Abstract
L-Glutamate (Glu) is the major excitatory neurotransmitter in the mammalian CNS and five types of high-affinity Glu transporters (EAAT1–5) have been identified. The transporters EAAT1 and EAAT2 in glial cells are responsible for the majority of Glu uptake while neuronal EAATs appear to have specialized roles at particular types of synapses. Dysfunction of EAATs is specifically implicated in the pathology of neurodegenerative conditions such as amyotrophic lateral sclerosis, epilepsy, Huntington's disease, Alzheimer's disease and ischemic stroke injury, and thus treatments that can modulate EAAT function may prove beneficial in these conditions. Recent advances have been made in our understanding of the regulation of EAATs, including their trafficking, splicing and post-translational modification. This article summarises some recent developments that improve our understanding of the roles and regulation of EAATs.
Keywords: L-Glutamate, transport, EAAT, trafficking, neurodegeneration, molecular pharmacology, splicing, glia, neurons
Introduction
All amino-acid and monoamine neurotransmitters possess specific, high-affinity transport mechanisms that have evolved to terminate the synaptic actions of the transmitter and to recycle the molecules involved. For the major excitatory transmitter of the mammalian central nervous system (CNS), L-glutamate (Glu), this transport is vital as high concentrations of Glu in the synaptic milieu can cause neuronal injury through a multifaceted process termed excitotoxicity. Transporters for Glu functionally play a role in preserving the local integrity of excitatory synaptic transmission (Marcaggi and Attwell, 2004). Extracellular levels of Glu are regulated by a family of transporters, which are quite distinct from those for the monoamine (noradrenaline, dopamine and 5-hydroxytryptamine) and other amino-acid (4-aminobutyric acid or glycine) transmitters. High-affinity transporters for Glu (excitatory amino-acid transporters (EAATs)) represent a unique family of proteins (reviewed by Kanai and Hediger, 2004) that display considerable homology (50–60% at the amino-acid level) and there has been considerable recent progress in our understanding of their pharmacology, although unlike the monoamine transporters, their physiological regulation is poorly understood. While many clinically effective drugs act via monoamine transporters (Torres et al., 2003), molecules displaying selectivity for the various subtypes of EAATs are only just beginning to emerge. Moreover, there are likely to be considerable advances in our knowledge of the structure–activity relationships governing EAAT function with the recent description of the crystal structure of a bacterial EAAT, which resembles the mammalian proteins (Yernool et al., 2004). Many membrane-associated proteins are internalized quickly and trafficked via intracellular compartments, but such mechanisms are poorly understood for EAATs and likely to be complex as the vast majority of Glu transport occurs into glia, which undergo extensive cytoskeletal reorganization during development and injury of the brain (Pekny and Nilsson, 2005). This minireview focuses on these emerging issues and more extensive reviews can be found elsewhere (Bridges et al., 1999; Danbolt, 2001; Balcar, 2002; O'Shea, 2002; Campiani et al., 2003; Kanai and Hediger, 2004; Shigeri et al., 2004; Bridges and Esslinger, 2005).
Characteristics, function and localization of EAATs
Over the past 15 years, five major subtypes of EAAT have been identified (nomenclature in human EAAT1-5; for simplicity, this paper will use this terminology regardless of the species being investigated). EAATs are members of the solute carrier family 1 (SLC1) that also includes the two neutral amino-acid transporters, ASCT1 and ASCT2 (Kanai and Hediger, 2004). EAATs are distinct from the family of proteins responsible for vesicular Glu transport, which have been reviewed elsewhere (Hisano, 2003; Fremeau et al., 2004; Shigeri et al., 2004). EAATs possess distinct localizations at both the cellular and regional level as well as distinct molecular and pharmacological characteristics. EAAT1 (SLC1A3, the human homologue of L-glutamate/L-aspartate transporter (GLAST)) is present in glial cells throughout the CNS and at high levels in Bergmann glia of the cerebellum (reviewed by Danbolt, 2001; O'Shea, 2002; Kanai and Hediger, 2004). EAAT2 (SLC1A2, human homologue of glutamate transporter 1 (GLT-1)) is almost exclusively glial, and is widespread and highly abundant throughout the CNS (reviewed by Danbolt, 2001; O'Shea, 2002; Kanai and Hediger, 2004). The transporters EAAT3 (SLC1A1, human homologue of EAAC1) and EAAT4 (SLC1A6) are present predominantly in neurons; EAAT3 is abundant throughout the CNS, whereas EAAT4 is predominantly localized to cerebellar Purkinje cells (reviewed by Danbolt, 2001; O'Shea, 2002; Kanai and Hediger, 2004), with low levels of expression also present in the forebrain (Dehnes et al., 1998; Massie et al., 2001). EAAT5 (SLC1A7) is present in rod photoreceptor and bipolar cells of the retina (Arriza et al., 1997; Pow and Barnett, 2000).
