIDS patients, adefovir dipivoxil for the treatment of chronic HBV infections, and tenofovir disoproxil fumarate for the treatment of HIV infections. Cidofovir has proved efficacious in delaying the progression of CMV retinitis in patients with AIDS (119, 204) and has been approved and marketed worldwide (Vistide) for the treatment of this disease. Figure 3 presents the core data on which the approval of cidofovir for the treatment of CMV retinitis in AIDS patients was based. The compound has to be given intravenously at a dose of 5 mg/kg once weekly for 2 weeks followed by 5 mg/kg intravenously once every other week. Strict adherence to monitoring of renal function before the initiation of cidofovir therapy and concomitant administration of oral probenecid and intravenous hydration are required to minimize drug-related nephrotoxicity (119, 204). Intravenous hydration is achieved with 2 liters of normal saline, and probenecid must be given at a dose of 2 g 3 h before each cidofovir infusion, 1 g 2 h after each infusion and 1 g 8 h after the completion of each infusion. Oral probenecid coadministration has been shown to effectively protect the host against nephrotoxicity in monkeys receiving chronic intravenous cidofovir (117). Nephrotoxicity is the dose-limiting adverse effect in the clinical use of cidofovir. It is due to the accumulation of cidofovir in the renal proximal tubules; this accumulation is mediated by a specific organic anion transporter (46) and can be counteracted by probenecid (117).
TABLE 2.
Major and potential indications for the clinical use of the acyclic nucleoside phosphonates
| Drug | Route of administration | Clinical indication |
|---|---|---|
| Cidofovir (Vistide) | Systemic (intravenous) | CMV retinitis in patients with AIDSa |
| HSV-1, HSV-2, and VZV infections (particularly those that are resistant to acyclovir) | ||
| CMV, EBV, HHV-6, HHV-7 and HHV-8 (i.e., KS) infections | ||
| Polyomavirus infections (e.g., PML) | ||
| Systemic adenovirus infections | ||
| Topical (gel or cream) | Mucocutaneous HSV-1 or HSV-2 infections (particularly when resistant to acyclovir) | |
| Topical (gel, cream, or injection) | HPV-associated papillomatous lesions (recurrent laryngeal papillomas, anogenital warts, cervical intraepithelial neoplasia grade III) | |
| Systemic (intravenous) or topical (gel or cream) | Molluscum contagiosum, orf, and, if necessary, other poxvirus infections (vaccinia, monkeypox, smallpox, etc.) | |
| Topical (eyedrops) | Keratoconjunctivitis due to HSV or adenovirus | |
| Topical (intravitreal) | CMV retinitis | |
| Adefovir dipivoxil (Hepsera) | Systemic (oral) | HBV infections (hepatitis B)a |
| Tenofovir DF (Viread) | Systemic (oral) | HIV infections (AIDS)a |
| HBV infections |
The clinical use has been officially licensed for cidofovir (Vistide) for the treatment of CMV retinitis in AIDS patients, adefovir dipivoxil (Hepsera) for the treatment of chronic hepatitis B, and tenofovir DF (Viread) for the treatment of HIV infections.
FIG. 3.
Time to progression of CMV retinitis: immediate treatment with cidofovir versus deferred treatment with cidofovir. Cidofovir was given intravenously at 5 mg/kg once weekly for 2 weeks followed by 5 mg/kg every other week. Administration of cidofovir was accompanied by intravenous hydration with 2 liters of normal saline and 4 g of probenecid orally (2 g at 3 h before, 1 g at 2 h after, and 1 g at 8 h after each cidofovir infusion). (Reprinted from reference 119 with permission of the publisher.)
Pharmacokinetic data following a single intravenous infusion at the recommended dosage regimen (5 mg/kg, with concomitant oral administration of probenecid) have been determined (Cmax [peak serum drug concentration] = 19.6 mg/liter; AUC [area under the serum concentration-time curve] = 40.8 mg/liter; CLS [clearance of drug from serum] = 0.138 liter/h/kg; CLR [renal clearance] = 0.096 liter/h/kg; VSS [volume of distribution at steady state] = 0.53 liter/kg; t1/2β [elimination half-life] = 2.2 h) (54, as quoted in reference 164). Cidofovir is poorly bound to plasma proteins and is excreted almost entirely as unchanged drug in the urine (>90% within 24 h). However, conventional pharmacokinetic measurements do not accurately reflect the duration of action of cidofovir, since the antiviral effect is dependent on the intracellular concentrations of the active phosphorylated metabolites within cells. As noted above, these metabolites have a long intracellular half-life (48 h for the HPMPCp-choline adduct [49, 100]), and this may contribute to the prolonged antiviral action of cidofovir.
CMV retinitis.
In the study reported by Lalezari et al. (119), cidofovir was found to be efficacious in delaying the progression of CMV retinitis (in patients with AIDS) when given intravenously at 5 mg/kg once weekly for 2 weeks (induction therapy) followed by once every other week (maintenance therapy). Treatment was associated with manageable side effects. Strict adherence to monitoring of renal function before cidofovir was administered and concomitant administration of probenecid and saline hydration appeared to minimize drug-related nephrotoxicity (119). In the SOCA/ACTG trial (209a), cidofovir at two treatment regimens (induction therapy, as indicated above; maintenance therapy at 5 mg/kg every other week [high dose] or 3 mg/kg every other week [low dose]) effectively slowed the progression of CMV retinitis (in patients with AIDS), with the high-dose regimen being clearly more efficacious than the low-dose regimen (209a). Further follow-up over a long-term period (210) showed similar rates of progression and median times to progression with both low maintenance and high maintenance doses of cidofovir to those reported in the initial study (210). From the long-term follow-up study (21), it was concluded that cidofovir is effective for the treatment of CMV retinitis but that it has potential for toxicity (both renal and ocular), which typically resolves on discontinuation of therapy.
The efficacy of cidofovir therapy for AIDS-associated CMV retinitis has been confirmed in patients receiving highly active antiretroviral therapy (HAART), although in this study a higher incidence of iritis was noted, probably as a consequence of the patients' enhanced ability to mount an inflammatory response (21). In fact, anterior uveitis occurs after a mean of 8.5 intravenous infusions of cidofovir (52); it responds to treatment with topical corticosteroids and mydriatics and does not preclude the continuation of cidofovir therapy (3) unless ocular hypotony develops (13).
To assess the effect of intravenous cidofovir on delaying the progression of previously treated, relapsing CMV retinitis, a randomized, controlled comparison of two maintenance doses of cidofovir was conducted: AIDS patients with CMV retinitis that had progressed despite treatment with ganciclovir, foscarnet, or both, were randomized to receive induction cidofovir (5 mg/kg once weekly for 2 weeks) and then maintenance therapy with either 5 mg/kg or 3 mg/kg once every other week (118). The median time to retinitis progression as assessed by retinal photography was not reached in the 5-mg/kg dose group and was 49 days in the 3-mg/kg dose group (118).
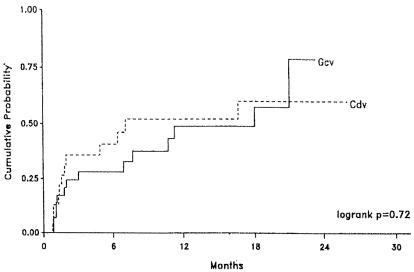
Recent studies have indicated that the regimens of, on the one hand, ganciclovir implantation (i.e., a surgically placed intraocular implant) plus oral ganciclovir and, on the other hand, intravenous cidofovir are equally effective in slowing the progression of CMV retinitis (Fig. 4) and preventing visual loss (Fig. 5) (211). A phaseI study indicated that combination therapy of intravenous cidofovir (5 mg/kg intravenously every 2 weeks) with oral ganciclovir (1 g orally three times a day) may further enhance clinical efficacy (104).
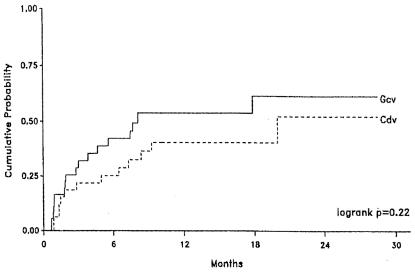
FIG. 4.
Kaplan-Meier curves showing the probability of retinitis progression in AIDS patients with CMV retinitis who were randomized to a regimen of ganciclovir implant plus oral ganciclovir (Gcv) or intravenous cidofovir (Cdv). Cidofóvir was administered according to the dosage schedule explained in the legend to Fig. 3. Ganciclovir was administered as a surgical intraocular implant together with an oral dosage regimen of 1 g three times daily. (Reprinted from reference 211 with permission of the publisher.)
FIG. 5.
Kaplan-Meier curves showing the probability of a 15-letter or greater loss of visual acuity in AIDS patients with CMV retinitis who were randomized to a regimen of ganciclovir implant plus oral ganciclovir (Gcv) or intravenous cidofovir (Cdv). Treatment and dosage regimens were as explained in the legends to Fig. 3 and 4. (Reprinted from reference 211 with permission of the publisher.)
Cidofovir has also proved efficacious, on intravitreal injection, against CMV retinitis (in patients with AIDS) (112, 113), although intravitreal injection of HPMPC should be used with caution because of the risk of anterior uveitis and decrease of intraocular pressure (15).
CMV disease.
In clinical trials cidofovir has proven efficacious not only against CMV retinitis but also against other manifestations of CMV disease, e.g., intravenously against asymptomatic CMV infection in HIV-infected patients (121, 165). Cidofovir can be recommended as a preemptive treatment for CMV disease (i.e., treatment initiated if CMV antigen or DNA is detected in the blood) after allogeneic blood stem cell transplantation (163). In the study reported by Platzbecker et al. (163), 9 (90%) of 10 patients showed a response to cidofovir treatment, with 7 of the 9 experiencing a complete clearance of the virus (pp65 negative, PCR negative); treatment-related toxicity was moderate, with 4 patients developing reversible renal impairment.
In another study (129), half of the patients who were treated for CMV disease after allogeneic stem cell transplantation responded to cidofovir therapy, as did 66% of the patients who had failed or relapsed after previous preemptive therapy with ganciclovir or foscarnet and 62% of the patients in whom cidofovir was used as the primary preemptive therapy (129). Similar results were obtained in another prospective study of primary preemptive therapy with cidofovir (31).
Cidofovir should also be considered as second-line therapy in patients with CMV disease failing to respond to ganciclovir or foscarnet (54, 129). According to a case report (28), cidofovir effected a complete resolution of CMV retinitis, CMV encephalitis, and CMV esophagitis after only 2 months of intravenous therapy and after initial attempts to stop the disease with ganciclovir and foscarnet had been unsuccessful.
Herpesvirus infections other than CMV infections.
Cidofovir has proven efficacious not only against CMV disease but also against HSV infections, e.g., when injected intravenously (115, 122) or applied topically (120, 195) to treat acyclovir-resistant mucocutaneous HSV infections. Such acyclovir-resistant HSV infections may be quite severe in immunosuppressed (e.g., AIDS) patients. As described in a recent case report, a severe perianal HSV-2 infection in an AIDS patient that was refractory to both ganciclovir and acyclovir treatment healed virtually completely within 30 days of treatment with intravenous cidofovir at a dose of 5 mg/kg once weekly for a total of 3 weeks (115). In a child with AIDS presenting with a facial HSV ulcer that had developed resistance to acyclovir and did not respond to foscavir either, local application of cidofovir 1% led to a prompt recovery (123).
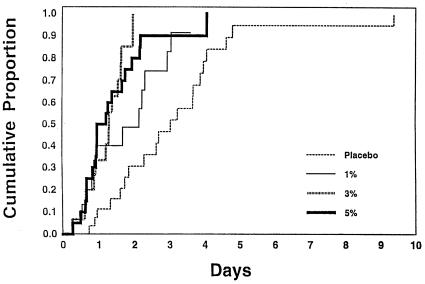
The safety and efficacy of single topical applications of 1, 3, and 5% cidofovir gel have been monitored in a multicenter double-blind, randomized phase I/II dose-escalating study for the treatment of early, lesional, recurrent genital herpes at five Canadian outpatient sites (178). Cidofovir gel at all strengths significantly decreased the median time to negative virus culture: 3.0 days in the placebo group versus 2.2, 1.3, and 1.1 days in the 1, 3, and 5% cidofovir gel treatment groups, respectively (Fig. 6). Application site reactions occurred in 3, 5, 19, and 22% of the patients in these four groups, respectively. Additional studies are warranted to further identify the optimal efficacious dose of cidofovir that can be tolerated (178).
FIG. 6.
Cumulative proportion of patients with genital herpes who converted to HSV culture negativity following a single topical application of placebo, or 1, 3, or 5% cidofovir gel. (Reprinted from reference 178 with permission.)
The emergence of acyclovir- and/or foscarnet-resistant HSV-1 infections after allogeneic stem cell transplantation has become an increasing concern (38); since these HSV strains retain sensitivity to cidofovir, the use of cidofovir in such cases should be advocated. In fact, in a specific case of a foscarnet- and acyclovir-resistant HSV infection after an unrelated bone marrow transplantation for a relapse of acute myeloblastic leukemia, cidofovir effected spectacular improvements after each intravenous dose (5 mg/kg) and complete healing of the herpetic lesions after the seventh injection (29). In another patient, who had undergone umbilical cord stem cell transplantation and who had severe unremitting HSV-1 mucositis of the oropharynx, which was unresponsive to acyclovir and foscarnet therapy, the mucositis cleared after three consecutive once-weekly doses of cidofovir (intravenously at 5 mg/kg once weekly) and the patient was able to tolerate oral nutrition (130).
There is anecdotal evidence for the efficacy of cidofovir in the treatment of oral hairy leukoplakia (which is presumably due to EBV infection) (121). According to a case report, treatment with cidofovir together with rituximab (anti-CD20 monoclonal antibody) led to complete remission of an EBV-associated lymphoma involving the central nervous system (95). Also, in another case of EBV-associated lymphoma, intralesional injections of cidofovir led to a marked regression of the lesions (A. Meerbach, M. Schacke, P. Hyckel, H. Kosmehl, and P. Wutzler, Abstr. 15th Int. Conf. Antiviral Res., abstr. 91, 2002). Cidofovir may also be expected to be effective in the adjunctive treatment of nasopharyngeal carcinoma since it strongly inhibits the growth of nasopharyngeal carcinoma xenografts in nude mice, apparently through the induction of apoptosis (142, 154).
Whether cidofovir would be effective in the treatment of KS máy depend on the interplay of different factors. In two patients with AIDS, an important regression of all cutaneous KS lesions was noted after 3 months of cidofovir treatment (5 mg/kg intravenously at 1-week intervals for the first two injections and every 2 weeks thereafter) (136). A clinical response of KS lesions to intravenous cidofovir treatment has also been noted in an HIV-negative homosexual man (84). In another patient without AIDS, classical KS was not affected by intralesional injections of cidofovir (186). Successful treatment of KS in a HIV-negative man was observed with intravenous cidofovir treatment added to liposomal daunorubicin (12). Abatement of cutaneous KS was noted in a patient with AIDS and CMV retinitis who was treated with cidofovir (94). Since opportunistic infections such as those due to CMV may trigger the development of KS through the release of cytokines and growth factor, inhibition of CMV replication by cidofovir may contribute to the abatement of KS lesions (185).
Adenovirus infections.
Adenovirus infection can be particularly severe in allogeneic stem cell transplant recipients. Intravenous cidofovir (with concomitant probenecid) at a dose of 5 mg/kg/week for 2 weeks and then every 2 weeks for a total of five doses proved successful in suppressing the manifestations of adenovirus disease in an allogeneic stem cell transplant recipient (174).
In five children who had developed a systemic adenovirus infection after bone marrow transplantation for leukemia, cidofovir (administered intravenously at 5 mg/kg once weekly for 3 weeks, then every 2 weeks) effected clinical improvement of diarrhea, cystitis, and fever and a concomitant disappearance of the virus, as assessed by PCR and cell culture (124).
From a retrospective analysis of 35 patients who were identified with adenovirus infection after having undergone allogeneic hematopoietic stem cell transplantation, it appeared that only cidofovir and donor leukocyte infusion were effective options whereas ribavirin and vidarabine were not (30).
In a prospective trial initiated to evaluate cidofovir in the treatment of adenovirus infections in hematopoietic stem cell transplant recipients, eight patients were enrolled on a dosage schedule of 1 mg/kg (intravenously) three times weekly (101). All of these patients eventually achieved long-term viral suppression and clinical improvement, although six patients needed prolonged cidofovir therapy for up to 8 months before the cidofovir administration could be stopped without adenovirus recurrence; no dose-limiting nephrotoxicity was observed, and discontinuation of the drug was not required in any patients (101).
Although topical cidofovir has proven to be effective against adenovirus and HSV keratoconjunctivitis in animal models (79, 108, 175-177), it has not been intensively pursued for the corresponding indications in humans. There is an anecdotal report of the potential effectiveness of 0.2% cidofovir eye drops in a patient with adenoviral conjunctivitis (91), but a larger study failed to show a statistically significant effect of topical 0.2% cidofovir on the course of the acute phase of adenoviral conjunctivitis (98). Eye drops of 1% cidofovir did prove effective in the prevention of severe corneal opacities and, concomitantly, caused inflammation of the eyelids and conjunctiva (99). This local toxicity is probably related to the too frequent administration (4 or 10 times daily) of the 1% cidofovir eye drops; further clinical studies are needed to delineate the optimal treatment regimen for adenovirus keratoconjunctivitis.
Polyomavirus infections.
There are a vast number of reports pointing to the efficacy of cidofovir (given at the dosage regimen recommended for the treatment of CMV retinitis in AIDS patients) in the treatment of PML in AIDS patients. For a series of 12 patients with AIDS-associated PML, a significant correlation was found between JC virus load and survival time (206). In one case, the JC virus load in CSF, which can be considered a prognostic parameter for the clinical outcome of PML, decreased and then became undetectable after cidofovir treatment; this was associated with clinical improvement (206). This patient had been receiving HAART for 2 months without experiencing a modification in JC virus load, before the cidofovir therapy was started (206).
