Abstract
Through mutagenesis by P-element transposition, we identified a series of mutants with deletions in topoisomerase 3β gene (top3β) and an adjacent, previously uncharacterized gene CG15897, here named wuho (wh). Whereas top3β truncation does not affect viability or fertility, wh null mutants display male sterile and female semi-sterile phenotypes. Furthermore, wh mutants can be fully rescued by wh transgenes, but not by top3β transgenes, suggesting that the fertility phenotypes are caused by wh deletion. The alignment of WH protein sequence with other eukaryotic putative homologues shows they are evolutionarily conserved proteins with 5 WD40 repeats in the middle portion of the protein, and a bipartite nuclear localization signal at the carboxyl terminus. Yeast homologue with 5 WD40 repeats, Trm82, is the non-catalytic subunit of a tRNA methylase. Immunostaining shows that WH has the highest expression in hub cells, a niche for germline stem cells of testis. However, WH is not required for the maintenance of hub cells or the germline stem cells. In wh mutant males, spermatogenesis is arrested at the elongating stage of the developing spermatids, resulting in an absence of mature sperms in the seminal vesicles. The decreased fertility in wh mutant females is mostly due to defects in oogenesis. There are abnormal egg chambers present in the mutant females, in which the cystocytes fail to arrest their cell division at the fourth mitotic cycle, resulting in more than 16 cells in a single egg chamber. Additionally, these abnormal cystocytes do not undergo multiple rounds of endoreplication as the nurse cells do in a normal egg chamber. Therefore, the cytological analyses demonstrate that wh has a critical function in cellular differentiation for germline cells during gametogenesis.
Keywords: Gametogenesis, Drosophila, Hub cells, WD40 repeats, wh, Male sterile
Introduction
Gametogenesis provides a unique system to study cell division, differentiation and morphogenesis. At the initial stage of gametogenesis in Drosophila melanogaster, female and male share many key characteristics (Fuller, 1993; King, 1970; Lindsley and Tokuyasu, 1980; Spradling, 1993). Germline stem cells (GSCs) are positioned at the anterior tip of both ovarioles and testes. Each GSC undergoes a stereotypical asymmetric cell division, which gives rise to one daughter stem cell that continues to undergo asymmetric cell divisions and the other that commits to differentiation.
In female, each ovariole, the functional unit of ovary, contains 2–3 GSCs at its apex in a specialized structure called the germarium. The microenvironment in GSCs, known as niche, is composed of terminal filament cells at the anterior tip of the germarium and cap cells at the base of the terminal filament, both of which are in direct contact with the underlying GSCs. Each GSC divides asymmetrically with respect to the niche, producing one daughter stem cell that remains in contact with the cap cell. The other daughter cell which leaves the niche and differentiates into a cystoblast, undergoes four synchronous, incomplete divisions to form a 16-cell germline cyst (Lin, 2002; Spradling, 1993). These cystocytes in a cyst will have different developmental fates in the subsequent cellular differentiation (Lin and Spradling, 1995; Spradling, 1993). A cystocyte from the first cystoblast mitotic division will develop into an oocyte and undergo partial meiotic division, arresting at the metaphase of meiosis I. The meiotic arrest will be maintained throughout the development from the oocyte to mature egg, and meiosis II will only complete after fertilization. The rest of the 15 cystocytes will develop into nurse cells and undergo multiple rounds of endoreplication. The developing egg chamber with nurse cells and oocyte is surrounded by the follicle cells that are of somatic origins. The cytoplasmic contents of the nurse cells, along with those from follicle cells, will be transferred into the mature egg. Mutations affecting any step in the process of oogenesis will result in female sterile phenotype (Mahowald, 1980; Spradling, 1993). Because the first 14 mitotic cycles during embryogenesis depends exclusively on the maternal endowment (Merrill et al., 1988; Wieschaus and Sweeton, 1988; Zalokar, 1976), any mutations affecting the transfer and storage of maternal components will also lead to embryonic lethal, and consequently female sterile phenotype. Mutants with defective germ cells differentiation can give rise to sterile phenotypes. Mutations in bam and bgcn will result in the over-proliferation of small cells with characteristics of undifferentiated germ cells (Gonczy et al., 1997; Lavoie et al., 1999; McKearin and Spradling, 1990). In bam mutants, the undifferentiated germ cells appear to behave like stem cells (Gonczy et al., 1997; McKearin and Spradling, 1990). bam mRNA is absent in GSCs, but accumulates in cystoblasts and mitotic cysts (McKearin and Spradling, 1990). The functions of bam are in promoting the elimination of GSCs and differentiation of cystoblasts (Chen and McKearin, 2003; Ohlstein and McKearin, 1997; Song et al., 2004).
In male, the testes are two tubules where different developmental stages of spermatogenesis are laid out in chronological order from the GSCs at the anterior tip to mature spermatozoa at the end. It has become an excellent system for investigating the molecular mechanisms governing stem cell proliferation and germline cell differentiation (Kiger et al., 2000, 2001; Tulina and Matunis, 2001). At the anterior tip of testis, there are two types of stem cells, GSCs and cyst progenitor cells (somatic stem cells). GSCs are attached to a cluster of somatic cell called hub cells (Hardy et al., 1979). Recent studies showed that hub cells function as a niche for GSCs (Kiger et al., 2001; Tulina and Matunis, 2001). The hub cells produce the Unpaired (Upd), a secreted ligand that locally activates the Jak-STAT pathway within GSCs to maintain stem cell fate (Kiger et al., 2001; Tulina and Matunis, 2001). Division of GSCs produces one daughter cell remaining in stem cell fate and the other differentiates into a primary spermatogonial cell. Each primary spermatogonial cell undergoes four synchronous mitotic divisions to give rise to a cyst with 16 spermatocytes (Fuller, 1993; Lindsley and Tokuyasu, 1980; Yamashita et al., 2003). The 16 spermatocytes then go through two consecutive meiotic divisions to generate 64 spermatids. Following the second meiosis, the flagellar axonemes are assembled from basal bodies embedded in each spermatid nucleus. As the axonemes elongate, nuclear shape is streamlined and the spermatid nuclei are driven toward the end of the testis. After elongation, the syncytial spermatids are separated into individual sperms by an individualization complex, and the coiled and motile sperms are moved into the seminal vesicles. Mutations interrupting any step in this progression of spermatogenesis can lead to male sterility (Fuller, 1993, 1998). Spermatogenesis may also share some of the same genes that are required for oogenesis. For example, bam and bgcn have the same role in restricting the proliferation of the germline cells in the spermagonia cyst (Gonczy et al., 1997; McKearin and Spradling, 1990).
In this paper, we present the isolation and characterization of a novel Drosophila male-sterile and female semi-sterile mutant caused by mutations in a Drosophila gene CG15897, which we named wh (wuho, meaning no progeny). wh encodes a highly conserved WD40-repeat protein, which has known homologues in human and mouse (WDR4) (Michaud et al., 2000). The homologue in baker’s yeast is Trm82, which is a part of the complex that confers m7G methyltransferase activity on pre-tRNAPhe, and associates with Trm8, the catalytic subunit of the complex (Alexandrov et al., 2002). Although both Trm82 and Trm8 are required for the methyltransferase activity, neither TRM82 nor TRM8 is essential for the yeast viability (Alexandrov et al., 2002). However, the mutants have growth defects under restrictive nutrient conditions. Strains lacking functional TRM82 or TRM8 are temperature-sensitive for growth in synthetic media containing glycerol as a sole carbon source (Alexandrov et al., 2005). To date, there is no functional study on any member of this conserved family of proteins in a metazoan system. Interestingly, the data presented here suggest that the Drosophila homologue in this family, wh, has a specific and critical function in the development of germline cells during spermatogenesis and oogenesis.
