Abstract
The physiological regulation of the immune system encompasses comprehensive anti-inflammatory mechanisms that can be harnessed for the treatment of infectious and inflammatory disorders. Recent studies indicate that the vagal nerve, involved in control of heart rate, hormone secretion and gastrointestinal motility, is also an immunomodulator. In experimental models of inflammatory diseases, vagal nerve stimulation attenuates the production of proinflammatory cytokines and inhibits the inflammatory process. Acetylcholine, the principal neurotransmitter of the vagal nerve, controls immune cell functions via the alpha7 nicotinic acetylcholine receptor (alpha7nAChR). From a pharmacological perspective, nicotinic agonists are more efficient than acetylcholine at inhibiting the inflammatory signaling and the production of proinflammatory cytokines. This ‘nicotinic anti-inflammatory pathway' may have clinical implications as treatment with nicotinic agonists can modulate the production of proinflammatory cytokines from immune cells. Nicotine has been tested in clinical trials as a treatment for inflammatory diseases such as ulcerative colitis, but the therapeutic potential of this mechanism is limited by the collateral toxicity of nicotine. Here, we review the recent advances that support the design of more specific receptor-selective nicotinic agonists that have anti-inflammatory effects while eluding its collateral toxicity.
Keywords: acetylcholine, nicotine, cholinergic, acetylcholine, NF-κB, sepsis, nicotinic agonist, STAT, macrophage
Introduction
Survival is impossible without the precise regulation of the immune system. The production of proinflammatory cytokines is a critical physiological process to orchestrate immune and metabolic responses during development, tissue regeneration, healing, trauma or infection, and to protect our bodies against hemorrhage, ischemia, cancer and sepsis. A controlled production of proinflammatory cytokines, such as interleukins (ILs), tumor necrosis factor-alpha (TNF-α) and high-mobility group box (HMGB)-1, triggers beneficial inflammatory responses that promote local coagulation to confine infection and tissue damage (Ulloa and Tracey, 2005). However, the unrestricted production of these cytokines is more dangerous than the original injury and it is one of the principal causes of human morbidity and mortality. One of the most dramatic examples of this process is ‘severe sepsis', the leading cause of death in intensive care units and one of the principal causes of death in developed societies (Martin et al., 2003). Severe sepsis is characterized by an overwhelming production of proinflammatory cytokines that causes systemic inflammation, cardiovascular dysfunction and lethal multiple organ failure (van der Poll and Lowry, 1995; Hotchkiss and Karl, 2003; Rice and Bernard, 2005). Its pathogenesis is complex and requires dedicated treatment. This effect is illustrated by the studies indicating that neutralizing pro-inflammatory cytokines (monoclonal anti-TNF antibodies, IL-1 receptor antagonists and TNF-receptor fusion proteins) have proven to be successful in inflammatory conditions such as rheumatoid arthritis, Crohn's disease, ankylosing spondylitis and psoriasis (Feldmann, 2002; Ulloa and Tracey, 2005; Rutgeerts et al., 2006; Ulloa and Messmer, 2006), but fail to produce significant effects in the treatment of severe sepsis (Abraham et al., 1998). A potential explanation for this conundrum is that the pathogenesis of sepsis is not mediated by a single cytokine and hence a successful treatment may require a comprehensive strategy to inhibit several, rather than just single, inflammatory cytokine. Recent studies indicated that the nervous system controls the immune system through a composite mechanism that modulates the production of multiple inflammatory cytokines. These studies suggest that this mechanism can provide a therapeutic advantage for the treatment of complex inflammatory disorders similar to severe sepsis. In agreement with this hypothesis, recent studies in experimental sepsis indicated that stimulation of the efferent vagal nerve prevented systemic inflammation and reduced lethality (Borovikova et al., 2000; Wang et al., 2003). The parasympathetic nervous system attenuated serum levels of diverse proinflammatory cytokines including TNF and HMGB1 (Figure 1). Beyond its physiological implications, this mechanism provides major advantage for the design of novel pharmacological anti-inflammatory strategies. This article reviews the current understanding of the regulation of innate immune responses by the parasympathetic nervous system and its pharmacological translation for the treatment of infectious and inflammatory disorders.
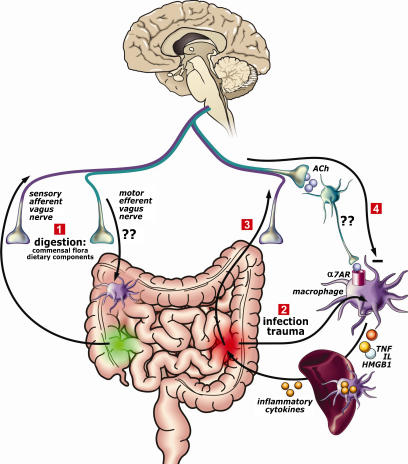
Figure 1.
The cholinergic anti-inflammatory surveillance. Hypothetical scheme of the vagus nerve continuously monitoring and modulating innate immune activation following ingestion, infection, and trauma. (1) During digestion, the commensal flora and dietary components activate the sensory afferent vagus nerve, which will transmit the information to the brain. In return, the brain may activate the efferent vagus nerve to modulate gastrointestinal macrophages. (2) The efferent vagus nerve also modulates systemic inflammatory responses through a mechanism involving an intact spleen. Upon infection or trauma, bacterial components or intracellular mediators (HMGB1, heat shock proteins, etc) activate macrophages to produce proinflammatory cytokines. (3) This will trigger afferent vagus nerve signaling. (4) In return, the brain will activate efferent vagus nerve to release acetylcholine, which can bind to the α7 acetylcholine receptor on macrophages and inhibit the production of proinflammatory cytokines. Interrogation marks indicate that although macrophages are found in the proximity of cholinergic fibers in the spleen and the intestine (De Jonge et al., 2005) there is currently no evidence demonstrating that parasympathetic neurons indeed innervate immune cells and further studies are needed to determine the physiological interaction between the vagus nerve and immune cells. HMGB1, high-mobility group box 1.
A neuronal strategy for inflammation
Vagal modulation of immune responses may have particular implications in the densely innervated gastrointestinal tract. The intestinal lumen contains massive numbers of commensal gut microbes (estimated to amount 1011 to 1012 per gram stool in the colon (Sansonetti, 2006)) and the immune system must display a regulated response to either beneficial or pathogenic microbes (Backhed et al., 2005). The parasympathetic nervous system, which classically assigned function is to control heart rate, hormone secretion, gastrointestinal peristalsis and digestion during ingestion, may also control immune responses to commensal flora and dietary components. Ingestion of dietary fat stimulates the production of cholecystokinin (CCK), which is a characteristic neuropeptide released during ingestion to trigger several digestive functions, including exocrine pancreas secretion and activation of afferent vagus nerve signals to induce satiety (Figure 1). A recent study indicated that CCK, released as a result of high-fat enteral nutrition, inhibited hemorrhagic shock-induced TNF-α and IL-6 release (Luyer et al., 2005). This anti-inflammatory effect of CCK is mediated by the vagus nerve because surgical or chemical vagotomy abrogates the anti-inflammatory effect of both high-fat diet and CKK (Luyer et al., 2005). In accordance, activation of the vagus nerve prevents manipulation-induced inflammation of the intestinal muscularis externa and ameliorates postoperative ileus (De Jonge et al., 2005), a typical pathological condition resulting from intestinal manipulation during abdominal surgery. However, with respect to cholinergic anti-inflammatory effects of vagus nerve signaling in the GI-tract, it should be kept in mind that vagal afferents are thought to be involved in maintenance of intestinal mucosal barrier function (reviewed in Downing and Miyan, 2000), plausibly via modulation of mast-cell activity (Theodorou et al., 1996; Stead et al., 2006). Hence, the pro-inflammatory effect of vagotomy on intestinal and peritoneal inflammation could be indirect and involve other than direct cholinergic mechanisms (Figure 1).
The anti-inflammatory effects of efferent vagus nerve activity on the GI-tract are in agreement with previous studies indicating that surgical vagotomy increases the susceptibility of rodents to systemic inflammation in septic and hemorrhagic shock (Borovikova et al., 2000). Hence, the vagus nerve can function as a physiological anti-inflammatory system and the clinical potential of this mechanism is not necessarily limited to the gastrointestinal tract, but extends to several clinical scenarios including injury, trauma, hemorrhage and severe sepsis. Electrical stimulation of the vagus nerve inhibits systemic levels of proinflammatory mediators such as TNF, IL6, IL1β and HMGB-1 in experimental models of endotoxemia, hemorrhagic shock and polymicrobial sepsis. Recent studies indicated that the spleen is crucial in mediating these systemic effects, as electrical stimulation of the vagus nerve fails to attenuate serum TNF levels in splenectomized animals (Huston et al., 2006). Moreover, selective lesioning of the common celiac nerve abolishes TNF suppression by vagus nerve stimulation (Huston et al., 2006). This connection between the common celiac nerve and the spleen reveals that the parasympathetic nervous system may modulate systemic inflammation by regulating immune cells in the spleen.
In addition to its physiological implications, recent studies reveal a previously unrecognized role of the vagal nerve in mediating the anti-inflammatory potential of specific pharmacological agents. The vagal nerve seems to contribute to the anti-inflammatory potential of a wide diversity of compounds such as many nonsteroidal anti-inflammatory drugs, semapimod (CNI-1493) and melanocortin peptides. Similar to CCK, leptin, melanocortin peptides and some nonsteroidal anti-inflammatory drugs appear to control the production of pro-inflammatory cytokines through an anti-inflammatory mechanism mediated by the vagus nerve (Ulloa, 2005). Among others, semapimod and adrenocorticotropic hormone (1–24) activate the efferent vagus nerve and limit circulating serum TNF levels during endotoxemia through a mechanism that requires an intact vagus nerve as surgical vagotomy abrogates their anti-inflammatory potential (Bernik et al., 2002).