In HIV-infected hosts, the clinical course of PML is almost invariably fatal and there is no proven effective therapy for this condition. Initial anecdotal reports of responses to cytarabine (cytosine arabinoside) have not been confirmed in larger or randomized trials (78). If, however, cytarabine was combined with cidofovir, a remarkable turnaround of PML was noted in a patient with advanced HIV disease (27); cidofovir may well have been the major factor contributing to the successful resolution of PML in this patient (27).
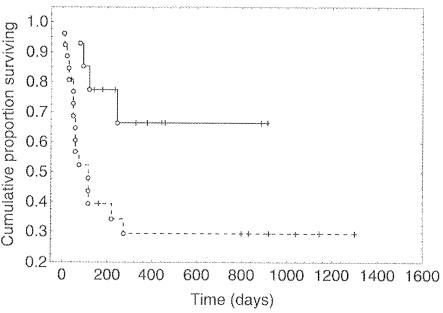
In a multicenter study of consecutive HIV-positive patients with histologically or virologically proven PML, 26 patients were treated with HAART only while 14 patients were treated with HAART plus cidofovir (5 mg/kg intravenously per week for the first 2 weeks and alternate weeks thereafter) (77). Cidofovir added to HAART was associated with a more effective control of JC virus replication, with improved neurological outcome and survival compared with that due to HAART alone (Fig. 7) (77).
FIG. 7.
Efficacy of cidofovir combined with HAART in AIDS-associated PML. Kaplan-Meier curves show the survival of patients receiving HAART plus cidofovir (continuous line) versus patients receiving HAART only (dashed line) (log rank, P = 0.01). Cidofovir was administered intravenously at 5 mg/kg once weekly for the first 2 weeks and on alternate weeks thereafter (see also the legend to Fig. 3). (Reprinted from reference 77 with permission of the publisher.)
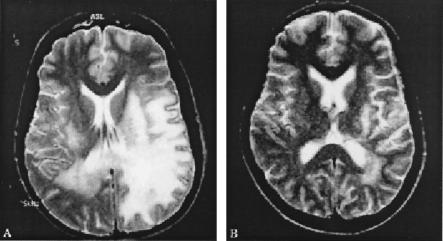
Substantial clinical, virological, and neuroradiological improvement has also been reported in various other AIDS patients with PML following the addition of cidofovir to the HAART regimen (32, 75, 166, 170, 232). This suggests that the combination of HAART with cidofovir improves the outcome of AIDS-related PML (232). For a typical example of the neuroradiological improvement following cidofovir therapy, see Fig. 8 (75). Also, the efficacy of cidofovir in the treatment of JC virus-associated PML has been demonstrated in a patient with systemic lupus erythematosus (179).
FIG. 8.
Response of PML to cidofovir after failure of HAART alone. T2-weighted axial brain magnetic resonance imaging scan shows marked neuroradiologic improvement 12 weeks after addition of cidofovir to HAART. Cidofovir was administered intravenously at the dosage schedule explained in the legend to Fig. 3. (Reprinted from reference 75 with permission of the publisher.)
In a patient with idiopathic CD4+ lymphocytopenia and PML, cidofovir was found to be apparently inefficacious (93). However, in this particular case (93), it was not demonstrated whether the disease was actually caused by, or associated with, JC virus (93). Similarly, no clinical benefit was noted with cidofovir in the treatment of PML in a child with hyperimmunoglobulin E recurrent infection syndrome (9), although in this patient a CSF sample obtained after the first two cidofovir infusions was still positive for JC virus DNA (9).
In a follow-up study of the multicenter observational study mentioned above (77), it was ascertained that the 1-year cumulative probability of survival was 0.61 with cidofovir and 0.29 without. After adjusting for baseline CD4 counts, the JC virus load in the CSF, Karnofsky score, and use of HAART prior to the onset of PML, the use of cidofovir was independently associated with a reduced risk of death (76).
In another monocenter observational study of the effect of cidofovir on AIDS-associated PML, the 1-year cumulative probability of being active was 62% in the cidofovir-plus-HAART group, compared to 53% in the HAART group (87). An additional benefit with respect to survival was observed in patients who were given cidofovir after adjustment to the baseline variables CSF JC virus load, CD4 cell count, and expanded disability status scale (87).
Although there is overwhelming evidence to suggest that cidofovir offers at least partial benefit in the treatment of JC virus-associated PML (see above), its role in the treatment of BK polyomavirus-associated hemorrhagic cystitis is less well established. In a patient with BK polyomavirus-associated acute hemorrhagic cystitis who had received an allogeneic bone marrow transplant, cidofovir proved efficacious (90), but in another case of BK virus-associated hemorrhagic cystitis in an HIV-infected patient, there was apparently no response to cidofovir (17). BK virus-associated nephropathy has been increasingly recognized as an important cause of renal transplant dysfunction, and cidofovir (at a dose as low as 0.25 to 1 mg/kg) was found to reduce BK viruria to undetectable levels, accompanied by stable renal function for 6 to 26 months post-therapy.
HPV infections.
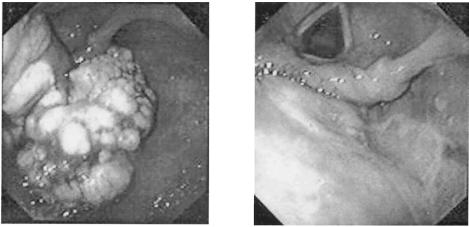
Complete and permanent remissions of papillomatous lesions have been achieved following either topical gel application or direct intralesional injections of cidofovir. The first case of a hypopharyngeal/esophageal papilloma (due to HPV-16) that showed complete regression after topical cidofovir therapy was reported several years ago (Fig. 9) (216).
FIG. 9.
Hypopharyngeal papilloma before and after treatment with cidofovir. Cidofovir was administered by local injection (directly into the tumor) at 1.25 mg/kg at weekly intervals. Complete regression of the tumor was achieved after seven injections (216).
The efficacy of cidofovir in the topical treatment (as a 1% gel) of anogenital HPV-16 infections was first shown in three AIDS patients with severe relapsing penile, perigenital, intra-anal or cervical/vulvar condylomata (198); following topical cidofovir treatment, the lesions disappeared, and the patients remained free of disease during the 6- to 12-month follow-up period (198). Recalcitrant condyloma, unresponsive to any other form of treatment, disappeared after topical application of cidofovir 1.5% in a viscous gel (97); when applied topically at 1% in amphiphile cream once daily for 2 weeks, cidofovir effected a virtually total disappearance of extensive penile condyloma acuminata (181). A randomized, placebo-controlled trial indicated that 1% cidofovir cream was significantly more effective than vehicle cream in the treatment of external anogenital warts in HIV-infected patients (135).
In another double-blind, placebo-controlled, study of 1% cidofovir gel in the treatment of anogenital HPV infections, a partial to complete response was observed in 84.2% of the cidofovir-treated patients compared to 18.2% of the placebo-treated patients (197).
An open randomized prospective study indicated that topical 1% cidofovir gel was more effective than electrocautery in preventing recurrences of genital warts in HIV-infected patients (relapse rates, 35.29 and 73.68%, respectively) and that the two procedures, if combined, effected a complete response (158).
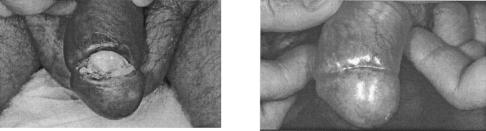
Initial clinical trials with 1% cidofovir topical gel also point to its efficacy in the treatment of cervical intraepithelial neoplasia grade III (196): partial to complete responses were observed histologically in the majority of the patients and were confirmed by PCR, and this effect was seen after only three applications (every other day) (196). Complete and permanent eradication of vulvar intraepithelial neoplasia grade III was achieved with topical 1% cidofovir in a patient who did not respond to interferon and isotretinoin (114). Also, bowenoid papulosis (perianal intraepithelial neoplasia) in an AIDS patient was found to respond to cidofovir: after three treatment cycles (0.4% cidofovir cream twice daily for five consecutive days every 15 weeks), the lesions completely disappeared (80). A similar, complete cure was seen in an AIDS patient with bowenoid papulosis of the penis after three rounds of 1% cidofovir cream applications (once daily for 5 days) (Fig. 10) (201).
FIG. 10.
Topical cidofovir in the treatment of Bowenoid papulosis of the penis. Cidofovir was administered topically at 1% in Beeler base. Two months after initiation of treatment (three courses of once-daily applications for 5 days), the lesion had completely disappeared (201).
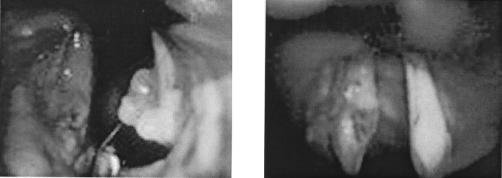
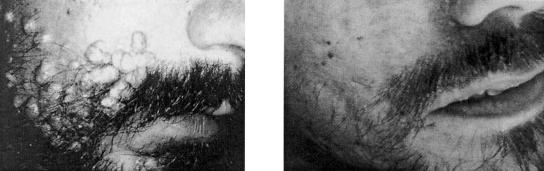
The potential of cidofovir to treat of laryngeal papillomatosis has been clearly demonstrated by studies of a number of patients with severe recurrent laryngeal papillomatosis (200), where local intralesional injections of cidofovir (diluted in saline at 2.5 mg/ml) caused a complete and durable regression of the papillomatous lesions in the majority of the patients (Fig. 11). Although minor papilloma recurrences may arise following cidofovir treatment, these recurrences seem to respond to additional intralesional injections, so that eventually complete cures are achieved (225). Percutaneous injection of cidofovir has been recommended for office-based treatment of adult patients with anterior laryngeal papillomatosis, since it reduces the need for repeated direct laryngoscopy and laser ablation under general anesthesia (42). Intralesional cidofovir therapy has been advocated as an excellent treatment option for laryngeal papillomas in adults, although it requires perseverance from both the patient and the surgeon to achieve remission of the disease (22).
FIG. 11.
Laryngeal papilloma before and after treatment with cidofovir. Cidofovir (2.5 mg/ml) was injected directly into the tumor. Complete regression of the tumor was achieved after 15 injections over 9 months (200).
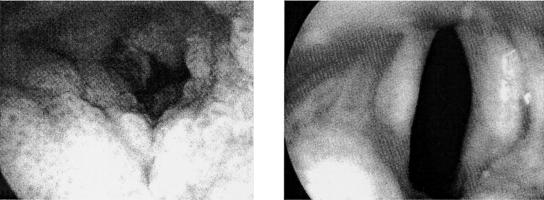
The potential benefit of intralesional administration of cidofovir (2.5 mg/ml) has also been assessed in children with severe recurrent respiratory papillomatosis: they demonstrated a dramatic response at the follow-up visit 9 months after the last injection of cidofovir (i.e., complete regression of the tumor after 15 injections every 2 to 3 weeks) (Fig. 12) (167), which led the authors (167, 168) to conclude that intralesional injection of cidofovir may be of benefit in the treatment of severe respiratory papillomatosis in pediatric patients.
FIG. 12.
Intralesional cidofovir for recurrent respiratory papillomatosis in children. Cidofovir (2.5 mg/ml) was injected into the tumor. Complete regression of the tumor was achieved after 15 injections every 2 to 3 weeks. (Reprinted from reference 167 with permission of the publisher.)
From three case studies, it now appears that systemic (intravenous) cidofovir, whether combined with alpha interferon or used alone, would also be effective against recurrent respiratory papillomatosis with involvement of the lung parenchyma (11, 58, 221); under these conditions, laryngeal and tracheal lesions may regress or even disappear and pulmonary lesions may undergo consolidation.
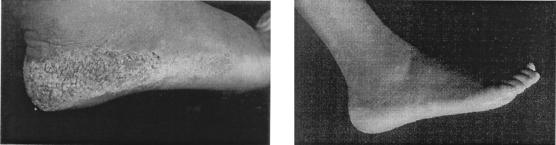
Cidofovir has also proved successful in the topical treatment of verruca vulgaris: two patients with a 2- to 3-year history of recurrent verruca vulgaris that did not respond to repeated conventional therapies (electrodesiccation, liquid nitrogen, cryosurgery, CO2 laser ablation, etc.) experienced complete disappearance of the verruca vulgaris lesions following once- or twice-daily therapy with 3% topical cidofovir (229). These patients have remained completely free of lesions for more than 40 weeks (230). A large plantar wart (caused by HPV-66) in an AIDS patient completely resolved within 3 to 4 weeks following two courses of topical 3% cidofovir therapy (Fig. 13) (60).
FIG. 13.
Topical cidofovir in the treatment of a large plantar wart (caused by HPV-66). Cidofovir was administered topically at 3% in emollient cream twice daily. The wart disappeared within 3 to 4 weeks. (Reprinted from reference 60 with permission of the publisher.)
In another AIDS patient, recalcitrant HPV gingival infection, which did not respond to conventional therapies, healed within 2 weeks after treatment with 1% cidofovir cream (once daily for 5 consecutive days, for 2 weeks) (35). Topical cidofovir (1% cream) has proved effective in the treatment of cutaneous HPV lesions, refractory to conventional methods, in a number of patients (203), including patients with AIDS (36). Inflammation and/or erosion may occur at the site of application of the cidofovir cream (36). While such erosions invariably heal and may actually reflect an effective response of the HPV lesions to cidofovir treatment, care should be taken not to apply cidofovir on abraded skin. Also, for topical application, a concentration of 1% (wt/wt) cidofovir should not be exceeded. If applied at too high a concentration (e.g., 4%) over a too large surface of abraded skin for too long a period (daily for 12 days), there is a risk of systemic toxicity, as illustrated by a case of acute renal failure in a bone marrow transplant recipient with chronic renal failure who developed genital condylomas and was treated with topical 4% cidofovir for 12 days (23).
In view of the circumstantial evidence for the effectiveness of cidofovir in the treatment of virtually all HPV-associated diseases, it is intriguing that in one particular patient with epidermodysplasia verruciformis, topical and systemic treatment of cidofovir had no apparent effect (169). On the other hand, cidofovir injected intralesionally proved successful in the treatment of squamous cell carcinoma, a disease that is supposedly not related to HPV (37). This antineoplastic activity may be based on the same mechanism, i.e., induction of apoptosis, that underlies the inhibitory effect of cidofovir on the growth of HPV-associated tumors.
Poxvirus infections.
Cidofovir is effective in the treatment of molluscum contagiosum, a cutaneous skin growth caused by the poxvirus MCV. Recalcitrant molluscum contagiosum in three AIDS patients resolved completely and permanently after either topical cidofovir treatment (3% cream, applied once daily) or intravenous cidofovir treatment (5 mg/kg each week for 2 weeks, followed by 5 mg/kg once every 2 weeks) (137). A similar complete and permanent remission of recalcitrant molluscum contagiosum was seen in an AIDS patient following nine cycles of intravenous cidofovir treatment (5 mg/kg once every 2 weeks) (Fig. 14) (103). Similar complete and durable resolution of molluscum contagiosum lesions was noted in children following topical treatment with cidofovir (at either 1% or 3%), e.g., in a boy with Wiskott-Aldrich syndrome (59), in two otherwise healthy children (231), and in two HIV-infected children (212).
FIG. 14.
Intravenous cidofovir therapy in the treatment of molluscum contagiosum in AIDS patients. Cidofovir was administered intravenously (at 5 mg/kg once per week followed by 5 mg/kg once every 2 weeks); after nine cycles (4 months), the lesions had disappeared. (Reprinted from reference 103 with permission of the publisher.)
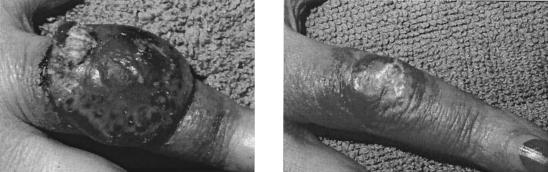
We recently reported the case of a 39-year-old renal transplant patient (under immunosuppression with cyclosporine, methylprednisolone, and mycophenolate mofetil) who developed a giant orf (ecthyma contagiosum) lesion, which continued to grow to dramatic proportions (88). Topical treatment with 1% cidofovir cream (five cycles of 5 days with and 5 days without treatment) effected a complete resolution, with only granulation tissue left; after some signs of recurrence, the lesion was treated with another two courses of cidofovir cream, after which a complete cure ensued (Fig. 15) (88).
FIG. 15.
Orf (ecthyma contagiosum) before and after treatment with cidofovir. Cidofovir was administered topically as a 1% cream in Beeler base once daily for repeated courses (5-days-on/5-days-off therapy), with complete resolution of the lesions after seven courses (2 to 3 months) (88).
Cidofovir has not been clinically used in the treatment of poxvirus infections other than orf or molluscum contagiosum. Based on the data obtained with cidofovir in the treatment of vaccinia virus and cowpox virus infections in experimental animal model infections (33, 34, 149, 188-190), cidofovir may be expected to be effective in the therapy as well as the pre- and postexposure prophylaxis of smallpox, monkeypox, and vaccinia virus infections in humans, whether administered intravenously or perorally (in its oral prodrug form [HDP-CDV]) or aerosolized (against an aerosolized virus infection) (68).
Should smallpox vaccination, based on the use of the live vaccinia virus vaccine, be reinstalled, it would be formally contraindicated to use this vaccine in immunocompromised patients, whatever the cause of their immunodeficiency (primary immune deficiency, HIV infection, immunosuppressive therapy, etc.). Inadvertent use of the live vaccinia virus vaccine in such patients may lead to a serious, life-threatening, disseminated, and progressive vaccinia (111, 171). The therapy or prophylaxis of such complications may well represent a primary indication for the use of cidofovir.
Adefovir
HIV infections.
