Abstract
Using ex vivo antigen-specific T-cell analysis, we found that symptomatic cytomegalovirus recrudescence in transplant recipients was coincident with reduced expression of gamma interferon (IFN-γ) by virus-specific CD8+ T cells and an up-regulation of CD38 expression on these T cells, although there was no significant change in the absolute number of virus-specific cells (as assessed by major histocompatibility complex-peptide multimers). In contrast, HLA class I-matched transplant patients with asymptomatic viral recrudescence showed increased expansion of antigen-specific T cells and highly stable IFN-γ expression by epitope-specific T cells. These studies suggest that a strong functional T-cell response plays a crucial role in defining the clinical outcome of acute viral recrudescence.
The clinical presentation of acute latent viral infections and its interaction with host T-cell responses have recently been investigated in order to understand the dynamics of immune regulation and to develop better therapeutic strategies (5, 9, 10, 20). Previous studies have proposed a role for a number of potential factors, such as viral load and cytokine dysregulation, in controlling the symptoms of acute viral infection (1, 17). It is entirely feasible that the dynamics of emergence of the virus-specific T-cell response during the early stages of viral recrudescence may delimitate the patterns of clinical symptoms in different individuals (11, 13, 16). Indeed, massive expansion of CD8+ T cells specific for Epstein-Barr virus latent and lytic antigens, which is often a feature of acute Epstein-Barr virus infection, suggests that these T-cell responses are recruited to control the active viral infection (2). However, understanding the biological significance and the longitudinal dynamics of these T cells during acute viral infections in humans is often difficult and is complicated by the nature of immune responses in naturally outbred individual patients. We have addressed some of these limitations by analyzing the dynamics of T-cell responses to a panel of CD8+ T-cell epitopes in a group of HLA class I-matched unrelated human subjects undergoing acute human cytomegalovirus (HCMV) infection with contrasting clinical symptoms. We studied three broad groups of transplant patients: (i) individuals with asymptomatic viral recrudescence, (ii) individuals with symptomatic viral recrudescence, and (iii) individuals with no evidence of viral recrudescence. In each of these groups of patients we longitudinally analyzed CD8+ T-cell responses using ex vivo ELISPOT assays and major histocompatibility complex (MHC)-peptide multimer analysis. In addition, we also assessed the viral load in these individuals to determine whether there was any correlation with T-cell dynamics and/or clinical symptoms.
Peripheral blood samples from a cohort of 15 HLA class I-matched solid-organ transplant (SOT) patients (renal or heart and/or lung) were collected into EDTA collection tubes. These blood samples were collected at multiple time points (see Fig. 1), cryopreserved, and used for T-cell assays and viral load analysis. All blood samples were collected following informed consent, and the study was approved by the relevant human ethics committees. Clinical diagnosis of symptomatic viral recrudescence was based on laboratory diagnosis (pp65 antigenemia; ≥10 positive cells/106 peripheral blood mononuclear cells [PBMC]) and previously published clinical criteria outlined by the American Society of Transplantation (8). Patients with symptomatic HCMV disease were treated with oral and intravenous ganciclovir (the exact period of HCMV disease and treatment is indicated in Fig. 1 as a shaded area) with the exception of patient N, who received cidofovir. Patient L also received foscarnet and valganciclovir. The transplant immunosuppressive regimens have been outlined elsewhere (14). Briefly, these patients received cyclosporine, mycophenolate mofetil, and prednisolone.
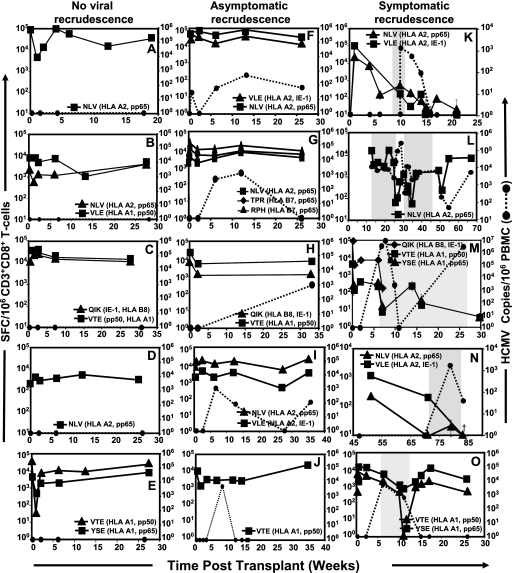
FIG. 1.