Materials and methods
Generation of deficiency strains
We acquired a P-element insertion mutant top3βEP(X)1432 from Berkeley Drosophila Genome Project and mapped the insertion to the 5′ untranslated region, 29 bp upstream to the translational initiation codon (Wilson et al., 2000). We also showed that although this insertion strain is a hypomorph for top3β, it is viable and fertile. To create more severe alleles of top3β, we carried out mutagenesis through imprecise excision of the inserted P-element (Engels et al., 1990) and screened the progeny that have lost w+ marker. Five truncation mutants were recovered (Fig. 1A). top3β16, top3β26 and top3β37 have deletions affecting only top3β and are top3β nulls. At the original P-element insertion site, top3β16, top3β26 and top3β37 have an insert of 3 kb, 170 bp and 2.3 kb, respectively. top3β16 has a deletion from the nucleotide 36 bp upstream to the translation initiation codon to the nucleotide 2758 bp downstream to the translation initiation codon. top3β26 has a deletion from the nucleotide 495 bp to nucleotide 894 bp downstream from the translation start site. top3β37 has a deletion from the nucleotide 36 bp upstream to 672 bp downstream of the translation start site. Two of the mutants, having deletions extended beyond the 5′ end of the top3β and removing part of the coding sequence from the neighboring gene CG15897, were named wh7 and wh56. wh7 has 632 bp insert at the original P-element insertion site and deletion from the nucleotide 385 bp upstream to the nucleotide 1038 bp downstream to the translation initiation codon of CG15897. wh56 has deletion from nucleotide 3648 bp upstream to nucleotide 365 bp downstream of translation start. Both were nulls for CG15897 and had similar phenotypes in all the analysis to be presented in this paper.
Fig. 1.
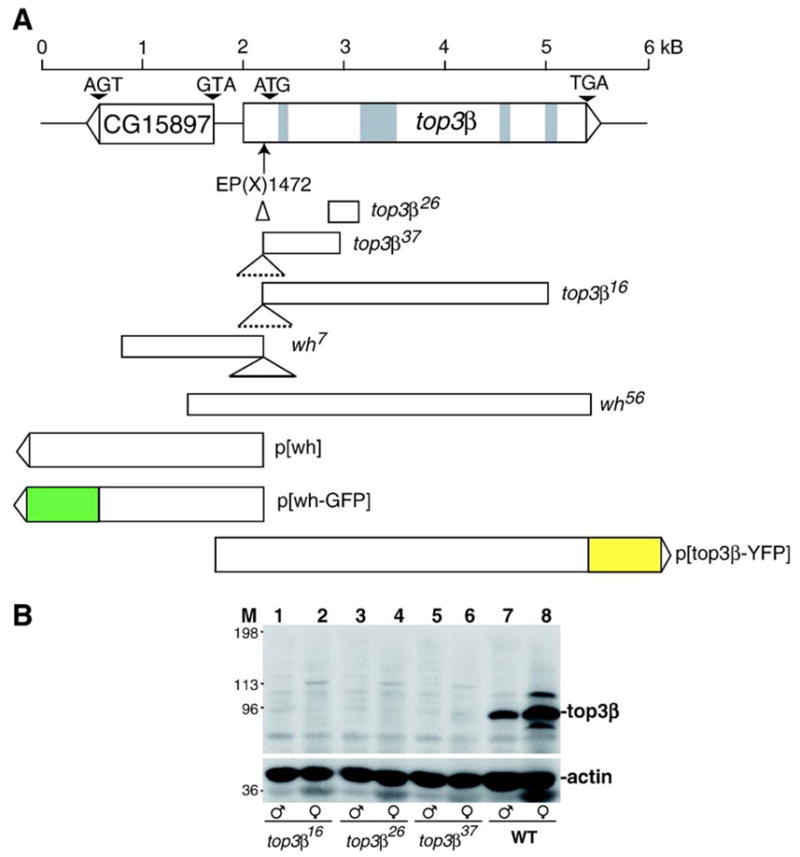
Deficiency mutants of wh and top3β. (A) Schematic diagram of the genomic structure of wh and top3β genes, their deficiency mutants and transgenic constructs. The P-element insertion mutant top3βEP(X)1432 was described previously (Wilson et al., 2000). At the P-element insertion site, top3β26, top3β37, top3β16 and wh7 have an insert of 170 bp, 2.3 kb, 3 kb and 632 bp, respectively. top3β26 has 400 bp deletion in the second exon. top3β37 has 712 bp deletion from the first exon through most of the second exon. top3β16 has 2798 bp deletion from the first exon through first 3 bp of the fourth intron. wh7 has 1426 bp deletion beyond the P-element insertion site, including 1041 bp of WH coding region. wh56 had 4016 bp deletion, including 368 bp of WH coding sequence, and the whole coding region of top3β gene. The transgenic construct diagrams show the start and end point of wh or top3β in the genomic DNA. Green and yellow box indicate the in-frame fusion of GFP and YFP, respectively. (B) top3β16, top3β26 and top3β37 are top3β null mutants. Adult males (lanes 1, 3, 5 and 7) and females (lanes 2, 4, 6 and 8) were homogenized with SDS sample buffer, subjected to SDS–PAGE, transferred to a nitrocellulose membrane and detected with rabbit anti-top3β (top panel), or rabbit anti-actin (bottom panel) serving as a loading control. M marks the lane for molecular mass standard, WT for precise excision wild-type control.
Generation of WH antibody
We used two primers 5′-ACTCggatccAATGTGCACAACAATTT-CGTTCGC-3′ and 5′-ACTCgtcgacGCCGCACTTCTGCTGCTGCTG-3′ to amplify the WH coding region, with a unique BamHI and SalI site at the end (lower case in the primer sequence). The resulting fragment was inserted into pET23b (Novagen, Madison, WI) vector to generate a hexahistidine tag at the C-terminus of the recombinant protein. The expressed WH protein was insoluble and was purified under denaturing conditions using a Ni-NTA column (Qiagen, Valencia, CA). We used the purified protein as an antigen to immunize a rabbit and as the ligand for affinity purification of the antibody. Affinity purified antibody was used to detect WH by Western blot or immunostaining.