Adefovir dipivoxil has been initially pursued for the treatment of HIV infections. In a randomized, double-blind, placebo-controlled dose escalation study (16), adefovir dipivoxil, at three dose levels (125, 250, or 500 mg once daily) was found to effect a significant decrease in serum p24 antigen levels and HIV RNA copy numbers. Adefovir dipivoxil was best tolerated at the lowest dose studied (125 mg daily), and its anti-HIV activity was already evident after 8 days of dosing (16). In another randomized, double-blind, placebo-controlled study (71), adefovir dipivoxil at the two dose levels studied (125 mg and 250 mg) was found to effect a significant decrease in HIV-1 RNA plasma load and a significant increase in CD4+ T-cell counts throughout the 12 weeks of treatment. The drug was determined to be safe and well tolerated when administered for 12 weeks (71).
Single-dose pharmacokinetics and the safety of oral adefovir dipivoxil have also been established in children infected with HIV-1 (102), and the results of this study were quoted as providing sufficient information to warrant proceeding to a multidose phase II study to further evaluate the safety, pharmacokinetics, and efficacy of adefovir dipivoxil in children. Hughes et al. (102) noted that in children the oral clearance (CL/F) of adefovir (dipivoxil) was greater than in adults (when a dose of 3.0 mg/kg in children was compared with a 60-mg dose in adults).
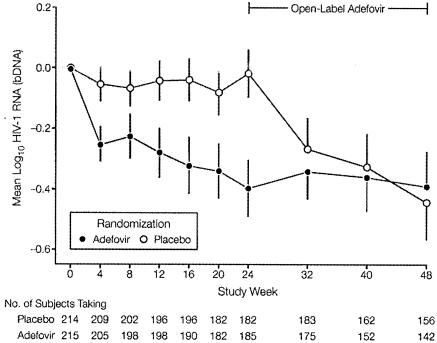
In one 24-week, randomized, double-blind, placebo-controlled multicenter study, adefovir dipivoxil, administered as a single daily oral dose of 120 mg added to stable antiretroviral therapy, effected a 0.4-log10/ml decline from baseline in the HIV RNA plasma load, compared with no change in the placebo group (Fig. 16) (107). This reduction in viral load extended beyond 24 weeks but was accompanied by (reversible) nephrotoxicity (107). In another randomized, double-blind, placebo-controlled multicenter trial, no virologic or immunologic benefit was observed when oral adefovir dipivoxil (120 mg once daily) was added to background antiretroviral therapy in treating advanced HIV disease (85). The difference in the virologic results between the two studies (85, 107) may be explained by the fact that the patients in the latter study (85) had more advanced disease (lower CD4+ cell counts) and were more drug experienced than those in the former study (107). In the latter study (85), the use of adefovir dipivoxil (120 mg daily) was associated with considerable nephrotoxicity.
FIG. 16.
Changes from baseline in HIV-1 RNA levels in patients is a 24-week, randomized, double-blind, placebo-controlled multicenter study. Patients with HIV-1 infection were randomized to receive either a single daily dose of 120 mg of adefovir dipivoxil or placebo for 24 weeks. Open-label adefovir was offered after 24 weeks. (Reprinted from reference 107 with permission of the publisher.)
HIV-infected patients harboring the lamivudine-associated RT M184V mutation showed the largest decline in viral load (almost −1.0 log10 unit from baseline) (157), which seems to relate to the fact that the M184V mutation results in increased in vitro susceptibility to adefovir (140).
Combination therapy with adefovir dipivoxil plus efavirenz, in highly treatment-experienced patients, resulted in a marked viral load suppression (>2.0 log10 units at 24 weeks) but only in patients who had no prior experience with nonnucleoside RT inhibitors (184).
In view of the rather modest reduction in viral load observed with adefovir dipivoxil (only a 0.4-log10/ml decline in viral load [Fig. 16] [107]) and the accompanying risk for nephrotoxicity (development of a Fanconi-like renal syndrome consisting of acidosis, proteinuria, glucosuria, hypophosphatemia, and elevated creatinine levels at the dosage used [120 mg once daily]), adefovir dipivoxil has not further been pursued for the treatment of HIV infections.
HBV infections.
Adefovir dipivoxil has been actively pursued for the therapy of chronic hepatitis B. In a placebo-controlled phase I/II study, adefovir dipivoxil given orally at 125 mg as a single daily dose for 28 days effected a 1.8-log10 pg/ml reduction in HBV DNA levels, compared to an increase of 0.01 log10 pg/ml in the control patients with chronic HBV infection (89).
Lamivudine, the current drug of choice for the treatment of chronic hepatitis B, has been shown to elicit resistance in 20, 38, 49, 66, and 69 of patients after 1, 2, 3, 4, or 5 years of lamivudine therapy, respectively (C. E. Westland, C. S. Gibbs, M. D. Miller, M. Sullivan, J. Fry, C. L. Brosgart, M. Wulfsohn, and S. Xiong, Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Diseases, abstr. 568, 2002). A 48-week, randomized, double-blind, placebo-controlled, multicenter study was designed to evaluate the efficacy of adefovir dipivoxil in patients with chronic hepatitis B due to lamivudine-resistant HBV (M. Peters, H. W. Hann, P. Martin, E. Heathcote, P. Buggisch, A. E. Moorat, M. Sullivan, K. Kleber, R. Ebrahimi, S. Xiong, and S. Brosgart, Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 646, 2002). Patients were randomized to receive either adefovir dipivoxil (10 mg), or adefovir dipivoxil (10 mg) plus lamivudine (100 mg), or lamivudine (100 mg). After 16 weeks, patients in both the adefovir dipivoxil monotherapy and combination adefovir dipivoxil plus lamivudine groups achieved a similar reduction in serum HBV DNA levels from baseline (2.86 and 2.87 log10 copies per ml), compared to no change in patients treated with 100-mg lamivudine monotherapy (Fig. 17). After 32 weeks of treatment, 32% of the patients who had received adefovir dipivoxil monotherapy had lost the lamivudine resistance mutation M204V/I (located within the YMDD motif).
FIG. 17.
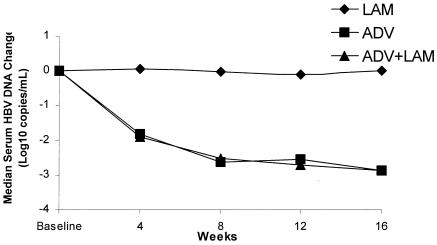
Median changes in serum HBV DNA levels in patients in a 48-week, randomized, double-blind, placebo-controlled multicenter study (study 451). Patients with chronic hepatitis B due to lamivudine-resistant HBV were randomized to receive either 10 mg of adefovir dipivoxil (ADV), 10 mg of adefovir dipivoxil plus 100 mg of lamivudine (ADV + LAM), or 100 mg of lamivudine (LAM) (Peters et al., Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 646, 2002).
An open-label pilot study with oral adefovir dipivoxil (10 mg once daily) in patients coinfected with HIV-1 and lamivudine-resistant HBV indicated significant declines in serum HBV DNA concentrations from baseline (8.64 log10 copies per ml): −3.40 log10 copies per ml at week 24 and −4.01 log10 copies per ml at week 48 (Fig. 18) (20). This treatment was well tolerated and did not produce significant side effects, except for a transient increase in alanine aminotransferase (ALT) levels in some patients. The concomitant use of adefovir dipivoxil and antiretroviral regimens in these HIV-1/HBV-coinfected patients did not influence either HIV-1 replication or the development of HIV-1 mutations (20).
FIG. 18.
Mean changes from baseline in serum HBV DNA concentration in patients coinfected with HIV-1 and lamivudine-resistant HBV. Patients received lamivudine (150 mg twice daily) as part of their HIV-1 antiretroviral regimen. They were given adefovir dipivoxil (10 mg once daily for 48 weeks). Error bars indicate standard errors. (Reprinted from reference 20 with permission of the publisher.)
Successful treatment with adefovir dipivoxil (10 mg once daily, orally) has been reported in several anecdotal cases of lamivudine-resistant HBV infections (161), including patients with fulminant hepatic failure (162) or fibrosing cholestatic hepatitis (223). Acute liver graft failure due to the emergence of lamivudine-resistant HBV promptly resolved during treatment with adefovir dipivoxil (143).
In patients with HBV e antigen (HBeAg)-positive chronic hepatitis B, 48 weeks of 10 or 30 mg of adefovir dipivoxil per day resulted in histologic liver improvement, reduced serum HBV DNA levels, reduced serum ALT levels, and increased rates of HBeAg seroconversion. Since there was a higher frequency of adverse events and renal abnormalities in the group given 30 mg of adefovir dipivoxil per day, the 10-mg dose was considered to have the better risk-benefit profile for long-term treatment (132).
Adefovir dipivoxil has also been the subject of a double-blind, placebo-controlled, multicenter phase III clinical trial in patients with precore mutant (e-antigen-negative) HBV patients (92). Patients were randomized to receive either 10 mg of adefovir dipivoxil or placebo once daily for 48 weeks. Treatment with adefovir dipivoxil resulted in a median reduction in the serum HBV DNA level from baseline of 3.91 log10 copies per ml, compared with a median reduction of 1.35 log10 copies per ml in patients who received placebo (Fig. 19). ALT levels normalized in 72% of the adefovir dipivoxil patients compared to 29% in the placebo group. Of the patients treated with adefovir dipivoxil, 64% exhibited significant improvement in liver histology, compared to 33% of the patients on placebo.
FIG. 19.
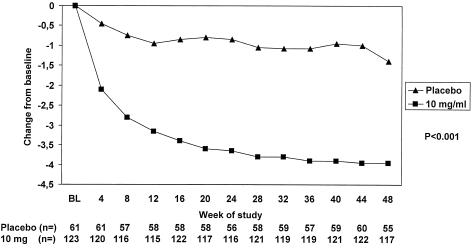
Median changes in serum HBV DNA (log10 copies per milliliter) in patients from a double-blind, placebo-controlled multicenter study (study 438). Patients with precore mutant (e-antigen negative) HBV infection were randomized to receive either 10 mg of adefovir dipivoxil or placebo once daily for 48 weeks (92).
cccDNA is a key intermediate in HBV replication is and considered the reservoir responsible for the persistence of chronic HBV infection. Treatment with adefovir dipivoxil reduced the level of HBV cccDNA in the liver by 89% from baseline at 48 weeks, whereas placebo treatment resulted in no reduction (B. Werle, K. Wursthorn, J. Petersen, S. Bowden, S. Locarnini, C. James, C. Brosgart, S. Xiong, W. Delaney, C. Gibbs, and F. Zoulim, Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 638, 2002). Preliminary data also point to the regression of cirrhosis is 5 of 11 patients following adefovir dipivoxil treatment, compared to 0 of 12 patients following placebo treatment (P. Marcellin, Z. Goodman, T. T. Chang, S. G. Lim, M. Tong, W. Sievert, M. Schiffman, L. Jeffers, M. Wulfsohn, R. Fallis, J. Fry, and C. Brosgart, Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dise. abstr. 560, 2002).
The antiviral efficacy of adefovir dipivoxil in hepatitis B patients is independent of the HBV genotype: after 48 weeks, treatment with adefovir dipivoxil (10 mg once daily, orally) resulted in a significant decrease in serum HBV DNA levels across all HBV genotypes (A, B, C, D, E, F, and G), with mean reductions ranging from 3.4 to 4.2 log10 copies per ml (C. Westland, W. Delaney, H. Yang, J. Fry, C. Brosgart, C. Gibbs, M. Miller, and S. Xiong, Poster Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Diseases, abstr. 273, 2002).
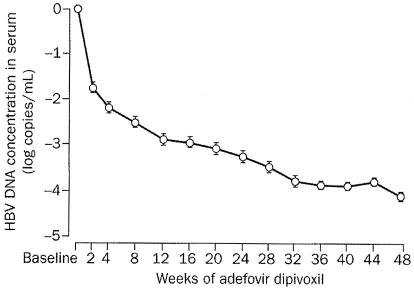
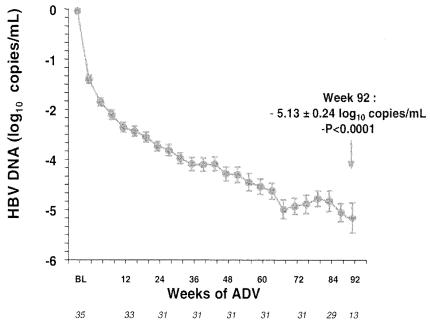
As an extension to the open-label pilot study in patients coinfected with HIV-1 and lamivudine-resistant HBV (20), these patients were further treated with adefovir dipivoxil (10 mg once daily, orally) beyond 48 weeks (Y. Benhamou, M. Bochet, V. Thibault, V. Calvez, M. H. Fievet, M. Sullivan, C. Brosgart, H. Namini, T. Poynard, and C. Katlama, Abstr. 9th Conf. Retroviruses Opportunistic Infect. abstr. 123, 2002; Y. Benhamou, M. Bochet, V. Thibault, V. Calvez, M. H. Fievet, M. Sullivan, C. Brosgart, H. Namini, T. Poynard, and C. Katlama, Poster Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 245, 2002). The mean decrease in serum HBV DNA from baseline (8.64 log10 copies per ml) was −3.40 log10 copies per ml at week 48, −4.77 log10 copies per ml at week 72, and −5.13 log10 copies per ml at week 92 (Fig. 20). Following long-term therapy with adefovir dipivoxil for chronic hepatitis B, HBV DNA was undetectable (<400 copies per ml) by week 100 in 70% of the patients (Fig. 21) (E. J. Heathcote, L. Jeffers, R. Perrillo, T. Wright, M. Sherman, H. Namini, S. Xiong, C. James, V. Ho, J. Fry, and C. Brosgart, Poster Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 294, 2002). Over the course of the study, 21% of the patients achieved seroconversion of the HBV e antigen.
FIG. 20.
Mean changes in serum HBV concentrations following long-term adefovir dipivoxil treatment (ADV) for lamivudine-resistant HBV in patients coinfected with HIV (this is a follow-up of the study in Fig. 18) (Benhamou, et al., Poster Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 245, 2002).
FIG. 21.
Kaplan-Meier plot of the percentage of patients with undetectable HBV DNA levels following long-term adefovir dipivoxil therapy (ADV) for chronic hepatitis B (Heathcote et al., Poster Presentations 37th Annu. Meet. Eur. Asso. Study Liver Dis. abstr. 294, 2002).
Adefovir dipivoxil has also been evaluated for treatment of lamivudine-resistant HBV infection of patients with liver failure requiring liver transplantation and patients with chronic hepatitis after liver transplantation. Although not placebo controlled, adefovir dipivoxil treatment was associated with impressive and favorable evolution of the clinical, virological, and biochemical parameters. In all studies, adefovir dipivoxil proved safe and was well tolerated. At a dose of 10 mg/day, significant renal abnormalities were not observed (D. Mutimer, Abstract, Antiviral Ther. 7:L84, 2002).
The safety and efficacy of adefovir dipivoxil were further assessed in a study of 40 patients (26 without and 14 with liver transplantation) with decompensated chronic hepatitis B, YMDD variant, and reduced clinical and virological response to lamivudine. Addition of adefovir dipivoxil to ongoing lamivudine therapy in these patients was well tolerated and led to significant inhibition of viral replication, and improvement of both clinical status and biochemical parameters (such as ALT levels) (D. Mutimer, H.-W. L. Hann, M. Buti, S. Strasser, K. Watkins, M. Woessner, C. Brosgart, E. Bourne, D. Tait, and R. Perrillo, Abstract, Antiviral Ther. 7:L90, 2002).
Adefovir dipivoxil can be considered a safe drug for the treatment of chronic HBV infections: an integrated analysis of two phase III studies with HBeAg+ and HBeAg− patients revealed a safety profile for adefovir dipivoxil at 10 mg/day that was similar to that of placebo throughout 48 weeks; there was no evidence of renal laboratory abnormalities in either the adefovir dipivoxil or placebo groups (E. J. Heathcote, T. T. Chang, S. G. Lim, S. Hadziyannis, N. Tassopoulos, M. Tong, W. Sievert, M. Wollman, S. Van Doren, J. Fry, and C. Brosgart, Abstract, Antiviral Ther. 7: L105, 2002).
While there has been general concern in the past about the potential nephrotoxicity of adefovir dipivoxil when used at a daily dose of 60 mg (and, a fortiori, at 120 mg), it should be pointed out that in the pivotal phase III clinical trial involving 172 HBV-infected patients, 93% of whom were treated with 10 mg of adefovir dipivoxil daily for 48 weeks, there was a mean reduction in viral titer of 3.52 log10 units and a mean reduction in the ALT level of 49 IU/liter, with no patients having creatinine level increases of >0.5 mg/dl or phosphorus levels of <1.5 mg/dl (18). Benhamou et al. (20) have supported these findings and furthermore described the complications of interpreting the causes of renal toxicity during multiple treatment regimens.
Tenofovir
HIV infections.
In a randomized, double-blind, placebo-controlled, dose escalation clinical trial, intravenous tenofovir monotherapy at two doses (1 and 3 mg/kg/day), administered on days 1 and 8 through 14, effected a significant decline in plasma HIV-1 RNA levels, with the reduction in HIV-1 RNA being sustained for at least 7 days after completion of dosing in the 3-mg/kg/day dose cohort (72). From this study (72) and the pharmacokinetics study in dogs, it was ascertained that tenofovir has a terminal half-life of approximately 10 h and is mainly-excreted unchanged in the urine (57). As with adefovir, several potentially orally bioavailable prodrugs of tenofovir were evaluated for anti-HIV activity, chemical and intestinal stability, and oral bioavailability (10). From a series of alkyl methyl carbamate prodrugs of PMPA, the bis(isopropoxycarboxymethyl)ester of PMPA, termed tenofovir disoproxil, was chosen as the clinical candidate, its oral bioavailability in dogs being 30% (183). Meanwhile, tenofovir disoproxil has been approved and is marketed worldwide as its fumarate salt (Viread; 300 mg once daily) for the treatment of HIV infections.