Longitudinal functional analysis of HCMV-specific T cells in HLA class I-matched SOT recipients using IFN-γ ELISPOT assays and peptide epitopes from HCMV antigens (Table 1). Data from an individual recipient are presented in each panel. Data from individuals with no evidence of viral recrudescence are presented in panels A to E, those from individuals with asymptomatic recrudescence are presented in panels F to J, and those from individuals with symptomatic recrudescence are presented in panels K to O. Data presented in panels A to E, K, L, and N are based on SOT patients who received heart and/or lung transplants, while data in panels F to J, M, and O are based on renal transplant recipients. Gray-shaded areas in panels K to O indicate the time period when clinically active HCMV disease was diagnosed and the patient was being treated with antiviral medication (Table 2). Patients B, D, and E received antiviral prophylaxis based on oral ganciclovir. The results are expressed as spot-forming cells (SFC) per 106 CD3+/CD8+ T cells. Also shown in panels F to O is the HCMV load in peripheral blood of these patients as HCMV copies/106 PBMC. Blood sample collection time points (weeks) for each of the donors are indicated on the x axis.
In the first set of studies, we longitudinally analyzed the HCMV-specific T-cell responses using ELISPOT assays and MHC-peptide pentamer/tetramer staining in these transplant patients as described previously (3, 4). For these assays, HCMV epitopes restricted through various HLA class I alleles (HLA-A1, HLA-A2, HLA-B7, and HLA-B8) were used (Table 1). Data from each of these SOT recipients are presented in Fig. 1A to O. Longitudinal analysis of immune responses clearly illustrated that those SOT patients who either showed no evidence of viral recrudescence (Fig. 1A to E) or showed asymptomatic viral recrudescence (Fig. 1F to J) maintained a stable virus-specific gamma interferon (IFN-γ) expression by CD8+ T cells throughout the follow-up period. These responses were towards epitopes derived from both structural and/or IE-1 antigens. In contrast, HLA-matched SOT recipients who were diagnosed with symptomatic viral recrudescence (Fig. 1K to O; Table 2) showed significant fluctuations of their virus-specific IFN-γ expression by CD8+ T cells. These fluctuations ranged from a 3- to 120,000-fold reduction in the virus-specific CD8+ T-cell responses. One of the interesting aspects of this longitudinal analysis was that patients with symptomatic viral recrudescence showed reduced IFN-γ expression (>5-fold reduction) by virus-specific CD8+ T cells in >70% of their blood samples, while <10% of the blood samples from SOT recipients with no viral recrudescence or with asymptomatic viral recrudescence showed reduced IFN-γ expression by virus-specific CD8+ T cells. Furthermore, T-cell responses towards multiple epitopes showed a contemporaneous pattern of fluctuation which is consistent with our previous findings for healthy virus carriers (3). Most importantly, the reduction in the IFN-γ expression by antigen-specific T cells in four of the five symptomatic recipients preceded the clinical diagnosis of active disease. It is important to mention here that the viral load in asymptomatic or symptomatic recipients showed no correlation with either IFN-γ expression by HCMV-specific T cells or clinical symptoms. Taken together, these analyses strongly suggest that a stable antigen-specific CD8+ T-cell response is crucial to prevent symptomatic clinical syndromes following latent viral recrudescence.
TABLE 1.