A number of elegant studies have shown that effective EAATs are essential for the maintenance of normal excitatory synaptic transmission and thus, for example, Glu clearance from the synaptic milieu determines the time course of Glu receptor activation (Diamond and Jahr, 1997; Takayasu et al., 2006) and its dysfunction may result in excitotoxicity (reviewed by Danbolt, 2001; O'Shea, 2002; Kanai and Hediger, 2004). The majority of synapses in the CNS are in close apposition with glia, and glial EAATs are responsible for the bulk of Glu uptake (reviewed by Danbolt, 2001; O'Shea, 2002; Kanai and Hediger, 2004), whereas neuronal EAATs appear to have more specialized roles, some of which are discussed below. Presumably, the existence of multiple EAATs (with subtypes and splice variants distributed both in the same and different cells) reflects the evolutionary development of tightly controlled regulatory processes needed to ensure the efficient maintenance of synaptic transmission for the major mammalian excitatory transmitter. Numerous in vitro and in vivo studies have demonstrated that transgenic ablation, antisense downregulation or pharmacological inhibition of glial EAATs results in increased extracellular Glu and neuronal death. Furthermore, immunocytochemical and physiological studies on the distribution and function of different EAAT subtypes confirm the functional dominance of the glial transporters (reviewed by Danbolt, 2001). Glial EAATs also serve another vital role in the CNS by supplying Glu for metabolic processes such as the glutamate–glutamine cycle. Thus, a range of evidence suggests that alterations in Glu metabolism or glial viability resulting from impaired Glu transport may also contribute to neuronal damage (Rae et al., 2000; Had-Aissouni et al., 2002; Ré et al., 2003; Aoyama et al., 2006). As well as reducing the potentially toxic build-up of extracellular Glu, it is likely that Glu uptake by glial EAATs also signals the energy needs of nearby neurons via activation of the Na+/K+ ATPase and glucose transporters, and changes in the levels of ATP and lactate (Voutsinos-Porche et al., 2003). This growing body of evidence suggests an interactive role between glial EAATs and intermediary metabolism that has both physiological and pathological relevance.
The existence of Glu transporters on presynaptic neuronal terminals has been suspected for at least 30 years (Beart, 1976; Storm-Mathisen and Iversen, 1979), but the subtype of EAAT responsible for this uptake has remained elusive. Recent evidence suggests the presence of a variant form of EAAT2 in neurons (Schmitt et al., 2002; Chen et al., 2004) that may account for presynaptic Glu uptake. The difficulty of demonstrating such presynaptic localizations could relate to various issues, including the relatively low abundance of EAATs on these terminals (compared to nearby glial cells) and problems of antibody access and geometry in a relationship that is tipped heavily in favour of astrocytes tightly juxtaposed to nerve terminals (cf. Danbolt, 2001).
While astrocytic EAATs are generally responsible for the majority of Glu uptake (see above), neuronal transporters assume greater significance at certain specialized synapses, particularly in the cerebellum, where the spatial relationship between EAATs and Glu receptors is altered and where many peri- or extrasynaptic Glu receptors are present (Huang and Bergles, 2004). Although physiological roles for neuronal EAATs are poorly documented, the localization of EAAT3 and EAAT4 at these synapses allows the selective modulation of Glu signalling at particular Glu receptors. For example, in the cerebellum where EAAT4 is abundant on Purkinje neurons, signalling at metabotropic glutamate receptors (mGluRs) on these cells is limited by Glu uptake (Wadiche and Jahr, 2005). Similarly, at parallel fibre and climbing fibre synapses on Purkinje neurons, EAATs limit mGluR responses under resting conditions (Otis et al., 2004). Furthermore, inhibition of neuronal EAATs in the cerebellum facilitates mGluR-mediated long-term depression (Brasnjo and Otis, 2001). Although EAATs play only a minor role in regulating the synaptic activation of Glu receptors at most central synapses (Chen and Diamond, 2002), their role is more prominent at specialized types of synapses. In the hippocampus, inhibition of glial (but not neuronal) EAATs potentiates activation of postsynaptic mGluRs, resulting in enhanced inhibition of pyramidal neurons (Huang et al., 2004). A further example of EAAT inhibition promoting the recruitment of extrasynaptic receptors occurs at N-methyl-D-aspartate (NMDA) receptors on retinal ganglion cell synapses, where EAATs play a critical role in limiting the synaptic activation of NMDA receptors by Glu (Chen and Diamond, 2002).
Modulation of EAAT activity occurs via both short-term (due to altered trafficking and post-translational modifications such as phosphorylation) (Robinson, 2002; Vermeiren et al., 2005) and longer-term (due to altered expression and abundance) mechanisms (Rothstein et al., 2005; Ganel et al., 2006; O'Shea et al., 2006). A further mechanism for the regulation for EAAT expression, particularly for the glial transporters, exists in the form of alternative splicing of transcripts. Numerous splice variants of EAAT2 have been identified, showing differential (tissue- and species-dependent) patterns of expression. Despite their existence, functional differences in transport activity have not been identified in the major EAAT2 splice variants that are functional (Utsunomiya-Tate et al., 1997; Chen et al., 2002; Sullivan et al., 2004), suggesting that this alternative splicing may be more important in the differential targeting of transporters to specific cell types or localizations than in regulating the properties of Glu uptake. Interestingly, Sullivan et al. (2004) have suggested the differential localization of an EAAT2 splice variant relative to EAAT2 itself could indicate its role in regulating Glu spill over in proximal processes of astrocytes. The presence of an EAAT2 splice variant in neurons (Schmitt et al., 2002; Chen et al., 2004) may go some way towards explaining earlier difficulties in identifying the EAAT subtype responsible for presynaptic Glu uptake, although some debate remains as to the identity of this transcript (Chen et al., 2004; Sullivan et al., 2004). A much smaller number of studies have identified splice variants in EAAT1 (Huggett et al., 2000; Vallejo-Illarramendi et al., 2005a) and neuronal EAAT3 (Matsumoto et al., 1999), whereas a variant form of EAAT3 (‘EAAC2'), transcribed from an independent promoter, has also been reported (Jin et al., 2002). While little is yet known about these transcripts, the form of human EAAT1 lacking exon 9 appears to be retained in the endoplasmic reticulum and exert a dominant-negative effect on full-length EAAT1 (Vallejo-Illarramendi et al., 2005a). The involvement of EAAT splice variants in pathological conditions is discussed later in this paper.
