Abstract
Cancer is a disease of aging. The accumulation of mutations in individual cells over a lifetime is thought to be the reason. In this work, we explored an additional hypothesis: could p53 function decline with age, which would contribute to an enhanced mutation frequency and tumorigenesis in the aging process? The efficiency of the p53 response to γ-irradiation was found to decline significantly in various tissues of aging mice from several inbred strains, including lower p53 transcriptional activity and p53-dependent apoptosis. This decline resulted from a decreased stabilization of the p53 protein after stress. The function of the Ataxia-telangiectasia mutated (ATM) kinase declined significantly with age, which may then be responsible for the decline of the p53 response to radiation. Declining p53 responses to other stresses were also observed in the cultured splenocytes from aging mice. Interestingly, the time of onset of this decreased p53 response correlated with the life span of mice; mice that live longer delay their onset of decreased p53 activity with time. These results suggest an enhanced fixation of mutations in older individuals because of the declining fidelity of p53-mediated apoptosis or senescence in response to stress, and they suggest a plausible explanation for the correlation between tumorigenesis and the aging process.
Keywords: apoptosis, Ataxia-telangiectasia mutated (ATM), tumorigenesis
Cancer is largely a disease of the elderly (1). Most tumors arise in the last quarter of life, with the frequency increasing exponentially with time. Mice (2- to 3-year mean life spans) frequently acquire tumors after 1.5–2 years, whereas dogs (12- to 16-year mean life spans) acquire most tumors after 10 years, and humans (75-year mean life spans) acquire tumors with an increased frequency after 50–55 years of age (1–3). This observation has been thought to be caused by the accumulation of DNA mutations in oncogenes or tumor suppressor genes in individual cells over a lifetime (the somatic tissues) (4). This hypothesis is supported by ample evidence from both humans and animal models showing that many tumors contain mutations in crucial tumor suppressor genes and oncogenes. The tumor suppressor p53 gene is the most frequently mutated gene in human tumors, and >50% of tumors harbor mutations in the p53 gene or the p53 pathway (5). The p53 protein can be activated by a wide variety of stress signals and results in various cellular responses, including apoptosis, cell cycle arrest, or senescence through transcriptional regulation of its target genes (5). Disruption of normal p53 function is in some circumstances a prerequisite for the development or progression of tumors. By preventing these tumors early in life, p53 is also an important longevity assurance gene. For example, p53-null mice all succumb to tumors within several months, and heterozygous p53 mutant mice develop tumors over a period of a year or more (6). Li–Fraumeni syndrome patients with heterozygous p53 gene display a 50% cancer incidence by the age of 30 (7).
Aging is a natural process with gradual decline of many normal biological functions of cells or organisms. It has been reported that the function of DNA repair, regulation of cell proliferation, and immune response decrease with age in both animals and humans (8, 9). These findings together raised an interesting hypothesis: could the function of the p53 protein decline with age, which may then contribute to the enhanced mutation frequency and tumorigenesis in the aging process along with the accumulation of DNA mutations? To explore this hypothesis, in this work we investigated the activity of the p53 protein and the efficiency of p53 responses to various stresses in several inbred mouse strains as a function of their ages.
Results
Decreased p53 Transcriptional Activity in Aging Mice.
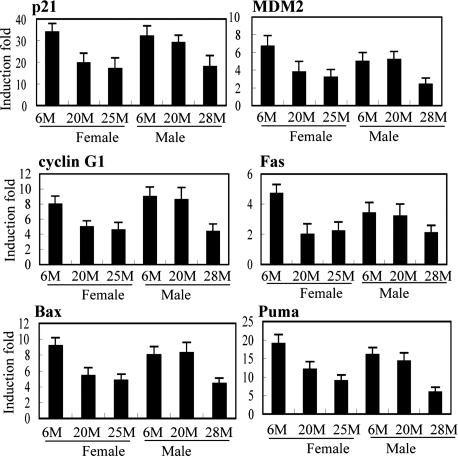
As a transcription factor, the p53 protein regulates the transcription of many target genes (5). To explore whether the p53 function declines with age, the p53 transcriptional activity was tested in young and older mice. Sex-matched C57BL/6 mice of different ages were irradiated with γ-irradiation (IR, 5 Gy) and killed at 6 h after IR to collect different tissues. The mRNA levels of a group of p53 target genes, including p21, Puma, Bax, Fas, MDM2, and cyclin G1, were examined before and after IR by real-time PCR. They are involved in the regulation of cell cycle arrest (p21), apoptosis (Puma, Bax, and Fas), and negative regulation of the p53 pathway (MDM2 and cyclin G1). The induction of these genes by IR was p53-dependent because they were not induced or induced to significantly lesser extents in various tissues of p53−/− mice compared with p53+/+ mice (data not shown). In spleen, which is a radio-sensitive tissue and has a high p53 response to IR, these genes were clearly induced by IR (Fig. 1). Interestingly, the induction folds of these genes were consistently (for six p53-regulated genes) almost two times lower in aging mice (in both 20- or 25-month-old females and 28-month-old males) compared with 6-month-old mice. Similar results were also observed in a wide variety of tissues in addition to spleen, including kidney, thymus, heart, lung, brain, muscle, small intestine, and skin. The induction levels by IR were constantly lower in all of these tissues for p21 and were lower in the majority of tissues for MDM2 and cyclin G1 in older mice, although the degree of difference varied among different tissues (Table 1). These results demonstrate the decline of p53 transcription activity in the aging mice, which is not tissue-specific but rather a general phenomenon. The kinetics of induction for these six genes at different time points (6, 12, and 24 h) after IR was measured. Significantly higher gene inductions in the spleen were observed consistently at all time points after IR in 6-month-old female C57BL/6 mice compared with the older mice (20- or 25-month-old) (data not shown), excluding the possibility that the induction of these p53 target genes was not actually lower but only slower in aging mice.