List of HLA class I-restricted HCMV epitopes used in this study
| Epitope sequence (code) | HLA restriction | Antigen
|
Assay used:
|
||
|---|---|---|---|---|---|
| Name | Expression kinetics | ELISPOT | MHC-peptide tetramer | ||
| YSEHPTFTSQL (YSE) | A1 | pp65 | Late/structural | X | |
| RPHERNGFTVL (RPH) | B7 | pp65 | Late/structural | X | |
| NLVPMVATV (NLV) | A2 | pp65 | Late/structural | X | X |
| VTEHDTLLY (VTE) | A1 | pp50 | Late/structural | X | X |
| TPRVTGGAM (TPR) | B7 | pp65 | Late/structural | X | X |
| VLEETSVML (VLE) | A2 | IE-1 | Immediate-early | X | |
| ELRRKMMYM (ELR) | B8 | IE-1 | Immediate-early | X | |
| QIKVRVDMV (QIK) | B8 | IE-1 | Immediate-early | X | |
TABLE 2.
Clinical syndrome in active HCMV disease
| Patient codea | Clinical syndrome | Antiviral therapyb |
|---|---|---|
| K (D−/R−) | Systemic HCMV infection without defined end organ involvement | Treatment with ganciclovir (i.v., 400 mg b.i.d.) followed by oral ganciclovir (1,000 mg t.i.d.) |
| L (D+/R−) | Clinical disease with very high viral load and antigenemia | Treatment with ganciclovir (i.v., 400 mg b.i.d., followed by valganciclovir at 450 mg b.i.d.); also treatment with a combination of foscarnet (4 g), i.v. ganciclovir (460 mg), and CMV hyperimmunoglobulin |
| M (D+/R−) | Systemic HCMV infection with gastric involvement | Treatment with ganciclovir (i.v., 160 mg b.i.d.) followed by oral ganciclovir (1,000 mg t.i.d.) |
| N (D+/R−) | HCMV syndrome with end organ disease (liver, pancreas, brain, and gut) | Treatment with cidofovir (200 mg weekly) |
| O (D+/R+) | HCMV syndrome without defined end organ involvement | Treatment with ganciclovir (i.v., 325 mg b.i.d.) followed by oral ganciclovir (1,000 mg t.i.d.) |
Patient code refers to the panels in Fig. 1.
Abbreviations: i.v., intravenous(ly); b.i.d., twice daily; t.i.d., three times daily; D, donor; R, recipient.
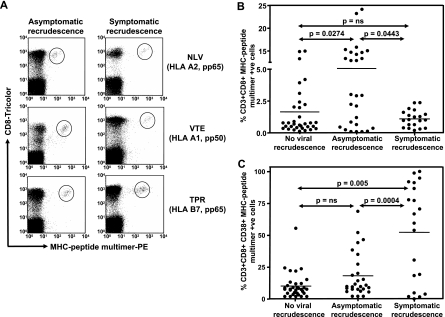
To determine whether the reduced IFN-γ expression by virus-specific T cells in individuals with symptomatic viral recrudescence was due to the loss of HCMV-specific T cells, PBMC from all SOT recipients were stained with MHC-peptide tetramers/pentamers and anti-CD8 antibody (Fig. 2A). Data presented in Fig. 2B show that although there was very little difference in the overall numbers of HCMV-specific T cells between SOT recipients who showed no viral recrudescence and those who showed symptomatic recrudescence, a significant increase in the number of HCMV-specific T cells was observed in individuals with asymptomatic recrudescence. We hypothesize that this increase in the HCMV-specific T cells in these individuals is not unexpected, as the viral recrudescence would provide increased stimulation to these cells, resulting in their expansion, and thus provide protection from clinical disease. It is highly likely that in addition to the reduced IFN-γ expression, the lack of expansion of HCMV-specific T cells in individuals with symptomatic recrudescence may also contribute towards the development of clinical disease. Furthermore, we also observed a significant increase in the expression of CD38 on the HCMV-specific CD8+ T cells in SOT recipients with symptomatic viral recrudescence compared to recipients who either showed no viral recrudescence or showed asymptomatic recrudescence (Fig. 2C). No significant difference in the expression of CD62L or CD27 on HCMV-specific CD8+ T cells was observed during or after antiviral prophylaxis (data not shown). Previous studies of human immunodeficiency virus-infected individuals have shown that increased expression of CD38 on CD8+ T cells is coincident with chronic human immunodeficiency virus disease progression to AIDS (12, 15).