Acetylcholine and inflammation
At molecular level, most of the studies about the anti-inflammatory potential of the vagus nerve have been based on the effects of acetylcholine, the principal neurotransmitter of the parasympathetic nervous system. Acetylcholine receptors are prominently expressed in immune cells, and those cells derived from bone marrow (lymphoid and myeloid cells). The functional implications have become clear after the finding that acetylcholine controls the production of proinflammatory cytokines from macrophages (Borovikova et al., 2000). Since acetylcholine signals through either muscarinic (G-protein-coupled receptors) or nicotinic (ligand-gated ion channels) receptors (Miyazawa et al., 2003), selective cholinergic agonists and antagonists were used to identify the receptors involved in the control of macrophage activation. Muscarine slightly inhibited macrophage activation at supraphysiological levels, but nicotine was more efficient than acetylcholine at inhibiting the production of pro-inflammatory cytokines from macrophages (Wang et al., 2003). These effects were specific for proinflammatory cytokines and neither acetylcholine nor nicotine inhibited the production of anti-inflammatory cytokines such as transforming growth factor β (TGF-β) or IL-10. Hence, the anti-inflammatory effects of acetylcholine on macrophages seem to be mediated through nicotinic receptors, and nicotine is a more selective pharmacological agonist to control the production of pro-inflammatory cytokines. Transcripts for nicotinic acetylcholine receptors (nAChR) subunit α7, β2, as well as α4 have been detected in multiple inflammatory cell types, including macrophages derived from various tissues (Borovikova et al., 2000; Matsunaga et al., 2001). The finding of distinct nAChR subtypes expressed on immune cells suggests that nicotine may affect distinct inflammatory cells its based on receptor affinity (Fujii et al., 1999; Sato et al., 1999; Gahring and Rogers, 2005). However, most evidences point towards a crucial role for the α7 homopentamer nAChR in the cholinergic regulation of macrophage activity (Wang et al., 2003). The α7-subunit is expressed by macrophages and its expression is crucial for the anti-inflammatory effect of vagal nerve signaling. Nicotine exerts anti-inflammatory effects on macrophages that can be counteracted by selective α7-antagonists (Tracey, 2002; Wang et al., 2003; Ulloa, 2005). Selective α7 nAChR agonists have proven effective in reducing macrophage cytokine production and inflammation in animal models of pancreatitis (van Westerloo et al., 2006), dextran sulfate sodium (DSS)-induced colitis (Ghia et al., 2006) and intestinal ileus (The et al., unpublished).
Structural and functional aspects of α7 nicotinic acetylcholine receptors (α7 nAChR)
In general, neuronal nAChRs are pentameric complexes of α- and β-subunits that form a ligand-gated ion channel. The large number of different subunits (nine α- and four β-subunits) provides a large variation in possible nAChRs with different physiological function and ligand affinity (Lukas et al., 1999; Drisdel and Green, 2000; Lloyd and Williams, 2000). The α7 nAChR is a well-characterized member of the neurotransmitter-gated ion channel superfamily (Lukas et al., 1999). Expression of the α7-subunit gene in several cell types has revealed that assembly of the protein to functional nAChR is complex (Figure 2). In neurons, α7 proteins assemble as a homopentamer composed of five individual α7-subunits (Rangwala et al., 1997; Drisdel and Green, 2000), although α7-subunits seem to form functional heteropentamers with β2-subunits if overexpressed in Xenopus leavis oocytes or human epithelial kidney cells tsA201 (Khiroug et al., 2002). However, assembly of α7-subunits to a functional homopentameric receptor requires assembly protein Ric-3 (Williams et al., 2005) and/or post-translational processing of α7-subunit (Rakhilin et al., 1999).
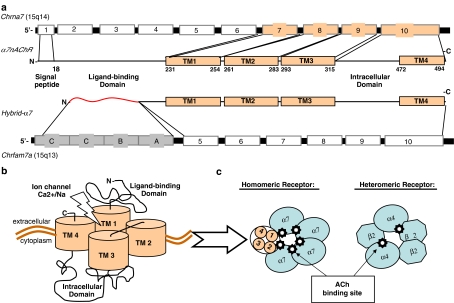
Figure 2.
The α7 nicotinic acetylcholine receptor (α7 nAChR) structure. (a) The Chrna7 is located at the 15q14 chromosomal region. The gene comprises ten exons encompassing 138.5 kb that codifies for the α7 nAChR protein with an estimated MW 50 kDa. Six mRNA splice variants have been described in addition to the wild type α7-gene, though it is uncertain whether any of these transcripts are processed to functional protein. The gen Chrfam7a represents a partial duplication of the human Chrna7 exons 5 to 10. This partial duplicated gene is combined with four novel exons (A to D) to comprise a new gene named ‘Cholinergic Receptor FAMily with sequence similarity 7A' (Chrfam7a) or ‘hybrid α7'. To date, there is evidence that this gene is transcribed and processed to form a functional receptor. (b) The α7 nAChR has an N-terminal signal peptide and ligand-binding domain, four transmembrane domains (TM1-4), and a regulatory intracellular domain located between TM3 and TM4. (c) Nicotinic acetylcholine receptors (nAChRs) are a family of ligand-gated ion channels created by a diversity of subunits that form homo- or heteropentameric receptors with distinct pharmacological properties. Nicotinic receptors can functionally be differentiated into two principal classes that differ in their affinity for nicotine and α-bungarotoxin. Receptors that bind nicotine with high affinity, contain α2–α6 as ligand-binding subunits, and require β-subunits for proper activation. A second class of receptors (α7–α10) binds nicotine with low affinity; has high affinity for α-bungarotoxin, and functions as homomeric or heteromeric ion channels in vitro. The α7 nAChR forms homo-pentameric ion channel receptors and they appears to be the only α-bungarotoxin receptor identified in macrophages and mammalian brain, as α8-subunits appear to be expressed only in chick, and α9,10-subunits expression is limited to mechanosensory cochlear hair cells and the pituitary.
The α7-subunit gene includes 10 exons, with 4 transmembrane domains encoded by exons 7–10, 3 putative glycosylation and ligand-binding sites located extracellularly in exons 2–6 (Boyd, 1997; Colquhoun and Patrick, 1997; Lloyd and Williams, 2000). The α7-subunit is approximately 56 kDa and is composed of 502 aa, including a 22 aa N-terminal signal peptide followed by an extracellular 200-aa ligand-binding domain (Galzi et al., 1991; Bertrand et al., 1993). The snake toxin α-bungarotoxin is a selective antagonist with high affinity for the α7 nAChR, functionally differentiating this receptor subtype from other neuronal nAChRs (Rangwala et al., 1997; Drisdel and Green, 2000). The binding domain of this toxin is contained in the extracellular N terminus, encoded by exons 1–5 (Galzi et al., 1991; Drisdel and Green, 2000). The assembled subunits form a central pore with ligand binding at subunit junctions responsible for changes in the state of the receptor. The transmembrane domains are composed of four α-helices that cross the lipid bilayer and form the ion channel (Boyd, 1997). Folding and interaction of the extracellular ACh-binding protein loops determine pore opening in response to ACh binding (Bouzat et al., 2004). Although nicotinic receptors are originally described as sodium channels, the α7 homopentamer is highly permeable for Ca2+ as well (Berg and Conroy, 2002). Phosphorylation of α7 nAChR upon nicotine binding decreases ACh-evoked currents (Charpantier et al., 2005). In neuronal cells, the activation of α7 nAChR itself is negatively regulated by phosphorylation at Tyr-386 and Tyr-442 by Src-family kinases that interact directly with its cytoplasmic loop (Charpantier et al., 2005). Mutant α7 nAChRs lacking cytoplasmic loop tyrosine residues because of alanine replacement of Tyr-386 and Tyr-442 were more active than wild-type receptors and insensitive to kinase or phosphatase inhibition (Charpantier et al., 2005).
The human α7-nAChR gene has been mapped to chromosome 15q14. Six mRNA splice variants of the α7 gene have been described in human brain as well as leukocytes (Gault et al., 1998; Villiger et al., 2002; Gault et al., 2003; Severance et al., 2004), although it is uncertain whether any of these transcripts are processed to functional protein (Villiger et al., 2002; Severance et al., 2004). Interestingly, the human α7-nAChR has been described to be partially duplicated on this chromosome. Exons 5–10 of the gene have been duplicated in a ‘tail-to-head' orientation and this partially duplicated gene is combined with four novel exons (A to D) to comprise a new gene, the ‘hybrid α7' or the ‘cholinergic receptor family with sequence similarity 7A' (CHRFAM7A) (Gault et al., 1998). Although it is reported that this gene is transcribed as a 45 kDa protein (that is, in human leukocytes) (Villiger et al., 2002), it remains unclear whether this hybrid transcript is appropriately translated and processed to form a functional receptor. In cells expressing this hybrid receptor rather than the α7 containing all exons, no binding of α-bungarotoxin and no nicotine-induced electrical current could be demonstrated (Villiger et al., 2002), possibly explained by the fact that the hybrid gene is mutated in the binding site for nicotine and α-bungarotoxin. Future studies are needed to determine whether the various gene products of α7 comprise nonfunctional splice variants, or have a role in the cellular responses to ACh.
Functional aspects of α7 ACh receptor activation on immune cells
The bone marrow is innervated by parasympathic cholinergic nerve fibers and several types of bone marrow-progenitor-and other myeloid and lymphoid cells (B-cells, microglia, monocytes and dendritic cells (DC)) are functionally responsive to nicotine and express the α7 receptor.