Molecular structure and new functional implications
A major advance in late 2004 was the successful determination of the crystal structure of a Glu transporter homologue from Pyrococcus horikoshii (Yernool et al., 2004). Although our interpretation of these landmark observations should be tempered with some caution as this transporter shares only 37% homology with human EAAT2, the predictions arising from these findings are especially exciting. The authors employed sequence alignments involving EAAT1–3, reflecting the significant relationships throughout the entire polypeptides of prokaryotic and eukaryotic Glu transporters, and the analyses were in the context of the commonly accepted topology of EAATs (reviewed by Kanner and Borre, 2002). Although earlier observations based on freeze fracture electron microscopy suggested a pentamer (Eskandari et al., 2000), recent molecular and biophysical evidence (Gendreau et al., 2004; Yernool et al., 2004; Grewer et al., 2005) favours a functional transporter with a stoichiometry of three identical subunits. Yernool et al., (2004) suggest that the oligomeric transporter is bowl-shaped with a solvent-filled extracellular basin extending halfway across the membrane. The structure of this large aqueous basin fits with the idea that the activity of EAATs, and other Na+/Cl− co-transporters (Hilgemann and Lu, 1999; Ryan et al., 2004), is likely to be exemplified by the alternating-access model allowing access of the substrate to either intracellular or extracellular solution. Based upon recent fluorescence resonance energy transfer analysis with fluorescently labelled human EAAT3, relatively small conformational changes may occur in each subunit in the coupled transport of substrate – here a hybrid version of the ‘rocker-switch model' of alternating access was proposed for EAAT3 (Koch and Larsson, 2005). Other work with EAAT3 shows that the transporter undergoes three conformational changes during a cycle and that important Na+-dependent conformational changes precede Glu binding (Larsson et al., 2004). It is particularly interesting that evidence supports the concept that each subunit of the trimer functions independently (Yernool et al., 2004; Grewer et al., 2005). Of course, much now remains to be resolved about the fine details of the molecular events that subserve each step in the transport process. Key issues to be addressed here include where and how subunit oligomerization is regulated, and how assembly responds to synthesis, intracellular compartmentalization and trafficking events regulating EAAT activity.
Transport of Glu by EAATs is well documented to involve the co-transport of three Na+ and one H+, and the counter transport of one K+ enabling EAATs to maintain a large concentration gradient across the cell membrane (Danbolt, 2001; Kanai and Hediger, 2003; Shigeri et al., 2004). There now seems a general consensus from several laboratories that EAATs are likely to subserve at least dual functions both as a transporter and ion channel, whereby residues in the carboxyl-terminal and amino-terminal portions, respectively, are the molecular determinants critical for these different functions (Slotboom et al., 2001; Amara and Fontana, 2002; Kanner and Borre, 2002). Put simply, these two functions are Glu transport (i.e. substrate recognition, binding, transport and ion coupling) and chloride flux. A recent elegant study by Vandenberg and co-workers (Ryan et al., 2004) has shown this convincingly for EAAT1. Results over some 10 years have demonstrated through the use of site-directed mutagenesis and chimeric transporters that mutations that disrupt chloride permeation do not alter transport, and vice versa. Furthermore, while Glu can be transported in the absence of a change in chloride conductance, the latter cannot occur without activation of the transporter, with other evidence suggesting an inverse relationship between transporter rate and anion conductance (Grewer and Rauen, 2005). This functional duality has been best described for the dopamine transporter where presynaptic excitability is regulated by substrate-activated chloride conductance (Mortensen and Amara, 2003). This phenomenon is likely to have physiological relevance for EAAT4/5, which possess greater chloride conductance than EAAT1–3 (Grewer and Rauen, 2005). For example, Arriza et al. (1997) suggest that the channel-like properties of EAAT5 may indicate a role in retinal physiology distinct from neurotransmitter clearance. Whether EAATs play such roles at other specialized synapses (vide supra) requires further investigation. However, as most Glu transport is into astrocytes, which possess EAATs displaying relatively low chloride conductance (Grewer and Rauen, 2005), the functional relevance of this duality in astrocytes remains to be determined.