FIG. 2.
Ex vivo enumeration of HCMV-specific CD8+ T cells using MHC-peptide multimers. PBMC from SOT recipients were costained with anti-human CD8 tricolor-labeled antibody and phycoerythrin (PE)-labeled MHC-peptide tetramers/pentamers. The fluorescence intensity was assessed with a FACSCalibur, and data were analyzed using Cellquest software. Representative data plots for three different HCMV epitopes, NLV (HLA A2 restricted), VTE (HLA A1 restricted), and TPR (HLA B7 restricted), are shown in panel A. Panel B shows percentages of MHC-peptide multimer-positive CD8+ T cells in samples from individuals with no evidence of viral recrudescence, with asymptomatic recrudescence, and with symptomatic recrudescence. Panel C shows the phenotypic analysis of HCMV-specific T cells in these individuals. HCMV-specific T cells were costained with anti-human CD38 antibody. Data presented in panels B and C are based on all the blood samples collected at different time points as indicated in Fig. 1. P values were calculated using the nonparametric Mann-Whitney test. ns, not significant.
In conclusion, this study is the first to demonstrate directly that a broadly directed CD8+ T-cell response with strong functional activity was coincident with the protection from symptomatic viral recrudescence. In addition, we have also shown that terminal differentiation/exhaustion of antigen-specific T cells (as indicated by the increased CD38 expression) may contribute towards the development of clinical symptoms following viral recrudescence. These conclusions are strongly supported by previous studies of other viral infections which have shown that the breadth of the cytotoxic T-lymphocyte response may be important in preventing viral pathogenesis (1, 18). Another important implication of this study relates to the potential use of T-cell functional analysis as a diagnostic tool for identifying the levels of virus-specific T-cell responses which would predict the increased or decreased risk of symptomatic HCMV recrudescence (6, 7, 19). The diagnostic application of this technology will require an extended analysis of a larger cohort of transplant patients in various clinical settings to determine if the protection from HCMV disease in transplant patients is dependent on T-cell responses directed against a broad range of antigens rather than any single antigen.
Acknowledgments
This work was supported by research funding from the Prince Charles Hospital Foundation, Cooperative Research Centre for Vaccine Technology, and the National Health and Medical Research Council (Australia), James S. McDonnell Foundation 21st Century Research Award/Studying Complex Systems, Cooperative Research Centre for Vaccine Technology. R.K. is supported by a Principal Research Fellowship from the National Health and Medical Research Council. M.P.D. is a Sylvia and Charles Viertel Senior Medical Research Fellow.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Bharadwaj, M., S. R. Burrows, J. M. Burrows, D. J. Moss, M. Catalina, and R. Khanna. 2001. Longitudinal dynamics of antigen-specific CD8+ cytotoxic T lymphocytes following primary Epstein-Barr virus infection. Blood 98:2588-2589. [DOI] [PubMed] [Google Scholar]
- 2.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crough, T., J. M. Burrows, C. Fazou, S. Walker, M. P. Davenport, and R. Khanna. 2005. Contemporaneous fluctuations in T cell responses to persistent herpes virus infections. Eur. J. Immunol. 35:139-149. [DOI] [PubMed] [Google Scholar]