Generation of transgenes
We constructed transgenes P[top3β-YFP], P[wh] and P[wh-GFP] using pCaSpeR2 vector (Thummel et al., 1988). P[top3β-YFP] is a transgene with the insertion of a coding sequence for YFP (yellow fluorescent protein) after the last codon of top3β and before the termination codon. The 5′ portion of top3β gene, up to the last codon, was cloned by using two primers 5′-GAAtctagaGCGCG-TATCTTATCATCCCGAT-3′ and 5′-TCAacgcgtAACGAAATACGAGGC-CAGTTG-3′ to amplify a DNA fragment from Drosophila genomic DNA by PCR. This top3β genomic fragment contains a unique restriction site XbaI and MluI (lower case in the sequence) at its 5′ and 3′ end, respectively. The 3′ portion of top3β including the termination codon and 3′ untranslated region was amplified by primers 5′-CTTgcggccgcGTTTGAGGACACTGAATTGCA-3′ and 5′-CTGgaattcACAGCTTATGATTCGTTTCGC-3′, resulting a DNA fragment with a unique restriction site NotI and EcoRI (lower case in the sequence) at its 5′ and 3′ end, respectively. The YFP fragment has MluI site at its 5′ end and NotI at its 3′ end. The tripartite, ligated DNA fragments containing top3β, YFP and top3β 3′ untranslated region were inserted into the XbaI/EcoRI site of the pCaSpeR2 vector DNA, generating a genomic top3β transgene with an in-frame fusion of YFP at its C-terminus.
We used a pair of primers 5′-GAAtctagaGGCCTATATAATTGATGATGA-3′ and 5′-GAAgcggccgcTTGGACAGGAAGATCGAG-3′ to amplify wh gene from fly genomic DNA, with a unique restriction site of XbaI and Not I (lower case in the sequence) at the end. PCR fragment was inserted into the P-element vector at the XbaI/NotI site.
To construct the p[wh-GFP], a wh transgene with an in-frame fusion of GFP to its C-terminus, we used primers 5′-GAAtctagaGGCCTATATAATTGAT-GATGA-3′ and 5′-GAAgcgcgcGCCGCACTTCTGCTGCTGCTG to amplify the complete wh gene except for terminating right before the stop codon. The amplified fragment has a unique restriction site XbaI and BssHI (lower case in the sequence) at its 5′ and 3′ end, respectively, and was subsequently ligated to a GFP gene. The in-frame fusion gene of wh and GFP was inserted into the pCaSpeR2 vector. All the constructs were first sequenced to confirm the recombinant clones, and the purified DNA was microinjected into Drosophila embryos (Rubin and Spradling, 1982) with a helper plasmid expressing transposase.
Adult fly Western blot
Two- to five-day-old adult flies were homogenized with SDS–PAGE sample buffer. Proteins were separated by standard SDS–PAGE, transferred to nitrocellulose membrane and detected by SuperSignal West Dura reagents (Pierce, Rockford, IL) according to the manufacturer’s manual. Rabbit anti-actin antibody (Sigma, St. Louis, MO) was used at 1:4000 dilution to detect actin as loading controls.
Fertility assay
Newly eclosed virgins were fed for 2 days before crosses were set up. Each cross had 3 virgins and 3 males. After 3 days, we removed parents and scored the progeny. Because the mutant strains were generated through mutagenesis mediated by P-element transposition, we used a wild-type strain, with a precise excision event from the same mutagenesis steps, as the control for these experiments. DNA sequence surrounding the P-element insertion site in the top3βEP(X)1432 was determined to confirm that the control strain has a precise excision. In the genetic tests performed in this paper, the control strain also behaves like a laboratory wild-type strain Oregon R P2.
Conventional microscopy
Testes were isolated from adult males, transferred to a small drop of 1× EBR buffer (Lin et al., 1994) on a slide and covered with cover glass. Kimwipes was used to absorb the buffer until cells were released. Samples were examined using phase contrast or Nomarski differential interference contrast (DIC) optics. The seminal vesicles were examined for the presence of mature sperms with phase contrast with an objective lens of 40×, and the fine structures of developing cysts with spermatids were observed with 63× objective lens.
Immunofluorescence microscopy
Testes and ovaries were dissected from 2- to 5-day-old flies in 1× EBR and stained as described (Lin et al., 1994). The rabbit anti-GFP (Molecular Probes, Eugene, OR) was used at 1:2000 dilution. Monoclonal antibodies of anti-Fasciclin III (Brower et al., 1981; Gonczy et al., 1992) and anti-adducin (Zaccai and Lipshitz, 1996) were purchased from the Developmental Studies Hybridoma Bank at the University of Iowa (Iowa City, IA). Both were used at 1:100 dilution. Rabbit anti-vasa was a gift from Haifan Lin and was used at 1:100 dilution. DAPI, 4,6-diamidino-2-phenylindole (Sigma, St. Louis, MO), was used at 1 μg/ml. Fluorophore-labeled goat anti-mouse and goat anti-rabbit antibodies (Molecular Probes, Eugene, OR) were used at 1:500 dilution. Images were collected on a Zeiss LSM-410 confocal microscope and processed with Adobe Photoshop 7.0.
Results
Drosophila top3β is a non-essential gene
We identified a fly strain with P-element insertion in X chromosome, EP(X)1432, located at the 5′ untranslated region of top3β, which greatly reduced top3β expression, but has no apparent phenotype (Wilson et al., 2000). To create truncation mutations in top3β for further analysis of its function, we carried out mutagenesis through imprecise excision of the P-element in EP(X)1432. Five alleles of top3β mutants were recovered, and they all have significant deletions in top3β coding region (Fig. 1A). Three of the alleles, top3β16, top3β26 and top3β37, have deletions affecting only top3β, and there was no detectable top3β protein in homozygous mutant males or females (Fig. 1B), confirming that these alleles are nulls. They are homozygous viable and fertile for both males and females. These results suggest that top3β is not an essential gene. It is interesting to note that top3β gene is also dispensable in mice (Kwan and Wang, 2001). However, the top3β knockout mice have a reduced lifespan and a rapidly declining fertility as the mutant animals age (Kwan et al., 2003; Kwan and Wang, 2001). We did not observe any dramatic phenotypes in viability, fertility or lifespan for the Drosophila top3β null mutants, and we were able to maintain the homozygous mutants for over 30 generations. However, we have not carried out experiments to monitor if these mutants have subtle changes in fertility.
wh is essential for male fertility
Among the mutant alleles we recovered, two have deletions extending beyond top3β, and removed part of the neighboring gene CG15897, which we named wh. These two mutants, in contrast to the top3β mutants, have atypical sex-specific phenotypes and were named wh7 and wh56 (Fig. 1A).
We maintained the wh mutant in a strain with a balancer X chromosome (FM7c). In an attempt to generate homozygous wh mutant, we crossed female wh heterozygote with FM7c male to generate wh mutant male and heterozygote wh female (cross a, Fig. 2A). However, wh mutant male is sterile and no progeny can be produced (cross b, Fig. 2A). This wh male sterile phenotype is not specific to wh/FM7c female, and in the presence of wild-type females wh male also fails to produce any progeny. However, a stable wh stock can be maintained with wh heterozygote female and FM7c male (cross c, Fig. 2A).
Fig. 2.