Fig. 1.
Decreased p53 transcriptional activity in aging mice. Sex-matched C57BL/6 mice of different ages (M, months) were treated with 5 Gy IR, and the mice were killed at 6 h after IR. The mRNA expression of p53 target genes before and after IR in the spleen was detected by real-time PCR. The relative induction fold of each gene was calculated as mRNA expression levels in irradiated mice compared with nonirradiated mice of the same age. All expression levels were normalized to actin. At least five mice were used for each group.
Table 1.
Decreased p53 transcriptional activity in various tissues of aging C57BL/6 female mice
| Tissue | Age, months | Gene |
||
|---|---|---|---|---|
| p21 | MDM2 | Cyclin G1 | ||
| Kidney | 6 | 22.4 ± 1.9 | 2.8 ± 0.4 | 5.8 ± 0.8 |
| 20 | 12.2 ± 4.3 | 1.9 ± 0.7 | 2.7 ± 1.1 | |
| Thymus | 6 | 17.4 ± 1.7 | 2.1 ± 0.3 | 6.2 ± 0.7 |
| 20 | 7.6 ± 2.3 | 1.8 ± 0.6 | 4.0 ± 1.0 | |
| Heart | 6 | 15.1 ± 0.7 | 2.2 ± 0.4 | 1.7 ± 0.3 |
| 20 | 6.0 ± 1.4 | 1.6 ± 0.8 | 1.8 ± 0.6 | |
| Lung | 6 | 10.2 ± 1.8 | 3.5 ± 0.7 | 7.4 ± 1.2 |
| 20 | 6.3 ± 1.6 | 2.4 ± 0.6 | 5.8 ± 1.7 | |
| Brain | 6 | 10.5 ± 1.1 | 1.6 ± 0.3 | 2.1 ± 0.3 |
| 20 | 5.4 ± 0.9 | 1.0 ± 0.2 | 1.3 ± 0.2 | |
| Small intestine | 6 | 4.1 ± 0.7 | 3.2 ± 0.6 | 5.7 ± 0.8 |
| 20 | 2.8 ± 1.2 | 2.2 ± 0.7 | 3.8 ± 1.0 | |
| Muscle | 6 | 9.1 ± 1.8 | 2.2 ± 0.3 | 1.4 ± 0.2 |
| 20 | 6.6 ± 2.0 | 2.1 ± 0.5 | 1.5 ± 0.2 | |
| Skin | 6 | 2.9 ± 0.7 | 2.7 ± 0.8 | 2.4 ± 0.6 |
| 20 | 2.0 ± 0.8 | 1.5 ± 0.7 | 1.4 ± 0.8 | |
Mice were treated with 5 Gy IR and killed at 6 h after IR. The mRNA levels of genes were determined by real-time PCR. All expression levels were normalized to actin. Shown are gene induction folds calculated as gene expression levels in irradiated mice compared with nonirradiated mice. At least five mice were used for each group. Data are presented as mean ± SD.
This decline of p53 transcriptional activity with age was also observed in other inbred mouse strains, including DBA2 and BALB/c. Consistent with the observations in C57BL/6 mice, the induction of these p53 target genes by IR was significantly lower in the spleen of both male and female older mice from both DBA2 and BALB/c strains (Table 2), indicating that this decrease of p53 transcriptional activity is a general phenomenon in aging mice. Together, these data clearly demonstrate that p53 transcriptional activity, or more accurately, the steady-state levels of mRNA, decreased significantly in aging mice.
Table 2.