GS-7340 represents another oral prodrug form of PMPA: it corresponds to 9-[(R)-[[[[(S)-1-(isopropoxycarbonyl)ethyl]amino]phenoxy-phosphinyl]methoxy]propyl]adenine (82): this prodrug of tenofovir, apart from being orally bioavailable, is stable in plasma and selectivity taken up by lymphatic tissue and PBMCs, where it achieves markedly increased levels of tenofovir compared with tenofovir disoproxil (W. Lee, G. He, A. Mulato, W. Delaney, E. Eisenberg, T. Cihlar, S. Xiong, M. Miller, S. Gill, R. Shibata, and C. Gibbs, Abstr. 9th Conf. Retroviruses Opportunistic Infect. abstr. 384-T, 2002).
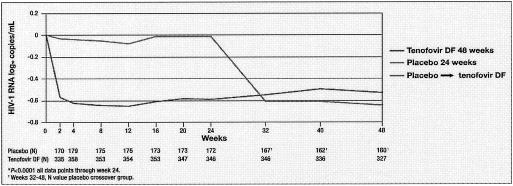
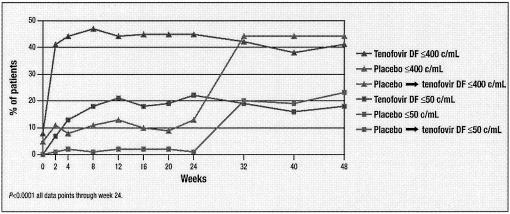
Tenofovir disoproxil fumarate (tenofovir DF) has been evaluated in a 48-week, randomized, double-blind, placebo-controlled study of treatment-experienced HIV-1-infected patients at three different dose levels, namely, 75, 150, and 300 mg daily (180). After 24 weeks, patients who were initially randomized to placebo were given tenofovir DF (300 mg) for another 24 weeks. In this study by Schooley et al. (180) tenofovir DF provided a dose-related durable reduction in the HIV-1 RNA load, with a safety profile similar to that of placebo.
To further assess the efficacy and safety of tenofovir DF in the treatment of HIV-1 infections, a randomized, double-blind, placebo-controlled phase III study was conducted with antiretroviral-experienced patients, whereby either placebo or 300 mg of tenofovir DF was administered orally once daily for 24 weeks, after which all patients received open-label drug for another 24 weeks (K. Squires, G. Pierone, D. Berger, C. Steinhart, N. Bellos, S. L. Becker, S. S. Chen, M. D. Miller, D. F. Coakley, and A. Cheng, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 413-W, 2002). Tenofovir DF effected a rapid antiviral response (0.6 log10 unit reduction in viral load) from week 2, which was maintained through week 48 (Fig. 22). This favorable response was also reflected by a significant increase in the percentage of patients with plasma HIV-1 RNA levels of ≤400 or ≤50 copies per ml following treatment with tenofovir DF (Fig. 23). The safety profile of tenofovir DF in this study was similar to that of placebo. The safety and efficacy of tenofovir DF were confirmed in an expanded-access program study conducted in the European Union, United States, Canada, and Australia, where viral load reductions of 1.2 log10 were noted (Fig. 24) (S. Follansbee, J. Reynes, M. Nelson, B. Clotet, A. Lazzarin, A. Adam, S. Van Doren, R. Buffels, S. Barriere, L. Zagury, I. Miranski, and J. Rooney, Abstr. 9th Conf. Retroviruses Opportunistic Infect. abstr. 415-W, 2002).
FIG. 22.
Mean change from baseline in plasma HIV-1 RNA levels in patients in a randomized, double-blind, placebo-controlled multicenter study (study 907). Antiretroviral-experienced patients with HIV-1 infection were randomized to receive either placebo or 300 mg of tenofovir DF once daily for 24 weeks, after which all patients received open-label drug for another 24 weeks (Squires et al., Abst. 9th Conf. Retroviruses Opportunistic Infect., abstr. 413-W, 2002).
FIG. 23.
Percentage of patients with HIV-1 RNA levels of ≤400 and ≤50 copies/ml for the study (study 907) in Fig. 22 (Squires et al., Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 413-W, 2002).
FIG. 24.
Viral load reductions, as noted in the Viread expanded-access program, after treatment with tenofovir DF (300 mg once daily, orally) in antiretroviral-experienced patients with HIV-1 infection (Follansbee et al., Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 415-W, 2002).
Adding tenofovir DF (300 mg) to existing antiretroviral therapy for highly treatment-experienced patients with preexisting resistance mutations has proved to achieve significant and durable reductions in HIV-1 RNA levels through week 96. Through 96 weeks of tenofovir DF therapy, there was infrequent development of RT mutations associated with tenofovir DF therapy (K65R mutation, 3%), consistent with the durability of the observed HIV-1 RNA reductions (134).
From an ongoing 3-year, randomized, double-blind, clinical trial being conducted at 81 sites in the United States, Europe, and South America and comparing the efficacy and safety of a treatment regimen of tenofovir DF, lamivudine, and efavirenz to a regimen of stavudine, lamivudine, and efavirenz in 600 antiretroviral-naive patients with HIV infection, the 48-week data and, subsequently, the 96-week data were divulged (S. Staszewski, J. Gallant, A. Pozniak, J. M. A. H. Suleiman, E. Dejesus, E. Koenig, S. Coleman, B. Lu, A. K. Cheng, and D. F. Coakley, Oral Presentations XIV Int. AIDS Conf., abstr. Or17, 2002; S. Staszewski, J. E. Gallant, A. L. Pozniak, J. M. A. H. Suleiman, E. DeJesus, J. Sayre, B. Lu, A. Cheng, Abstr. 10th Conf. Retroviruses Opportunistic Infect., abstr. 564b, 2003). Both treatment groups showed a mean reduction in HIV RNA load of 3.09 log10 copies per ml (baseline, 4.9 log10 copies per ml) after 48 weeks; 95% of the patients in the tenofovir DF arm compared to 91% in the stavudine arm had reductions in HIV RNA levels to below 50 copies/ml after 96 weeks. Thus, the stavudine and tenofovir DF arms showed similar, high efficiencies. However, lipid abnormalities (increase in triglyceride and cholesterol levels) were significantly lower (P < 0.001) in the tenofovir DF arm than in the stavudine arm. Also, the toxicities (peripheral neuropathy, lipodystrophy, lactic acidosis, and pancreatitis) associated with mitochondrial dysfunction through week 96 were markedly lower in the tenofovir DF arm than in the stavudine arm, while the two arms showed a similar renal safety profile. Although this study is still under way, the ad interim results point to a similar efficiency but better safety profile of tenofovir DF compared to stavudine.
Genotypic analyses of HIV samples from patients enrolled in two placebo-controlled clinical trials have demonstrated that most patients who received tenofovir DF in addition to their existing combination antiretroviral regimen achieved significant viral RNA load reductions relative to placebo, regardless of the number and type of thymidine analogue mutations (TAMs) (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) present in the viral reverse transcriptase (studies 902 and 907; (M. D. Miller, N. A. Margot, A. K. Cheng, B. Lu, Oral Presentations XIV Int. AIDS Conf. abstr. B1390, 2002); changes in HIV RNA levels at 24 weeks were −0.80, −0.66, −067, and −0.21 log10 copy per ml if the number of baseline TAMs was zero, one to two, three or more (without M41L or L210W), or three or more (including M41L or L210W). Even the last patients, with the most advanced pattern of TAM resistance, showed a significant reduction in viral load after treatment with tenofovir DF. Although tenofovir DF may still work in these patients with advanced TAMS, investigators should be striving for treatment approaches that do not lead to such extensive TAM development.
Not only may tenofovir DF be expected to be active in vivo against HIV-1 strains that are resistant to various nucleoside analogues, but also it should be effective against non-B subtypes of HIV-1. Indeed, tenofovir and adefovir were found to be equally active in vitro against a panel of HIV-1 subtypes A, B, C, D, E, F, and G and group O primary HIV-1 isolates (160).
Monotherapy with oral tenofovir DF (300 mg, once daily) in chronically HIV-1-infected antiretroviral-naive individuals has also been studied; after 3 weeks of therapy, a reduction of 1.5 log10 units in plasma HIV-1 RNA levels was noted, an antiviral response that was as robust as previously noted with ritonavir monotherapy (131). These data support further pursuit of tenofovir DF in simplified combinations of antiviral agents as initial treatment for chronic HIV-1 infection.
It should be noted that in comparison with cidofovir, tenofovir has substantially weaker effects on the proliferation and viability of renal proximal tubule epithelial cells (47). It is also less toxic toward erythroid and myeloid progenitor cells than are the nucleoside RT inhibitors zidovudine, stavudine, and zalcitabine (47). Furthermore, tenofovir at concentrations that greatly exceed those required for anti-HIV activity in peripheral blood mononuclear cells is not associated with mitochondrial toxicity (26); this is most probably due to the low efficiency of tenofovir incorporation by mitochondrial DNA polymerase (105) and, again, contrasts with the observations made for the nucleoside analogues zidovudine, stavudine, didanosine, and zalcitabine (26).
HAART is being received by an increasing percentage of pregnant women, pregnancy being of little impediment to its use, despite few published studies regarding the safety of antiretroviral drugs for mother and fetus. Tenofovir was assessed for its safety and efficacy during pregnancy and postnatally in gravid rhesus monkeys whether infected or not infected with SIV (207, 208). Efficient placental transport of tenofovir and significant reduction of viral load in SIV-infected fetuses were shown to result in healthy newborns (208), although the maternal dose used (30 mg/kg/day) throughout gestation transiently affected maternal bone biomarkers and altered some fetal parameters, the long-term ramifications of which need further follow-up (207).
HBV infections.
In patients coinfected with HIV and HBV, a once-daily dose of 300 mg of tenofovir DF effected a reduction in serum HBV DNA levels of 3.34 log10 copies per ml after 12 weeks and of 4.63 log10 copies per ml after 24 weeks. This response was similar for both wild-type and lamivudine-resistant HBV and thus points to the potential of tenofovir as a treatment modality not only for patients infected with HIV but also for those coinfected with HIV and HBV (D. Cooper, A. Cheng, D. Coakley, J. Sayre, L. Zhong, S. S. Chen, C. Westland, M. Miller, and C. Brosgart, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 124, 2002; M. Bochet, R. Tubiana, Y. Benhamou, V. Thibault, L. Suffisseau, C. Brosgart, J. Rooney, M. Sullivan, T. Poynard, F. Bricaire, and C. Katlama, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 675-M, 2002).
RESISTANCE DEVELOPMENT
Could acyclic nucleoside phosphonates, like any other specific antiviral compounds, be expected to give rise to the development of resistance through mutations in the target enzyme (the herpesvirus DNA polymerase for cidofovir and the HIV RT for adefovir and tenofovir)? The development of resistance is a well-known phenomenon for the RT inhibitors and protease inhibitors that are being used for the treatment of HIV infections (65). However, although cidofovir-resistant CMV strains (carrying the K513N mutation in the DNA polymerase gene) and adefovir-resistant HIV-1 strains (carrying the K65R or K70E mutation in the reverse transcriptase gene) (40, 86) have been found to emerge in vitro after prolonged exposure of the viruses to the drugs, neither cidofovir, adefovir, nor tenofovir, has been found to select in vivo for virus mutant strains that have been shown to compromise their clinical efficacy.
Cidofovir
To date, there is no evidence that, in vivo, CMV resistance to cidofovir may develop as the result of cidofovir treatment. Earlier work indicated that cidofovir sensitivity remained unchanged for semen CMV samples isolated from AIDS patients with asymptomatic CMV shedding after limited in vivo exposure to the drug (41). Later, CMV isolates were obtained from patients enrolled in the cidofovir CMV retinitis trials: isolates obtained from patients receiving first-line cidofovir all showed unaltered susceptibility to cidofovir; however, some isolates from patients who had received second-line cidofovir (following ganciclovir) did show reduced susceptibility to cidofovir and high-level resistance to ganciclovir (39).
Whereas low-level ganciclovir-resistant CMV isolates were associated with mutations in the phosphotransferase gene UL97, high-level ganciclovir-resistant CMV isolates were associated with both phosphotransferase gene UL97 and DNA polymerase UL54 mutations: only the high-level ganciclovir-resistant mutants showed decreased sensitivity to cidofovir (193). Precidofovir therapy isolates were not available for many of these patients, so that it is not possible to determine whether cidofovir or ganciclovir selected for these resistant strains. Importantly, though, there was no difference in time to retinitis progression in patients receiving cidofovir therapy when patients with isolates that were equally sensitive or less sensitive to cidofovir were compared. This is in contrast to the findings for ganciclovir, where the emergence of drug-resistant CMV strains has been associated with clinical failure (39).
Cidofovir may be ineffective in the treatment of CMV retinitis in patients who have received long-term treatment with ganciclovir and have developed high-level ganciclovir-resistant CMV disease (182). Thus, the primary use of this drug may be first-line treatment of CMV infection, particularly since resistance to cidofovir does not emerge easily (39).
In vitro, in cell culture, cidofovir may select for CMV strains with decreased susceptibility toward cidofovir. One such strain, selected in vitro had a single-base substitution resulting in a K513N mutation at the viral DNA polymerase (48). Although the K513N virus had decreased susceptibility to cidofovir as well as ganciclovir in vitro, compared with the wild-type virus, the K513N mutation significantly decreased the CMV replication capacity (48), which means that if such mutants were to arise in vivo, they may go unnoticed because of impaired replicative ability.
As with CMV, there is no documented evidence that any other virus may develop resistance to cidofovir in vivo as a result of cidofovir treatment. However, in vitro resistance of both herpesviruses and poxviruses to cidofovir can be generated by prolonged passage of the virus in the presence of the drug (5, 192). These mutant viruses have been well characterized, both genotypically (5) and phenotypically (192). It was inferred that should such cidofovir-resistant mutant viruses arise in vivo, which is not obvious, they may be untreatable with cidofovir but their virulence may be attenuated (192).
Adefovir
In vitro, adefovir selects for the mutations K65R and K70E in the HIV-1 RT (40). However, these mutations did not develop in any of the AIDS patients following 24 to 48 weeks of adefovir dipivoxil therapy (141). The HIV RT changes that arose during adefovir dipivoxil therapy appeared attributable to the background therapy used for the patients. Adefovir dipivoxil therapy may have influenced the pattern of zidovudine-associated resistance mutations that developed, but this did not result in an observed loss of viral load suppression. In some patients there was a decreased susceptibility to adefovir, but no decreased susceptibility to tenofovir was observed in any of the patients treated with adefovir dipivoxil (141).
The dose of adefovir dipivoxil (10 mg once daily) used for the treatment of HBV infections is suboptimal for the treatment of HIV infections and may therefore be suspected to select for possible resistance mutations in the HIV RT. However, adefovir dipivoxil administered at this dose to HIV-1/HBV-coinfected patients did not select for K65R, K70E, or any other mutations in the HIV RT, even in the setting of uncontrolled HIV replication (73).
The M204V/I mutation (located within the YMDD motif of the HBV DNA polymerase), which has been previously referred to as M550V/I (for HBV) and is analogous to the M589V mutation in WHV, is known to confer resistance to lamivudine but does not confer any cross-resistance to adefovir (43, 209). In contrast, adefovir dipivoxil wiped out the M204V/I mutation in a certain percentage of patients with chronic HBV, as mentioned above (Peters et al., Oral Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 646, 2002).
In HBV resistance surveillance studies (92), adefovir-associated resistance mutations were not observed in any of the 467 patients which were evaluated after 48 weeks of adefovir dipivoxil therapy, and even in patients who had received up to 136 weeks of adefovir dipivoxil treatment no adefovir-associated resistance mutations could be witnessed (Benhamou et al., Poster Presentations 37th Annu. Meet. Eur. Assoc. Study Liver Dis., abstr. 245, 2002).
Week 48 resistance surveillance in two phase III clinical trials of adefovir dipivoxil in chronic hepatitis B patients (467 adefovir dipivoxil-treated and 228 placebo-treated patients) revealed four substitutions (S119A, H133L, V214A and H234Q), each at conserved sites of the HBV DNA polymerase, in four patients treated with adefovir dipivoxil; the HBV mutants encoding the four substitutions remained fully susceptible to adefovir in vitro and the four patients had HBV DNA reductions of 3.3 to 5.9 log10 copies per ml by week 48 with no rebound (224). When resistance surveillance was extended to 60 weeks of therapy with adefovir dipivoxil, still no emergence of resistant virus was noted (228).
Follow-up studies have ascertained that after 48 weeks, 0 of 629 HBeAg-negative chronic hepatitis B patients taking adefovir dipivoxil developed resistance mutations; after 96 weeks, however, 2 of 124 patients developed a novel HBV DNA polymerase mutation (N236T) conferring reduced susceptibility to adefovir (C. S. Gibbs, S. Xiong, H. Yang, C. E. Westland, W. E. Delaney IV, D. Colledge, A. Bartholomeusz, V. Thibault, Y. Benhamou, P. Angus, M. Wulfsohn, J. Fry, C. L. Brosgart, and S. Locarnini, Abstr. 16th Int. Conf. Antiviral Res., abstr. 12, 2003). It was concluded that adefovir has a favorable resistance profile, with infrequent and delayed emergence of HBV with reduced susceptibility (0 of 629 patients at 48 weeks; 2 of 124 patients at 96 weeks).
Tenofovir
As with adefovir, HIV-1 variants with reduced susceptibility to tenofovir have been isolated in vitro, the only reproducible mutation generating resistance to tenofovir being the K65R substitution in the reverse transcriptase. However, tenofovir disoproxil was still able to inhibit the replication of the K65R virus at submicromolar, nontoxic concentrations (202).
The K65R mutation and additional mutations (N69T, R82K, A158S, and S211N) have also been noted in vivo in SIV-infected newborn macaques treated with tenofovir (217). Despite a fivefold-reduced susceptibility to tenofovir, viral replication was efficiently suppressed by the compound and the animals remained healthy for a prolonged time course (219). The authors concluded that prophylactic administration of tenofovir to human newborns or adults following exposure to HIV would still be beneficial even in the presence of viral variants with reduced susceptibility to tenofovir.