EAATs, targeting and trafficking
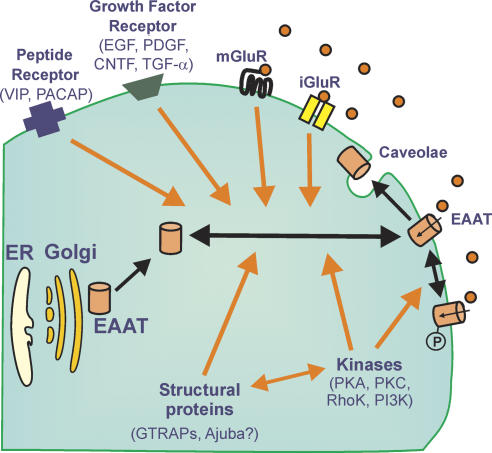
By analogy to monoamine transporters (Torres et al., 2003), a variety of evidence indicates that the intrinsic activity of EAATs and cell surface trafficking are regulated, not only by kinases/phosphatases and scaffolding proteins, but also by diverse influences (e.g. endothelin B, vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, platelet-derived growth factor, thyroid hormone and trophic factors) that appear to affect trafficking to and from the plasma membrane to intracellular pools involving lipid rafts and caveolae (Figure 1). In general, the transport of oligomeric membrane proteins from intracellular pools to the cell surface seems to be highly organized and subject to quality control involving the endoplasmic reticulum and/or the Golgi apparatus – presumably this is also the case for Na+/Cl− co-transporters as cells have inherent systems for minimizing errors and maintaining efficiency (Ellgaard and Helenius, 2003). For EAATs, the story in this area is only just beginning to emerge, with the initial series of investigations implicating various kinases (protein kinases C and B, phosphoinositol-3-kinase, mitogen-activated protein kinase, serum and glucocorticoid inducible kinase 1) and calcineurin (Aronica et al., 2003; González et al., 2003; Boehmer et al., 2006; Li et al., 2006), with this phosphatase perhaps linked to the involvement of the immunophilin, FK506 (Labrande et al., 2006) (Figure 1). Indeed, based upon early observations, the cell-surface expression and trafficking of EAATs may display appreciable heterogeneity between subtypes of transporter with ∼80% of EAAT2 expressed at the cell surface, whereas ∼70% of EAAT3 is found in the cytosol (Sheldon et al., 2006). Apparently a C-terminal motif in EAAT3 is important for its trafficking and constitutive sorting (Sheldon et al., 2006).
Figure 1.
Mechanisms contributing to short- and long-term regulation of EAAT activity. Cell-surface expression and activity of EAATs is regulated by diverse influences including various receptors, phosphorylation (‘P'), trafficking to and from intracellular pools and interactions with lipid rafts and structural proteins. This figure should be considered as a general schema only, in that it reflects the various processes involved. Whether individual effects occur independently of transcription, via various intracellular pools or purely at the level of the cell surface, has not always been elucidated.
Thus far all the scaffolding proteins clearly linked to EAATs (Glu transporter-associated proteins (GTRAPs) and Ajuba) (Jackson et al., 2001; Lin et al., 2001; Marie et al., 2002) seem to have some association with actin systems. Diverse molecules (e.g. integrins, tropomysosin, gelsolin and cofilin) are involved in regulating actin dynamics (Fass et al., 2004) and linkages to EAAT function remain to be fully elucidated. Given that the majority of Glu uptake is into astrocytes, rather than neurones, and these cells possess remarkable potential for cytoskeletal re-arrangement in response to diverse stimuli arising locally (Hughes et al., 2004; Pekny and Nilsson, 2005; Zagami et al., 2005), via the circulation or the cerebrospinal fluid, then Rho family small GTPases (Rho, Rac, etc.) may play a key role in regulating their actin organization (Hall, 1998; Abe and Misawa, 2003). A number of G-protein-coupled receptors including those for protease-activated receptors, lysophosphatidic acid, sphingosine-1-phosphate and mGluRs also influence astrocytic phenotype (Aronica et al., 2003; Sorensen et al., 2003), but possible links to EAAT function require further clarification. Moreover, astrocytes are known to possess caveolae, which have been implicated in cell migration, and structures positive for caveolin-1 are known to co-align with stress fibres (Navarro et al., 2004), which are well documented in astrocyte biology. Much remains to be discovered about the functional involvement of caveolae, but they are crucial regulators of signalling cascades and cholesterol-rich lipid-raft domains, and have a remarkable propensity to form supramolecular complexes (Parton, 2003).
Cholesterol-rich lipid rafts have recently been associated with EAAT2 (Butchbach et al., 2004), although cholesterol was found to be important for Glu uptake more than 15 years ago (Shouffani and Kanner, 1990). Treatment of mixed cortical cultures with the cholesterol-depleting agent methyl-β-cyclodextrin rapidly decreases Glu uptake and membrane cholesterol (<5 min) predominantly by an EAAT2 mechanism rather than an EAAT1 mechanism (Butchbach et al., 2004). Immunostaining revealed dispersion of the normal clustering of EAAT2 on the plasma membrane of astrocytes (Butchbach et al., 2004). EAAT3 staining within neurites of neurones in these cortical cultures was less sensitive to this treatment, although in vivo methyl-β-cyclodextrin reduces EAAT3-mediated Glu transport and induces the expression of GTRAP3–18 (Butchbach et al., 2003). EAAT3 too is probably associated with such lipid microdomains, as polarized distributions have been noted in other model systems (Cheng et al., 2002). Interestingly, protein kinase Cα, which is implicated in the redistribution of EAAT3 (González et al., 2003), regulates caveolae dynamics (Parton, 2003). Clustering of EAATs has been recognized for some time, although its significance remains unclear (Zhou and Sutherland, 2004), but with these recent insights, dynamic changes in the localization of Glu transporters are likely to reflect altered trafficking from intracellular compartments in concert with overall alterations in cytoskeletal motility. Recently, a further level of complexity has been described whereby EAAT1/2 in astrocytes undergo glycosylation in response to ciliary neurotrophic factor in a manner that effects transporter assembly and localization in raft microdomains (Escartin et al., 2006).