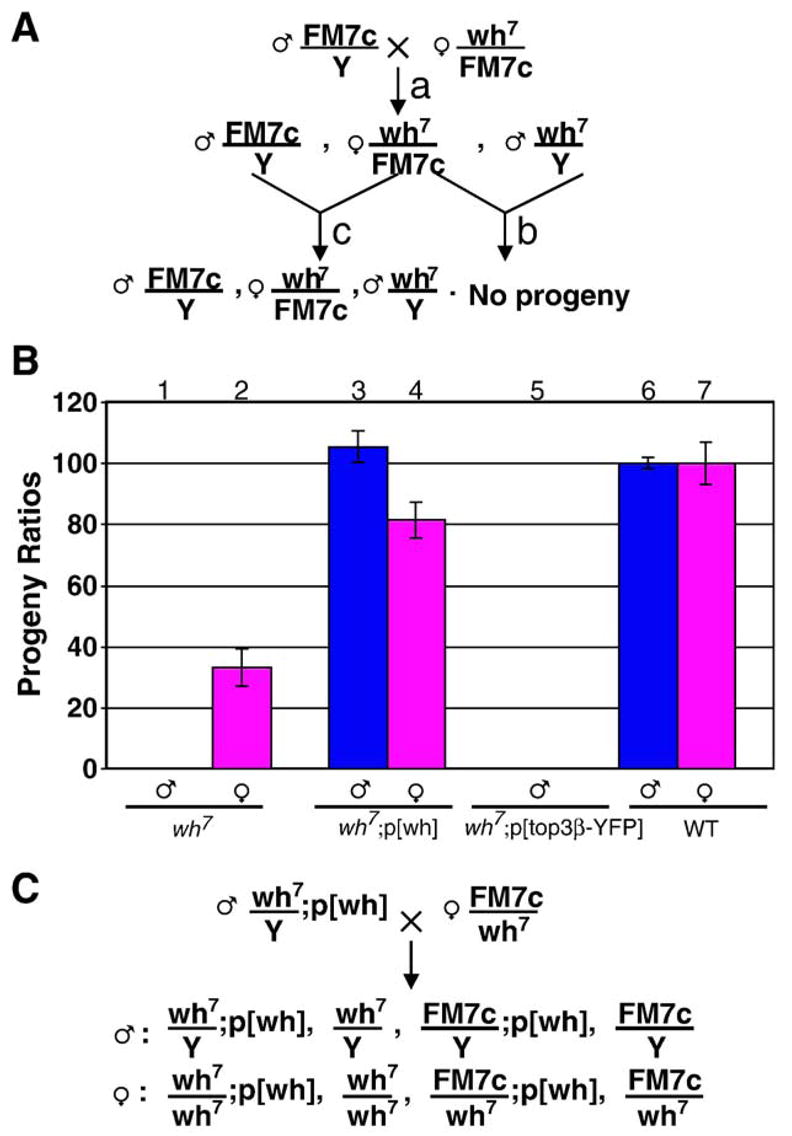
wh mutant males are sterile and can be rescued by wh transgene. (A) Males with wh7 mutation, generated from cross a, has no progeny (cross b). Thus, we cannot obtain wh7/wh7 homozygous female without the aid of a rescuing transgene. Stable stock of wh mutant can be maintained with cross c (or cross a). Similar phenotype was observed for wh56. (B) wh transgene can rescue the fertility of wh mutant, whereas the top3β transgene cannot. The male fertility was scored by mating the fly having the indicated genotype with wild-type females (columns 1, 3, 5 and 6), and female fertility was scored by mating the mutant females with wild-type males (columns 2, 4 and 7). wh mutant males are sterile, and mutant females have greatly reduced fertility (columns 1 and 2), when compared with wild-type controls (columns 6 and 7). wh transgene could rescue the fertility of both mutant males and females (compare columns 3 and 4 vs. columns 6 and 7). However, top3β transgene could not rescue the infertility of wh mutant males (column 5). (C) Genetic cross demonstrating that wh transgene can rescue wh mutant male to restore its fertility. From this cross, we could also generate wh homozygous females with or without a wh transgene, which were used in the experiments for monitoring effect of wh mutation on female fertility. Although the fly with wh7 mutant allele is shown here, similar cross was also used for wh56 mutant.
Because truncations limited to top3β gene produce no noticeable phenotypes, it is reasonable to assume that male sterile phenotype associated with wh7 and wh56 could be due to the deletions in the gene CG15897. To test this hypothesis, we introduced either wh or top3β transgene into wh mutant and examined the fertility by scoring the progeny when either mutant or control male was crossed with the wild-type female (columns 1, 3, 5 and 6, Fig. 2B). The transgene p[wh] could rescue the male fertility of wh mutant to the wild-type level (column 3 vs. 6, Fig. 2B), and top3β transgene, p[top3β-YFP], was ineffective (column 5, Fig. 2B). We have also tested p[wh-GFP] transgene, which could efficiently rescue wh mutant male (data no shown). Because wh transgenes could rescue the fertility of mutant males, we were able to design a genetic cross to obtain homozygous wh mutant females (Fig. 2C). The wh homozygous female mutant was used to investigate whether wh could affect fertility of females. Although wh is required for male fertility, it has an important but non-essential role in female fertility. Mutant females have one third of the fertility compared with precise excision control, and wh transgene can rescue their fertility to nearly the level of wild-type control (columns 2 and 4 vs. column 7, Fig. 2B). The fertility decrease in mutant female was based on scoring the adults emerged from these crosses, and this maternal effect could be due to the decrease of embryo yield, or lethality during embryogenesis, which will affect the embryo hatching. To address these possibilities, we mated wild-type males with virgin females of wh mutant, wh mutant containing a transgene or wild-type control. We scored from these crosses embryo yield (eggs laid) and embryo hatching (larvae emerged). In comparison with wild-type, wh mutant females yield only half the number of embryos and have about 80% of the embryos eventually hatched (columns 1 and 2 vs. columns 5 and 6, Fig. 3). Therefore, the reduced female fertility is largely due to a decrease in the eggs laid, presumably as a result of defective oogenesis in the mutant. The maternal defects in oogenesis likely contribute to the defects during embryogenesis. However, most of the matured eggs escaped from the mutant ovary can develop through embryogenesis. Interestingly, wh transgene can rescue both the yield and hatching to nearly the wild-type levels (columns 3 and 4 vs. columns 5 and 6, Fig. 3). The genetic results therefore indicate that wh is important for both male and female fertility, with its role in male fertility being essential.
Fig. 3.
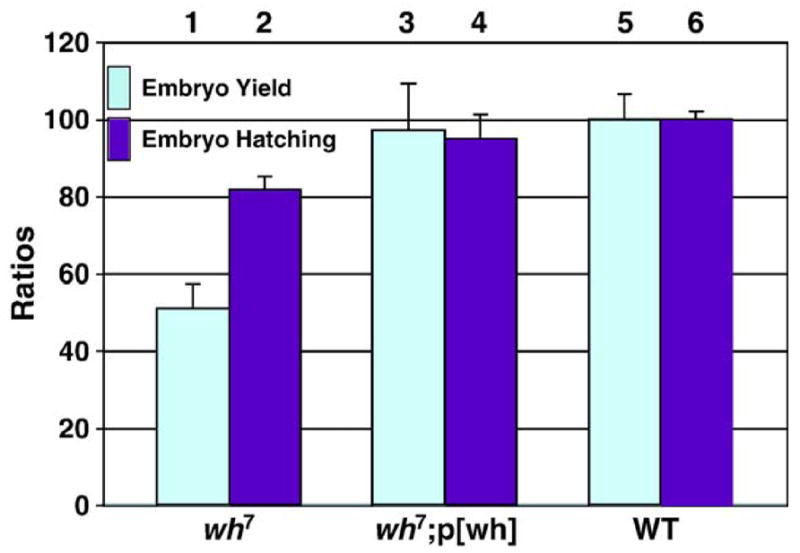
Both egg laying (embryo yield, columns 1, 3 and 5) and emerged larvae (embryo hatching, columns 2, 4 and 6) are reduced in wh female. The reduced fertility of wh mutant females is due to the decreased embryo yield and embryo hatching (columns 1 and 2) when compared with controls from either a transgene-rescued line (columns 3 and 4) or precise excision strain (columns 5 and 6).