As mentioned above, the lamivudine-associated HIV-1 RT M184V mutation results in increased susceptibility to adefovir (140). This mutation also ensures increased susceptibility to tenofovir, apparently through a decreased processivity of the M184V mutant RT and a decreased replicative ability of the M184V mutant virus (144).
Pyrophosphorolysis, i.e., the removal of a nucleoside chain terminator by a pyrophosphate (PP1) acceptor molecule (which corresponds to the reversal of the normal chain elongation reaction), and a similar nucleotide-dependent chain terminator removal with ATP as the acceptor molecule have been proposed as mechanisms of zidovudine and stavudine resistance. These mechanisms are less functional with tenofovir, which would explain why tenofovir is less efficiently removed once it has been incorporated into the DNA chain and why it is less likely to engender resistance development (145, 146). The minimal acyclic structure of tenofovir provides a greater degree of torsional freedom, resulting in multiple conformations at the RT active site, providing an unfavorable environment for excision (as seen with zidovudine) or for resistance development due to steric hindrance (as seen with lamivudine) (S. Tuske, S. Sarafianos, A. D. Clark, Jr., J. Ding, L. K. Naeger, M. D. Miller, C. Gibbs, D. M. Jerina, S. Hughes, and E. Arnold, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 44, 2002).
When the phase II 48-week, placebo-controlled clinical trial of tenofovir disoproxil fumarate in antiretroviral-experienced HIV-1-infected patients (180) was monitored genotypically and phenotypically for HIV drug resistance (133), only 2% of the patients were found to have developed the K65R mutation, with a three- to fourfold reduction in tenofovir susceptibility but no evidence of rebound viremia. Of the patients, 34% had developed TAMs, coincident with concurrent zidovudine or stavudine therapy, but also showed durable HIV-1 load reductions (133). Reductions in viral load were sustained on extended treatment with tenofovir DF for 96 weeks, with the K65R mutation being noted in only 3% of the patients (134).
A randomized, double-blind, phase III study enrolling 552 antiretroviral therapy-experienced patients, who received, in addition to their background regimen, 300 mg of tenofovir DF or placebo (2:1) for 24 weeks followed by open-label tenofovir disoproxil fumarate for another 24 weeks revealed that the incidence of the K65 mutation through week 48 was not higher than 3% and did not correlate with treatment failure. Treatment failure was associated with resistance to other agents in the background regimen. Development of most mutations in the patients receiving tenofovir disoproxil fumarate was due to the background regimen and was not associated with loss of HIV RNA suppression (N. A. Margot, A. Johnson, A. Cheng, D. F. Coakley, and M. D. Miller, Abstr. 9th Conf. Retroviruses Opportunistic Infect., abstr. 414-W, 2002; D. McColl, N. Margot, and M. D. Miller, Abstr. 15th Int. Conf. Antiviral Res., abstr. 9, 2002).
From a panel of over 1,000 HIV-1 isolates from treatment-naive patients, more than 97.5% had tenofovir susceptibilities less than 3-fold above those of the wild-type controls, and from a panel of nearly 5,000 HIV-1 isolates from predominantly treatment-experienced patients, more than 99% exhibited less than 10-fold-reduced susceptibility to tenofovir (96). These results suggest that the majority of treatment-naive and -experienced individuals harbor HIV that remains within the normal range of tenofovir susceptibilities (96).
CONCLUSION
In conclusion, acyclic nucleoside phosphonates (cidofovir, adefovir, and tenofovir) offer attractive perspectives for the treatment of DNA virus and retrovirus infections (Table 2). Cidofovir is active against virtually all DNA virus infections (polyomavirus, papillomavirus, adenovirus, herpesvirus, and poxvirus), including herpesvirus infections which have become resistant to other antiviral agents (such as TK− HSV, TK− VZV, and PK− CMV, which are resistant to acyclovir, penciclovir, and ganciclovir). Cidofovir (Vistide) has been formally approved for the treatment of CMV retinitis in AIDS patients but has also proven efficacious in many other DNA virus infections. Adefovir and tenofovir are inhibitory to both retrovirus (e.g., HIV) infections and hepadnavirus (e.g., HBV) infections; tenofovir DF (Vircad) has been licensed for the treatment of HIV infections (AIDS), whereas adefovir dipivoxil (Hepsera) has been approved for the treatment of chronic HBV infections. In comparison with the nucleoside analogues, the acyclic nucleoside phosphonates provide a much longer antiviral response. For adefovir dipivoxil and tenofovir DF, this allows administration on a once-daily base, and for cidofovir it allows once-weekly (or once every 2 weeks) administration, if given intravenously. A dose-limiting side effect of intravenous cidofovir administration is nephrotoxicity, whereas for adefovir dipivoxil and tenofovir DF no such (or other) toxic side effects have been noted at the approved dosage schedules. As a rule, the acyclic nucleoside phosphonates do not readily lead to the emergence of virus drug resistance, which may otherwise compromise their clinical effectiveness, even after administration for extended periods (months or years).
Acknowledgments
I thank Christiane Callebaut for her proficient editorial help.
REFERENCES
- 1.Abdulkarim, B., S. Sabri, E. Deutsch, H. Chagraoui, L. Maggiorella, J. Thierry, F. Eschwege, W. Vainchenker, S. Chouaïb, and J. Bourhis. 2002. Antiviral agent cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene 21:2334-2346. [DOI] [PubMed] [Google Scholar]
- 2.Aldern, K. A., S. L. Ciesla, K. L. Winegarden, and K. Y. Hostetler. 2003. Increased antiviral activity of 1-O-hexadecyloxypropyl-[2-14C]cidofovir in MRC-5 human lung fibroblasts is explained by unique cellular uptake and metabolism. Mol. Pharmacol. 63:678-681. [DOI] [PubMed] [Google Scholar]
- 3.Ambati, J., K. B. Wynne, M. C. Angerame, and M. R. Robinson. 1999. Anterior uveitis associated with intravenous cidofovir in patients with cytomegalovirus retinitis. Br. J. Ophthalmol. 83:1153-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrei, G., R. Snoeck, D. Schols, and E. De Clercq. 2001. Induction of apoptosis by cidofovir in human papillomavirus (HPV)-positive cells. Oncol. Res. 12:397-408. [DOI] [PubMed] [Google Scholar]
- 5.Andrei, G., R. Snoeck, E. De Clercq, R. Esnouf, P. Fiten, and G. Opdenakker. 2000. Resistance of herpes simplex virus type 1 against different phosphonylmethoxyalkyl derivatives of purines and pyrimidines due to specific mutations in the viral DNA polymerase gene. J. Gen. Virol. 81:639-648. [DOI] [PubMed] [Google Scholar]
- 6.Andrei, G., R. Snoeck, J. Piette, P. Delvenne, and E. De Clercq. 1998. Antiproliferative effects of acyclic nucleoside phosphonates on human papillomavirus (HPV)-harboring cell lines compared with HPV-negative cell lines. Oncol. Res. 10:523-531. [PubMed] [Google Scholar]
- 7.Andrei, G., R. Snoeck, J. Piette, P. Delvenne, and E. De Clercq. 1998. Inhibitory effects of cidofovir (HPMPC) on the growth of the human cervical carcinoma (SiHa) xenografts in athymic-nude mice. Oncol. Res. 10:533-539. [PubMed] [Google Scholar]
- 8.Andrei, G., R. Snoeck, M. Vandeputte, and E. De Clercq. 1997. Activities of various compounds against murine and primate polyomaviruses. Antimicrob. Agents Chemother. 41:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini, L., M. C. Pietrogrande, M. R. Delle Piane, F. Zibordi, P. Cinque, C. Maccagnano, and L. Vago. 2001. Progressive multifocal leukoencephalopathy in a child with hyperimmunoglobulin E recurrent infection syndrome and review of the literature. Neuropediatrics 32:250-255. [DOI] [PubMed] [Google Scholar]
- 10.Arimilli M. N., C. U. Kim, J. Dougherty, A. Mulato, R. Oliyai, J. P. Shaw, K. C. Cundy, and N. Bischofberger. 1997. Synthesis, in vitro biological evaluation, and oral bioavailability of 9-[2-(phosphonomethoxy)propyl]adenine prodrugs. Antiviral Chem. Chemother. 8:557-564. [Google Scholar]
- 11.Armbruster, C., A. Kreuzer, H. Vorbach, M. Huber, and C. Armbruster. 2001. Successful treatment of severe respiratory papillomatosis with intravenous cidofovir and interferon α-2b. Eur. Respir. J. 17:830-831. [DOI] [PubMed] [Google Scholar]
- 12.Badiaga, S., P. Parola, C. Zandotti, and P. Brouqui. 1998. Successful treatment of Kaposi's sarcoma with a combination of antiviral drug therapy and chemotherapy: two case reports. Clin. Infect. Dis. 27:1558. [DOI] [PubMed] [Google Scholar]
- 13.Bainbridge, J. W., J. Raina, S. M. Shah, J. Ainsworth, and A. J. Pinching. 1999. Ocular complications of intravenous cidofovir for cytomegalovirus retinitis in patients with AIDS. Eye 13:353-356. [DOI] [PubMed] [Google Scholar]
- 14.Balzarini J., J.-F. Navé, M. A. Becker, M. Tatibana, and E. De Clercq. 1995. Kinetic properties of adenine nucleotide analogues against purified 5-phosphoribosyl-1-pyrophosphate synthetases from E. coli, rat liver and human erythrocytes. Nucleosides Nucleotides 14:1861-1871. [Google Scholar]
- 15.Banker, A. S., J. F. Arevalo, D. Munguia, F. M. Rahhal, B. Ishimoto, C. Berry, E. De Clercq, R. Ochabski, I. Taskintuna, and W. R. Freeman. 1997. Intraocular pressure and aqueous humor dynamics in patients with AIDS treated with intravitreal cidofovir (HPMPC) for cytomegalovirus retinitis. Am. J. Ophthalmol. 124:168-180. [DOI] [PubMed] [Google Scholar]
- 16.Barditch-Crovo, P., J. Toole, C. W. Hendrix, K. C. Cundy, D. Ebeling, H. S. Jaffe, and P. S. Lietman. 1997. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)phosphonylmethoxy-ethyl]adenine) in HIV-infected patients. J. Infect. Dis. 176:406-413. [DOI] [PubMed] [Google Scholar]
- 17.Barouch, D. H., W. C. Faquin, Y. Che, I. J. Koralnik, G. K. Robbins, and B. T. Davis. 2002. BK virus-associated hemorrhagic cystitis in a human immunodeficiency virus-infected patient. Clin. Infect. Dis. 35:326-329. [DOI] [PubMed] [Google Scholar]
- 18.Beadle, J. R., C. Hartline, K. A. Aldern, N. Rodriguez, E. Harden, E. R. Kern, and K. Y. Hostetler. 2002. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob. Agents Chemother. 46:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendele, R. A., and F. C. Richardson. 2002. Adefovir nephrotoxicity and mitochondrial DNA depletion. Hum. Pathol. 33:574. [DOI] [PubMed] [Google Scholar]
- 20.Benhamou, Y., M. Bochet, V. Thibault, V. Calvez, M. H. Fievet, P. Vig, C. S. Gibbs, C. Brosgart, J. Fry, H. Namini, C. Katlama, and T. Poynard. 2001. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet 358:718-723. [DOI] [PubMed] [Google Scholar]
- 21.Berenguer, J., J. Mallolas, and the Spanish Cidofovir Study Group. 2000. Intravenous cidofovir for compassionate use in AIDS patients with cytomegalovirus retinitis. Clin. Infect. Dis. 30:182-184. [DOI] [PubMed] [Google Scholar]
- 22.Bielamowicz, S., V. Villagomez, S. V. Stager, and W. R. Wilson. 2002. Intralesional cidofovir therapy for laryngeal papilloma in an adult cohort. Laryngoscope 112:696-699. [DOI] [PubMed] [Google Scholar]
- 23.Bienvenu, B., F. Martinez, A. Devergie, M. Rybojad, J. Rivet, P. Bellenger, P. Morel, E. Gluckman, and C. Lebbé. 2002. Topical use of cidofovir induced acute renal failure. Transplantation 73:661-662. [DOI] [PubMed] [Google Scholar]
- 24.Birkus, G., I. Votruba, A. Holý, and B. Otová. 1999. 9-[2-(Phosphonomethoxy)ethyl]-adenine diphosphate (PMEApp) as a substrate toward replicative DNA polymerases α, β, ɛ, and ɛ*. Biochem. Pharmacol. 58:487-492. [DOI] [PubMed] [Google Scholar]
- 25.Birkus, G., M. Hájek, P. Kramata, I. Votruba, A. Holý, and B. Otová. 2002. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerases α, δ, and ɛ. Antimicrob. Agents Chemother. 46:1610-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkus, G., M. J. M. Hitchcock, and T. Cihlar. 2002. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 46:716-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blick, G., M. Whiteside, P. Griegor, U. Hopkins, T. Garton, and L. LaGravinese. 1998. Successful resolution of progressive multifocal leukoencephalopathy after combination therapy with cidofovir and cytosine arabinoside. Clin. Infect. Dis. 26:191-192. [DOI] [PubMed] [Google Scholar]
- 28.Blick, G., T. Garton, U. Hopkins, and L. LaGravinese. 1997. Successful use of cidofovir in treating AIDS-related cytomegalovirus retinitis, encephalitis, and esophagitis. J. Acquir. Immun. Defic. Syndr. Hum. Retrovirol. 15:84-85. [DOI] [PubMed] [Google Scholar]
- 29.Blot, N., P. Schneider, P. Young, C. Janvresse, D. Dehesdin, P. Tron, and J. P. Vannier. 2000. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant. 26:903-905. [DOI] [PubMed] [Google Scholar]
- 30.Bordigoni, P., A.-S. Carret, V. Venard, F. Witz, and A. Le Faou. 2001. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:1290-1297. [DOI] [PubMed] [Google Scholar]
- 31.Bosi, A., B. Bartolozzi, A. M. Vannucchi, A. Orsi, S. Guidi, and P. Rossi Ferrini. 2002. Polymerase chain reaction-based “pre-emptive” therapy with cidofovir for cytomegalovirus reactivation in allogeneic hematopoietic stem cells transplantation recipients: a prospective study. Haematologica 87:446-447. [PubMed] [Google Scholar]
- 32.Brambella, A. M., A. Castagna, R. Novati, P. Cinque, M. R. Terrini, M. C. Moioli, and A. Lazzarin. 1999. Remission of AIDS-associated progressive multifocal leukoencephalopathy after cidofovir therapy. J. Neurol. 246:723-725. [DOI] [PubMed] [Google Scholar]
- 33.Bray, M., M. Martinez, D. F. Smee, D. Kefauver, E. Thompson, and J. W. Huggins. 2000. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 181:10-19. [DOI] [PubMed] [Google Scholar]
- 34.Bray, M., M. Martinez, D. Kefauver, M. West, and R. Roy. 2002. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antiviral Res. 54:129-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calista, D. 2000. Resolution of recalcitant human papillomavirus gingival infection with topical cidofovir. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 90:713-715. [DOI] [PubMed] [Google Scholar]
- 36.Calista, D. 2000. Topical cidofovir for severe cutaneous human papillomavirus and molluscum contagiosum infections in patients with HIV/AIDS. A pilot study. J. Eur. Acad. Dermatol. Venereol. 14:484-488. [DOI] [PubMed] [Google Scholar]
- 37.Calista, D., L. Riccioni, and L. Coccia. 2002. Successful treatment of squamous cell carcinoma of the lower eyelid with intralesional cidofovir. Br. J. Ophthalmol. 86:932-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen, Y., C. Scieux, V. Garrait, G. Socié, V. Rocha, J.-M. Molina, D. Thouvenot, F. Morfin, L. Hocqueloux, L. Garderet, H. Espérou, F. Sélimi, A. Devergie, G. Leleu, M. Aymard, F. Morinet, E. Gluckman, and P. Ribaud. 2000. Resistant herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31:927-935. [DOI] [PubMed] [Google Scholar]
- 39.Cherrington, J. M. 1997. Human cytomegalovirus: resistance profile of cidofovir. Int. Antiviral News 5:91-92. [Google Scholar]
- 40.Cherrington, J. M., A. S. Mulato, M. D. Fuller, and M. S. Chen. 1996. Novel mutation (K70E) in human immunodeficiency virus type 1 reverse transcriptase confers decreased susceptibility to 9-[2-(phosphonomethoxy)ethyl]adenine in vitro. Antimicrob. Agents Chemother. 40:2212-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherrington, J. M., R. Miner, M. J. M. Hitchcock, J. P. Lalezari, and W. L. Drew. 1996. Susceptibility of human cytomegalovirus to cidofovir is unchanged after limited in vivo exposure to various clinical regimens of drug. J. Infect. Dis. 173:987-992. [DOI] [PubMed] [Google Scholar]
- 42.Chhetri, D. K., J. H. Blumin, N. L. Shapiro, and G. S. Berke. 2002. Office-based treatment of laryngeal papillomatosis with percutaneous injection of cidofovir. Otolaryngol. Head Neck Surg. 126:642-648. [DOI] [PubMed] [Google Scholar]