Decreased p53 transcriptional activity in the spleen of aging BALB/c and DBA2 mice
| Gene | BALB/c |
DBA2 |
||||||
|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|||||
| 6 months | 23 months | 6 months | 24 months | 6 months | 20 months | 6 months | 20 months | |
| p21 | 20.2 ± 3.7 | 12.4 ± 3.6 | 23.0 ± 2.1 | 10.2 ± 3.6 | 61.3 ± 5.1 | 35 ± 6.7 | 42.5 ± 4.9 | 16.0 ± 4.0 |
| MDM2 | 5.2 ± 0.7 | 2.1 ± 1.1 | 7.8 ± 1.0 | 2.5 ± 1.2 | 4.7 ± 0.6 | 3.0 ± 1.3 | 6.2 ± 0.8 | 3.9 ± 1.1 |
| Cyclin G1 | 6.3 ± 1.1 | 3.4 ± 1.0 | 7.3 ± 1.4 | 4.0 ± 1.5 | 8.9 ± 0.7 | 5.2 ± 1.6 | 10.8 ± 1.4 | 6.2 ± 1.8 |
| Puma | 11.6 ± 2.0 | 4.0 ± 1.7 | 12.2 ± 1.2 | 4.1 ± 2.0 | 17.4 ± 1.9 | 10.2 ± 3.2 | 12.9 ± 2.1 | 6.7 ± 2.3 |
| Bax | 4.6 ± 0.9 | 2.1 ± 0.8 | 6.4 ± 1.2 | 3.1 ± 1.8 | 7.8 ± 0.7 | 4.2 ± 1.7 | 6.5 ± 1.0 | 3.7 ± 1.3 |
| Fas | 4.8 ± 0.5 | 1.8 ± 0.7 | 4.6 ± 0.7 | 2.8 ± 0.8 | 5.2 ± 1.0 | 2.2 ± 0.8 | 4.7 ± 0.6 | 2.5 ± 1.0 |
Mice were treated with 5 Gy IR and killed at 6 h after IR. The mRNA levels of genes were determined by real-time PCR. All expression levels were normalized to actin. Shown are gene induction folds calculated as gene expression levels in irradiated mice compared with nonirradiated mice. At least five mice were used for each group. Data are presented as mean ± SD.
These data also clearly show that the onset age of declining p53 transcriptional activity was different between male and female C57BL/6 mice; it decreased at a later point in the life span in males than females. The decrease was observed at 28 months and not at 20 months in males, whereas it was already observed at 20 months in females (Fig. 1). Interestingly, several studies with large mouse populations have reported that C57BL/6 males live longer than females by 2–3 months (10, 11). The C57BL/6 mice used in this work from the National Institute on Aging/National Institutes of Health (Bethesda, MD) have this same sexual dimorphic life span (www.nia.nih.gov/researchinformation/scientificresources/agedrodentcolonieshandbook/strainsurvivalinformation.htm). This finding suggested that the onset age of this decreased p53 transcriptional activity correlates with the life spans of C57BL/6 mice and not just their chronological age: mice that live longer delay their onset of decreased p53 activity with time. A similar observation was made in DBA2 mice, which have a shorter life span than C57BL/6 (≈2 months); the p53 transcriptional activity decreased at an earlier age in DBA2 males (20 months old) than C57BL/6 males (Table 2).
Decreased p53-Dependent Apoptosis in Aging Mice.
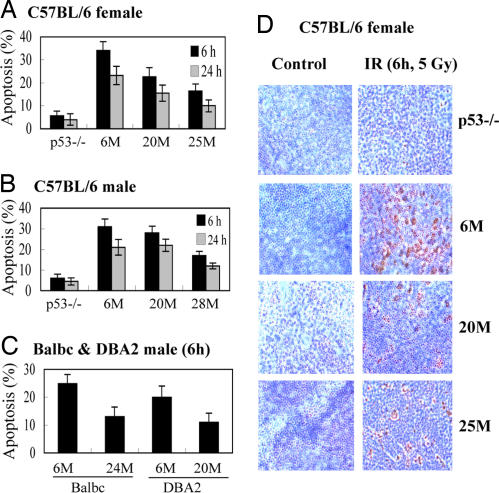
One of the major functions of p53 is to induce apoptosis in response to stress. To investigate whether the p53 function in inducing apoptosis also declines with age, young and aging mice were irradiated (5 Gy); splenocytes were then isolated, and apoptotic cells were measured by using Annexin V staining in a flow cytometer. Splenocytes underwent p53-dependent apoptosis in response to IR in 6-month-old C57BL/6 mice (30–35% and 20–25% apoptotic cells at 6 and 24 h after IR, respectively), and very few cells (<5%) died of apoptosis in p53−/− C57BL/6 mice (Fig. 2A and B). Significantly fewer apoptotic cells were observed in spleen in both 28-month-old male and 20- or 25-month-old female mice than young mice (Fig. 2 A and B). These results were confirmed by a TUNEL assay; the numbers of apoptotic cells at 6 h after IR were 45 ± 6% for 6-month-old, 25 ± 8% for 20-month-old, and 20 ± 8% for 25-month-old female mice, respectively (Fig. 2D). Similar results were observed in the older DBA2 and BALB/c mice (Fig. 2C). These results demonstrate that the p53 function in apoptosis declines with age. A clear difference of apoptosis in response to IR was observed between the 20-month-old male and female C57BL/6 mice (Fig. 2 A and B); whereas females had poorer apoptotic responses compared with young mice, males at 20 months but not 28 months still had an apoptosis response very close to the young ones. These data were consistent with the differences of the p53 transcriptional activity observed between 20-month-old male and female C57BL/6 mice, which further suggested that the onset age of declining p53 function correlates with the life spans of these mice.