T lymphocytes
Thymic epithelium as well as T cells in the thymus express nAChR as do mature lymphocytes (Fujii et al., 1999). The suppressive effect of nicotine on the immune response, as well as the expression of nicotinic receptors in T lymphocytes is documented (DeRosa et al., 2005; Kawashima and Fujii, 2003; Skok et al., 2006); however, the role of α7 nAChR is not clearly defined. Lymphocytes express most components of the cholinergic system including ACh, muscarinic and nicotinic ACh receptors (mAChRs and nAChRs, respectively), choline acetyltransferase (ChAT), high-affinity choline transporter and AChsterase (Fujii et al., 1999; DeRosa et al., 2005). Lymphocytes may produce ACh (Rinner et al., 1998), and α7 nAChR expression in T lymphocytes and T lymphocyte-derived cell lines has been reported (Kawashima and Fujii, 2004; Skok et al., 2006). On the basis of these reports, ACh has been proposed to affect the ability of T cells to participate in the immune response (Sopori et al., 1998; Sato et al., 1999). Stimulation of AChRs with ACh or nicotine causes Ca2+ signaling and upregulation of c-fos expression in T cells (DeRosa et al., 2005; Nizri et al., 2006), but this effect may involve muscarinic AChRs as well as α7 nAChRs (Kawashima and Fujii, 2004, Nizri et al, 2006). α7 nAChR activation is suggested to enhance cortisol-induced T-cell apoptosis (DeRosa et al., 2005), but the further functional consequences of α7 nAChR expression in T cells is largely unknown.
B lymphocytes
Antigen-dependent B-cell activation may also be affected by nicotinic agonists. Expression of α7 nAChRs in B lymphocytes has been reported (Fujii et al., 1999; Sato et al., 1999; Gahring and Rogers, 2005), in particular on re-circulating B cells (Skok et al., 2006). It should be noted however that in addition to α7, heteromeric α4/β2 nAChRs are found on B lymphocytes (Skok et al., 2005), and deficiency of those subunits affects both the pre-immune status of mice and their immune response. Both of these nicotinic receptors seem to affect signaling and expression of co-stimulatory CD40 on B-cells. The functional contribution of α7 or α4/β2 nAChR subtypes to the biological effects of nicotine or ACh is unclear. Studies indicate that both α4/β2 and α7 nAChRs may be involved in the maturation of pre/pro B cell in the bone marrow to develop into circulating B cells. Using bone-marrow reconstituted chimeras of genetically modified mice strains that lack either of the two receptors in their bone marrow progenitor cells, it was shown that both α7 or β2 nAChR stimulate B-cells development from the pre-B-cell stage (Skok et al., 2006). The lack of either of the 2 nAChRs, but in particular of α4/β2 nAChR, decreased peripheral B-cell number, whereas on the other hand, nicotine treatment increased B lymphocyte numbers in the bone marrow.
Dendritic cells
While macrophages initiate many inflammatory and innate immune functions, DCs are the principal antigen-presenting cells (APCs) and are critical cells for initiation of cell-mediated immunity against infection. A series of in vitro studies report that DCs express the α7 nAChR and that exposure to nicotine or ACh alters DC function (Aicher et al., 2003; Nouri-Shirazi and Guinet, 2003; Guinet et al., 2004). Hence, it should be kept in mind that some of the anti-inflammatory effects of vagal nerve stimulation, or administration of nicotinic agonists, may be mediated, at least in part, by altered DC function rather than by circulating or resident monocytes/macrophage activity. In vitro, immature monocyte-derived DCs undergo maturation in response to bacterial antigen triggers such as lipopolysaccharide. If DCs are matured in the presence of nicotine, mature DCs manifest lower endocytic and phagocytic activities if compared to vehicle. In matured DCs, nicotine exposure decreases the production of IL-12, and the capacity of DCs to induce APC-dependent T-cell responses (Nouri-Shirazi and Guinet, 2003; Guinet et al., 2004). Interestingly, however, others find that the effect of nicotine on matured DC function is pro-inflammatory in nature; exposure of mature DCs to low (nM) doses of nicotine induced an increased expression of CD86, CD54 and HLA-DR (Aicher et al., 2003). In addition, nicotine-induced expression of molecules with costimulatory activity toward antigen presentation and induced increased secretion of IL-12 by Th1T lymphocytes by seven fold. This effect was mediated by activation of phosphatidyl-inositol-3-kinase (PI3K), Akt and p38 mitogen-activated protein kinase (MAPK)-signaling pathways.
The reason for these opposite effects may involve DC maturation status at the time of assay, rendering the in vivo implications of cholinergic modulation of DCs on inflammatory disease hard to appreciate. Clearly, the expression of α7 nAChR in DCs is suggestive of vagal (cholinergic) regulation of their activity, but further, in vivo, studies are required to determine a role of the vagal nerve in modulating their function in animal models of inflammation.
Monocytes, macrophages and neutrophils
Macrophages are critical effector cells for early recognition and destruction of organisms invading through most surfaces including the gut, skin and lung. Studies reporting that nicotine and/or cigarette smoke alter macrophage functions, such as pinocytosis (Schwartz et al., 1972; Schwartz, 1976), endocytosis and intracellular degradation of phagocytosed protein (Thyberg et al., 1983) and killing of microbes date back some 25–40 years (Green and Carolin, 1967). However, most of these effects were attributed to accumulation of nicotine in the lysosomes leading to an impaired digestive capacity (Thyberg et al., 1983). In monocytes and macrophages of various species, nicotine causes alteration of macrophage functions such as microbial killing (Green and Carolin, 1967; Ortega et al., 1994; Matsunaga et al., 2001), inhibition of by lipopolysaccharide (LPS)-stimulated/-induced TNF, IL-6, IL-1β, IL-8, prostaglandin E2 (PGE2) and other pro-inflammatory mediators, and as recently shown expression of LPS-responsive receptors such as CD14 and Toll-like receptor 4 (Hamano et al., 2006). It is tempting to assume that this ‘deactivated state' resembles the effect of known humoral peptides such as IL-10 and TGF-β, but this awaits further investigation. Treatment with nicotine of a mouse alveolar macrophage cell line (expressing α4 and β2, but not α7) results in enhanced intracellular replication of Legionella pneumophila (Matsunaga et al., 2001). Further, the production of the inflammatory cytokines IL-6, TNF and IL-12 was downregulated by nicotine in these cells. A potential outcome of this effect may be that, on the one hand, the inflammatory responses become less, but on the other the anti-inflammatory properties of nicotine actually enhance the survival of invading microbes or viruses and induce significantly higher titers of virus following infection (Sopori, 2002). Of note, in this respect, may be that certain infections, such as pneumonia caused by Streptococcus pneumoniae, are more frequent in smokers (Nuorti et al., 2000). Hence, these studies suggest that an acute treatment with nicotinic agonists may provide a pharmacological strategy to restrain inflammation, but chronic exposure to nicotine or nicotinic agonists may provide a sustained anti-inflammatory potential that favors some lung infections as observed in tobacco smokers and their susceptibility to chronic obstructive pulmonary disease (Barnes, 2003). In addition to macrophages, nicotine alters various neutrophil functions such as superoxide anion production (Sorensen et al., 2004), chemotaxis (Totti III et al., 1984), integrin expression (Speer et al., 2002) and chemokine production (Iho et al., 2003), and suppresses apoptosis (Aoshiba et al., 1996). However, although neutrophils express nicotinic receptors (Davies et al., 1982), it is yet unclear whether these effects are mediated by the α7 nAChR.
Myeloid cells; microglia
Microglia cells represent the largest class of phagocytes within the central nervous system (CNS). In parallel to activation of phagocytes in non-neuronal tissues, the outcome of activation of microglia can be either neuroprotective or neurotoxic. It is generally recognized that healthy neurons and astrocytes in the microenvironment can regulate the magnitude of microglia-mediated innate immune responses via activation of α7 nAChRs, among other neurotransmitter receptors such as purinergic P2X7 receptors (Suzuki et al., 2006). Primary cultures of both resting and activated microglia and astrocytes show ChAT activity and synthesize ACh (DeRosa et al., 2005; De et al., 2005), suggesting that this neurotransmitter may act as a local hormone and contribute to the regulation of microglial functions.
In normal healthy brain, microglia show a typical resting phenotype when compared to other tissue macrophages, but they rapidly react in response to a number of acute and chronic insults. Activated microglia can be of crucial importance in CNS pathology via liberation of free radicals as well as cytokines and toxic factors (Streit, 2002). Alternatively, microglia can exert neuroprotective functions by secreting growth factors or diffusible anti-inflammatory mediators, which help to resolve inflammation and restore tissue homeostasis (Streit, 2002). Cultured microglia express α7 nAChR protein and mRNA, nicotine and ACh inhibit production of TNF in LPS-stimulated mouse microglial cultures (Shytle et al., 2004). This inhibition in TNF production is associated with a reduction in phosphorylation of p44/42 and p38 MAPK (Shytle et al., 2004; Suzuki et al., 2006). Nicotine also acts as an anti-inflammatory agent on microglia by increasing the expression of COX-2 and the synthesis of PGE2, known to downregulate microglial activation and expression of pro-inflammatory genes, including TNF (Zhang and Rivest, 2001; Suzuki et al., 2006). However, nicotine has no effect on the release of NO, IL-1β and IL-10. The effect of nicotine on the LPS-induced TNF production and PGE2 release is counteracted by the specific antagonist of the α7-subunit, α-bungarotoxin, demonstrating the requirement of α7 nAChR.