- 43.Chin, R., T. Shaw, J. Torresi, V. Sozzi, C. Trautwain, T. Bock, M. Manss, H. Isom, P. Furman, and S. Locarnini. 2001. In vitro susceptibilities of wild-type or drug-resistant hepatitis B virus to (−)-β-d-2,6-diaminopurine dioxolane and 2′-fluoro-5-methyl-β-l-arabinofuranosyluracil. Antimicrob. Agents Chemother. 45:2495-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christensen, N. D., M. D. Pickel, L. R. Budgeon, and J. W. Kreider. 2000. In vivo anti-papillomavirus activity of nucleoside analogues including cidofovir on CRPV-induced rabbit papillomas. Antiviral Res. 48:131-142. [DOI] [PubMed] [Google Scholar]
- 45.Christensen, N. D., R. Han, N. M. Cladel, and M. D. Pickel. 2001. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob. Agents Chemother. 45:1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cihlar, T., D. C. Lin, J. B. Pritchard, M. D. Fuller, D. B. Mendel, and D. H. Sweet. 1999. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol. Pharmacol. 56:570-580. [DOI] [PubMed] [Google Scholar]
- 47.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. M. Hitchcock. 2002. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antiviral Res. 54:37-45. [DOI] [PubMed] [Google Scholar]
- 48.Cihlar, T., M. D. Fuller, A. S. Mulato, and J. M. Cherrington. 1998. A point mutation in the human cytomegalovirus DNA polymerase gene selected in vitro by cidofovir confers a slow replication phenotype in cell culture. Virology 248:382-393. [DOI] [PubMed] [Google Scholar]
- 49.Cihlar, T., I. Votruba, K. Horská, R. Liboska, I. Rosenberg, and A. Holý. 1992. Metabolism of 1-(S)-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in human embryonic lung cells. Collect. Czech. Chem. Commun. 57:661-672. [Google Scholar]
- 50.Cihlar, T., and M. S. Chen. 1996. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 50:1502-1510. [PubMed] [Google Scholar]
- 51.Cihlar, T., and M. S. Chen. 1997. Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogues into DNA by human DNA polymerases α, β and γ. Antiviral Chem. Chemother. 8:187-195. [Google Scholar]
- 52.Cochereau; I., S. Doan, M.-C. Diraison, H. Mousalatti, N. Guvenisik, L. Ren, and T. Hoang-Xuan. 1999. Uveitis in patients treated with intravenous cidofovir. Ocul. Immunol. Inflammation 7:223-229. [DOI] [PubMed] [Google Scholar]
- 53.Connelly, M. C., B. L. Robbins, and A. Fridland. 1993. Mechanism of uptake of the phosphonate analog (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in Vero cells. Biochem. Pharmacol. 46:1053-1057. [DOI] [PubMed] [Google Scholar]
- 54.Crippa, F., L. Corey, E. L. Chuang, G. Sale, and M. Boeckh. 2001. Virological, clinical, and ophthalmologic features of cytomegalovirus retinitis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 32:214-219. [DOI] [PubMed] [Google Scholar]
- 55.Cullen, J. M., D. H. Li, C. Brown, E. J. Eisenberg, K. C. Cundy, J. Wolfe, J. Toole, and C. Gibbs. 2001. Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks. Antimicrob. Agents Chemother. 45:2740-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cundy, K. C. 1999. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin. Pharmacokinet. 36:127-143. [DOI] [PubMed] [Google Scholar]
- 57.Cundy, K. C., C. Sueoka, G. R. Lynch, L. Griffin, W. A. Lee, and J.-P. Shaw. 1998. Pharmacokinetics and bioavailability of the anti-human immunodeficiency virus nucleotide analog 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Antimicrob. Agents Chemother. 42:687-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dancey, D. R., D. W. Chamberlain, M. Krajden, J. Palefsky, P. W. Alberti, and G. P. Downey. 2000. Successful treatment of juvenile laryngeal papillomatosis-related multicystic lung disease with cidofovir. Chest 118:1210-1214. [DOI] [PubMed] [Google Scholar]
- 59.Davies, E. G., A. Thrasher, K. Lacey, and J. Harper. 1999. Topical cidofovir for severe molluscum contagiosum. Lancet 353:2042. [DOI] [PubMed] [Google Scholar]
- 60.Davis, M. D. P., B. S. Gostout, R. M. McGovern, D. H. Persin, R. L. Schut, and M. R. Pittelkow. 2000. Large plantar wart caused by human papillomavirus-66 and resolution by topical cidofovir therapy. J. Am. Acad. Dermatol. 43:340-343. [DOI] [PubMed] [Google Scholar]
- 61.De Clercq, E. 1991. Broad-spectrum anti-DNA virus and anti-retrovirus activity of phosphonylmethoxyalkylpurines and -pyrimidines. Biochem. Pharmacol. 42:963-972. [DOI] [PubMed] [Google Scholar]
- 62.De Clercq, E. 1993. Therapeutic potential of HPMPC as an antiviral drug. Rev. Med. Virol. 3:85-96. [Google Scholar]
- 63.De Clercq, E. 1995. Trends in the development of new antiviral agents for the chemotherapy of infections caused by herpesviruses and retroviruses. Rev. Med. Virol. 5:149-164. [Google Scholar]
- 64.De Clercq E. 1996. Therapeutic potential of cidofovir (HPMPC, Vistide™) for the treatment of DNA virus (i.e. herpes-, papova-, pox- and adenovirus) infections. Verh. K. Acad. Geneeskd. Belg. 58:19-49. [PubMed] [Google Scholar]
- 65.De Clercq, E. 1997. Development of resistance of human immunodeficiency virus (HIV) to anti-HIV agents: how to prevent the problem? Int. J. Antimicrob. Agents 9:21-36. [DOI] [PubMed] [Google Scholar]
- 66.De Clercq, E. 2001. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 14:382-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Clercq, E. 2002. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 55:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Clercq, E. 2002. Cidofovir in the therapy and short-term prophylaxis of poxvirus infections. Trends Pharmacol. Sci. 23:456-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Clercq, E., and A. Holý. 1991. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxy-propyl)cytosine in various models of herpes simplex virus infection in mice. Antimicrob. Agents Chemother. 35:701-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Clercq, E., L. Naesens, L. De Bolle, D. Schols, Y. Zhang, and J. Neyts. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381-395. [DOI] [PubMed] [Google Scholar]
- 71.Deeks, S. G., A. Collier, J. Lalezari, A. Pavia, D. Rodrigue, W. L. Drew, J. Toole, H. S. Jaffe, A. S. Mulato, P. D. Lamy, W. Li, J. M. Cherrington, N. Hellmann, and J. Kahn. 1997. The safety and efficacy of adefovir dipivoxil, a novel anti-HIV therapy, in HIV infected adults. A randomized, double-blind, placebo controlled trial. J. Infect. Dis. 176:1517-1523. [DOI] [PubMed] [Google Scholar]
- 72.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. O. Kahn. 1998. Safety, pharmacokinetics, and antiretroviral activity of intravenous 9-[2-(R)-phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delaugerre, C., A.-G. Marcelin, V. Thibault, G. Peytavin, T. Bombled, M.-V. Bochet, C. Katlama, Y. Benhamou, and V. Calvez. 2002. Human immunodeficiency virus (HIV) type 1 reverse transcriptase resistance mutations in hepatitis B virus (HBV)-HIV-coninfected patients treated for HBV chronic infection once daily with 10 milligrams of adefovir dipivoxil combined with lamivudine. Antimicrob. Agents Chemother. 46:1586-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delmas, J., O. Schorr, C. Jamard, C. Gibbs, C. Trépo, O. Hantz, and F. Zoulim. 2002. Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro. Antimicrob. Agents Chemother. 46:425-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Luca, A., M. Fantoni, T. Tartaglione, and A. Antinori. 1999. Response to cidofovir after failure of antiretroviral therapy alone in AIDS-associated progressive multifocal leukoencephalopathy. Neurology 52:891-892. [DOI] [PubMed] [Google Scholar]
- 76.De Luca, A., M. L. Giancola, A. Ammassari, S. Grisetti, A. Cingolani, D. Larussa, L. Alba, R. Murri, G. Ippolito, R. Cauda, A. d'Arminio Monte, and A. Antinori. 2001. Potent anti-retroviral therapy with or without cidofovir for AIDS-associated progressive multifocal leukoencephalopathy: extended follow-up of an observational study. J. Neurovirol. 7:364-368. [DOI] [PubMed] [Google Scholar]
- 77.De Luca, A., M. L. Giancola, A. Ammassari, S. Grisetti, A. Cingolani, M. G. Paglia, A. Govoni, R. Murri, L. Testa, A. d'Arminio Monforte, and A. Antinori. 2000. Cidofovir added to HAART improves virological and clinical outcome in AIDS-associated progressive multifocal leukoencephalopathy. AIDS 14:F117-F121. [DOI] [PubMed] [Google Scholar]
- 78.De Luca, A., M. L. Giancola, A. Cingolani, A. Ammassari, L. Gillini, R. Murri, and A. Antinori. 1999. Clinical and virological monitoring during treatment with intrathecal cytarabine in patients with AIDS-associated progressive multifocal leukoencephalopathy. Clin. Infect. Dis. 28:624-628. [DOI] [PubMed] [Google Scholar]
- 79.de Oliveira, C. B. R., D. Stevenson, L. LaBree, P. J. McDonnell, and M. D. Trousdale. 1996. Evaluation of cidofovir (HPMPC, GS-504) against adenovirus type 5 infection in vitro and in a New Zealand rabbit ocular model. Antiviral Res. 31:165-172. [DOI] [PubMed] [Google Scholar]
- 80.Descamps, V., X. Duval, M. Grossin, B. Crickx, and C. Leport. 2001. Topical cidofovir for bowenoid papulosis in an HIV-infected patient. Br. J. Dermatol. 144:628-650. [DOI] [PubMed] [Google Scholar]
- 81.Duan, J., W. Paris, J. De Marte, D. Roopchand, T.-L. Fleet, and M. G. Cordingley. 2000. Topical effects of cidofovir on cutaneous rabbit warts: treatment regimen and inoculum dependence. Antiviral Res. 46:135-144. [DOI] [PubMed] [Google Scholar]
- 82.Eisenberg, E. J., G.-X. He, and W. A. Lee. 2001. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids 20:1091-1098. [DOI] [PubMed] [Google Scholar]
- 83.Field, A. K., and K. K. Biron. 1994. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin. Microbiol. Rev. 7:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fife, K., J. Gill, D. Bourboulia, B. Gazzard, M. Nelson, and M. Bower. 1999. Cidofovir for the treatment of Kaposi's sarcoma in an HIV-negative homosexual man. Br. J. Dermatol. 141:1136-1153. [DOI] [PubMed] [Google Scholar]
- 85.Fisher, E. J., K. Chaloner, D. L. Cohn, L. B. Grant, B. Alston, C. L. Brosgart, B. Schmetter, W. M. El-Sadr, and J. Sampson. 2001. The safety and efficacy of adefovir dipivoxil in patients with advanced HIV disease: a randomized, placebo-controlled trial. AIDS 15:1695-1700. [DOI] [PubMed] [Google Scholar]
- 86.Foli, A., K. M. Sogocio, B. Anderson, M. Kavlick, M. W. Saville, M. A. Wainberg, Z. X. Gu, J. M. Cherrington, H. Mitsuya, and R. Yarchoan. 1996. In vitro selection and molecular characterization of human immunodeficiency virus type 1 with reduced sensitivity to 9-[2-(phosphonomethoxy)ethyl]adenine (PMEA). Antiviral Res. 32:91-98. [DOI] [PubMed] [Google Scholar]
- 87.Gasnault, J., P. Kousignian, M. Kahraman, J. Rahoiljaon, S. Matheron, J.-F. Delfraissy, and Y. Taoufik. 2001. Cidofovir in AIDS-associated progressive multifocal leuko-encephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J. Neuro virol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 88.Geerinck, K., G. Lukito, R. Snoeck, R. De Vos, E. De Clercq, Y. Vanrenterghem, H. Degreef, and B. Maes. 2001. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J. Med. Virol. 64:543-549. [DOI] [PubMed] [Google Scholar]
- 89.Gilson, R. J., K. B. Chopra, A. M. Newell, I. M. Murray-Lyon, M. R. Nelson, S. J. Rice, R. S. Tedder, J. Toole, H. S. Jaffe, and I. V. D. Weller. 1999. A placebo-controlled phase I/II study of adefovir dipivoxil in patients with chronic hepatitis B virus infection. J. Viral Hepatitis 6:387-395. [DOI] [PubMed] [Google Scholar]
- 90.González-Fraile, M. I., C. Canizo, D. Caballero, R. Hernández, L. Vázquez, C. López, A. Izarra, J. L. Arroyo, A. de la Loma, M. J. Otero, and J. F. San Miguel. 2001. Cidofovir treatment of human polyomavirus-associated acute haemorrhagic cystitis. Transplant. Infect. Dis. 3:44-46. [DOI] [PubMed] [Google Scholar]
- 91.Gordon, Y. J., L. Naesens, E. De Clercq, P. C. Maudgal, and M. Veckeneer. 1996. Treatment of adenoviral conjunctivitis with topical cidofovir. Cornea 15:546. [DOI] [PubMed] [Google Scholar]
- 92.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T.-T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. New Engl. J. Med. 348:800-807. [DOI] [PubMed] [Google Scholar]
- 93.Haider, S., D. Nafziger, J. A. Gutierrez, I. Brar, N. Mateo, and J. Fogle. 2000. Progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia: a case report and review of reported cases. Clin. Infect. Dis. 31:e20-e22. [DOI] [PubMed] [Google Scholar]
- 94.Hammoud, Z., D. M. Parenti, and G. L. Simon. 1998. Abatement of cutaneous Kaposi's sarcoma associated with cidofovir treatment. Clin. Infect. Dis. 26:1233. [DOI] [PubMed] [Google Scholar]
- 95.Hänel, M., F. Fiedler, and C. Thorns. 2001. Anti-CD20 monoclonal antibody (rituximab) and cidofovir as successful treatment of an EBV-associated lymphoma with CNS involvement. Onkologie 24:491-494. [DOI] [PubMed] [Google Scholar]
- 96.Harrigan, P. R., M. D. Miller, P. McKenna, Z. L. Brumme, and B. A. Larder. 2002. Phenotypic susceptibilities to tenofovir in a large panel of clinically derived human immunodeficiency virus type 1 isolates. Antimicrob. Agents Chemother. 46:1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hengge, U. R., and G. Tietze. 2000. Successful treatment of recalcitrant condyloma with topical cidofovir. Sex. Transm. Infect. 76:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hillenkamp, J., T. Reinhard, R. S. Ross, D. Bohringer, O. Cartsburg, M. Roggendorf, E. De Clercq, E. Godehardt, and R. Sundmacher. 2001. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine: a controlled clinical pilot study. Arch. Ophthalmol. 119:1487-1491. [DOI] [PubMed] [Google Scholar]
- 99.Hillenkamp, J., T. Reinhard, R. S. Ross, D. Bohringer, O. Cartsburg, M. Roggendorf, E. De Clercq, E. Godehardt, and R. Sundmacher. 2002. The effects of cidofovir 1% with and without cyclosporin A 1% as a topical treatment of acute adenoviral keratoconjunctivitis: a controlled clinical pilot study. Ophthalmology 109:845-850. [DOI] [PubMed] [Google Scholar]
- 100.Ho, H.-T., K. L. Woods, J. J. Bronson, H. De Boeck, J. C. Martin, and M. J. M. Hitchcock. 1992. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine. Mol. Pharmacol. 41:197-202. [PubMed] [Google Scholar]
- 101.Hoffman, J. A., A. J. Shah, L. A. Ross, and N. Kapoor. 2001. Adenoviral infections and a prospective trial of cidofovir in pediatric hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 7:388-394. [DOI] [PubMed] [Google Scholar]
- 102.Hughes, W. T., J. L. Shenep, J. H. Rodman, A. Fridland, R. Willoughby, S. Blanchard, L. Purdue, D. F. Coakley, K. C. Cundy, M. Culnane, B. Zimmer, S. Burchett, J. S. Read, and the Pediatric AIDS Clinical Trials Group. 2000. Single-dose pharmacokinetics and safety of the oral antiviral compound adefovir dipivoxil in children infected with human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 44:1041-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ibarra, V., J. R. Blanco, J. A. Oteo, and L. Rosel. 2000. Efficacy of cidofovir in the treatment of recalcitrant molluscum contagiosum in an AIDS patient. Acta Dermatol. Venereol. 80:315-316. [DOI] [PubMed] [Google Scholar]
- 104.Jacobson, M. A., S. Wilson, H. Stanley, C. Holtzer, J. Cherrington, and S. Safrin. 1999. Phase I study of combination therapy with intravenous cidofovir and oral ganciclovir for cytomegalovirus retinitis in patients with AIDS. Clin. Infect. Dis. 28:528-533 [DOI] [PubMed] [Google Scholar]
- 105.Johnson, A. A., A. S. Ray, J. Hanes, Z. Suo, J. M. Colacino, K. S. Anderson, and K. A. Johnson. 2001. Toxicity of antiviral nucleoside analogs and the human mitochondrial DNA polymerase. J. Biol. Chem. 276:40848-40857. [DOI] [PubMed] [Google Scholar]
- 106.Julander, J. G., R. W. Sidwell, and J. D. Morrey. 2002. Characterizing antiviral activity of adefovir dipivoxil in transgenic mice expressing hepatitis B virus. Antiviral Res. 55:27-40. [DOI] [PubMed] [Google Scholar]