The homozygous wh mutants made available in the cross (Fig. 2C) allowed us to test if they were indeed null. We carried out Western blot using the antibody raised against the recombinant WH protein. No WH protein could be detected in either female or male of wh7 and wh56 mutants, in contrast to these mutants with a rescuing transgene, and the precise excision control (Fig. 4A). The Coomassie blue-stained gel was used as a control for loading of the proteins and their resolution in SDS–PAGE (Fig. 4B). The biochemical data therefore confirmed the molecular genetic results that wh7 and wh56 are wh null mutants. The Western blot data also indicate the specificity of WH antibody.
Fig. 4.
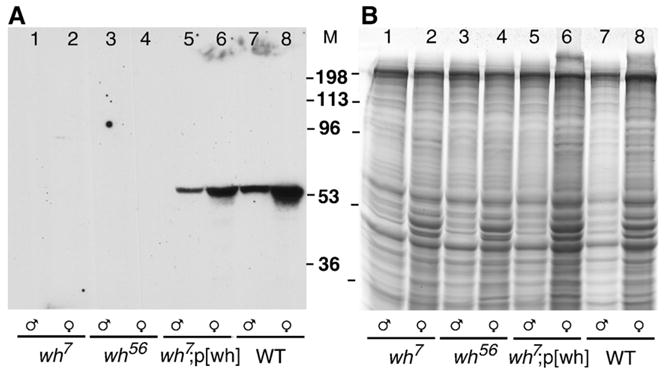
wh7 and wh56 are wh null mutants. Adult males (lanes 1, 3, 5 and 7) and females (lanes 2, 4, 6 and 8) were homogenized with SDS sample buffer, subjected to SDS–PAGE, transferred to a nitrocellulose membrane and detected with rabbit anti-WH (A). Because the molecular mass of WH and actin is similar, a duplicate gel stained with Coomassie blue serves as a loading control (B). There is no WH expression in wh7 (lanes 1 and 2) or wh56 mutants (lanes 3 and 4), and p[wh] transgene can restore the WH expression in the null mutant background (lanes 5 and 6). WH expression in the precise excision strain is used as a control (lanes 7 and 8).
WH belongs to a conserved family of proteins with 5 WD40 repeats
wh gene is located on the X chromosome at the cytological location 5E5, neighboring the top3β gene, and with their directions of transcription being divergent (Fig. 1A). This gene is compact, with no introns, and predicted to encode a protein with 424 amino acids (Fig. 5A). It has a high abundance of acidic amino residues, with 12.7% of the total residues being Asp and Glu, and a predicted pI of 5.0. The predicted molecular mass is 46 kDa. Based on the Western blot, the measured, apparent molecular mass is 56 kDa (Fig. 4A). The difference in the apparent molecular mass measured by SDS–PAGE and the predicted one could be due to either post-translational modification or a bias in amino acid distribution in the protein, for example, an abundance in acidic residues.
Fig. 5.
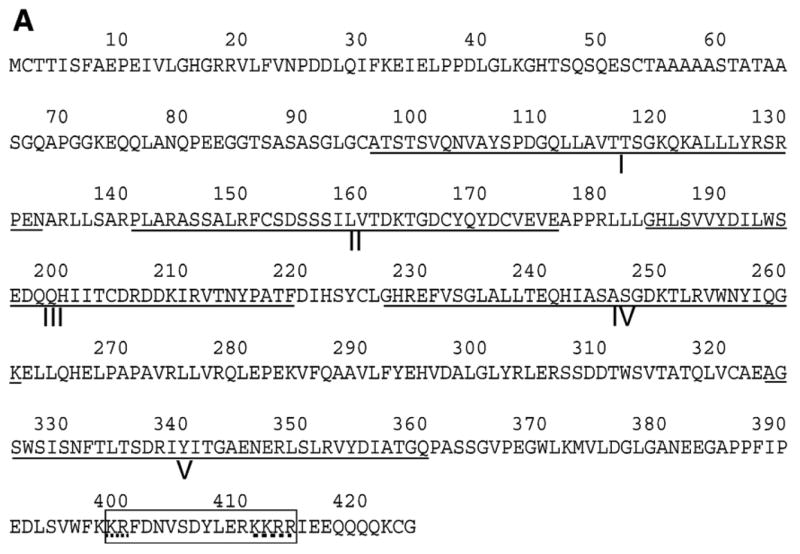

WH is an evolutionarily conserved WD40-repeat protein. (A) Protein sequence analysis (Stultz et al., 1997) of the deduced WH amino acid sequence showed WH had 5 WD40 repeats (underlined, I–V). A typical nuclear localization signal (NLS) was boxed at carboxyl terminus. The bipartite signal is composed of two basic sequence elements (dashed lines) separated by a spacer of ten residues (Dingwall et al., 1988). (B) Alignment of the WH deduced amino acid sequence and other putative homologues from human, mouse, rat, chicken, frog, worm (Caenorhabditis elegans), mosquito (Anopheles gambiae), fission yeast and baker’s yeast. Light shadow indicates the similarities and dark shadow indicates the identities. Five WD40 repeats (I–V) of WH are marked with black lines and NLS with a gray line.
Sequence analysis revealed that WH has homologues in many eukaryotic species, including human, chicken, frog, worm, mosquito and yeast (Fig. 5B). There is a bipartite nuclear localization signal (Robbins et al., 1991) at the carboxyl termini (Figs. 5A and B). A unique feature of this family of proteins is that they contain 5 repeats of WD40 sequence motifs (Figs. 5A and B). These repeats were found in approximately 1–2% of all proteins in eukaryotic species (Madrona and Wilson, 2004). The WD40 motif has been identified in a range of proteins serving a vast array of physiological roles including chromatin modification, cell cycle regulation, cell division, cytokinesis, RNA processing and signal transduction (Madrona and Wilson, 2004; Neer et al., 1994; van Nocker and Ludwig, 2003).
wh mutant females have defects in oogenesis
To probe the WH function in oogenesis, we first examined the WH expression patterns by immunofluorescence in ovarioles of p[wh-GFP] transgenic females with rabbit anti-GFP antibody. WH-GFP fusion protein is functional because p [wh-GFP] transgene could rescue the fertility of wh mutant (data not shown). WH-GFP was localized in the nuclei of nurse cells, follicle cells and oocytes at early stages, from germarium to Stage 4 egg chambers (Fig. 6). The expression of WH is diminishing in the egg chambers at and after Stage 4 (Fig. 6 and data not shown).
Fig. 6.