Some of these considerations may only be answered by use of caveolin knockdown (Ge and Pachter, 2004) or of appropriate knockout mice. At present, there are many unknowns in this area, as cyclodextrins do not distinguish between lipid rafts and caveolae (Sowa et al., 2001), lipid rafts are difficult to study in isolation and data emerging from investigations in heterologous expression systems may not adequately reflect the true biology of intact physiological cells. Certainly, more complexities will emerge here as there is preliminary evidence that EAAT2 clustering may be influenced by a clathrin- and dynamin-dependent pathway (Zhou and Sutherland, 2004) and EAAT3 may interact with caveolin-1/2 (Gonzalez et al., 2004).
New molecules targeting EAATs
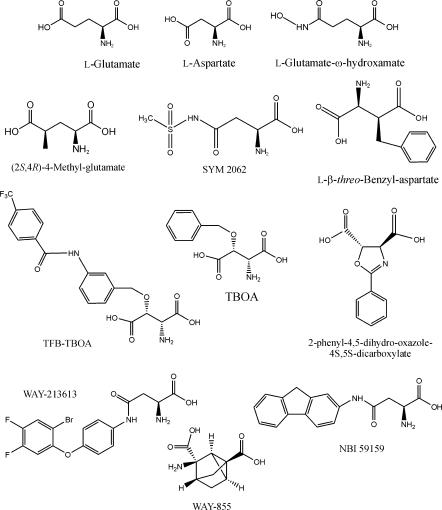
For over 30 years, virtually all drugs acting at EAATs were structural analogues of Glu or L/D-aspartate (Balcar and Johnston, 1972), and included various cyclic molecules such as L-trans-2,4-pyrrollidinedicarboxylate, kainate and dihydrokainate (DHK) (Bridges et al., 1999; Balcar, 2002). Many of the early analogues studied proved to be transportable blockers (i.e. substrates) and produced release of radiolabelled substrates. Moreover, among these many compounds, very little pharmacological selectivity existed across EAAT1–5, although DHK and L-serine-O-sulphate displayed some selectivity for EAAT2 and EAAT1, respectively. Studies of L-threo-β-benzyloxyaspartate (TBOA) (Shimamoto et al., 1998), L-anti-endo-3,4-methanopyrollidinedicarboxylate (Chamberlin et al., 1998) and their analogues revealed non-transportable blockers providing some of the first clues to the design of blockers versus substrates (Figure 2). More recent studies have revealed potent analogues of TBOA such as the blocker (2S,3S)-3-{3-[4-(trifluoromethyl)benzoylamino]benzyloxy}aspartate (Shimamoto et al., 2004) and caged derivatives that can be released by photolysis (Takaoka et al., 2004). Bridges and co-workers (Bridges et al., 1999; Koch et al., 1999) undertook pharmacophore modelling and more recently these ideas have been extended by the rational design and proof-of-principle syntheses of blockers active at EAAT3 (Campiani et al., 2001). These guidelines for SAR can be summarized as follows: (1) steric excess favours inhibitors versus substrates, (2) among constrained molecules, generally folding results in blockers, while unfolded molecules tend to be substrates and (3) conformational restriction per se does not differentiate transportable from non-transportable inhibitors. There have been a number of advances in this area driven by the premise that EAAT blockers, rather than substrates, would not release Glu into the extracellular space by heteroexchange (facilitated exchange–diffusion) exacerbating excitotoxicity and thus might have therapeutic potential (Dunlop et al., 1999).
Figure 2.
Structure of EAAT ligands. TBOA, L-threo-β-benzyloxyaspartate; TFB-TBOA, (2S,3S)-3-{3-[4-(trifluoromethyl)benzoylamino]benzyloxy}aspartate.
Generally, progress in the development of pharmacological agents targeting EAATs has been hampered by the absence of new subtype-selective radioligands for EAATs as the development of [3H]D-aspartate in 1976. (2S,4R)-4-methylglutamate (4MG) displayed some differences in its interactions with EAAT1/2 (Vandenberg et al., 1997) encouraging Beart and O'Shea to employ [3H]4MG in a high-throughput assay (Apricò et al., 2001). Screening of analogues of 4MG revealed a number of active, new molecules (∼IC50 values 150–400 μM) with aromatic hydrophobic substituents (phenyl, benzyl, etc.) in the 4-position of Glu. Rather surprisingly, a new family of sulphamido analogues (e.g. SYM 2062) was revealed with replacement of the ω- or α-carboxyl group of Glu and aspartate, allowing retention of activity (∼IC50 values 5–100 μM; Beart et al., 2002) (Figure 2). These sulphamido analogues question the need for two acidic biosteres to bind to the transporters (Bridges et al., 1999; Campiani et al., 2003). However, we should recall that many years ago Roberts and Watkins (1975) demonstrated that the ω-hydroxamates of Glu and L-aspartate possessed activity versus Glu uptake. In a synthetic chemistry programme directed at EAATs, Wyeth firstly reported a conformationally restricted Glu analogue (WAY-855) that was an EAAT2-preferring non-substrate inhibitor (Dunlop et al., 2003). More recently, Dunlop and co-workers found a series of amido and ureido derivatives of aspartate that possessed high affinities for Glu uptake, and appreciable selectivity for EAAT2 (Dunlop et al., 2005) and EAAT3 (Coon et al., 2004), respectively. These studies represent major advances as not only were both families of molecules non-substrate inhibitors, but the most potent members (WAY-213613 and NBI 59159, respectively) had affinities of ∼100 nM (Figure 2). Importantly, distal lipophilic pockets were hypothesized to be key contributors to the resultant pharmacological activity, suggesting that existent pharmacophore models need updating. Clearly, these new lead structures offer great opportunities for the development of novel subtype-selective radioligands. A positive development here has been the recent synthesis and characterization of the non-transportable ligand [3H]ETB-TBOA (2S,3S)-3-{3-[4-ethylbenzoylamino]benzyloxy}aspartate (Shimamoto et al., 2006) (Figure 2).