- 107.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. J. Toole, and D. Coakley. 1999. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy. JAMA 282:2305-2312. [DOI] [PubMed] [Google Scholar]
- 108.Kaufman, H. E., E. D. Varnell, and H. W. Thompson. 1999. Cidofovir and experimental herpetic stromal disease. Arch. Ophthalmol. 117:925-928. [DOI] [PubMed] [Google Scholar]
- 109.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. J. Clin. Investig. 99: 2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46:991-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kesson, A. M., J. K. Ferguson, W. D. Rawlinson, and A. L. Cunningham. 1997. Progressive vaccinia treated with ribavirin and vaccinia immune globulin. Clin. Infect. Dis. 25:911-914. [DOI] [PubMed] [Google Scholar]
- 112.Kirsch, L. S., J. F. Arevalo, E. Chavez-de-la-Paz, D. Munguia, E. De Clercq, and W. R. Freeman. 1995. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 102:533-542. [DOI] [PubMed] [Google Scholar]
- 113.Kirsch, L. S., J. F. Arevalo, E. De Clercq, E. Chavez-de-la-Paz, D. Munguia, R. Garcia, and W. R. Freeman. 1995. Phase I/II study of intravitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am. J. Ophthalmol. 119:466-476. [DOI] [PubMed] [Google Scholar]
- 114.Koonsaeng, S., C. Verschraegen, R. Freedman, M. Bossens, A. Kudelka, J. Kavanagh, T. Sittisomwong, E. De Clercq, and R. Snoeck. 2001. Successful treatment of recurrent vulvar intraepithelial neoplasia resistant to interferon and isotretinoin with cidofovir. J. Med. Virol. 64:195-198. [DOI] [PubMed] [Google Scholar]
- 115.Kopp, T., A. Geusau, A. Rieger, and G. Stingl. 2002. Successful treatment of an aciclovir-resistant herpes simplex type 2 infection with cidofovir in an AIDS patient. Br. J. Dermatol. 147:134-138. [DOI] [PubMed] [Google Scholar]
- 116.Kramata, P., I. Votruba, B. Otová, and A. Holý. 1996. Different inhibitory potencies of acyclic phosphonomethoxyalkyl nucleotide analogs toward DNA polymerases α, λ and ɛ. Mol. Pharmacol. 49:1005-1011. [PubMed] [Google Scholar]
- 117.Lacy, S. A., M. J. M. Hitchcock, W. A. Lee, P. Tellier, and K. C. Cundy. 1998. Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys. Toxicol. Sci. 44:97-106. [DOI] [PubMed] [Google Scholar]
- 118.Lalezari, J. P., G. N. Holland, F. Kramer, G. F. McKinley, C. A. Kemper, D. V. Ives, R. Nelson, W. D. Hardy, B. D. Kuppermann, D. W. Northfelt, M. Youle, M. Johnson, R. A. Lewis, D. V. Weinberg, G. L. Simon, R. A. Wolitz, A. E. Ruby, R. J. Stagg, and H. S. Jaffe. 1998. Randomized, controlled study of the safety and efficacy of intravenous cidofovir for the treatment of relapsing cytomegalovirus retinitis in patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17:339-344. [DOI] [PubMed] [Google Scholar]
- 119.Lalezari, J. P., R. J. Stagg, B. D. Kuppermann, G. N. Holland, F. Kramer, D. V. Ives, M. Youle, M. R. Robinson, W. L. Drew, and H. S. Jaffe. 1997. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. Ann. Intern. Med. 126:257-263. [DOI] [PubMed] [Google Scholar]
- 120.Lalezari, J., T. Schacker, J. Feinberg, J. Gathe, S. Lee, T. Cheung, F. Kramer, H. Kessler, L. Corey, W. L. Drew, J. Boggs, B. McGuire, H. S. Jaffe, and S. Safrin. 1997. A randomized, double-blinded, placebo-controlled trial of cidofovir gel for the treatment of acyclovir unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J. Infect. Dis. 176:892-898. [DOI] [PubMed] [Google Scholar]
- 121.Lalezari, J. P., W. L. Drew, E. Glutzer, C. James, D. Miner, J. Flaherty, P. E. Fisher, K. Cundy, J. Hannigan, and J. C. Martin. 1995. (S)-1-[3-Hydroxy-2-(phosphonylmethoxy)-propyl]cytosine (cidofovir): results of a phase I/II study of a novel antiviral nucleotide analogue. J. Infect. Dis. 171:788-796. [DOI] [PubMed] [Google Scholar]
- 122.Lalezari, J. P., W. L. Drew, E. Glutzer, D. Miner, S. Safrin, W. F. Owen, Jr., J. M. Davidson, P. E. Fisher, and H. S. Jaffe. 1994. Treatment with intravenous (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]-cytosine of acyclovir-resistant mucocutaneous infection with herpes simplex virus in a patient with AIDS. J. Infect. Dis. 170:570-572. [DOI] [PubMed] [Google Scholar]
- 123.Lateef, F., P. C. Don, M. Kaufmann, S. M. White, and J. M. Weinberg. 1998. Treatment of acyclovir-resistant, foscarnet-unresponsive HSV infection with topical cidofovir in a child with AIDS. Arch. Dermatol. 134:1169-1170. [DOI] [PubMed] [Google Scholar]
- 124.Legrand, F., D. Berrebi, N. Houhou, F. Freymuth, A. Faye, M. Duval, J. F. Mougenot, M. Peuchmaur, and E. Vilmer. 2001. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 27:621-626. [DOI] [PubMed] [Google Scholar]
- 125.Lenzo, J. C., G. R. Shellam, and C. M. Lawson. 2001. Ganciclovir and cidofovir treatment of cytomegalovirus-induced myocarditis in mice. Antimicrob. Agents Chemother. 45:1444-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liekens, S., E. Verbeken, E. De Clercq, and J. Neyts. 2001. Potent inhibition of hemangiosarcoma development in mice by cidofovir. Int. J. Cancer 92:161-167. [DOI] [PubMed] [Google Scholar]
- 127.Liekens, S., G. Andrei, M. Vandeputte, E. De Clercq, and J. Neyts. 1998. Potent inhibition of hemangioma formation in rats by the acyclic nucleoside phosphonate analogue cidofovir. Cancer Res. 58:2562-2567. [PubMed] [Google Scholar]
- 128.Liekens, S., J. Neyts, E. De Clercq, E. Verbeken, D. Ribatti, and M. Presta. 2001. Inhibition of fibroblast growth factor-2-induced vascular tumor formation by the acyclic nucleoside phosphonate cidofovir. Cancer Res. 61:5057-5064. [PubMed] [Google Scholar]
- 129.Ljungman, P., G. L. Deliliers, U. Platzbecker, S. Matthes-Martin, A. Bacigalupo, H. Einsele, J. Ullmann, M. Musso, R. Trenschel, P. Ribaud, M. Bornhäuser, S. Cesaro, B. Crooks, A. Dekker, N. Gratecos, T. Kingebiel, E. Tagliaferri, A. J. Ullmann, P. Wacker, and C. Cordonnier. 2001. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. Blood 97:388-392. [DOI] [PubMed] [Google Scholar]
- 130.LoPresti, A. E., J. F. Levine, G. B. Munk, C. Y. Tai, and D. B. Mendel. 1998. Successful treatment of an acyclovir- and foscarnet-resistant herpes simplex virus type 1 lesion with intravenous cidofovir. Clin. Infect. Dis. 26:512-513. [DOI] [PubMed] [Google Scholar]
- 131.Louie, M., C. Hogan, A. Hurley, V. Simon, C. Chung, N. Padte, P. Lamy, J. Flaherty, D. Coakley, M. Di Mascio, A. S. Perelson, and M. Markowitz. 2003. Determining the antiviral activity of tenofovir disoproxil fumarate in treatment-naive chronically HIV-1-infected individuals. AIDS 17:1151-1157. [DOI] [PubMed] [Google Scholar]
- 132.Marcellin, P., T.-T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 133.Margot, N. A., E. Isaacson, I. McGowan, A. K. Cheng, R. T. Schooley, and M. D. Miller. 2002. Genotypic and phenotypic analyses of HIV-1 in antiretroviral-experienced patients treated with tenofovir DF. AIDS 16:1227-1235. [DOI] [PubMed] [Google Scholar]
- 134.Margot, N. A., E. Isaacson, I. McGowan, A. Cheng, and M. D. Miller. 2003. Extended treatment with tenofovir disoproxil fumarate in treatment-experienced HIV-1-infected patients: genotypic, phenotypic, and rebound analyses. J. Acquir. Immune Defic. Syndr. 33:15-21. [DOI] [PubMed] [Google Scholar]
- 135.Matteelli, A., A. Beltrame, S. Graifemberghi, M. A. Forleo, M. Gulletta, G. Ciravolo, S. Tedoldi, C. Casalini, and G. Carosi. 2001. Efficacy and tolerability of topical 1% cidofovir cream for the treatment of external anogenital warts in HIV-infected persons. Sex. Transm. Dis. 28:343-346. [DOI] [PubMed] [Google Scholar]
- 136.Mazzi, R., S. G. Parisi, L. Sarmati, I. Uccella, E. Nicastri, G. Carolo, F. Gatti, E. Concia, and M. Andreoni. 2001. Efficacy of cidofovir on human herpesvirus 8 viraemia and Kaposi's sarcoma progression in two patients with AIDS. AIDS 15:2061-2063. [DOI] [PubMed] [Google Scholar]
- 137.Meadows, K. P., S. K. Tyring, A. T. Pavia, and T. M. Rallis. 1997. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch. Dermatol. 133:987-990. [PubMed] [Google Scholar]
- 138.Medveczky, M. M., E. Horvath, T. Lund, and P. G. Medveczky. 1997. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 11:1327-1332. [DOI] [PubMed] [Google Scholar]
- 139.Merta, A., I. Votruba, J. Jindrich, A. Holý, T. Cihlar, I. Rosenberg, M. Otmar, and T. Y. Hervé. 1992. Phosphorylation of 9-(2-phosphonomethoxyethyl)-adenine by AMP(dAMP) kinase from L1210. Biochem. Pharmacol. 44:2067-2077. [DOI] [PubMed] [Google Scholar]
- 140.Miller, M. D., K. E. Anton, A. S. Mulato, P. D. Lamy, and J. M. Cherrington. 1999. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J. Infect. Dis. 179:92-100. [DOI] [PubMed] [Google Scholar]
- 141.Miller, M. D., N. A. Margot, P. D. Lamy, M. D. Fuller, K. E. Anton, A. S. Mulato, and J. M. Cherringon. 2001. Adefovir and tenofovir susceptibilities of HIV-1 after 24 to 48 weeks of adefovir dipivoxil therapy: genotypic and phenotypic analyses of study GS-96-408. J. Acquir. Immune Defic. Syndr. 27:450-458. [DOI] [PubMed] [Google Scholar]
- 142.Murono, S., N. Raab-Traub, and J. S. Pagano. 2001. Prevention and inhibition of nasopharyngeal carcinoma growth by antiviral phosphonated nucleoside analogs. Cancer Res. 61:7875-7877. [PubMed] [Google Scholar]
- 143.Mutimer, D., B. H. Feraz-Neto, R. Harrison, K. O'Donnell, J. Shaw, P. Cane, and D. Pillay. 2001. Acute liver graft failure due to emergence of lamivudine resistant hepatitis B virus: rapid resolution during treatment with adefovir. Gut 49:860-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Naeger, L. K., N. A. Margot, and M. D. Miller. 2001. Increased drug susceptibility of HIV-1 reverse transcriptase mutants containing M184V and zidovudine-associated mutations: analysis of enzyme processivity, chain-terminator removal and viral replication. Antiviral Ther. 6:115-126. [PubMed] [Google Scholar]
- 145.Naeger, L. K., N. A. Margot, and M. D. Miller. 2001. Tenofovir (PMPA) is less susceptible to pyrophosphorolysis and nucleotide-dependent chain-terminator removal than zidovudine or stavudine. Nucleosides Nucleotides Nucleic Acids 20:635-639. [DOI] [PubMed] [Google Scholar]
- 146.Naeger, L. K., N. A. Margot, and M. D. Miller. 2002. ATP-dependent removal of nucleoside reverse transcriptase inhibitors by human immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 46:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Naesens, L., R. Snoeck, G. Andrei, J. Balzarini, J. Neyts, and E. De Clercq. 1997. HPMPC (cidofovir), PMEA (adefovir) and related acyclic nucleoside phosphonate analogues: a review of their pharmacology and clinical potential in the treatment of viral infections. Antiviral Chem. Chemother. 8:1-23. [Google Scholar]
- 148.Nettleton, P. F., J. A. Gilray, H. W. Reid, and A. A. Mercer. 2000. Parapoxviruses are strongly inhibited in vitro by cidofovir. Antiviral Res. 48:205-208. [DOI] [PubMed] [Google Scholar]
- 149.Neyts, J., and E. De Clercq. 1997. Antiviral drug susceptibility of human herpesvirus type 8. Antimicrob. Agents Chemother. 41:2754-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neyts, J., and E. De Clercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 41:242-246. [DOI] [PubMed] [Google Scholar]
- 151.Neyts, J., and E. De Clercq. 1998. In vitro and in vivo inhibition of murine gamma herpesvirus 68 replication by selected antiviral agents. Antimicrob. Agents Chemother. 42:170-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Neyts, J., H. Sobis, R. Snoeck, M. Vandeputte, and E. De Clercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihdyroxy-2-propoxymethyl)guanine in the treatment of intracerebral murine cytomegalovirus infections in immunocompetent and immunodeficient mice. Eur. J. Clin. Microbiol. Infect. Dis. 12:269-279. [DOI] [PubMed] [Google Scholar]
- 153.Neyts, J., J. Balzarini, L. Naesens, and E. De Clereq. 1992. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for the treatment of murine cytomegalovirus infection in severe combined immunodeficiency mice. J. Med. Virol. 37:67-71. [DOI] [PubMed] [Google Scholar]
- 154.Neyts, J., R. Sadler, E. De Clercq, N. Raab-Traub, and J. S. Pagano. 1998. The antiviral agent cidofovir [(S)-1-(3-hydroxy-2-phosphonyl-methoxypropyl)cytosine] has pronounced activity against nasopharyngeal carcinoma grown in nude mice. Cancer Res. 58:384-388. [PubMed] [Google Scholar]
- 155.Neyts, J., R. Snoeck, D. Schols, J. Balzarini, and E. De Clercq. 1990. Selective inhibition of human cytomegalovirus DNA synthesis by (S)-1-(3-hydroxy-2-phosphonylmethoxy-propyl)cytosine [(S)-HPMPC] and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG). Virology 179:41-50. [DOI] [PubMed] [Google Scholar]
- 156.Neyts, J., R. Snoeck, J. Balzarini, and E. De Clercq. 1991. Particular characteristics of the anti-human cytomegalovirus activity of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in vitro. Antiviral Res. 16:41-52. [DOI] [PubMed] [Google Scholar]
- 157.Noble, S., and K. L. Goa. 1999. Adefovir dipivoxil. Drugs 58:479-487. [DOI] [PubMed] [Google Scholar]
- 158.Orlando, G., M. M. Fasolo, R. Beretta, S. Merli, and A. Cargnel. 2002. Combined surgery and cidofovir is an effective treatment for genital warts in HIV-infected patients. AIDS 16:447-450. [DOI] [PubMed] [Google Scholar]
- 159.Otten, R. A., D. K. Smith, D. R. Adams, J. K. Pullium, E. Jackson, C. N. Kim, H. Jaffe, R. Janssen, S. Butera, and T. M. Folks. 2000. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J. Virol. 74:9771-9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Palmer, S., N. Margot, H. Gilbert, N. Shaw, R. Buckheit, Jr., and M. Miller. 2001. Tenofovir, adefovir, and zidovudine susceptibilities of primary human immunodeficiency virus type 1 isolates with non-B subtypes of nucleoside resistance. AIDS Res. Hum. Retroviruses 17:1167-1173. [DOI] [PubMed] [Google Scholar]
- 161.Perrillo, R., E. Schiff, E. Yoshida, A. Statler, K. Hirsch, T. Wright, K. Gutfreund, P. Lamy, and A. Murray. 2000. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129-134. [DOI] [PubMed] [Google Scholar]
- 162.Peters, M. G., G. Singer, T. Howard, S. Jacobsmeyer, X. Xiong, C. S. Gibbs, P. Lamy, and A. Murray. 1999. Fulminant hepatic failure resulting from lamivudine-resistant hepatitis B virus in a renal transplant recipient. Transplantation 68:1912-1914. [DOI] [PubMed] [Google Scholar]
- 163.Platzbecker, U., D. Bandt, C. Thiede, A. Helwig, J. Freiberg-Richter, U. Schuler, R. Plettig, G. Geissler, A. Rethwilm, G. Ehninger, and M. Bornhäuser. 2001. Successful preemptive cidofovir treatment for CMV antigenemia after dose-reduced conditioning and allogeneic blood stem cell transplantation. Transplantation 71:880-885. [DOI] [PubMed] [Google Scholar]
- 164.Plosker, G. L., and S. Noble. 1999. Cidofovir. A review of its use in cytomegalovirus retinitis in patients with AIDS. Drugs 58:325-345. [DOI] [PubMed] [Google Scholar]
- 165.Polis, M. A., K. M. Spooner, B. F. Baird, J. F. Manischewitz, H. S. Jaffe, P. E. Fisher, J. Falloon, R. T. Davey, Jr., J. A. Kovacs, R. E. Walker, S. M. Whitcup, R. B. Nussenblatt, H. C. Lane, and H. Masur. 1995. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob. Agents Chemother. 39:882-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Portilla, J., V. Boix, F. Román, S. Reus, and E. Merino. 2000. Progressive multifocal leukoencephalopathy treated with cidofovir in HIV-infected patients receiving highly active anti-retroviral therapy. J. Infect. 41:182-184. [DOI] [PubMed] [Google Scholar]
- 167.Pransky, S. M., A. E. Magit, D. B. Kearns, D. R. Kang, and N. O. Duncan. 1999. Intralesional cidofovir for recurrent respiratory papillomatosis in children. Arch. Otolaryngol. Head Neck Surg. 125:1143-1148. [DOI] [PubMed] [Google Scholar]
- 168.Pransky, S. M., D. F. Brewster, A. E. Magit, and D. B. Kearns. 2000. Clinical update on 10 children treated with intralesional cidofovir injections for severe recurrent respiratory papillomatosis. Arch. Otolaryngol. Head Neck Surg. 126:1239-1243. [DOI] [PubMed] [Google Scholar]