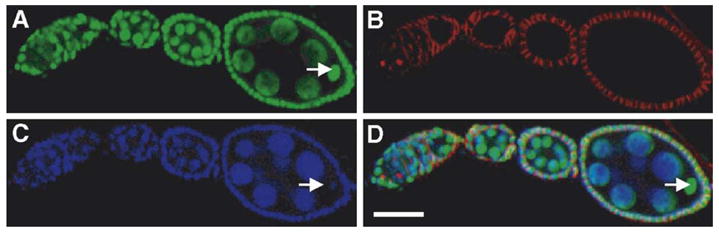
WH-GFP localization during oogenesis. Ovarioles were dissected from transgenic flies expressing WH-GFP fusion protein and stained with rabbit anti-GFP polyclonal antibody (A), mouse anti-adducin (B) and DAPI for DNA (C). Panel D is the merged image. The ovariole shown here has from left to right, germarium, and egg chambers at Stages 2–4. Arrow indicates the oocyte in Stage 4 egg chamber. Scale bar, 25 μm.
To determine whether the decreased embryo yield of wh mutant females is caused by deteriorated ovarioles, we isolated ovarioles from both wh mutant and rescued females and examined them by immunostaining with rabbit anti-vasa for visualizing germline cells, mouse anti-adducin for fusome and DAPI for DNA. In the wh mutant ovary, although about 80% of ovarioles appear to have normal oogenesis, over-proliferation of cystocytes occurs in 20% of ovarioles, resulting in more than 16 germ cells in a single egg chamber (Figs. 7B, D, F, H). These abnormal egg chambers could appear in consecutive order (Figs. 7B, D, F, H) or interspersed in between apparently normal ones (data not shown). In contrast, ovariole with wh transgene has normal oogenesis and no egg chambers with more than 16 germ cells were observed (Figs. 7A, C, E, G). Therefore, the germ cells in abnormal egg chambers do not arrest cell division after 4 mitotic cycles like the wild type. The DNA staining in each germ cell in the abnormal egg chamber is significantly less than the nurse cell (Fig. 7A vs. 7B). Therefore, the germ cells in the abnormal egg chamber do not undergo multiple cycles of endoreplication as the nurse cells in a normal egg chamber at the comparable stage. These results indicate that WH is involved in controlling the differentiation of germ cells during the process of oogenesis.
Fig. 7.
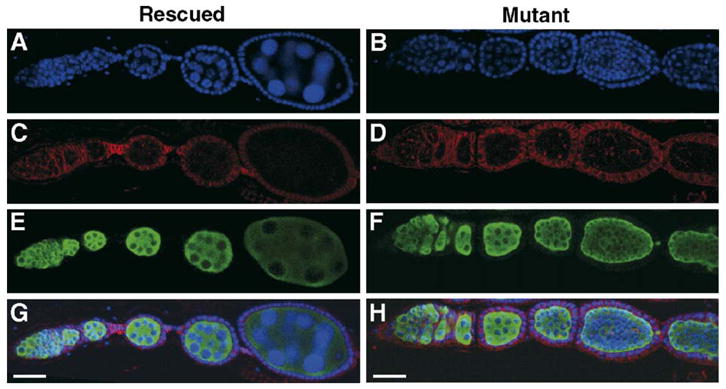
Oogenesis defects in wh mutant females. The same ovariole either from wh rescued transgenic line (A, C, E and G) or mutant line (B, D, F and H) is stained for DNA (blue, A and B), adducin (red, C and D) and vasa (green, E and F). The merged image is shown in panels G and H. Normal oogenesis is observed in wh transgenic females, whereas wh mutant ovariole has egg chambers with more than 16 germ cells. Notice that DNA staining in the mutant germ cells is significantly less than the nurse cells in wh rescued controls (compare A vs. B). Scale bar, 25 μm.
WH has the highest expression in hub cells but is non-essential for hub cell maintenance
To probe the role of WH in male fertility, we examined the localization of WH-GFP protein in the testes of males with p [wh-GFP] transgene. In addition to the nuclei of spermatocytes, we observed intense staining in the apical cells of the testes (Fig. 8A). We assumed these apical cells were hub cells because of their position and compact morphology. Hub cells are a group of 8–16 small somatic cells at the tip of testis and serve as a niche for GSCs (Hardy et al., 1979; Kiger et al., 2001; Tulina and Matunis, 2001). Fasciclin III is a specific marker for hub cells (Brower et al., 1981; Gonczy et al., 1992) and is clearly colocalized with WH-GFP (panels A, B and D, Fig. 8), indicating that the apical cells with high concentration of WH-GFP are indeed hub cells. Although it has the highest expression in hub cells, WH-GFP is also expressed at a relatively lower level at the other stages of spermatogenesis (panel A, and data not shown). This expression pattern of WH in the testis, with the maximal amount in the hub cells, was also confirmed with immunofluorescence experiments using the antibody against WH (data not shown).
Fig. 8.
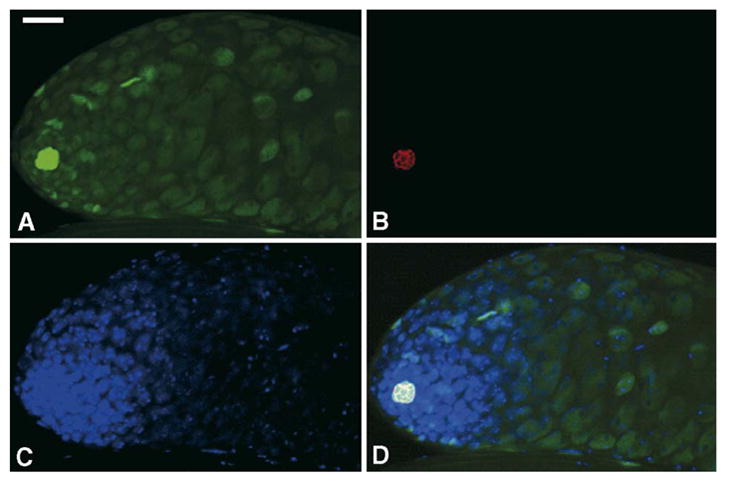
WH-GFP has the highest expression in hub cells. Testes were dissected from transgenic flies expressing WH-GFP fusion protein and stained with rabbit anti-GFP polyclonal antibody (A), mouse anti-Fasciclin III for marking hub cells (B) and DAPI for DNA (C). WH-GFP is colocalized with Fasciclin III (D, merged image). Scale bar, 25 μm.
Because there is a high concentration of WH in the hub cells, it could have a role in maintaining this niche or germline stem cells. Immunostaining analysis using monoclonal anti-Fasciclin III antibody revealed the characteristic staining of Fasciclin III in wh mutant testis in comparison with a wild-type control testis (Fig. 9B vs. 9A), indicating the existence of hub cells in the testes of wh mutant. This result suggests that WH is not essential for maintaining hub cells in the testis. The maintenance of hub cells and the associated germline stem cells in wh mutant is also supported by the presence of cysts of pre-meiotic spermatocytes (Supplementary Fig. S1) and the bundles of spermatids arrested at the elongating stage (see the following section).
Fig. 9.