The strategy of enhancing Glu transport to expedite its removal from the synaptic milieu during a possible excitotoxic event has also received some attention because, in theory, such drugs could prove beneficial in ischaemic stroke injury and amyotrophic lateral sclerosis (ALS). Hypothetically, such enhancement of EAAT activity could be achieved by drugs acting either directly on the transporter proteins or indirectly on trafficking mechanisms. One such molecule is MS-153 (Shimada et al., 1999), which acts by a kinase-related mechanism to potentiate Glu uptake and to provide benefit in experimental stroke injury (Umemura et al., 1996). A purified component of the venom from the spider, Parawixia bistriata, provides neuroprotection via a non-competitive mechanism in a retinal model of injury (Fontana et al., 2003). Enhanced EAAT activity could also be achieved by increasing transcription or altering the stability of existent transcripts. Thus, a particularly exciting development is the recent report by Rothstein et al. (2005) that β-lactam antibiotics (e.g. ceftriaxone) were found in a screen of more than a thousand Food and Drug Administration-approved drugs and nutritionals to selectively elevate EAAT2 expression. Ceftriaxone appeared to be cytoprotective both in vitro and in vivo in a mouse model of ALS (Rothstein et al., 2005), and further insights into the mechanism underlying the apparent two- to five-fold increase in EAAT2 gene activation will be eagerly awaited. A further recent study demonstrated that the neuroimmunophilin ligand GPI-1046 selectively induced expression of EAAT2 in vitro and in vivo, as well as protecting motor neurons in cell culture and in a mouse model of ALS (Ganel et al., 2006). Taken together, these two recent studies provide strong support for the hypothesis that enhancement of EAAT activity could provide clinical benefit in neuropathological conditions involving excitotoxicity. Additional recent evidence suggests the potential for modulation of EAATs by pathways involving growth factors, neurotrophic factors and peptides (Figure 1) (Figiel et al., 2003; Escartin et al., 2006). As yet, allosteric modulators of EAAT function have not been identified that might produce therapeutic beneficial effects, but given the success in this area with many other proteins (Christopoulos, 2002), the site for Zn2+ binding distinct from the substrate binding site (Mitrovic et al., 2001) may be such a target.
EAATs in pathological conditions
Although a very strong case exists for EAAT function being altered in several neurological conditions, often it proves difficult to discern cause and effect in terms of dysfunction of Glu transporters. Nevertheless, excellent work on ALS (Rothstein et al., 2005) and spinocerebellar ataxia (Ikeda et al., 2006) has provided persuasive evidence for seminal roles for EAAT2 and EAAT4, respectively, in these degenerative conditions. One mechanism attracting attention as likely to contribute to EAAT dysfunction is altered splicing of EAATs and/or altered expression of splice variants. Despite an initial report of aberrant splicing of EAAT2 in ALS patients resulting in non-functional transporters (Lin et al., 1998), later studies have identified similar variations in control patients (Nagai et al., 1998; Meyer et al., 1999; Honig et al., 2000; Flowers et al., 2001), suggesting that this altered splicing may not be a cause of ALS. A more recent study in the G93A-superoxide dismutase1 mouse model of ALS identified differential distribution of various splice variants of EAAT2 in pre-symptomatic transgenic animals (Münch et al., 2002), and a similar result has been reported in human motor cortex from ALS patients (Maragakis et al., 2004). Altered splicing of EAAT2 has also been found in brains from mice fed washed cycad flour containing the suspected neurotoxin that induces features like those of the Guamanian disorder, ALS–Parkinson's dementia complex (Wilson et al., 2003). An intriguing report by Guo et al. (2002) demonstrated that some aberrantly spliced forms of EAAT2 mRNA suppressed expression of normal EAAT2 protein in vitro, and this mechanism may explain the reduction in EAAT expression in human gliomas (Ye et al., 1999). Aberrant splicing of EAAT2 has also been detected in glial cells infected with an enterovirus that has been detected in the spinal cord of ALS patients (Legay et al., 2003).