Nicotine may be protective against the development of neurodegenerative diseases, such as Alzheimer's and Parkinson's disease, in which a local inflammatory response is sustained by microglial cells (Streit, 2002; Hellstrom-Lindahl et al., 2004; Wang et al., 2000a, 2000b). The potential of the α7 nAChR in mediating CNS inflammation has gained clinical interest since the implication of α7 nAChR in β-amyloid (Abeta (1–42)) neurotoxicity, a critical factor in the pathology of Alzheimer's disease. One of the major features of it is the reduction of nAChRs in disease-relevant brain regions such as the cerebral cortex and hippocampus. This loss is explained by the loss of cholinergic cells, which contributes to the cognitive dysfunction. The most vulnerable neurons appear to be those that abundantly express the α7 nAChR. In vivo studies clearly indicate that α7 nAChRs play a critical role in protection from cholinergic lesions and enhancing cognitive function (Hellstrom-Lindahl, 2000). Many data point towards binding of Abeta (1–42) to the α7 nAChR as the triggering factor in neuronal cell death and Alzheimer's pathology. The high-affinity binding of Abeta (1–42) to the α7 nAChR on neuronal cell surfaces leads to internalization of the nAChR complex and its accumulation within the lysosomal compartment (Wang et al., 2000a, 2000b). Because this α7 nAChR–Abeta (1–42) interaction also leads to the inhibition of ACh release and calcium flux, and even cell death in vitro, it is speculated that this interaction may be one of the key events in the pathogenesis of Alzheimer's disease.
Evidence is provided that α7 nicotinic agonists are neuroprotective against Abeta (1–42) toxicity in various culture systems. This effect appears to be mediated by α7 subtype nAChRs, since the protection is blocked by α-bungarotoxin and is mimicked by α7 selective agonists (Shaw et al., 2002, 2003; Marrero et al., 2004). In the neuronal cell line PC12, nicotine interaction with the α7 nAChR inhibits binding of Abeta (1–42) to the same receptor, and prevents the Abeta (1–42)-induction of caspase 3 and apoptosis. The latter seems to be the result of nicotinic activation of tyrosine kinase Janus kinase 2 (Jak2) and activation of phosphatidylinositol 3-kinase and Akt-signaling pathways rather than blockade of Abeta (1-42) binding to the α7 nAChR (Shaw et al., 2002). In turn, nicotine-induced Jak2 kinase activity is negatively mediated by activity of the cytoplasmic phosphatase Src-homology phosphatase-1 (Shaw et al., 2003). Notably, phosphorylation of the α7 nAChR (Charpantier et al., 2005) and activation of Jak2 by nicotinic binding to α7 nAChR has also been demonstrated in non-neuronal cell types such has macrophages (De Jonge et al., 2005; Arredondo et al., 2006) and oral keratinocytes (Arredondo et al., 2006). Together, these findings support the idea that Jak2 plays a central role in the α7 nAChR-induced neuroprotection against Abeta (1-42). Hence, this strengthens the potential role of α7-agonists as drug target in the control of neuronal apoptosis and α7-mediated neuroprotection in Alzheimer's disease.
α7 nAChR activation and subcellular signaling
How does acetylcholine modulate the activation of immune cells? Recent reports advance our understanding on the intracellular signaling pathways involved in the anti-inflammatory potential of acetylcholine, but the exact mechanism remains to be established. The classically assigned cellular effects of α7 nAChR activation are mediated via ion channel fluxes, although α7 nAChR also activates alternative signaling pathways in neuronal and non-neuronal cells. In different non-neuronal cell types, such as microglia and macrophages (Blanchet et al., 2006), nicotinic activation of the α7 nAChR elicit an increase in intracellular Ca2+ concentration. However, this increase may arise from release of intracellular Ca2+ following nicotinic PI3K and phospholipase-C (PLC) activation rather than from α7 nAChR-mediated Ca2+ membrane fluxes. In human leukocytes, neither nicotine or acetylcholine elicit detectable membrane currents (Villiger et al., 2002). Alternatively, in neuronal as well as non-neuronal cells, agonists of α7 nAChR trigger phosphorylation of Akt (Shaw et al., 2002; Arredondo et al., 2006) via activation of Jak2 and PI3K. Previous studies indicate that among several pathways, cholinergic agonists interfere with two critical signaling routes required for immune cell activation: the Jak/STAT (signal transducer and activator of transcription) and the nuclear factor kappaB (NF-κB) pathways (Figure 3).
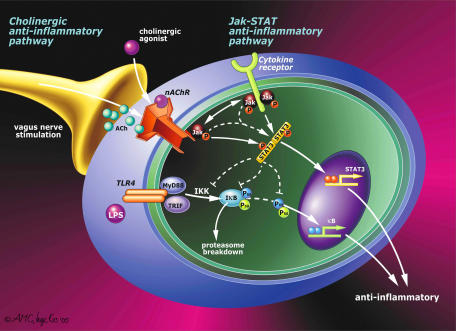
Figure 3.
The cellular ‘nicotinic anti-inflammatory pathway'. Nicotine has been successfully used in clinical trials for the treatment of ulcerative colitis. Despite its pharmacological interest, little is known about the ‘nicotinic anti-inflammatory pathway' triggered by the α7 nAChR in immune cells. Two major pathways have been recently reported in macrophages. During infection or trauma, bacterial components (LPS, PGN, CpG-DNA, etc) or intracellular mediators (HMGB1, heat shock proteins, etc) trigger pro-inflammatory pathways that converge in the activation of the NF-κB, which will translocate into the nucleus and induce the transcriptional activation of a variety of inflammatory cytokines. Recent studies indicate that nicotine inhibits the production of pro-inflammatory cytokines in macrophages by preventing the activation of the NF-κB through a mechanism dependent on the α7 nAChR. In peritoneal macrophages, the α7 nAChR can recruit the tyrosine kinase Jak2 and promotes STAT3 phosphorylation and subsequent activation. The Jak2-STAT3 pathway can contribute to the anti-inflammatory potential of the α7 nAChR by inducing the expression of anti-inflammatory proteins such as SOCS3.
In different cell types including monocytes, macrophages and endothelial cells, studies have indicated that the anti-inflammatory potential of the α7-nAChR is mediated by the inhibition of the transcription factor, NF-κB (Sugano et al., 1998; Wang et al., 2004; Saeed et al., 2005). Activation of the NF-κB requires ubiquitination of the IκB. This process will allow the nuclear translocation of the p65 and/or p50 subunits in order to modulate the transcription of NF-κB responsive genes such as IL6 and iNOS (Sugano et al., 1998; Rioux and Castonguay, 2000). Activation of the α7 nAChR may prevent IκB breakdown and p65 nuclear translocation and this mechanism explains the anti-inflammatory potential of nicotinic agonists in monocytes, macrophages and endothelial cells. Indeed, it has been shown that the anti-inflammatory action of ACh is associated with the inhibition of the LPS-induced activation of the NF-κB (Wang et al., 2004, Saeed et al., 2005). Another critical component of the nicotinic anti-inflammatory pathway appears to be mediated by the Jak2/STAT3 signaling in non-neuronal cells. The α7 nAChR triggers activation of its catalytic intracellular domain leading to recruitment and phosphorylation of tyrosine kinase Jak2, and subsequent activation of the transcription factor STAT3 (De Jonge et al., 2005; Arredondo et al., 2006). Activation of STAT3 signaling is crucial for the anti-inflammatory potency of nicotine, as nicotine fails to reduce TNF production in cells that express STAT3 mutated in the phosphorylation domain, or the DNA-binding domain (De Jonge et al., 2005). Accordingly, vagal nerve stimulation inhibits intestinal inflammation in wild-type mice, but failed to do so in mice devoid of STAT3 in their macrophages (LysM-STAT3fl/fl) (De Jonge et al., 2005). Hence, the anti-inflammatory activity of vagus nerve activity involves Jak2/STAT3 signaling. Indeed, STAT3 is generally recognized as an anti-inflammatory transcription factor (Takeda et al., 1999; Levy and Lee, 2002) contributing to the anti-inflammatory effects of IL-6 and IL-10 (Takeda et al., 1999; Levy and Lee, 2002; Williams et al., 2004). However, STAT3 seems to exert its anti-inflammatory action indirectly, and does not directly inhibit the transcription of pro-inflammatory genes such as TNF, IL-6 and iNOS (Murray, 2005).
Most likely, nAChR activation interferes with other signaling pathways and transcription factors via activation of Jak/STAT signaling, such as NF-κB or TGF-β (Ulloa et al., 1999; Jenkins et al., 2005). Under physiological conditions, immune cells are generally regulated by the action of many cytokines and it has become clear that crosstalk between different cytokine-signaling pathways is involved in the regulation of the Jak/STAT pathway. It should be mentioned that the NF-κB and the STAT3 pathway may converge and cooperate to relay inflammatory signaling, as the NF-κB p65 homodimer recruits and associates with STAT3 upon activation (Yu et al., 2002; Yoshida et al., 2004; Hoentjen et al., 2005).
In addition to the Jak/STAT and NF-κB pathways, cholinergic agonists can modulate several other signaling pathways such as extracellular signal-regulated kinases (ERK; p44/p42 MAPK) (Arredondo et al., 2006; Hamano et al., 2006). These serine/threonine terminal protein kinases are activated downstream of a variety of transmembrane receptors and are part of the MAPK. Recent studies also indicate that nicotine induces PGE2 production through the upregulation of cyclooxygenase (COX)-2 expression in human monocytes (Heeschen et al., 2001; Takahashi et al., 2006). PGE2 elicits an increase in cyclic AMP levels and protein kinase A (PKA) activity. Consistent with these results, COX-2- and PKA inhibitors prevent the effects of nicotine on adhesion molecule expression and cytokine production, indicating that the mechanism of action of nicotine may be via endogenous PGE2 production (Heeschen et al., 2001; Takahashi et al., 2006). In conclusion, α7 nAChR activation triggers a spectrum of signaling mechanisms that in inflammatory cells, generally, are anti-inflammatory directly or indirectly leading to inhibited NF-κB activation and/or Jak/STAT signal transduction.