Fig. 2.
Decreased p53-dependent cellular apoptosis in aging mice. Sex-matched C57BL/6, BALB/c, and DBA2 mice of different ages (M, months) were treated with 5 Gy IR. The mice were killed, and spleen tissues were collected at 6 and 24 h after IR. (A–C) Splenocytes were isolated, stained with Annexin V, and analyzed in a flow cytometer to detect apoptotic cells. (D) TUNEL assay was used to detect apoptotic cells in the formalin-fixed spleen tissues of C57BL/6 mice.
Decreased p53 Protein Accumulation in Response to IR in Aging Mice.
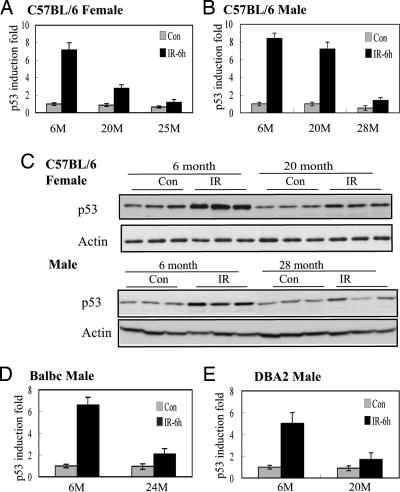
These results demonstrate that both p53-mediated transcription and apoptosis decrease in older mice, and so the efficiency of the p53 response to stress declines with age. To study further the mechanisms of the decreased p53 function in aging mice, the p53 protein levels were measured by ELISA and Western blotting. The ability of p53 protein stabilization in response to IR decreased greatly in older mice; the p53 protein levels were induced by ≈7–8 fold at 6 h after IR in spleen in young C57BL/6 mice but only 2–3 fold in the older mice (Fig. 3 A–C). Similar results were observed in both DBA2 and BALB/c mice (Fig. 3 D and E). Again, these data demonstrated a clear difference in the p53 protein stabilization between male and female C57BL/6 mice at 20 months of age.
Fig. 3.
Decreased p53 protein accumulation after IR in aging mice. Sex-matched C57BL/6, BALB/c, and DBA2 mice of different ages (M, months) were treated with 5 Gy IR and killed at 6 h after IR. ELISA (A, B, D, and E) and Western blotting (C) were used to measure the p53 protein levels in the spleen before (Con) and after IR. The relative induction fold was calculated as p53 protein levels in irradiated mice compared with 6-month-old nonirradiated mice of the same sex.
The p53 basal protein levels stayed at the same level between 6-month-old and 20-month-old C57BL/6 mice and declined (≈40–50%) at the later time points of their life spans, which was observed at 25 months in females and at 28 months in males (Fig. 3 A and B). The basal expression levels of MDM2, a key negative regulator of the p53 protein, showed no significant difference between young and aging mice at the levels of protein (Fig. 4) or mRNA (data not shown). No difference in the basal mRNA levels of either Cop1 or Pirh2, two p53 ubiquitin ligases (12, 13), was observed between young and aging C57BL/6 mice (data not shown). These results indicated that the decline of p53 function in the aging process was not caused by the up-regulation of the basal expression of p53-negative regulators, including MDM2, Cop1, or Pirh2.