Recent investigations have also shed light on the expression of EAAT2 splice variants under conditions relevant to other pathological states. Thus, altered splicing of EAAT2 has been reported in human astrocytoma (Münch et al., 2001). The expression of EAAT2 splice variants is altered in the brains of mice subjected to chemical hypoxia (Münch et al., 2003) and hypoxia was shown to induce the expression of a splice variant of EAAT2 in neurons of pigs (Pow et al., 2004). Fluid-percussion injury, a model of traumatic brain injury, was shown to alter EAAT2 splicing in rat brains (Yi et al., 2005), reducing the expression of the GLT-1v form in a number of brain regions. A higher frequency of altered EAAT2 splice variants was also detected in brains of epileptic patients (Hoogland et al., 2004), whereas the abundance of EAAT2b-immunoreactive protein was found to be reduced in the cortex of Alzheimer's disease patients (Maragakis et al., 2004). The overall hypothesis here could be that exaggerated expression of EAAT2 splice variants is a pathological response. At this point, it is notable that alterations in the expression of splice variants of other EAATs in response to pathological stimuli have not yet been reported.
There is an extensive literature on the alterations in Glu transport reported across a range of pathologies that affect the CNS. Some of these reports are more anecdotal in nature and here we have attempted a synthesis of recent or key findings where there is strong evidence for the involvement of EAATs and which is supported by a substantial body of supportive data, either from animal models or in vitro systems (Table 1). Interpretation of these data should always be carried out bearing in mind the issue of cause and effect, especially given the remarkable propensity of neurones and glia to display temporally dependent adaptative responses. Thus, for example, an elevation in extracellular Glu might elicit an early rise in EAAT activity by a genomic or trafficking mechanism to normalize synaptic function, or a later rise or fall due to astrogliosis or cell death, respectively. Moreover, these alterations in EAAT function could be in neurons, astrocytes, oligodendrocytes and/or microglia, which are all capable of displaying such responses (Matute et al., 2006; O'Shea et al., 2006).
Table 1.
Involvement of EAATs in neurological disorders
| Human tissue | Experimental models | |
|---|---|---|
| ALS | [Glu] ↑ in CSF.1 | ALS mutant SOD1 inactivates EAAT2.4 |
| ↓ Uptake in CNS tissue.2 | Extracellular [Glu] ↑ in the cortex of ALS-SOD1 transgenic mice.5 | |
| ↓ GLT-1 in CNS tissue.3 | Focal EAAT2 loss in ALS-SOD1 transgenic mice.6 | |
| Epilepsy | ↑Plasma [Glu] in epileptic patients.7 | Fatal seizures in EAAT2 knockout mice.11 |
| ↑Hippocampal [Glu] in seizures.8 | ↓ EAAT1, 2 and 3 protein in GLAST, GLT-1 and EAAC1 protein in the brain of genetically epileptic rats.12 | |
| Temporal lobe epilepsy: EAAT3 ↑ in hippocampal granule cells, EAAT2 ↓ in hilus and CA1, EAAT1 ↑ in CA2/3.9 | ||
| ↑Incidence of aberrant EAAT2 splicing in temporal lobe epilepsy patients.10 | ||
| Huntington's disease | EAAT2 mRNA ↓ in neostriatum, but the number of cells expressing EAAT2 mRNA ↑.13 | EAAT2 mRNA and uptake down in the striatum and cortex of R6 transgenic mice.15 |
| Uptake ↓ in the caudate and putamen.14 | Impaired Glu metabolism in R6/2 mouse brains.16 | |
| Alzheimer's disease | Uptake ↓ in the cortex17 and astrocytes18 and EAAT2 protein ↓19 in AD patients. | Glu uptake and EAAT1, EAAT2 protein ↓ in APP transgenic mice.22 |
| Aberrant neuronal expression of EAAT120 and EAAT221 associated with tau accumulation. | ↓ EAAT2 and Glu uptake in GFAP-tau transgenic mice.23 β-amyloid ↓ Glu uptake.24 | |
| Ischaemic stroke injury | EAAT2 promoter polymorphism associated with ↑ [Glu] and frequency of stroke.25 | EAAT reversal in severe ischaemia in vitro.27 |
| Neonatal hypoxic–ischaemic encephalopathy: EAAT1 ↓ in molecular layer, ↑ in Purkinje and inner granule cell layer; EAAT4 ↓ in Purkinje cells.26 | EAAT2/3 ↓ in piglet brain and neuronal expression of EAAT2 after hypoxia–ischaemia.28 | |
| EAAT2 ↑ in the cortex, ↓ in the striatum after hypoxia–ischaemia in rats.29 | ||
| White matter injury | ↑ Serum [Glu] in relapsing MS patients.30 | TNF-α ↓ EAAT-1 expression and Glu uptake in cultured oligodendrocytes.32 |
| ↑ [Glu] in white matter and acute lesions of MS brains.31 | Depolarization causes EAAT reversal in spinal white matter.35 | |
| ↓ EAAT1 and EAAT2 in oligodendrocytes in MS lesions.32 | ||
| ↓ EAAT1/2/3 in CNS tissue from MS patients.33 | ||
| ↑ EAAT1 and EAAT2 mRNA, protein and uptake in MS optic nerve.34 | ||
| Infection | ↑ Plasma [Glu] in HIV patients.36 | TNF-α ↓ uptake in primary human astrocytes.39 |