Pharmacological implications for nicotinic agonists
Nicotine is an alkaloid found in the nightshade family of plants (Solanaceae), predominantly in tobacco, and in lower quantities in tomato, potato, eggplant and green pepper. Nicotine is named after the tobacco plant Nicotiana tabacum, which in turn was named after Jean Nicot, a French ambassador in Portugal. In 1560, after treating the Queen of France, Catherine de Medici, for her migraine headaches, Nicot wrote of tobacco's therapeutic properties in cancer, healing and inflammation. Currently, the primary therapeutic use of nicotine is in treating smoking-induced addiction. The original characterization of nAChRs in neurons prompted the study of nicotine and its metabolites in a wide diversity of neurological disorders (depression, Tourette's syndrome, autosomal dominant frontal lobe epilepsy, attention-deficit hyperactivity disorder, Parkinson's disease, and Alzheimer's disease). Likewise, the recent characterization of nicotinic receptors in immune cells evoked the study of nicotinic agonists and their pharmacological and clinical implications in infectious and inflammatory disorders.
To date, chronic obstructive pulmonary disease (COPD) is probably one of the most significant clinical implications of nicotine in infectious and inflammatory disorders. COPD is one of the commonest causes of morbidity and mortality in the world and smoking cessation is the only strategy that reduces this decline in lung function (Barnes, 2003). The mainstay of treatment of COPD is bronchodilators for symptom relief and inhaled anticholinergics and β2-agonists are useful by reducing hyperinflation of the lungs. By contrast, inhaled corticosteroids are poorly effective and do not reduce disease profession (Barnes, 2003).These effects are in agreement with those studies suggesting that chronic exposure to nicotine provides a sustained anti-inflammatory potential that favors lung infections, such as pneumonia caused by S. pneumoniae, are more frequent in smokers (Nuorti et al., 2000). Likewise, one of the most compelling evidence supporting the therapeutic anti-inflammatory potential of nicotine is epidemiological studies of smokers in ulcerative colitis, a characteristic inflammatory bowel disorder that often results in colon cancer. Ulcerative colitis is a continuous idiopathic inflammation of the colonic or rectal mucosa that is normally associated with loss of tolerance to indigenous enteric flora, abnormal humoral and cell-mediated intestinal immunity, and/or generalized enhanced reactivity against intestinal bacterial antigens. About 90% of the victims of ulcerative colitis are nonsmokers. Among sectors, the rates of ulcerative colitis are five times higher among nonsmoking Mormons than in the general population. Patients with a history of smoking acquire their disease after they have stopped smoking (Pullan et al., 1994; Rubin and Hanauer, 2000; Ingram et al., 2005; Thomas et al., 2005). Patients who smoke intermittently often experience improvement in their colitis symptoms during the periods when smoking (Pullan et al., 1994; Mandavilli, 2004; Van Assche et al., 2005). In ex-smokers, onset is nearly always after quitting smoking. Smoking appears to have a protective effect against the development of this disease and also reduces its severity. This is important because the earlier it occurs, and the more extensive it is, the greater is the risk of future colorectal cancer. Colorectal cancer risk in ulcerative colitis is equal in men and women. But women and men with ulcerative colitis have respectively a 9.5 and 45.5 times greater risk of liver and gallbladder cancer, and 31 times the risk of primary sclerosing cholangitis, which are not influenced by medical or surgical treatment of ulcerative colitis. Overall cancer risk in patients with ulcerative colitis is 1.8 times that of the general population.
Following this reasoning and given the positive result of nicotine treatment in experimental models of DSS colitis (Ghia et al., 2006), nicotine treatment might well be beneficial in ulcerative colitis. However, the clinical trials using nicotine for the treatment of ulcerative colitis have provided different results. A recent clinical trial includes the treatment of 104 patients with active UC with either 6 mg nicotine enemas or placebo enemas for 6 weeks in a randomized double-blind study (Ingram et al., 2005). The enemas were well tolerated, but were not found to be efficacious for active ulcerative colitis. This study indicates that Clinical remission was achieved in 14 of 52 (27%) patients on active treatment and 14 of 43 (33%) patients on placebo (P=0.55). In 47 patients taking mesalamine only, active treatment conferred benefit that was not statistically significant; disease remission occurred in 9 of 25 patients on active therapy and 4 of 21 patients on placebo (P=0.20). Other studies indicated that the addition of transdermal nicotine to conventional therapy improves significantly the symptoms in patients with ulcerative colitis (Pullan et al., 1994; Van Assche et al., 2005). Seventy-seven patients diagnosed with active ulcerative colitis were treated with either transdermal nicotine or placebo patches for 6 weeks in a randomized, double-blind study (Pullan et al., 1994; Van Assche et al., 2005). The patients in the nicotine group had a statistically significant greater improvement in the histological and global clinical score of colitis, including lower abdominal pain, stool frequency and fecal urgency. Seventeen of the 30 patients that finished the nicotine treatment had complete remissions, as compared with 9 of the 37 patients in the placebo group. Since the trial included patients who had never smoked, the tolerance and pharmacokinetics of transdermal nicotine was first studied in normal subjects who were lifelong nonsmokers. The nicotine doses were increased in a stepwise manner over a period of 5 days to minimize side effects. Three out of 12 nonsmoker subjects were intolerant to nicotine, but the remaining 9 tolerated doses of 15 and 25 mg/day. Most patients with ulcerative colitis also tolerated similar doses of nicotine given in stepwise increments, although many had side effects. Five out of 35 patients in the nicotine group withdrew treatment because of intolerable side effects. The most common side effects were similar in both groups including nausea, lightheadedness, headache, sleep disturbance and dizziness. The occurrence of side effects in the nicotine group was related to the patients' smoking history; they occurred in 10 of the 11 lifelong nonsmokers as compared with 13 of the 24 former smokers. There were no significant correlations between side effects and plasma nicotine or cotinine concentrations. The total mean dose in the nicotine group was 17±6 mg, concentrations that were approximately 35% of the average for smokers. The cutoff point for distinguishing smokers from nonsmokers is 14 ng/ml. On average, each increase of 11 ng/ml in the plasma cotinine concentration reflects a nicotine intake of 1 mg (about one cigarette) in 24 h. During and after the trial, none reported a craving for smoking, suggesting that addiction may depend on the sharp increases in plasma nicotine concentrations that follow smoking, which are unlike the steady release from transdermal patches.
The potential therapeutic effects of nicotine are likely due to the binding to nicotine receptors in the gut immune cells and inhibiting inflammatory mediators like TNF and IL-8 or, alternatively, by changing adherent surface mucus in the colon (Zijlstra et al., 1994; Thomas et al., 2005). However, nicotine may be more effective in reducing the acute neuromotor symptoms of active colitis than in suppressing the underlying abnormality responsible for the relapsing nature of the condition (Thomas et al., 2005). Indeed, a randomized, double-blind clinical trial involving 80 patients with ulcerative colitis in remission indicated that transdermal nicotine alone was no better than placebo in maintaining the remission of ulcerative colitis (Thomas et al., 2005). The clinical and histological scores worsened over the 6 months of the study, and the number and the time course of the relapses were almost identical in the two groups. As compared with previous studies of acute colitis, this study presents several changes that may account for the clinical outcome. Transdermal nicotine was administered alone rather than in addition to a dose of mesalamine. Treatment began with a dose of only 2.5 mg, followed by a very slow buildup to a maintenance dose of 15 mg, instead of 25 mg. The patch was worn only during the day and removed at bedtime rather than being left on for 24 h. Although it is not possible to establish how any of these changes influenced the efficacy of treatment, the serum nicotine and cotinine concentrations of these patients were lower than expected. In other studies, the average daily intake of 15 mg of nicotine should produce cotinine concentrations of approximately 120 ng/ml; however, this study recorded concentrations of only 70 ng/ml. If future studies confirm nicotine to be ineffective as a maintenance therapy for ulcerative colitis, but effective for the active disease, then the situation is analogous to that of corticosteroids (Thomas et al., 2005). Although corticosteroids have been shown repeatedly to be effective for acute colitis, they are of little value for the maintenance of clinical remission. Although nicotine doses were increased stepwise over a period of several days, the treatment with nicotine was still associated with side effects including nausea lightheadedness, headache, sleep disturbance and dizziness. These studies suggested that, in contrast to the outcome of epidemiological studies of smokers suffering from colitis, nicotine administration may not lead to better therapeutic ratios as compared to the treatment with corticosteroids, presumably due to side effects.
The use of specific α7 nicotinic agonists is expected to bear potential as a maintenance therapy for active ulcerative disease. Such selective nicotinic agonists were originally designed to mimic the cognitive effects of nicotine in patients with neurological disorders while avoiding the toxicity of nicotine. The most characterized specific α7 nAChR-agonists are GTS21 (3-[(2,4-dimethoxy)benzylidene]-anabaseine), 4OHGTS (3-(4-hydroxy,2-methoxybenzylidene) anabaseine), ARR17779 ((-)-spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidin-2'-one]), CAP55, Exo2 (exo-2-(2-pyridyl)-7-azabicyclo[2.2.1] heptane) and PNU-282987 ([N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride]) (Ulloa, 2005). Among these, the most characterized is GTS-21, a partial α7 nAChR-agonist designed to compensate for loss of this receptor in the cerebral cortex and hippocampus of patients with Alzheimer's disease (Figure 4). In vitro, GTS-21 can protect neurons against damage induced by amyloid peptides, suggesting that α7 nAChRs may have a protective neuronal role. GTS-21 is a partial agonist that affects other nicotinic receptors including α4β2-nAChRs (Kitagawa et al., 2003; Ulloa, 2005). Treatment with GTS-21 produced beneficial effects in psychological and cognitive tests when given to healthy volunteers in clinical trials conducted by Taiho Pharmaceutical Co., Tokyo, Japan. However, GTS21 produced a limited therapeutic effect in clinical trials with Alzheimer's disease. Although the reasons for this effect remain unknown, some authors suggested that GTS21 may have limited capacity to cross the blood-brain-barrier. From an immunological perspective, this characteristic is an advantage to avoid potential secondary effects of GTS21 in the CNS. In contrast to nicotine, GTS21 has no effect on locomotor activity in mice or on dopamine turnover in rats indicating that it is less toxic than nicotine (Meyer et al., 1998; Kitagawa et al., 2003; Ulloa, 2005). Unlike trials using nicotine, patients tolerated doses of up to 450 mg/day of GTS21 well, and there were no clinically significant differences in adverse events between the treatment groups. Recent proof-of-concept trials indicated that GTS21 appears to have positive effects on neurocognition in persons with schizophrenia (Martin et al., 2004; Olincy et al., 2006). Future studies are needed to determine whether the cognitive potential of nicotinic agonists are based on their binding to neuronal receptors or whether their anti-inflammatory contribute to their therapeutic potential in neurological disorders.