- 4.Elkington, R., S. Walker, T. Crough, M. Menzies, J. Tellam, M. Bharadwaj, and R. Khanna. 2003. Ex vivo profiling of CD8+-T-cell responses to human cytomegalovirus reveals broad and multispecific reactivities in healthy virus carriers. J. Virol. 77:5226-5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandhi, M. K., and R. Khanna. 2004. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 4:725-738. [DOI] [PubMed] [Google Scholar]
- 6.Gerna, G., and D. Lilleri. 2006. Monitoring transplant patients for human cytomegalovirus: diagnostic update. Herpes 13:4-11. [PubMed] [Google Scholar]
- 7.Gerna, G., D. Lilleri, C. Fornara, G. Comolli, L. Lozza, C. Campana, C. Pellegrini, F. Meloni, and T. Rampino. 2006. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am. J. Transplant. 6:2356-2364. [DOI] [PubMed] [Google Scholar]
- 8.Humar, A., and M. Michaels. 2006. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am. J. Transplant. 6:262-274. [DOI] [PubMed] [Google Scholar]
- 9.Khanna, R., and D. J. Diamond. 2006. Human cytomegalovirus vaccine: time to look for alternative options. Trends Mol. Med. 12:26-33. [DOI] [PubMed] [Google Scholar]
- 10.Khanna, R., D. J. Moss, and M. Gandhi. 2005. Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat. Clin. Pract. Oncol. 2:138-149. [DOI] [PubMed] [Google Scholar]
- 11.Lacey, S. F., D. J. Diamond, and J. A. Zaia. 2004. Assessment of cellular immunity to human cytomegalovirus in recipients of allogeneic stem cell transplants. Biol. Blood Marrow Transplant. 10:433-447. [DOI] [PubMed] [Google Scholar]
- 12.Liu, Z., W. G. Cumberland, L. E. Hultin, H. E. Prince, R. Detels, and J. V. Giorgi. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83-92. [DOI] [PubMed] [Google Scholar]
- 13.Ljungman, P. 2006. Would monitoring CMV immune responses allow improved control of CMV in stem cell transplant patients. J. Clin. Virol. 35:493-495. [DOI] [PubMed] [Google Scholar]
- 14.McNeil, K., G. A. Wahlers, T. Knoop, C. Speich, R. Mamelok, R. D. Maurer, J. Ives, and P. A. Corris. 2006. Comparison of mycophenolate mofetil and azathioprine for prevention of bronchiolitis obliterans syndrome in de novo lung transplant recipients. Transplantation 81:998-1003. [DOI] [PubMed] [Google Scholar]
- 15.Paul, M. E., W. T. Shearer, C. A. Kozinetz, and D. E. Lewis. 2001. Comparison of CD8+ T-cell subsets in HIV-infected rapid progressor children versus non-rapid progressor children. J. Allergy Clin. Immunol. 108:258-264. [DOI] [PubMed] [Google Scholar]
- 16.Radha, R., S. Jordan, D. Puliyanda, S. Bunnapradist, A. Petrosyan, N. Amet, and M. Toyoda. 2005. Cellular immune responses to cytomegalovirus in renal transplant recipients. Am. J. Transplant. 5:110-117. [DOI] [PubMed] [Google Scholar]
- 17.Silins, S. L., M. A. Sherritt, J. M. Silleri, S. M. Cross, S. L. Elliott, M. Bharadwaj, T. T. Le, L. E. Morrison, R. Khanna, D. J. Moss, A. Suhrbier, and I. S. Misko. 2001. Asymptomatic primary Epstein-Barr virus infection occurs in the absence of blood T-cell repertoire perturbations despite high levels of systemic viral load. Blood 98:3739-3744. [DOI] [PubMed] [Google Scholar]
- 18.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker, S., C. Fazou, T. Crough, R. Holdsworth, P. Kiely, M. Veale, S. Bell, A. Gailbraith, K. McNeil, S. Jones, and R. Khanna. 2007. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl. Infect. Dis. 9:165-170. [DOI] [PubMed] [Google Scholar]
- 20.Zhong, J., and R. Khanna. 2007. Vaccine strategies against human cytomegalovirus infection. Expert Rev. Anti-Infect. Ther. 5:449-459. [DOI] [PubMed] [Google Scholar]