Arrest of spermatogenesis in wh mutant at the spermatids elongation stage. Confocal images were taken from fixed samples of testes (panels A and B; scale bar, 25 μm). Unfixed and squashed samples of testes were observed under a microscope with Nomarski differential interference contrast (panels C and D, with a scale bar of 50 μm) and phase-contrast (panels E–H; scale bar, 10 μm). (A and B) Testes were stained for Fasciclin III (red), vasa (green) and DNA (blue). No apparent defect was observed at the early stages of spermatogenesis when compared the mutant testis (B) with wh rescued testis as a wild-type control (A). Fasciclin III, a molecular marker of hub cell (Brower et al., 1981; Gonczy et al., 1992), is present in wh mutant testis (B), which indicates that the wh mutant testis contained the hub cells. Outlined are the presumed germline stem cells based on their morphology and location. (C) In the wh transgenic testis, elongated and bundled spermatids (arrow) are visible, and swollen seminal vesicles are filled with coiled mature sperms (arrowhead). (D) In wh mutant testis, no mature sperm bundles are visible, and seminal vesicles (arrowhead) are empty. (E and F) Spermatid bundles at the elongating stage (outlined). wh mutant spermatids are arrested at elongating step (F) as compared to the fully elongated spermatid bundles of wh transgenic males (E). (G and H) Developing spermatids at the early elongating stages. Arrowheads indicate the elongating mitochondria derivatives, which are morphologically distinct between the mutant and rescued control. Note the presence of vesicles (arrow) in wh mutant spermatids (H).
Spermatogenesis is arrested at spermatid elongating stage in wh mutant testis
To further determine the cause of infertility in wh mutant males, we examined the morphology of wh mutant testes. We analyzed the fresh samples under phase contrast or Nomarski differential interference contrast (DIC). Under the DIC microscopy, we observed no mature spermatozoa in seminal vesicles, nor any fully elongated spermatids bundle (Fig. 9D) in contrast to the mutant with wh tansgene (arrow, Fig. 9C) or wild-type control (data not shown). At the early stages of spermatogenesis, no apparent defect was observed, with cysts of different stages of pre-meiosis laid out in order (Fig. 9A vs. 9B). The defects of spermatogenesis in wh mutant testes first become apparent during the elongating stage of a developing cyst. No fully elongated spermatid was observed in wh mutant males in contrast to the mutant with wh transgene (outlined, Fig. 9E vs. 9F). The arrest of spermatogenesis at the elongating stage is also supported by the observation that there is an accumulation of post-meiotic cysts at elongating stage (data not shown). At the early elongating stage in wh mutant testis, we could observe the presence of abnormal vesicles (arrow, Fig. 9H) and mitochondria derivatives with a fibrous structure (arrowhead, Fig. 9H) in the spermatids, which were absent in the control testis (Fig 9G). These observations suggest that wh has an important role in the process of differentiation of spermatids. In the mutant male, the spermatids are arrested at a specific stage, and no mature sperms can be developed.
Discussion
Through mutagenesis by P-element transposition, we have isolated and characterized a new Drosophila gene, CG15897, which we named wh. Its null mutants have male sterile and female semi-sterile phenotypes. wh encodes a protein with five WD40 repeats. Interestingly, tandem copies of WD repeat with a length of 40 amino acids or more are present in approximately 1–2% of all proteins in typical eukaryotic cells (Madrona and Wilson, 2004). Proteomes with WD40 repeats have been found to contribute to over 30 functional subfamilies (Yu et al., 2000). This WD40 motif has been identified in a range of proteins serving a vast array of physiological roles including chromatin modification, cell cycle regulation, cell division, cytokinesis, RNA processing and signal transduction (Madrona and Wilson, 2004; Neer et al., 1994; van Nocker and Ludwig, 2003). Various structural studies on proteins with known WD40 domains have revealed a common seven-bladed propeller folding pattern for most WD40 domains (Madrona and Wilson, 2004). Despite their widespread occurrence in diverse physiological processes, the majority of WD40 proteins serve a common function as a mediator in protein–protein interactions (Madrona and Wilson, 2004; van Nocker and Ludwig, 2003). Although we do not have any information regarding the specific role of WH, it may function through interacting with one or more protein partners.
There are no genetic nor biochemical studies on this particular family of proteins with five WD40 repeats except in the budding yeast. The yeast homologue of this WD40 repeat protein is Trm82. Biochemical experiments showed that Trm82 forms a complex with Trm8, and Trm8 is the catalytic subunit of m7G46 tRNA methyltransferase (Alexandrov et al., 2002). Both Trm82 and Trm8 are required for the methyltransferase activity. Although neither TRM82 nor the gene for catalytic subunit TRM8 is essential for the yeast viability, they can affect yeast growth under restrictive conditions. Mutations in either TRM82 or TRM8 resulted in growth defects at 38°C in synthetic media containing glycerol as a sole carbon source (Alexandrov et al., 2005). It is not known whether Trm82 also serves as a mediator with other protein partners having different biochemical activities. For another tRNA modification enzyme, m22G26 tRNA dimethyl transferase, it also has a heterodimeric structure, a catalytic subunit Trm11 and a non-catalytic subunit Trm112 (Purushothaman et al., 2005). Trm112 is a Zn-finger protein, presumably serving a function as a mediator protein, and also interacts with a number of proteins other than Trm11 (Purushothaman et al., 2005). Interestingly, although TRM11 is not required for yeast growth, TRM112 is an essential gene, presumably because its interactions with cellular partner proteins other than the tRNA methylase have essential functions.
We do not know whether the function of wh is through its interaction with a tRNA methyltransferase or other cellular proteins. Because wh has specific and essential functions in germline cells development, it is less likely that these wh functions are through its association with a general biochemical activity like tRNA methyl transferase. Although wh is dispensable for the fruit fly viability, our experiments demonstrated that the lack of wh function is associated with dramatic germline-specific phenotypes: the spermatogenesis of wh mutant male is arrested at spermatid elongating stage, and 20% of the ovarioles in wh mutant female have apparent defects in oogenesis with an over-proliferation of cystocytes, resulting in more than 16 germ cells in a single egg chamber. These abnormal cystocytes also do not undergo the multiple rounds of endoreplication as the normal nurse cells. These data suggest that wh is important for the differentiation of germline cells to form mature gametes. The egg chambers in the wh mutant ovarioles have a similar phenotype to the bam and bgcn mutant egg chambers in the over-proliferation of germline cells and lack of endoreplication (Gonczy et al., 1997; Lavoie et al., 1999; McKearin and Spradling, 1990). However, unlike bam or bgcn mutant, not all the egg chambers displayed such a phenotype. Although wh mutant female is only semi-sterile, the egg chambers without the over-proliferation phenotype may still harbor other more subtle anomalies that were not detected with our current analysis. Another critical difference between wh and bam or bgcn is in the testes; wh mutant specifically arrests spermatogenesis at the elongating stage, and there is no over-proliferation in either post-mitotic or post-meiotic cells. In comparison with other female sterile mutants with ovarian tumorous phenotype like ovo or otu (Oliver et al., 1990, King, 1970), wh is also different in that the wh mutant is not penetrant and less dramatic in female and is completely arrested at a late stage of spermatogenesis. Therefore, wh can affect apparently distinct steps in the cellular differentiation processes in spermatogenesis and in oogenesis and belongs to a new class of mutants affecting fertility.