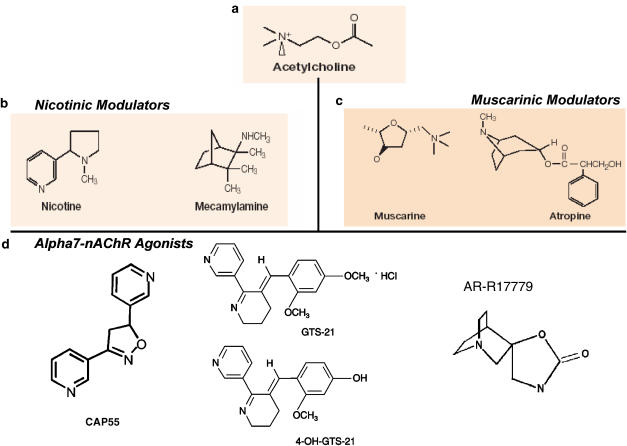
Figure 4.
Chemical structure of selective cholinergic agonists and antagonists. Nicotinic agonists specifically targeting the α7 nAChR have been developed. Acetylcholine (a) can signal through acetylcholine signals through either (c) muscarinic (G protein-coupled receptors) or (b) nicotinic (ligand-gated ion channels) receptors. These receptors are characterized by using selective pharmacological agonists and antagonists for nicotinic (nicotine vs mecamylamine) and muscarinic (muscarine vs atropine) receptors. Nicotine, a more selective and stable cholinergic agonist, is more efficient than acetylcholine as an anti-inflammatory pharmacological strategy. (d) The anti-inflammatory potential of nicotine appears to be mediated by the α7 nAChR, and recent studies are currently focused on designing selective α7-agonists as a anti-inflammatory pharmacological strategy for the treatment of infectious and inflammatory disorders.
The anti-inflammatory potential of GTS21 was analyzed in experimental models of pancreatitis (van Westerloo et al., 2006). Surgical vagotomy or pretreatment with mecamylamine resulted in an enhanced severity of pancreatitis, induced by 12-h intraperitoneal injections of cerulean in mice. The number of neutrophils migrating to the pancreas was increased in these mice, as shown by myeloperoxidase content and intrapancreatic staining of neutrophils. Conversely, GTS-21 pretreatment strongly decreased the severity of pancreatitis. Of note, pancreatitis-associated pulmonary inflammation was independent of the integrity of the vagus nerve and nicotinic receptors. These results are in agreement with other studies indicating the vagus nerve has a very limited anti-inflammatory potential in the lungs (Borovikova et al., 2000).
Other nicotinic agonists used in experimental models of inflammation include CAP55, which was used to study the potential anti-inflammatory potential of nicotinic agonists in endothelial cells (Saeed et al., 2005). CAP55 inhibited the LPS-induced production of TNF and TNF-induced production of chemokines in cultures of both human macrophages differentiated from peripheral blood mononuclear cells and microvascular endothelial cells (HUMVEC). In vitro, nicotine and CAP55 blocked TNF-induced adhesion molecule expression in HUMVEC and prevented monocyte and neutrophil adhesion to these cells. In the experimental carrageenan air pouch model, both vagus nerve stimulation and nicotinic agonists significantly blocked leukocyte migration through a mechanism dependent on nAChRs and inhibited by mecamylamine (Martin et al., 2004; Saeed et al., 2005). These results are in agreement with previous studies indicating that nicotine enhances the angiogenic response to inflammation. Nicotine increased endothelial-cell growth and tube formation in vitro, and accelerated fibrovascular growth in vivo. In mouse models of hind-limb ischemia, lung cancer and atherosclerosis, nicotine increased capillary and lesion growth in association with an increase in lesion vascularity. Increased vascularization of the tumor tissue was associated with a higher tumor growth in the nicotine group. Treatment with nicotine caused higher serum levels of VEGF and the angiogenic effect was abrogated with a COX-2 inhibitor (Heeschen et al., 2001). Although these studies indicated that nicotinic agonists can target endothelial cells in vitro, it is uncertain how the vagus nerve modulates the air pouch in the foot. According to the recent implication of the spleen in the anti-inflammatory potential of the vagus nerve (Huston et al., 2006), future studies are needed to determine whether the inhibition of the leukocyte migration in the carrageenan air pouch mode is due to the effect of vagus nerve on the foot or leukocyte differentiation in the spleen.
The therapeutic anti-inflammatory potential of nicotine in ulcerative colitis is not observed in other inflammatory bowel disorders. Treatment with nicotine induces no therapeutic effect on patients with Crohn's disease. Unlike ulcerative colitis, Crohn's disease is an inflammatory bowel disorder that is responsive to antibiotics such as Metronidazole and anti-TNF therapy. Obviously, these two inflammatory disorders have differential pathogenesis and a differential involvement of macrophage function. An alternative explanation for the disparate effect of nicotine on ulcerative colitis and Crohn's disease is that they might represent different levels of the cholinergic anti-inflammatory mechanism. Ulcerative colitis might be characterized by a defect in acetylcholine production that can be reversed by the administration of nicotine. However, Crohn's disease might be characterized by defects in the nicotinic receptor or signaling pathway that interfere with the potential of intestinal immune cells to respond to nicotine or acetylcholine. It is noteworthy that a similar mechanism might apply to different arthritic diseases. Smoking appears to confer some protection against osteoarthritis, but increases the risk of rheumatoid arthritis (Papadopoulos et al., 2005). If specific mutations in the nAChR may prevent the activation of these receptors by acetylcholine or nicotine, future studies will be needed to determine the potential effect of allosteric enhancers, specific agonists able activate these receptors without binding to the ACh-binding site (Martin et al., 2004; Akk and Steinbach, 2005). The most characteristic examples are physostigmine and galantamine (Reminyl; Janssen Pharmaceutica, Titusville, NJ, USA); both belong to a class of acetylcholinesterase inhibitors approved by the Food and Drug Administration for symptomatic treatment of schizophrenia and Alzheimer's disease. Their efficacy rests on enhancement of cholinergic signaling by inhibiting acetylcholinesterase and preventing acetylcholine degradation. However, the specificity of these compounds is questionable and its use has many neurotoxic side effects. Future studies are required to determine the anti-inflammatory potential of these compounds in immune cells and whether their effects are mediated by an allosteric regulation of nAChR.
Concluding remarks and future directions
The parasympathetic nervous system is a critical component of many pharmacological anti-inflammatory strategies against infectious and inflammatory disorders. One can distinguish two different types of anti-inflammatory compounds in which the efficacy may involve vagal nerve signaling. First, a group of compounds that either centrally or peripherally trigger vagal nerve activity including semapimod, CCK, leptin, melanocortin peptides, aspirin and other nonsteroidal anti-inflammatory drugs. This group is consistent with a previously unrecognized role of the vagus nerve in mediating the action of anti-inflammatory drugs and its pharmacological implications for the treatment of infectious and inflammatory disorders. A second group of anti-inflammatory compounds encompasses a variety of compounds, similar to acetylcholine and nicotinic agonists that are not dependent on an intact vagus nerve. Similar to the vagus nerve, specific nicotinic agonists can control systemic inflammation by regulating the production of proinflammatory cytokines in the spleen. However, unlike the vagus nerve, nicotinic agonists can induce an additional systemic effect and target other organs than the spleen. For instance, although vagus nerve has very limited anti-inflammatory potential in the lungs, nicotine induces a significant anti-inflammatory effect in the lung. This effect of nicotine in the lung is especially relevant in smokers. A similar reasoning also applies to the implications of nicotine for the treatment of inflammatory bowel diseases. Nicotine induces a therapeutic anti-inflammatory potential in the gastrointestinal tract that may be useful for the treatment of ulcerative colitis. As described previously, the release of acetylcholine from the vagus nerve in the intestine or the enteric nervous system is expected to inhibit cytokine production by macrophages and alter DC function. Conversely, modification of macrophage function could trigger enhanced reactivity against intestinal bacterial antigens in the gastrointestinal tract.
The study of the expression of specific nicotinic receptors in different cell types and organs, and the development of selective nicotinic agonists is critical to develop novel pharmacological strategies against neurological, infectious and inflammatory disorders. This consideration is especially relevant to avoid side effects of nicotine in other cell types. A characteristic example is based on the inhibition of the NF-κB pathway by nicotine. NF-κB not only contributes to pathological inflammatory responses, but also protects parenchyma cells from cytotoxic reagents. One of the most characteristic examples is that IKKβ- and p65RelA- knockout mice exhibit embryonic death resulting from extensive TNF- mediated fetal hepatocyte apoptosis. Consistently, disruption of the TNF signaling, by removing either TNF or TNF-R1, prevents this hepatocyte apoptosis in Rela−/− mice, allowing embryonic development to proceed through to birth.