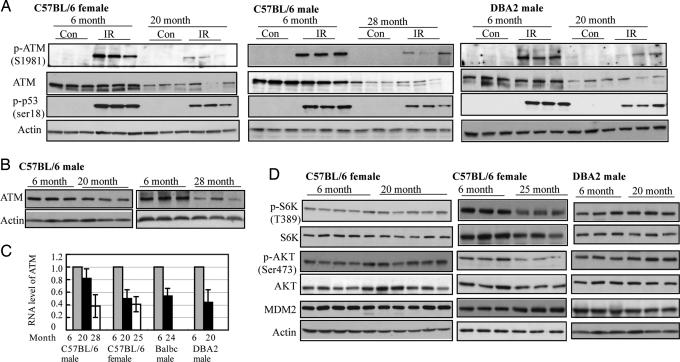
Fig. 4.
Decreased function of the ATM kinase in aging mice. (A) Sex-matched C57BL/6, BALB/c, and DBA2 mice of different ages were treated with 5 Gy IR, and protein levels of different genes in spleen tissues were measured by Western blotting before (Con) and after IR. (B–D) The basal protein levels of different genes (B and D) and the mRNA levels of ATM (C) were measured in spleen tissues in nonirradiated mice by Western blotting and real-time PCR, respectively. The expression levels of ATM were normalized to actin.
Decreased Ataxia-telangiectasia Mutated (ATM) Kinase Expression and Function in Aging Mice.
The ATM kinase is one of the important and most well studied upstream mediators of the p53 response on stress. Double-stranded DNA breaks induced by IR activate ATM by inducing autophosphorylation of the ATM kinase at Ser-1981 (14). The activated ATM kinase phosphorylates MDM2 at Ser-395 and p53 at Ser-15, the initial step of p53 activation in response to IR, which then leads to the stabilization of the p53 protein (15). An ATM functional deficiency in both humans and mice leads to a p53 functional deficiency and delay in the response to IR and also leads to premature aging (16). As shown in Fig. 4 A and B, the total ATM protein levels and the phosphorylation of Ser-1981 after IR, which determines the ATM kinase activity, decreased dramatically in both older male (28-month-old) and female (20-month-old) C57BL/6 mice. Consistent with this observation, the phosphorylation levels of p53 Ser-18 (equivalent to human Ser-15) after IR were significantly lower in older mice. Significantly lower mRNA levels of ATM (50–60% reduction) were detected in older C57BL/6 mice (Fig. 4C). Similar results were observed in both older DBA2 and BALB/c mice (Fig. 4 A and C). These data suggest that decreased ATM function has a significant impact on the efficiency of the p53 response to IR in the aging process. In C57BL/6 mice, consistent with the difference in p53 responses between 20-month-old males and females, the females had significantly lower levels of ATM protein and phosphorylation at Ser-1981 in response to IR compared with the males of the same age (Fig. 4 A and B), which further suggested that the declining ATM function is a major reason why the p53 response to IR declines with age.
The decline of the p53 pathway activity with age is not caused by a general decline of all signal transduction pathways with age. Two other signal transduction pathways, IGF-1-AKT and mTOR, which play roles in regulation of longevity (17) and tumorigenesis and are negatively regulated by p53 under stress conditions (18, 19), maintained the same activities at the same age when the efficiency of the p53 pathway already declined significantly. In 20-month-old female C57BL/6 mice, the levels of p-AKT (Ser-473) and p-S6K (Thr-389), which determine the activities of AKT and S6K, respectively, remained the same as those in 6-month-old mice, and so did the total protein levels of both AKT and S6K (Fig. 4D). Similar results were observed in DBA2 (Fig. 4D) and BALB/c mice. The activities of these two pathways did decrease with age but at much later times in the life spans of mice compared with the ATM and p53 pathway. The total protein levels of AKT and S6K and the levels of p-AKT (Ser-473) and p-S6K (Thr-389) were reduced significantly in 25-month-old female (Fig. 4D) and 28-month-old male C57BL/6 mice (data not shown). Clearly, the different signal transduction pathways that decline functionally with age do so at different rates. This observation tends to eliminate the explanation that all systems in mice decline with age and that this general inefficiency is responsible for the observed decline in p53 activity.
Decreased p53 Responses to Various Stress Signals in Aging Mice.
