Abstract
Mutation and overexpression are the main activating mechanisms for the ras family of genes in human cancer and the variable tandem repeat (VTR) located at the 3' end of H-ras has been associated with this risk. In the present study, we have analysed the relative levels of expression of H-ras mRNA in 26 samples of squamous cell carcinomas of the head and neck (SCCHN) by competitive reverse transcription-polymerase chain reaction (competitive RT-PCR) and also investigated whether there is an association between ras expression and alterations in the 3'-VTR region. In addition, we have studied the incidence of point mutations in codon 12 of H-ras, codons 12 and 13 of K-ras and codon 61 of N-ras in 120 SCCHN samples. Our results indicate that only two samples carry mutations, both of which are located in codon 12 of K-ras, but that overexpression of the H-ras proto-oncogene is a frequent event in SCCHN [54% (14/26)] and is associated with a favourable prognosis: 3 of 14 patients with H-ras overexpression have died, whereas 9 of 12 patients with low levels of H-ras expression have died. We have also undertaken an analysis of these results together with our previous investigations on microsatellite instability and loss of heterozygosity in SCCHN, but no associations were found. We therefore conclude that ras mutations are an infrequent event in the progression of the SCCHN in the Western world, whereas overexpression of the H-ras proto-oncogene is a common event.
Full text
PDF

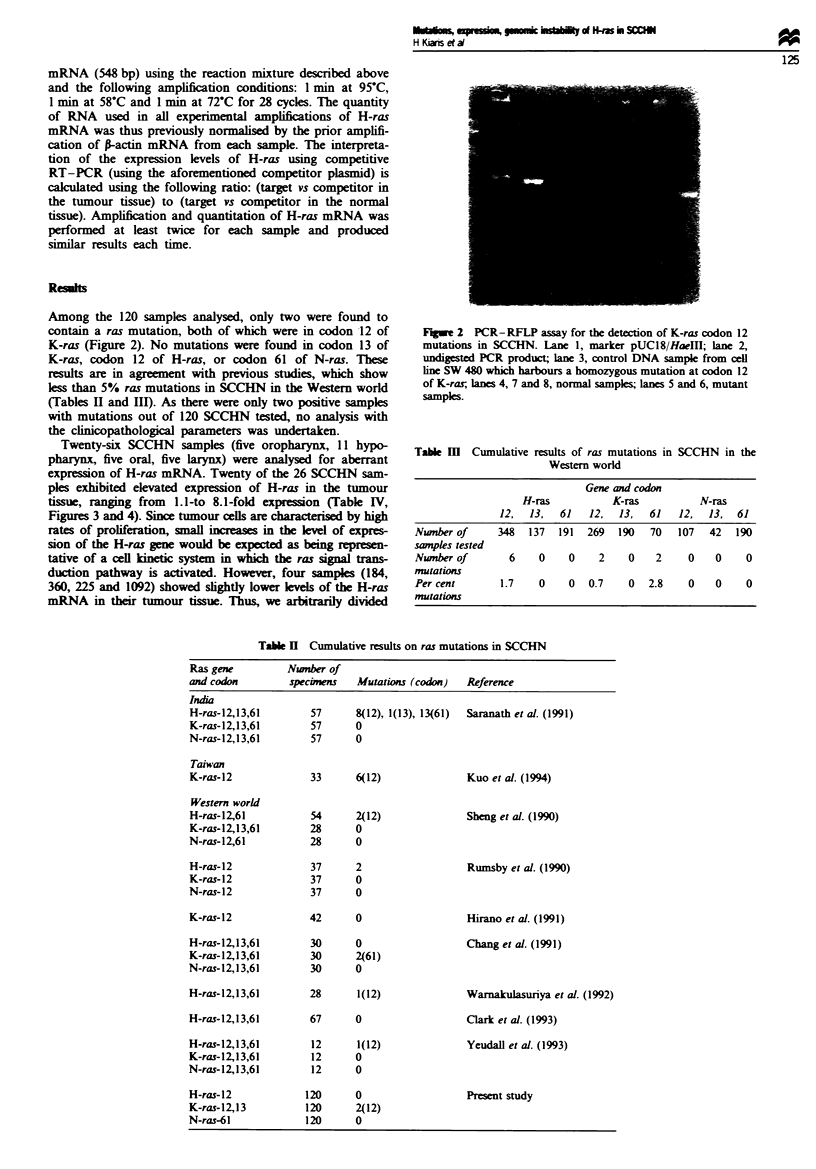



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., McCormick F. Proteins regulating Ras and its relatives. Nature. 1993 Dec 16;366(6456):643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Bhatia P., Johnson N. W., Morgan P. R., McCormick F., Young B., Hiorns L. Ras mutations in United Kingdom examples of oral malignancies are infrequent. Int J Cancer. 1991 May 30;48(3):409–412. doi: 10.1002/ijc.2910480318. [DOI] [PubMed] [Google Scholar]
- Clark L. J., Edington K., Swan I. R., McLay K. A., Newlands W. J., Wills L. C., Young H. A., Johnston P. W., Mitchell R., Robertson G. The absence of Harvey ras mutations during development and progression of squamous cell carcinomas of the head and neck. Br J Cancer. 1993 Sep;68(3):617–620. doi: 10.1038/bjc.1993.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman W. B., Throneburg D. B., Grisham J. W., Smith G. J. Overexpression of c-K-ras, c-N-ras and transforming growth factor beta co-segregate with tumorigenicity in morphologically transformed C3H 10T1/2 cell lines. Carcinogenesis. 1994 May;15(5):1005–1012. doi: 10.1093/carcin/15.5.1005. [DOI] [PubMed] [Google Scholar]
- Field J. K., Lamothe A., Spandidos D. A. Clinical relevance of oncogene expression in head and neck tumours. Anticancer Res. 1986 Jul-Aug;6(4):595–600. [PubMed] [Google Scholar]
- Field J. K. Oncogenes and tumour-suppressor genes in squamous cell carcinoma of the head and neck. Eur J Cancer B Oral Oncol. 1992 Jul;28B(1):67–76. doi: 10.1016/0964-1955(92)90016-t. [DOI] [PubMed] [Google Scholar]