- 169.Preiser, W., N. Kapur, R. Snoeck, R. W. Groves, and N. S. Brink. 2000. No apparent effect of cidofovir in epidermodysplasia verruciformis. J. Clin. Virol. 16:55-57. [DOI] [PubMed] [Google Scholar]
- 170.Razonable, R. R., A. J. Aksamit, A. J. Wright, and J. W. Wilson. 2001. Cidofovir treatment of progressive multifocal leukoencephalopathy in a patient receiving highly active antiretroviral therapy. Mayo Clin. Proc. 76:1171-1175. [DOI] [PubMed] [Google Scholar]
- 171.Redfield, R. R., D. C. Wright, W. D. James, T. S. Jones, C. Brown, and D. S. Burke. 1987. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N. Engl. J. Med. 316:673-676. [DOI] [PubMed] [Google Scholar]
- 172.Redondo, P., M. Idoate, J. C. Galofré, and T. Solano. 2000. Cidofovir inhibits growth of B16 melanoma cells in vivo. Br. J. Dermatol. 143:741-748. [DOI] [PubMed] [Google Scholar]
- 173.Reymen, D., L. Naesens, J. Balzarini, A. Holý, H. Dvoráková, and E. De Clercq. 1995. Antiviral activity of selected acyclic nucleoside analogues against human herpesvirus 6. Antiviral Res. 28:343-357. [DOI] [PubMed] [Google Scholar]
- 174.Ribaud, P., C. Scieux, F. Freymuth, F. Morinet, and E. Gluckman. 1999. Successful treatment of adenovirus disease with intravenous cidofovir in an unrelated stem-cell transplant recipient. Clin. Infect. Dis. 28:690-691. [DOI] [PubMed] [Google Scholar]
- 175.Romanowski, E. G., K. A. Yates, and Y. J. Gordon. 2001. Antiviral prophylaxis with twice daily topical cidofovir protects against challenge in the adenovirus type 5/New Zealand rabbit ocular model. Antiviral Res. 52:275-280. [DOI] [PubMed] [Google Scholar]
- 176.Romanowski, E. G., S. P. Bartels, and Y. J. Gordon. 1999. Comparative antiviral efficacies of cidofovir, trifluridine, and acyclovir in the HSV-1 rabbit keratitis model. Investig. Ophthalmol. Visual Sci. 40:378-384. [PubMed] [Google Scholar]
- 177.Romanowski, E. G., and Y. J. Gordon. 2000. Efficacy of topical cidofovir on multiple adenoviral serotypes in the New Zealand rabbit ocular model. Investig. Ophthalmol. Visual Sci. 41:460-463. [PubMed] [Google Scholar]
- 178.Sacks, S. L., S. D. Shafran, F. Diaz-Mitoma, S. Trottier, R. G. Sibbald, A. Hughes, S. Safrin, J. Rudy, B. McGuire, and H. S. Jaffe. 1998. A multicenter phase I/II dose escalation study of single-dose cidofovir gel for treatment of recurrent genital herpes. Antimicrob. Agents Chemother. 42:2996-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salmaggi, A., E. Maccagnano, A. Castagna, S. Zeni, F. Fantini, P. Cinque, and M. Savoiardo. 2001. Reversal of CSF positivity for JC virus genome by cidofovir in a patient with systemic lupus erythematosus and progressive multifocal leukoencephalopathy. Neurol. Sci. 22:17-20. [DOI] [PubMed] [Google Scholar]
- 180.Schooley, R. T., P. Ruane, R. A. Myers, G. Beall, H. Lampiris, D. Berger, S.-S. Chen, M. D. Miller, E. Isaacson, and A. K. Cheng. 2002. Tenofovir DF in antirctroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS 16:1257-1263. [DOI] [PubMed] [Google Scholar]
- 181.Schürmann, D., F. Bergmann, B. Temmesfield-Wollbrück, M. P. Grobusch, and N. Suttorp. 2000. Topical cidofovir is effective in treating extensive penile condylomata acuminata. AIDS 14:1075-1076. [DOI] [PubMed] [Google Scholar]
- 182.Schürmann, D., F. Bergmann, M. P. Grobusch, M. Behnsch, and A. Liekfeld. 1998. Lack of efficacy of cidofovir in treating AIDS-related cytomegalovirus retinitis after long-term treatment with ganciclovir. AIDS 12:678-679. [PubMed] [Google Scholar]
- 183.Shaw J.-P., C. M. Sueoka, R. Oliyai, W. A. Lee, M. N. Arimilli, and K. C. Cundy. 1997. Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res. 14:1824-1829. [DOI] [PubMed] [Google Scholar]
- 184.Shulman, N. S., A. R. Zolopa, D. J. Passaro, J. Murlidharan, D. M. Israelski, C. L. Brosgart, M. D. Miller, S. Van Doren, R. W. Shafer, and D. A. Katzenstein. 2000. Efavirenz- and adefovir dipivoxil-based salvage therapy in highly treatment-experienced patients: clinical and genotypic predictors of virologic response. J. Acquir. Immune Defic. Syndr. 23:221-226. [DOI] [PubMed] [Google Scholar]
- 185.Simonart, T., J.-C. Noel, E. De Clercq, and R. Snoeck. 1998. Abatement of cutaneous Kaposi's sarcoma associated with cidofovir treatment. Clin. Infect. Dis. 27:1562. [DOI] [PubMed] [Google Scholar]
- 186.Simonart, T., J.-C. Noel, G. De Dobbeleer, D. Parent, J.-P. Van Vooren, E. De Clercq, and R. Snoeck. 1988. Treatment of classical Kaposi's sarcoma with intralesional injections of cidofovir: report of a case. J. Med. Virol. 55:215-218. [PubMed] [Google Scholar]
- 187.Smee, D. F., J. L. B. Morris, J. A. Leonhardt, J. R. Mead, A. Holý and R. W. Sidwell. 1992. Treatment of murine cytomegalovirus infections in severe combined immunodeficient mice with ganciclovir, (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine, interferon, and bropirimine. Antimicrob. Agents Chemother. 36:1837-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smee, D. F., K. W. Bailey, M.-H. Wong, and R. W. Sidwell. 2001. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antiviral Res. 52:55-62. [DOI] [PubMed] [Google Scholar]
- 189.Smee, D. F., K. W. Bailey, M.-H. Wong, and R. W. Sidwell. 2001. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antiviral Res. 47:171-177. [DOI] [PubMed] [Google Scholar]
- 190.Smee, D. F., K. W. Bailey, and R. W. Sidwell. 2001. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antiviral Chem. Chemother. 12:71-76. [DOI] [PubMed] [Google Scholar]
- 191.Smee, D. F., R. A. Burger, R. P. Warren, K. W. Bailey, and R. W. Sidwell. 1997. An immunosuppressed mouse model of lethal murine gammaherpesvirus 68 infection for studying potential treatment of Epstein-Barr virus infection in man. Antiviral Chem. Chemother. 8:573-581. [Google Scholar]
- 192.Smee, D. F., R. W. Sidwell, D. Kefauver, M. Bray, and J. W. Huggins. 2002. Characterization of wild-type and cidofovir-resistant strains of camelpox, cowpox, monkeypox, and vaccinia viruses. Antimicrob. Agents Chemother. 46:1329-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Smith, I. L., J. M. Cherrington, R. E. Jiles, M. D. Fuller, W. R. Freeman, and S. A. Spector. 1997. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J. Infect. Dis. 176:69-77. [DOI] [PubMed] [Google Scholar]
- 194.Smith, M. S., L. Foresman, G. J. Lopez, J. Tsay, D. Wodarz, J. D. Lifson, A. Page, C. Wang, Z. Li, I. Adany, S. Buch, N. Bischofberger, and O. Narayan. 2000. Lasting effects of transient postinoculation tenofovir [9-R-(2-phosphonomethoxypropyl)adenine] treatment on SHIVKU2 infection of rhesus macaques. Virology 277:306-315. [DOI] [PubMed] [Google Scholar]
- 195.Snoeck, R., G. Andrei, E. De Clercq, M. Gerard, N. Clumeck, G. Tricot, and C. Sadzot-Delvaux. 1993. A new topical treatment for resistant herpes simplex infections. N. Engl. J. Med. 329:968-969. [DOI] [PubMed] [Google Scholar]
- 196.Snoeck, R., J. C. Noël, C. Muller, E. De Clercq, and M. Bossens. 2000. Cidofovir, a new approach for the treatment of cervix intraepithelial neoplasia grade III (CIN III). J. Med. Virol. 60:205-209. [DOI] [PubMed] [Google Scholar]
- 197.Snoeck, R., M. Bossens, D. Parent, B. Delaere, H. Degreef, M. Van Ranst, J. C. Noël, M. Wulfsohn, J. F. Rooney, H. S. Jaffe, and E. De Clercq. 2001. Phase II double-blind, placebo-controlled study of the safety and efficacy of cidofovir topical gel for the treatment of patients with human papillomavirus infection. Clin. Infect. Dis. 33:597-602. [DOI] [PubMed] [Google Scholar]
- 198.Snoeck, R., M. Van Ranst, G. Andrei, E. De Clercq, S. De Wit, M. Poncin, and N. Clumeck. 1995. Treatment of anogenital papillomavirus infections with an acyclic nucleoside phosphonate analogue. N. Engl. J. Med. 333:943-944. [DOI] [PubMed] [Google Scholar]
- 199.Snoeck, R., T. Sakuma, E. De Clercq, I. Rosenberg, and A. Holý. 1988. (S)-1(3-Hydroxy-2-Phosphonylmethoxyprophyl)cytosine, a potent and selective inhibitor of human cytomegalovirus replication. Antimicrob. Agents Chemother. 32:1839-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Snoeck, R., W. Wellens, C. Desloovere, M. Van Ranst, L. Naesens, E. De Clercq, and L. Feenstra. 1998. Treatment of severe laryngeal papillomatosis with intralesional injections of cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC]. J. Med. Virol. 54:219-225. [DOI] [PubMed] [Google Scholar]
- 201.Snoeck, R., Y. Van Laethem, E. De Clercq, J. De Maubeuge, and N. Clumeck. 2001. Treatment of a bowenoid papulosis of the penis with local applications of cidofovir in a patient with acquired immunodeficiency syndrome. Arch. Intern. Med. 161:2382-2384. [DOI] [PubMed] [Google Scholar]
- 202.Srinivas, R. V., and A. Fridland. 1998. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 42:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stragier, I., R. Snoeck, E. De Clercq, J. J. Van den Oord, M. Van Ranst, and H. De Greef. 2002. Local treatment of HPV-induced skin lesions by cidofovir. J. Med. Virol. 67:241-245. [DOI] [PubMed] [Google Scholar]
- 204.Reference deleted.
- 205.Takahashi, K., M. Suzuki, Y. Iwata, S. Shigeta, K. Yamanishi, and E. De Clercq. 1997. Selective activity of various nucleoside and nucleotide analogues against human herpesvirus 6 and 7. Antiviral Chem. Chemother. 8:24-31. [Google Scholar]
- 206.Taoufik, Y., J. Gasnault, A. Karaterki, M. P. Ferey, E. Marchadier, C. Goujard, A. Lannuzel, J. F. Delfraissy, and E. Dussaix. 1998. Prognostic value of JC virus load in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J. Infect. Dis. 178:1816-1820. [DOI] [PubMed] [Google Scholar]
- 207.Tarantal, A. F., A. Castillo, J. E. Ekert, N. Bischofberger, and R. B. Martin. 2002. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). J. Acquir. Immune Defic. Syndr. 29:207-220. [DOI] [PubMed] [Google Scholar]
- 208.Tarantal, A. F., M. L. Marthas, J. P. Shaw, K. Cundy, and N. Bischofberger. 1999. Administration of 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) to gravid and infant rhesus macaques (Macaca mulatta): safety and efficacy studies. J. Acquir. Immune Defic. Syndr. 20:323-333. [DOI] [PubMed] [Google Scholar]
- 209.Tatti, K. M., B. E. Korba, H. L. Stang, S. Peek, J. L. Gerin, B. C. Tennant, and R. F. Schinazi. 2002. Mutations in the conserved woodchuck hepatitis virus polymerase FLLA and YMDD regions conferring resistance to lamivudine. Antiviral Res. 55:141-150. [DOI] [PubMed] [Google Scholar]
- 209a.The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. 1997. Parenteral cidofovir for cytomegalovirus retinitis in patients with AIDS: the HPMPC peripheral cytomegalovirus retinitis trial. Ann. Intern. Med. 126:264-274. [DOI] [PubMed] [Google Scholar]
- 210.The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. 2000. Long-term follow-up of patients with AIDS treated with parenteral cidofovir for cytomegalovirus retinitis: the HPMPC peripheral cytomegalovirus retinitis trial. AIDS 14:1571-1581. [PubMed] [Google Scholar]
- 211.The Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group. 2001. The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: the ganciclovir cidofovir cytomegalovirus retinitis trial. Am. J. Ophthalmol. 131:457-467. [DOI] [PubMed] [Google Scholar]
- 212.Toro, J. R., L. V. Wood, N. K. Patel, and M. L. Turner. 2000. Topical cidofovir. A novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus type 1. Arch. Dermatol. 136:983-985. [DOI] [PubMed] [Google Scholar]
- 213.Tsai, C.-C., K. E. Follis, A. Sabo, T. W. Beck, R. F. Grant, N. Bischofberger, R. E. Benveniste, and R. Black. 1995. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 270:1197-1199. [DOI] [PubMed] [Google Scholar]
- 214.Tsai, C.-C., K. E. Follis, T. W. Beck, A. Sabo, N. Bischofberger, and P. J. Dailey. 1997. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res. Hum. Retroviruses 13:707-712. [DOI] [PubMed] [Google Scholar]
- 215.Tsai, C.-C., P. Emau, J. C. Sun, T. W. Beck, C.-A. Tran, K. E. Follis, N. Bischofberger, and W. R. Morton. 2000. Post-exposure chemoprophylaxis (PECP) against SIV infection of macaques as a model for protection from HIV infection. J. Med. Primatol. 29:248-258. [DOI] [PubMed] [Google Scholar]
- 216.Van Cutsem, E., R. Snoeck, M. Van Ranst, P. Fiten, G. Opdenakker, K. Geboes, J. Janssens, P. Rutgeerts, G. Vantrappen, and E. De Clercq. 1995. Successful treatment of a squamous papilloma of the hypopharynx-esophagus by local injections of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine. J. Med. Virol. 45:230-235. [DOI] [PubMed] [Google Scholar]
- 217.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 219.Van Rompay, K. K. A., M. D. Miller, M. L. Marthas, N. A. Margot, P. J. Dailey, D. R. Canfield, R. P. Tarara, J. M. Cherrington, N. L. Aguirre, N. Bischofberger, and N. C. Pedersen. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]-adenine (PMPA)administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van Rompay, K. K. A., P. J. Dailey, R. P. Tarara, D. R. Canfield, N. L. Aguirre, J. M. Cherrington, P. D. Lamy, N. Bischofberger, N. C. Pedersen, and M. L. Marthas. 1999. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 73:2947-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Van Valckenborgh, I., W. Wellens, K. De Boeck, R. Snoeck, E. De Clercq, and L. Feenstra. 2001. Systemic cidofovir in papillomatosis. Clin. Infect. Dis. 32:e62-e64. [DOI] [PubMed] [Google Scholar]
- 222.Vats, A., R. Shapiro, P. S. Randhawa, V. Scantlebury, A. Tuzuner, M. Saxena, M. L. Moritz, T. J. Beattie, T. Gonwa, M. D. Green, and D. Ellis. 2003. Quantitative viral load monitoring and cidofovir therapy for the management of BK virus-associated nephropathy in children and adults. Transplantation 75:105-112. [DOI] [PubMed] [Google Scholar]
- 223.Walsh, K. M., T. Woodall, P. Lamy, D. G. D. Wight, S. Bloor, and G. J. M. Alexander. 2001. Successful treatment with adefovir dipivoxil in a patient with fibrosing cholestatic hepatitis and lamivudine resistant hepatitis B virus. Gut 49:436-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Westland, C. E., H. Yang, W. E. Delaney IV, C. S. Gibbs, M. D. Miller, M. Wulfsohn, J. Fry, C. L. Brosgart, and S. Xiong. 2003. Week 48 resistance surveillance in two phase 3 clinical studies of adefovir dipivoxil for chronic hepatitis B. Hepatology 38:96-103. [DOI] [PubMed] [Google Scholar]
- 225.Wilson, W. R., R. Hashemiyoon, and A. Hawrych. 2000. Intralesional cidofovir for recurrent laryngeal papillomas: preliminary report. ENT—Ear Nose Throat J. 79:236-240. [PubMed] [Google Scholar]
- 226.Xiong, X., J. L. Smith, and M. S. Chen. 1997. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob. Agents Chemother. 41:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yang, H., and R. Datema. 1991. Prolonged and potent therapeutic and prophylactic effects of (S)-1-[(3-hydroxy-2-phosphonylmethoxy)propyl]cytosine against herpes simplex virus type 2 infections in mice. Antimicrob. Agents Chemother. 35:1596-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yang, H., C. E. Westland, W. E. Delaney IV, E. J. Heathcote, V. Ho, J. Fry, C. Brosgart, C. S. Gibbs, M. D. Miller, and S. Xiong. 2002. Resistance surveillance in chronic hepatitis B patients treated with adefovir dipivoxil for up to 60 weeks. Hepatology 36:464-473. [DOI] [PubMed] [Google Scholar]
- 229.Zabawski, E. J., B. Sands, D. Goetz, M. Naylor, and C. J. Cockerell. 1997. Treatment of verruca vulgaris with topical cidofovir. JAMA 278:1236. [PubMed] [Google Scholar]
- 230.Zabawski, E. J., and C. J. Cockerell. 1998. Topical and intralesional cidofovir: a review of pharmacology and therapeutic effects. J. Am. Acad. Dermatol. 39:741-745. [DOI] [PubMed] [Google Scholar]
- 231.Zabawski, E. J., Jr., and C. J. Cockerell. 1999. Topical cidofovir for molluscum contagiosum in children. Pediatr. Dermatol. 16:414-415. [PubMed] [Google Scholar]
- 232.Zimmermann, T., K. Stingele, M. Hartmann, J. Haas, R. von Einsiedel, and B. Wildemann. 2001. Succesful treatment of AIDS related PML with HAART and cidofovir. Eur. J. Med. Res. 6:190-192. [PubMed] [Google Scholar]