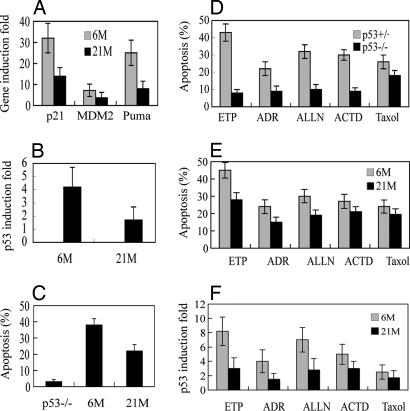
The p53 protein can be activated by various stress signals. The nature of the stress signal shapes the gene expression responses and ultimately the outcome of exposure. Therefore, it was of interest to study in the aging process whether the p53 function was also compromised in response to stress signals other than IR. We first tested whether the cultured splenocytes isolated from mice could be used to mimic the experimental results with p53 responses using whole mouse IR. A clear p53-dependent induction of p53 target genes and apoptosis was observed in IR-treated splenocytes from young wild-type mice (Fig. 5 A–C); no induction or marginal induction (<2-fold) of those genes and significantly fewer (<5%) apoptotic cells were observed in p53−/− splenocytes (data not shown). The p53 function in transcription (Fig. 5A) and apoptosis (Fig. 5C) decreased significantly in the splenocytes from older mice, which correlated well with a significant decrease of the levels of p53 protein accumulation (Fig. 5B). These results are consistent with the experiments carried out in whole mice, indicating that the cultured splenocytes could be used to mimic in vivo experiments on p53 functions. These data strongly suggested that the different efficiency of the p53 response to IR between young and older mice resulted from the different nature of cells but not the different in vivo microenvironment of cells or tissues. This experiment also excluded the possibility that the difference in body size or body fat between young and older mice affects the efficiency of IR.
Fig. 5.
Decreased p53 responses to various stress signals in the cultured splenocytes isolated from aging mice. The splenocytes were isolated from 6-month-old and 21-month-old female C57BL/6 mice and exposed to various stress signals. (A–C) Cells were treated with IR (5 Gy) and collected at 6 h after IR. (A) The mRNA levels were measured by real-time PCR. The gene induction fold was calculated as gene expression levels in irradiated cells compared with nonirradiated cells after normalization with actin. (B) The p53 protein levels were measured by ELISA. (C) Apoptotic cells were detected by Annexin V staining in a flow cytometer. (D–F) Splenocytes were isolated from 2-month-old p53−/− or p53+/+ (D) and 6-month-old or 21-month-old (E and F) female wild-type C57BL/6 mice and exposed to etoposide (ETP, 10 μM for 10 h), adriamycin (ADR, 200 ng/ml for 24 h), ALLN (10 μM for 12 h), actinomycin D (ACTD, 2 nM for 24 h), or Taxol (20 nM for 24 h). Apoptotic cells were detected by Annexin V staining in a flow cytometer (D and E), and the p53 protein levels were measured by ELISA (F). The p53 protein induction fold was calculated as the p53 levels in treated cells compared with untreated cells from the same mice.
Splenocytes were treated with various stress signals to investigate the p53 responses in aging mice. The stress signals included: (i) etoposide and (ii) adriamycin, both of which induce double-stranded DNA breaks as does IR; (iii) low levels of actinomycin D, which blocks ribosomal RNA synthesis; (iv) ALLN, an inhibitor of ubiquitin-mediated degradation by the 26 S proteasome; and (v) Taxol, which disrupts microtubules. The apoptosis induced by each of those stress signals was p53-dependent: a significantly higher percentage of cells underwent apoptosis after stress in p53+/+ splenocytes compared with p53−/− ones isolated from C57BL/6 female mice (2 months old) (Fig. 5D). The number of apoptotic cells (Fig. 5E), the levels of p53 protein accumulation (Fig. 5F) after stress were all significantly lower in the splenocytes isolated from older female C57BL/6 mice compared with young mice. Interestingly, the difference in p53 responses, including apoptosis and p53 protein accumulation, between young and older mice varied for different stress signals. The greatest difference was seen for IR, etoposide, adriamycin, and ALLN, whereas less difference was observed for actinomycin D and Taxol. These data suggest that the extents of decline in the efficiency for different signaling pathways were different in the aging process, which once again demonstrates that the aging process does not act equally on all p53-mediated responses in a cell and that the observations made here are not the result of a general decline in all functions of the mice. The splenocytes were further treated with glucose starvation or AICAR, an AMP kinase activator, to shut down the mTOR pathway (20). The S6K activity (the levels of p-Thr-389 detected by Western blotting) were decreased to similar extents in the splenocytes from both young and older mice (data not shown), which indicated that the mTOR activity in response to nutrition starvation remained normal in 21-month-old mice when the p53 responses to many other p53-mediated stresses were already significantly decreased.