- Field J. K., Spandidos D. A. The role of ras and myc oncogenes in human solid tumours and their relevance in diagnosis and prognosis (review). Anticancer Res. 1990 Jan-Feb;10(1):1–22. [PubMed] [Google Scholar]
- Field J. K., Yiagnisis M., Spandidos D. A., Gosney J. R., Papadimitriou K., Vaughan E. D., Stell P. M. Low levels of ras p21 oncogene expression correlates with clinical outcome in head and neck squamous cell carcinoma. Eur J Surg Oncol. 1992 Apr;18(2):168–176. [PubMed] [Google Scholar]
- Foley K. P., Leonard M. W., Engel J. D. Quantitation of RNA using the polymerase chain reaction. Trends Genet. 1993 Nov;9(11):380–385. doi: 10.1016/0168-9525(93)90137-7. [DOI] [PubMed] [Google Scholar]
- Garcia I., Dietrich P. Y., Aapro M., Vauthier G., Vadas L., Engel E. Genetic alterations of c-myc, c-erbB-2, and c-Ha-ras protooncogenes and clinical associations in human breast carcinomas. Cancer Res. 1989 Dec 1;49(23):6675–6679. [PubMed] [Google Scholar]
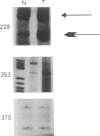
- Green M., Krontiris T. G. Allelic variation of reporter gene activation by the HRAS1 minisatellite. Genomics. 1993 Aug;17(2):429–434. doi: 10.1006/geno.1993.1343. [DOI] [PubMed] [Google Scholar]
- Hirano T., Steele P. E., Gluckman J. L. Low incidence of point mutation at codon 12 of K-ras proto-oncogene in squamous cell carcinoma of the upper aerodigestive tract. Ann Otol Rhinol Laryngol. 1991 Jul;100(7):597–599. doi: 10.1177/000348949110000716. [DOI] [PubMed] [Google Scholar]
- Honkawa H., Masahashi W., Hashimoto S., Hashimoto-Gotoh T. Identification of the principal promoter sequence of the c-H-ras transforming oncogene: deletion analysis of the 5'-flanking region by focus formation assay. Mol Cell Biol. 1987 Aug;7(8):2933–2940. doi: 10.1128/mcb.7.8.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell R. E., Wong F. S., Fenwick R. G. Loss of Harvey ras heterozygosity in oral squamous carcinoma. J Oral Pathol Med. 1989 Feb;18(2):79–83. doi: 10.1111/j.1600-0714.1989.tb00741.x. [DOI] [PubMed] [Google Scholar]
- Krontiris T. G., Devlin B., Karp D. D., Robert N. J., Risch N. An association between the risk of cancer and mutations in the HRAS1 minisatellite locus. N Engl J Med. 1993 Aug 19;329(8):517–523. doi: 10.1056/NEJM199308193290801. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Jeng J. H., Chiang C. P., Hahn L. J. Mutations of Ki-ras oncogene codon 12 in betel quid chewing-related human oral squamous cell carcinoma in Taiwan. J Oral Pathol Med. 1994 Feb;23(2):70–74. doi: 10.1111/j.1600-0714.1994.tb00259.x. [DOI] [PubMed] [Google Scholar]