In vivo, inhibition of NF-κB after partial hepatectomy results in massive hepatocyte necrosis associated with impaired liver function and decreased survival. Moreover, treatment with anti-HMGB1 antibodies to prevent hepatic injury in response to ischemic insult is associated with enhanced activation of the NF-κB pathway. However, anti-HMGB1 antibodies abrogate the activation of NF-κB in HMGB1-challenged enterocytes and prevent intestinal derangements. In agreement with this reasoning, recent studies indicate that treatment with nicotine prevents systemic inflammation in healthy animals, but worsens the clinical outcome in splenectomized mice, probably by inhibiting the NF-κB in other organs including the liver. This doubly edged sword makes it challenging to predict the clinical outcome of nonspecific inhibition of NF-κB in human inflammatory diseases and injuries. Unless therapy is specifically targeted to specific immune and cytokine-producing cells, inhibition of NF-κB activity may not generate an overall beneficial effect, especially in tissue injuries such as acute lung injury or hepatic injury induced by ischemia/reperfusion. Future studies will have to determine whether α7 nAChR-agonists can control NF-κB activity in specific cytokine-producing cells avoiding collateral effects in other cell types.
Acknowledgments
LU is supported by grants from the Faculty Award Program of the North Shore-LIJ Health System, Department of Surgery at UMDNJ, the US Army Medical Research Command (USAMRMC), and the American Heart Association.WdJ is supported by a Marie Curie grant within the 6th European Community Framework Programme, and by the Dutch Society of Digestive Diseases. Professor Siamon Gordon is gratefully acknowledged for critical reading of the manuscript. Mrs IE Kos-Oosterling (Academic Medical Center, Amsterdam) is gratefully acknowledged for illustrations.
Abbreviations
- CCK
cholecystokinin
- DC
dendritic cell
- HMGB
high-mobility group box
- IL
interleukin
- Jak
janus kinase
- nAChR
nicotinic acetylcholine receptor
- NF-κB
nuclear factor kappa B
- PI3K
phosphatidyl-inositol-3-kinase
- PLC
phospholipase-C
- STAT
signal transducer and activator of transcription
- TNF
tumor necrosis factor
- TGF
transforming growth factor
Conflict of interest
The authors state no conflict of interest.
References
- Abraham E, Anzueto A, Gutierrez G, Tessler S, San PG, Wunderink R, et al. Double-blind randomised controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. vNORASEPT II Study Group. Lancet. 1998;351:929–933. [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation. 2003;107:604–611. doi: 10.1161/01.cir.0000047279.42427.6d. [DOI] [PubMed] [Google Scholar]
- Akk G, Steinbach JH. Galantamine activates muscle-type nicotinic acetylcholine receptors without binding to the acetylcholine-binding site. J Neurosci. 2005;25:1992–2001. doi: 10.1523/JNEUROSCI.4985-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoshiba K, Nagai A, Yasui S, Konno K. Nicotine prolongs neutrophil survival by suppressing apoptosis. J Lab Clin Med. 1996;127:186–194. doi: 10.1016/s0022-2143(96)90077-3. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- Berg DK, Conroy WG. Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–523. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Villers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci USA. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet MR, Israel-Assayag E, Daleau P, Beaulieu MJ, Cormier Y. Dimethyphenylpiperazinium, a nicotinic receptor agonist, downregulates inflammation in monocytes/macrophages through PI3 K and PLC chronic activation. Am J Physiol Lung Cell Mol Physiol. 2006;291:L757–L763. doi: 10.1152/ajplung.00409.2005. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, et al. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. doi: 10.1038/nature02753. [DOI] [PubMed] [Google Scholar]
- Boyd RT. The molecular biology of neuronal nicotinic acetylcholine receptors. Crit Rev Toxicol. 1997;27:299–318. doi: 10.3109/10408449709089897. [DOI] [PubMed] [Google Scholar]
- Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, et al. Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases. J Neurosci. 2005;25:9836–9849. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun LM, Patrick JW. Pharmacology of neuronal nicotinic acetylcholine receptor subtypes. Adv Pharmacol. 1997;39:191–220. doi: 10.1016/s1054-3589(08)60072-1. [DOI] [PubMed] [Google Scholar]
- Davies BD, Hoss W, Lin JP, Lionetti F. Evidence for a noncholinergic nicotine receptor on human phagocytic leukocytes. Mol Cell Biochem. 1982;44:23–31. doi: 10.1007/BF00573842. [DOI] [PubMed] [Google Scholar]
- De Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- De SR, Jmone-Cat MA, Carnevale D, Minghetti L. Activation of alpha7 nicotinic acetylcholine receptor by nicotine selectively up-regulates cyclooxygenase-2 and prostaglandin E2 in rat microglial cultures. J Neuroinflammation. 2005;2:4. doi: 10.1186/1742-2094-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosa MJ, Esandi MC, Garelli A, Rayes D, Bouzat C. Relationship between alpha 7nAChR and apoptosis in human lymphocytes. J Neuroimmunol. 2005;160:154–161. doi: 10.1016/j.jneuroim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Downing JE, Miyan JA. Neural immunoregulation: emerging roles for nerves in immune homeostasis and disease. Immunol Today. 2000;21:281–289. doi: 10.1016/s0167-5699(00)01635-2. [DOI] [PubMed] [Google Scholar]
- Driessler F, Venstrom K, Sabat R, Asadullah K, Schottelius AJ. Molecular mechanisms of interleukin-10-mediated inhibition of NF-kappaB activity: a role for p50. Clin Exp Immunol. 2004;135:64–73. doi: 10.1111/j.1365-2249.2004.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Neuronal alpha-bungarotoxin receptors are alpha7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- Fujii T, Tajima S, Yamada S, Watanabe Y, Sato KZ, Matsui M, et al. Constitutive expression of mRNA for the same choline acetyltransferase as that in the nervous system, an acetylcholine-synthesizing enzyme, in human leukemic T-cell lines. Neurosci Lett. 1999;259:71–74. doi: 10.1016/s0304-3940(98)00921-5. [DOI] [PubMed] [Google Scholar]
- Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS J. 2005;7:E885–E894. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi JL, Bertrand D, Villers-Thiery A, Revah F, Bertrand S, Changeux JP. Functional significance of aromatic amino acids from three peptide loops of the alpha 7 neuronal nicotinic receptor site investigated by site-directed mutagenesis. FEBS Lett. 1991;294:198–202. doi: 10.1016/0014-5793(91)80668-s. [DOI] [PubMed] [Google Scholar]
- Gault J, Hopkins J, Berger R, Drebing C, Logel J, Walton C, et al. Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am J Med Genet B Neuropsychiatr Genet. 2003;123:39–49. doi: 10.1002/ajmg.b.20061. [DOI] [PubMed] [Google Scholar]
- Gault J, Robinson M, Berger R, Drebing C, Logel J, Hopkins J, et al. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7) Genomics. 1998;52:173–185. doi: 10.1006/geno.1998.5363. [DOI] [PubMed] [Google Scholar]
- Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Green GM, Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967;276:421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- Guinet E, Yoshida K, Nouri-Shirazi M. Nicotinic environment affects the differentiation and functional maturation of monocytes derived dendritic cells (DCs) Immunol Lett. 2004;95:45–55. doi: 10.1016/j.imlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Hamano R, Takahashi HK, Iwagaki H, Yoshino T, Nishibori M, Tanaka N. Stimulation of alpha7 nicotinic acetylcholine receptor inhibits CD14 and the toll-like receptor 4 expression in human monocytes. Shock. 2006;26:358–364. doi: 10.1097/01.shk.0000228168.86845.60. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Jang JJ, Weis M, Pathak A, Kaji S, Hu RS, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E. Modulation of beta-amyloid precursor protein processing and tau phosphorylation by acetylcholine receptors. Eur J Pharmacol. 2000;393:255–263. doi: 10.1016/s0014-2999(00)00028-5. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Mousavi M, Ravid R, Nordberg A. Reduced levels of Abeta 40 and Abeta 42 in brains of smoking controls and Alzheimer's patients. Neurobiol Dis. 2004;15:351–360. doi: 10.1016/j.nbd.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Hoentjen F, Sartor RB, Ozaki M, Jobin C. STAT3 regulates NF-kappaB recruitment to the IL-12p40 promoter in dendritic cells. Blood. 2005;105:689–696. doi: 10.1182/blood-2004-04-1309. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iho S, Tanaka Y, Takauji R, Kobayashi C, Muramatsu I, Iwasaki H, et al. Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-kappaB. J Leukoc Biol. 2003;74:942–951. doi: 10.1189/jlb.1202626. [DOI] [PubMed] [Google Scholar]
- Ingram JR, Thomas GA, Rhodes J, Green JT, Hawkes ND, Swift JL, et al. A randomized trial of nicotine enemas for active ulcerative colitis. Clin Gastroenterol Hepatol. 2005;3:1107–1114. doi: 10.1016/s1542-3565(05)00849-9. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Grail D, Nheu T, Najdovska M, Wang B, Waring P, et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-beta signaling. Nat Med. 2005;11:845–852. doi: 10.1038/nm1282. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sci. 2003;74:675–696. doi: 10.1016/j.lfs.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Front Biosci. 2004;9:2063–2085. doi: 10.2741/1390. [DOI] [PubMed] [Google Scholar]
- Khiroug SS, Harkness PC, Lamb PW, Sudweeks SN, Khiroug L, Millar NS, et al. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J Physiol. 2002;540:425–434. doi: 10.1113/jphysiol.2001.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- Levy DE, Lee CK. What does Stat3 do. J Clin Invest. 2002;109:1143–1148. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd GK, Williams M. Neuronal nicotinic acetylcholine receptors as novel drug targets. J Pharmacol Exp Ther. 2000;292:461–467. [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandavilli A. Nicotine fix. Nat Med. 2004;10:660–661. doi: 10.1038/nm0704-660. [DOI] [PubMed] [Google Scholar]