Discussion
Several different theories have been suggested to explain why all animals and plants age with time. The genetic theory proposes that the life span of an organism is programmed by a combination of different allelic forms of many genes (21). It has been possible with worms, flies, and mice to increase their mean life spans by mutations that lower the activity of the IGF pathway (17). These observations link the well characterized role of this network of genes (IGF-1, AKT, and mTOR) with energy metabolism, metabolic rates, and caloric restriction, which also extends the life span of an animal (17, 22). Similarly, increased levels of the Sir-2 orthologs extend longevity and link metabolic processes to genetic control of gene expression patterns (22). If wild strains of Drosophila are bred at a later time during their life span, then that population will have an increased life span (23). Thus, the age of producing offspring can feed back and select populations with alleles that confer increased longevity. Similarly, diseases such as tumors arise in the last quarter of the life spans of many organisms, including mice, dogs, and humans (1). There is little evidence that this observation is the result of the metabolic rates of these animals or even the number of different mutations that must occur in oncogenes or tumor suppressor genes to cause a cancer. Rather, DNA repair processes that impact the mutation rate and apoptotic processes that can eliminate cells with mutations and the immune system, which may help to eliminate or prevent some (viral-induced) tumors, all decline with age especially during the last quarter of an organism's life span (8, 9).
One of the major arguments against a genetic determination of life span is the fact that evolutionary selection ceases to act after the production of offspring, which results in both environmental and internal entropic forces, making the organism less efficient and prone to diseases. Thus, in this theory, stochastic processes determine life span. Each species has a similar function that describes a curve for the life span of a population with only the difference in mean age of death being species-specific. In this case, the strategy used by vertebrates to live longer is for the adult to retain the ability to regenerate many tissues by using a pool of stem cells. Only when this stem cell population declines does an organism age. Mice that express higher than wild-type levels of the p53 protein are more resistant to cancer, but they die at younger ages because of higher rates of apoptosis in these stem cell compartments (24, 25). Mice with too little p53 protein die of cancers at an early age because of a failure to eliminate stem cells that contain mutations (6).
Cancer is thought to be largely a disease of the elderly because it takes time to accumulate a series of mutations in a single cell of the body that then can develop into a clonal tumor. Hundreds of errors have been detected in the genome of these tumor cells, some subset of which presumably cause the tumor (26). This work provides a second reason why cancers arise late in life. The efficiency of the p53 pathway declines with age as a function of the life span of the organism. The p53 pathway ensures the fidelity of events during cell cycle division and responds to stresses that would result in mutations and errors in cell division (5). Loss of the p53 protein results in genomic instability and tumors. After DNA damage (IR) the ATM kinase detects DNA breaks and signals, via phosphorylation, to both p53 and MDM2, which results in lower MDM2 activities and increased p53 levels and activities (14, 15). This activated p53 transcribes a series of genes that lead to cell cycle arrest, senescence, or apoptosis. This work demonstrates that some types of DNA damage are not efficiently detected in older mice because of a lower activity of the ATM kinase that results in less p53 Ser-15 phosphorylation. The transcription of six different genes by p53 all reduced significantly, including genes involved in apoptosis, cell cycle arrest, and senescence, demonstrating the reproducibility of these data. These results provide a mechanism for the reduced apoptosis in cells from older individuals (27), which is a general phenomenon with a wide variety of tissues from several inbred strains of mice displaying the same result and several diverse stress signals all failing to activate p53 functions robustly. Interestingly, among the whole panel of tissues tested, including spleen, heart, lung, thymus, kidney, liver, and skin, spleen of aging C57BL/6 mice showed the most severe reduction in both levels of ATM mRNA expression and p53 protein accumulation in response to IR (data not shown), which could be one of the reasons why splenic lymphomas is the most frequent tumors observed in aging C57BL/6 mice.
This decline is not simply because of entropic forces acting on all signal transduction pathways of the mouse equally. The efficiency of the p53 pathway declines at an earlier age than the IGF-1-AKT-mTOR pathways. Furthermore, the decline in the efficiency of the p53 pathway in C57BL/6 mice is linked to the life span of these mice; males live longer than females, and the decline of the p53 function occurs later in chronological age in males than females. This sexual dimorphism involved in the decline of the p53 function suggests hormonal regulation of this process. In humans, women live longer and develop cancers at an older age than men, suggesting similar links between the age of onset of cancer and life span. The results in this work demonstrate that the efficiency of the p53 pathway declines with age, predicting the increased rates of mutation (caused by a decline in DNA repair) and fixation of mutations (caused by a decline in p53-mediated apoptosis) in older individuals, especially in response to stress. This decline of p53 function could, along with the accumulated mutations over a lifetime, explain why older individuals have a lower rate of fidelity of cell division, a higher error rate, and a higher frequency of tumors.
Materials and Methods
Mouse Strains and IR.
The C57BL/6, BALB/c, and DBA2 mice were purchased from the National Institute on Aging/National Institutes of Health (Bethesda, MD). The age- and sex-matched mice were subjected to 5 Gy of total-body IR with a 137 Cs γ source. Mice were killed at different times after IR, and different tissues were harvested for further experiments. At least five mice were used for each group.
Preparation and Treatment of Splenocytes.