The physiological functions of wh in the somatic cells, if any, are not essential. First, even though wh mutant female has a greatly reduced capacity in oogenesis, most of the eggs that a reproduced by the mutant mother can develop into larvae, suggesting that wh is not required for early embryogenesis. Second, adult flies with wh null mutations are apparently normal except for fertility, suggesting that wh is dispensable for most of the somatic functions post-embryonic development. The biochemical basis of the specific functions of wh in germline cells remains to be elucidated. The genetic and immunochemical reagents characterized in this paper should provide useful tools for future investigation into the pathway of the cellular differentiation during gametogenesis.
Supplementary Material
Acknowledgments
We thank Haifan Lin for helpful discussion, a gift of vasa antibody, and the use of a fluorescence microscope, Larry Lee and Carrie Reardon for technical assistance. The use of Confocal Microscope Facility and the microinjection by Model System Genomics Facility are acknowledged here. This work is supported by a grant from National Institute of Health (GM29006).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: 10.1016/j.ydbio.2006.04.459.
References
- Alexandrov A, Martzen MR, Phizicky EM. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA. 2002;8:1253–1266. doi: 10.1017/s1355838202024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov A, Grayhack EJ, Phizicky EM. tRNA m7G methyl-transferase Trm8p/Trm82p: evidence linking activity to a growth phenotype and implicating Trm82p in maintaining levels of active Trm8p. RNA. 2005;11:821–830. doi: 10.1261/rna.2030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower DL, Smith RJ, Wilcox M. Differentiation within the gonads of Drosophila revealed by immunofluorescence. J Embryol Exp Morphol. 1981;63:233–242. [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Dingwall C, Robbins J, Dilworth SM, Roberts B, Richardson WD. The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. J Cell Biol. 1988;107:841–849. doi: 10.1083/jcb.107.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels WR, Johnson-Schlitz DM, Eggleston WB, Sved J. High-frequency P element loss in Drosophila is homolog dependent. Cell. 1990;62:515–525. doi: 10.1016/0092-8674(90)90016-8. [DOI] [PubMed] [Google Scholar]
- Fuller MT. Spermatogenesis. In: Michael Bate AMA., editor. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Laboratory Press; 1993. pp. 71–148. [Google Scholar]
- Fuller MT. Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Semin Cell Dev Biol. 1998;9:433–444. doi: 10.1006/scdb.1998.0227. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- Gonczy P, Matunis E, DiNardo S. bag-of-marbles and benign gonial cell neoplasm act in the germline to restrict proliferation during Drosophila spermatogenesis. Development. 1997;124:4361–4371. doi: 10.1242/dev.124.21.4361. [DOI] [PubMed] [Google Scholar]
- Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M. The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res. 1979;69:180–190. doi: 10.1016/s0022-5320(79)90108-4. [DOI] [PubMed] [Google Scholar]
- Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- King R. Ovarian Development in Drosophila melanogaster. Academic Press; New York: 1970. [Google Scholar]
- Kwan KY, Wang JC. Mice lacking DNA topoisomerase III beta develop to maturity but show a reduced mean lifespan. Proc Natl Acad Sci U S A. 2001;98:5717–5721. doi: 10.1073/pnas.101132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Moens PB, Wang JC. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase III beta. Proc Natl Acad Sci U S A. 2003;100:2526–2531. doi: 10.1073/pnas.0437998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie CA, Ohlstein B, McKearin DM. Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol. 1999;212:405–413. doi: 10.1006/dbio.1999.9346. [DOI] [PubMed] [Google Scholar]
- Lin H. The stem-cell niche theory: lessons from flies. Nat Rev, Genet. 2002;3:931–940. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- Lin H, Spradling AC. Fusome asymmetry and oocyte determination in Drosophila. Dev Genet. 1995;16:6–12. doi: 10.1002/dvg.1020160104. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Tokuyasu KT. Spermatogenesis. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. 2d. Academic Press; 1980. pp. 225–294. [Google Scholar]
- Madrona AY, Wilson DK. The structure of Ski8p, a protein regulating mRNA degradation: implications for WD protein structure. Protein Sci. 2004;13:1557–1565. doi: 10.1110/ps.04704704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald A. Oogenesis. In: Ashburner WTM, editor. The Genetics and Biology of Drosophila. 2d. Academic Press; New York: 1980. pp. 141–224. [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Merrill PT, Sweeton D, Wieschaus E. Requirements for autosomal gene activity during precellular stages of Drosophila melanogaster. Development. 1988;104:495–509. doi: 10.1242/dev.104.3.495. [DOI] [PubMed] [Google Scholar]
- Michaud J, Kudoh J, Berry A, Bonne-Tamir B, Lalioti MD, Rossier C, Shibuya K, Kawasaki K, Asakawa S, Minoshima S, Shimizu N, Antonarakis SE, Scott HS. Isolation and characterization of a human chromosome 21q22.3 gene (WDR4) and its mouse homologue that code for a WD-repeat protein. Genomics. 2000;68:71–79. doi: 10.1006/geno.2000.6258. [DOI] [PubMed] [Google Scholar]
- Neer EJ, Schmidt CJ, Nambudripad R, Smith TF. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Oliver B, Pauli D, Mahowald AP. Genetic evidence that the ovo locus is involved in Drosophila germ line sex determination. Genetics. 1990;125:535–550. doi: 10.1093/genetics/125.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman SK, Bujnicki JM, Grosjean H, Lapeyre B. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol Cell Biol. 2005;25:4359–4370. doi: 10.1128/MCB.25.11.4359-4370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J, Dilworth SM, Laskey RA, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Michael Bate AMA, editor. The Development of Drosophila melanogaster. Vol. 1. Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Stultz CM, Nambudripad R, Lathrop RH, White JV. Predicting protein structure with probabilistic models. In: Allewell N, Woodward C, editors. Protein Structural Biology in Bio-Medical Research. JAI Press; Greenwith, CT: 1997. pp. 447–506. [Google Scholar]
- Thummel CS, Boulet AM, Lipshitz HD. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- van Nocker S, Ludwig P. The WD-repeat protein superfamily in Arabidopsis: conservation and divergence in structure and function. BMC Genomics. 2003;4:50. doi: 10.1186/1471-2164-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Sweeton D. Requirements for X-linked zygotic gene activity during cellularization of early Drosophila embryos. Development. 1988;104:483–493. doi: 10.1242/dev.104.3.483. [DOI] [PubMed] [Google Scholar]
- Wilson TM, Chen AD, Hsieh T. Cloning and characterization of Drosophila topoisomerase III beta. Relaxation of hypernegatively super-coiled DNA. J Biol Chem. 2000;275:1533–1540. doi: 10.1074/jbc.275.3.1533. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- Yu L, Gaitatzes C, Neer E, Smith TF. Thirty-plus functional families from a single motif. Protein Sci. 2000;9:2470–2476. doi: 10.1110/ps.9.12.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote. 1996;4:159–166. doi: 10.1017/s096719940000304x. [DOI] [PubMed] [Google Scholar]
- Zalokar M. Autoradiographic study of protein and RNA formation during early development of Drosophila eggs. Dev Biol. 1976;49:425–437. doi: 10.1016/0012-1606(76)90185-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