- Marrero MB, Papke RL, Bhatti BS, Shaw S, Bencherif M. The neuroprotective effect of 2-(3-pyridyl)-1-azabicyclo[3.2.2]nonane (TC-1698), a novel alpha7 ligand, is prevented through angiotensin II activation of a tyrosine phosphatase. J Pharmacol Exp Ther. 2004;309:16–27. doi: 10.1124/jpet.103.061655. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Klein TW, Friedman H, Yamamoto Y. Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J Immunol. 2001;167:6518–6524. doi: 10.4049/jimmunol.167.11.6518. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-Methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. J Pharmacol Exp Ther. 1998;287:918–925. [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Brenner T. Anti-inflammatory properties of cholinergic up-regulation: a new role for acetylcholinesterase inhibitors. Neuropharmacology. 2006;50:540–547. doi: 10.1016/j.neuropharm.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuorti JP, Butler JC, Farley MM, Harrison LH, McGeer A, Kolczak MS, et al. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N Engl J Med. 2000;342:681–689. doi: 10.1056/NEJM200003093421002. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Ortega E, Barriga C, Rodriguez AB. Decline in the phagocytic function of alveolar macrophages from mice exposed to cigarette smoke. Comp Immunol Microbiol Infect Dis. 1994;17:77–84. doi: 10.1016/0147-9571(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Papadopoulos NG, Alamanos Y, Voulgari PV, Epagelis EK, Tsifetaki N, Drosos AA. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients. Clin Exp Rheumatol. 2005;23:861–866. [PubMed] [Google Scholar]
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- Rakhilin S, Drisdel RC, Sagher D, McGehee DS, Vallejo Y, Green WN. alpha-bungarotoxin receptors contain alpha7 subunits in two different disulfide-bonded conformations. J Cell Biol. 1999;146:203–218. doi: 10.1083/jcb.146.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, et al. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J Neurosci. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice TW, Bernard GR. Therapeutic intervention and targets for sepsis. Annu Rev Med. 2005;56:225–248. doi: 10.1146/annurev.med.56.082103.104356. [DOI] [PubMed] [Google Scholar]
- Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol. 1998;81:31–37. doi: 10.1016/s0165-5728(97)00155-0. [DOI] [PubMed] [Google Scholar]
- Rioux N, Castonguay A. The induction of cyclooxygenase-1 by a tobacco carcinogen in U937 human macrophages is correlated to the activation of NF-kappaB. Carcinogenesis. 2000;21:1745–1751. doi: 10.1093/carcin/21.9.1745. [DOI] [PubMed] [Google Scholar]
- Rubin DT, Hanauer SB. Smoking and inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2000;12:855–862. doi: 10.1097/00042737-200012080-00004. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P, Van AG, Vermeire S. Review article: infliximab therapy for inflammatory bowel disease – seven years on. Aliment Pharmacol Ther. 2006;23:451–463. doi: 10.1111/j.1365-2036.2006.02786.x. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng-Nemeroff T, Sherry B, Balakhaneh D, Huston J, et al. Cholinergic stimulation blocks endothelial cell activation and leukocyte recruitment during inflammation. J Exp Med. 2005;201:1113–1123. doi: 10.1084/jem.20040463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti PJ. The innate signaling of dangers and the dangers of innate signaling. Nat Immunol. 2006;7:1237–1242. doi: 10.1038/ni1420. [DOI] [PubMed] [Google Scholar]
- Sato KZ, Fujii T, Watanabe Y, Yamada S, Ando T, Kazuko F, et al. Diversity of mRNA expression for muscarinic acetylcholine receptor subtypes and neuronal nicotinic acetylcholine receptor subunits in human mononuclear leukocytes and leukemic cell lines. Neurosci Lett. 1999;266:17–20. doi: 10.1016/s0304-3940(99)00259-1. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Schwartz SL. Interaction of nicotine and other amines with the endocytic and exocytic functions of macrophages. Fed Proc. 1976;35:85–88. [PubMed] [Google Scholar]
- Schwartz SL, Evans DE, Lundin JE, Bond JC. Inhibition of pinocytosis by nicotine. J Pharmacol Exp Ther. 1972;183:370–377. [PubMed] [Google Scholar]
- Severance EG, Zhang H, Cruz Y, Pakhlevaniants S, Hadley SH, Amin J, et al. The alpha7 nicotinic acetylcholine receptor subunit exists in two isoforms that contribute to functional ligand-gated ion channels. Mol Pharmacol. 2004;66:420–429. doi: 10.1124/mol.104.000059. [DOI] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem. 2002;277:44920–44924. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB. Angiotensin II blocks nicotine-mediated neuroprotection against beta-amyloid (1-42) via activation of the tyrosine phosphatase SHP-1. J Neurosci. 2003;23:11224–11228. doi: 10.1523/JNEUROSCI.23-35-11224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Skok M, Grailhe R, Agenes F, Changeux JP. The role of nicotinic acetylcholine receptors in lymphocyte development. J Neuroimmunol. 2006;171:86–98. doi: 10.1016/j.jneuroim.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Skok M, Grailhe R, Changeux JP. Nicotinic receptors regulate B lymphocyte activation and immune response. Eur J Pharmacol. 2005;517:246–251. doi: 10.1016/j.ejphar.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- Sopori ML, Kozak W, Savage SM, Geng Y, Soszynski D, Kluger MJ, et al. Effect of nicotine on the immune system: possible regulation of immune responses by central and peripheral mechanisms. Psychoneuroendocrinology. 1998;23:189–204. doi: 10.1016/s0306-4530(97)00076-0. [DOI] [PubMed] [Google Scholar]
- Sorensen LT, Nielsen HB, Kharazmi A, Gottrup F. Effect of smoking and abstention on oxidative burst and reactivity of neutrophils and monocytes. Surgery. 2004;136:1047–1053. doi: 10.1016/j.surg.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Speer P, Zhang Y, Gu Y, Lucas MJ, Wang Y. Effects of nicotine on intercellular adhesion molecule expression in endothelial cells and integrin expression in neutrophils in vitro. Am J Obstet Gynecol. 2002;186:551–556. doi: 10.1067/mob.2002.121106. [DOI] [PubMed] [Google Scholar]
- Stead RH, Colley EC, Wang B, Partosoedarso E, Lin J, Stanisz A, et al. Vagal influences over mast cells. Auton Neurosci. 2006;125:53–61. doi: 10.1016/j.autneu.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- Sugano N, Shimada K, Ito K, Murai S. Nicotine inhibits the production of inflammatory mediators in U937 cells through modulation of nuclear factor-kappaB activation. Biochem Biophys Res Commun. 1998;252:25–28. doi: 10.1006/bbrc.1998.9599. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Hide I, Matsubara A, Hama C, Harada K, Miyano K, et al. Microglial alpha7 nicotinic acetylcholine receptors drive a phospholipase C/IP3 pathway and modulate the cell activation toward a neuroprotective role. J Neurosci Res. 2006;83:1461–1470. doi: 10.1002/jnr.20850. [DOI] [PubMed] [Google Scholar]
- Takahashi HK, Iwagaki H, Hamano R, Yoshino T, Tanaka N, Nishibori M. alpha7 Nicotinic Acetylcholine Receptor Stimulation Inhibits Lipopolysaccharide-Induced Interleukin-18 and-12 Production in Monocytes. J Pharmacol Sci. 2006;102:143–146. doi: 10.1254/jphs.sc0060074. [DOI] [PubMed] [Google Scholar]
- Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- Theodorou V, Fioramonti J, Bueno L. Integrative neuroimmunology of the digestive tract. Vet Res. 1996;27:427–442. [PubMed] [Google Scholar]
- Thomas GA, Rhodes J, Ingram JR. Mechanisms of disease: nicotine-a review of its actions in the context of gastrointestinal disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:536–544. doi: 10.1038/ncpgasthep0316. [DOI] [PubMed] [Google Scholar]
- Thyberg J, Hedin U, Stenseth K, Nilsson J. Effects of nicotine on the fine structure of cultivated mouse peritoneal macrophages. Acta Pathol Microbiol Immunol Scand [A] 1983;91:23–30. doi: 10.1111/j.1699-0463.1983.tb02722.x. [DOI] [PubMed] [Google Scholar]
- Totti N, III, McCusker KT, Campbell EJ, Griffin GL, Senior RM. Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science. 1984;223:169–171. doi: 10.1126/science.6318317. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Tracey KJ. The ‘cytokine profile': a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Van Assche G, Vermeire S, Rutgeerts P. Medical treatment of inflammatory bowel diseases. Curr Opin Gastroenterol. 2005;21:443–447. [PubMed] [Google Scholar]
- van der Poll T, Lowry SF. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense. Shock. 1995;3:1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]
- van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Villiger Y, Szanto I, Jaconi S, Blanchet C, Buisson B, Krause KH, et al. Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J Neuroimmunol. 2002;126:86–98. doi: 10.1016/s0165-5728(02)00057-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1-42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. J Biol Chem. 2000a;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Wang HY, Lee DH, Davis CB, Shank RP. Amyloid peptide Abeta(1-42) binds selectively and with picomolar affinity to alpha7 nicotinic acetylcholine receptors. J Neurochem. 2000b;75:1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation - a continuing puzzle. Immunology. 2004;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Burton B, Urrutia A, Shcherbatko A, Chavez-Noriega LE, Cohen CJ, et al. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J Biol Chem. 2005;280:1257–1263. doi: 10.1074/jbc.M410039200. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Kumar A, Koyama Y, Peng H, Arman A, Boch JA, et al. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique T. J Biol Chem. 2004;279:1768–1776. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]
- Yu Z, Kone BC. The STAT3 DNA-binding domain mediates interaction with NF-kappaB p65 and iuducible nitric oxide synthase transrepression in mesangial cells. J Am Soc Nephrol. 2004;15:585–591. doi: 10.1097/01.asn.0000114556.19556.f9. [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rivest S. Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J Neurochem. 2001;76:855–864. doi: 10.1046/j.1471-4159.2001.00080.x. [DOI] [PubMed] [Google Scholar]
- Zijlstra FJ, Srivastava ED, Rhodes M, van Dijk AP, Fogg F, Samson HJ, et al. Effect of nicotine on rectal mucus and mucosal eicosanoids. Gut. 1994;35:247–251. doi: 10.1136/gut.35.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]