Splenocytes were isolated by teasing apart of spleens between the frosted ends of two ground-glass slides. The erythrocytes were depleted with Tris·NH4Cl solution (17 mM Tris·HCl/0.83% NH4Cl), and cells were cultured in RPMI medium 1640 containing 10% FBS and 5 × 10−3 mM 2-mercaptoethanol. ALLN was purchased from Calbiochem (La Jolla, CA). Actinomycin D, adriamycin, and Taxol were purchased from Sigma (St. Louis, MO). Cells were treated with various doses of these chemicals or IR for various times.
Western Blot Assay and ELISA.
Standard Western blotting was used to analyze protein expression. MDM2 antibody (2A10) was prepared in this laboratory. Anti-p53 (FL393) (sc-6243) and anti-ATM (sc-23921) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-ATM (Ser-1981) (4526), anti-phospho-p53 (Ser-15) (9284), anti-S6K (9202), and anti-phospho-S6K (Thr-389) (9206) were purchased from Cell Signaling (Danvers, MA). Anti-β-actin (A5441) was purchased from Sigma. The mouse total p53 ELISA kit (R&D Systems, Minneapolis, MN) was used to detect the p53 protein levels in mouse tissues according to the manufacturer's instructions.
Quantitative Real-Time PCR.
Total RNA was prepared from cells or mouse tissues with the RNeasy kit (Qiagen, Valencia, CA) and treated with DNase I to remove residual genomic DNA. Real-time PCR was performed in triplicate with Taqman PCR Mix (Applied Biosystems, Foster City, CA) in the 7000 ABI Sequence Detection System. All primers were purchased from Applied Biosystems. The expression of genes was normalized to β-actin gene.
Measurement of Cellular Apoptosis.
To detect apoptotic cells, the splenocytes were stained with Annexin V from a Nexin kit (Guava Technologies, Inc., Hayward, CA) and analyzed in a flow cytometer (Guava Technologies, Inc.) according to the manufacturer's instructions. A TUNEL assay was used to detect apoptotic cells in the spleen tissues fixed in 10% neutral buffered formalin solution for 24 h.
Acknowledgments
This work was supported by grants from the Breast Cancer Research Foundation and National Cancer Institute (CA087497).
Abbreviations
- ATM
Ataxia-telangiectasia mutated
- IR
irradiation.
Footnotes
The authors declare no conflict of interest.
References
- 1.Campisi J. In Vivo. 2000;14:183–188. [PubMed] [Google Scholar]
- 2.Anisimov VN. Exp Gerontol. 2001;36:1101–1136. doi: 10.1016/s0531-5565(01)00114-0. [DOI] [PubMed] [Google Scholar]
- 3.Albert RE, Benjamin SA, Shukla R. Mech Ageing Dev. 1994;74:149–159. doi: 10.1016/0047-6374(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 5.Levine AJ. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 6.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 7.Malkin D, Li FP, Strong LC, Fraumeni JF, Jr, Nelson CE, Kim DH, Kassel J, Gryka MA, Bischoff FZ, Tainsky MA, et al. Science. 1990;250:1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 9.Woodland DL, Blackman MA. Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodrick CL. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- 11.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 12.Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, O'Rourke K, Koeppen H, Dixit VM. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 13.Leng RP, Lin Y, Ma W, Wu H, Lemmers B, Chung S, Parant JM, Lozano G, Hakem R, Benchimol S. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 14.Bakkenist CJ, Kastan MB. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 15.Khosravi R, Maya R, Gottlieb T, Oren M, Shiloh Y, Shkedy D. Proc Natl Acad Sci USA. 1999;96:14973–14977. doi: 10.1073/pnas.96.26.14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA. Nature. 2003;421:643–648. doi: 10.1038/nature01385. [DOI] [PubMed] [Google Scholar]
- 17.Kenyon C. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Feng Z, Zhang H, Levine AJ, Jin S. Proc Natl Acad Sci USA. 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine AJ, Feng Z, Mak TW, You H, Jin S. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 20.Kahn BB, Alquier T, Carling D, Hardie DG. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Geiger-Thornsberry GL, Mackay TF. Mech Ageing Dev. 2004;125:179–189. doi: 10.1016/j.mad.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Guarente L. Mech Ageing Dev. 2005;126:923–928. doi: 10.1016/j.mad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Sgro CM, Partridge L. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 24.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, et al. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 25.Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 27.Camplejohn RS, Gilchrist R, Easton D, McKenzie-Edwards E, Barnes DM, Eccles DM, Ardern-Jones A, Hodgson SV, Duddy PM, Eeles RA. Br J Cancer. 2003;88:487–490. doi: 10.1038/sj.bjc.6600767. [DOI] [PMC free article] [PubMed] [Google Scholar]