- Rumsby G., Carter R. L., Gusterson B. A. Low incidence of ras oncogene activation in human squamous cell carcinomas. Br J Cancer. 1990 Mar;61(3):365–368. doi: 10.1038/bjc.1990.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranath D., Chang S. E., Bhoite L. T., Panchal R. G., Kerr I. B., Mehta A. R., Johnson N. W., Deo M. G. High frequency mutation in codons 12 and 61 of H-ras oncogene in chewing tobacco-related human oral carcinoma in India. Br J Cancer. 1991 Apr;63(4):573–578. doi: 10.1038/bjc.1991.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scambia G., Catozzi L., Benedetti Panici P., Ferrandina G., Almadori G., Paludetti G., Cadoni G., Distefano M., Piffanelli A., Mancuso S. Expression of ras oncogene p21 protein in normal and neoplastic laryngeal tissues: correlation with histopathological features and epidermal growth factor receptors. Br J Cancer. 1994 Jun;69(6):995–999. doi: 10.1038/bjc.1994.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z. M., Barrois M., Klijanienko J., Micheau C., Richard J. M., Riou G. Analysis of the c-Ha-ras-1 gene for deletion, mutation, amplification and expression in lymph node metastases of human head and neck carcinomas. Br J Cancer. 1990 Sep;62(3):398–404. doi: 10.1038/bjc.1990.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi M., Morinaga S., Noguchi M., Shimosato Y., Sekiya T. Loss of genes on the short arm of chromosome 11 in human lung carcinomas. Jpn J Cancer Res. 1987 Dec;78(12):1302–1308. [PubMed] [Google Scholar]
- Spandidos D. A., Frame M., Wilkie N. M. Expression of the normal H-ras1 gene can suppress the transformed and tumorigenic phenotypes induced by mutant ras genes. Anticancer Res. 1990 Nov-Dec;10(6):1543–1554. [PubMed] [Google Scholar]
- Spandidos D. A., Holmes L. Transcriptional enhancer activity in the variable tandem repeat DNA sequence downstream of the human Ha-ras 1 gene. FEBS Lett. 1987 Jun 22;218(1):41–46. doi: 10.1016/0014-5793(87)81014-1. [DOI] [PubMed] [Google Scholar]
- Spandidos D. A., Lamothe A., Field J. K. Multiple transcriptional activation of cellular oncogenes in human head and neck solid tumours. Anticancer Res. 1985 Mar-Apr;5(2):221–224. [PubMed] [Google Scholar]
- Spandidos D. A., Wilkie N. M. Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature. 1984 Aug 9;310(5977):469–475. doi: 10.1038/310469a0. [DOI] [PubMed] [Google Scholar]
- Vachtenheim J., Horáková I., Novotná H. Hypomethylation of CCGG sites in the 3' region of H-ras protooncogene is frequent and is associated with H-ras allele loss in non-small cell lung cancer. Cancer Res. 1994 Mar 1;54(5):1145–1148. [PubMed] [Google Scholar]
- Vokes E. E., Weichselbaum R. R., Lippman S. M., Hong W. K. Head and neck cancer. N Engl J Med. 1993 Jan 21;328(3):184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- Warnakulasuriya K. A., Chang S. E., Johnson N. W. Point mutations in the Ha-ras oncogene are detectable in formalin-fixed tissues of oral squamous cell carcinomas, but are infrequent in British cases. J Oral Pathol Med. 1992 May;21(5):225–229. doi: 10.1111/j.1600-0714.1992.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Yeudall W. A., Torrance L. K., Elsegood K. A., Speight P., Scully C., Prime S. S. Ras gene point mutation is a rare event in premalignant tissues and malignant cells and tissues from oral mucosal lesions. Eur J Cancer B Oral Oncol. 1993 Jan;29B(1):63–67. doi: 10.1016/0964-1955(93)90012-4. [DOI] [PubMed] [Google Scholar]