| Strong expression of EAAT1 in activated macrophages/ microglia of HIV-infected brains.37 | TNF-α, interferon-γ and interleukin-1β ↓ uptake in cultures of rat hippocampus.40 | |
| Uptake ↓ by >50% in AIDS dementia brains.38 | HIV-1 or gp120 ↓ EAAT2 and uptake in astrocytes.41 | |
| Microglia and macrophages express EAAT2 in SIV-infected primates.42 | ||
| LPS ↑ EAAT2 in cultured astrocytes and microglia.43 | ||
| Retinal disease/Glaucoma | ↑ [Glu] in vitreous body of glaucoma44 and diabetic retinopathy patients.45 | ↑ Intraocular [Glu] after optic nerve lesion.48 |
| ↑ [Glu] in aqueous humor in retinal artery occlusion.46 | ↓ EAAT1 activity after retinal ischaemia.49 | |
| ↓ EAAT1 in glaucomatous eyes.47 | ||
| Neuropsychiatric disorders | ↑ EAAT1 and EAAT2 mRNA in the thalamus of schizophrenia.50 | Altered expression of mRNA for EAAT-interacting proteins in clozapine-treated rats.52 |
| ↓ Striatal EAAT3/4 mRNA in bipolar disorder,51 ↓ striatal EAAT3 mRNA in schizophrenia,51 ↓ striatal EAAT4 mRNA in major depression.51 | Clozapine and haloperidol ↓ EAAT2/3 mRNA in regions of rat brain.54 | |
| ↑ mRNA for EAAT3- and EAAT4-interacting proteins in the thalamus in schizophrenia.52 | Clozapine ↓ EAAT2 and uptake in cultured astrocytes.55 | |
| ↑ mRNA for EAAT2 in prefrontal cortex of untreated schizophrenics, reduced by antipsychotic treatment.53 |
Abbreviations: AD, Alzheimer's disease; ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; EAAT, excitatory amino-acid transporter; GFAP, glial fibrillary acidic protein; MS, multiple sclerosis; SOD, superoxide dismutase; TNF-α, tumour necrosis factor-α.
1 Rothstein et al. (1990); 2 Rothstein et al. (1992); 3 Rothstein et al. (1995); 4 Trotti et al. (1999); 5 Alexander et al. (2000); 6 Howland et al. (2002); 7 Janjua et al. (1992); 8 During and Spencer (1993); 9 Mathern et al. (1999); 10 Hoogland et al. (2004); 11 Tanaka et al. (1997); 12 Dutuit et al. (2002); 13 Arzberger et al. (1997); 14 Cross et al. (1986); 15 Liévens et al. (2001); 16 Behrens et al. (2002); 17 Masliah et al. (1996); 18 Liang et al. (2002); 19 Li et al. (1997); 20 Scott et al. (2002); 21 Thai (2002); 22 Masliah et al. (2000); 23 Dabir et al. (2006); 24 Keller et al. (1997); Lauderback et al. (1999); 25 Mallolas et al. (2006); 26 Inage et al. (1998); 27 Rossi et al. (2000); 28 Martin et al. (1997); Pow et al. (2004); 29 Cimarosti et al. (2005); 30 Westall et al. (1980); 31 Srinivasan et al. (2005); 32 Pitt et al. (2003); 33 Werner et al. (2001); 34 Vallejo-Illarramendi et al. (2006); 35 Li et al. (1999); Li and Stys (2001); 36 Droge et al. (1993); 37 Vallat-Decouvelaere et al. (2003); 38 Sardar et al. (1999); 39 Fine et al. (1996); 40 Ye and Sontheimer (1996); 41 Wang et al. (2003); 42 Chretien et al. (2002); 43 O'Shea et al. (2006); Persson et al. (2005); 44 Dreyer et al. (1996); 45 Ambati et al. (1997); 46 Wakabayashi et al. (2006); 47 Naskar et al. (2000); 48 Yoles and Schwartz (1998); 49 Barnett et al. (2001); 50 Smith et al. (2001); 51 McCullumsmith and Meador-Woodruff (2002); 52 Huerta et al. (2006); 53 Matute et al. (2005); 54 Schmitt et al. (2003); 55 Vallejo-Illarramendi et al. (2005b).
While Table 1 specifically refers to neurological and psychiatric conditions where there are multiple reports on the involvement of EAATs, there are a number of other conditions worthy of mention where substantial alterations in Glu transport may have effects determining the clinical progression of the condition. Thus, changes in EAATs are reported to occur in alcoholism (Flatscher-Bader et al., 2005), spinal cord trauma (Vera-Portocarrero et al., 2002) and cancer (Ye et al., 1999; Markert et al., 2001).
Conclusions and summary
In summary, it is clear that EAATs play key roles in the maintenance of excitatory synaptic transmission and are variously affected in a range of neuropathological conditions. Advances in our understanding of EAAT regulation suggest a number of therapeutic strategies for the management of CNS disorders, and such strategies may be of a traditional pharmacological or genetic nature. Therapeutic modulation of EAATs will be greatly expedited by a better understanding of the basic regulation of these transporters.
Acknowledgments
We thank numerous colleagues for their input in the preparation of this review and regret being unable to cite many outstanding publications in this area due to space limitations. This work was supported by an NHMRC (Australia) Program Grant (No. 236805). PMB acknowledges support from Ecole Superiere Physique et Chemie Industrielle and hospitality of CNRS UMR 7637, 75005, Paris.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- DHK
dihydrokainate
- EAAT
excitatory amino-acid transporter
- ETB-TBOA
(2S,3S)-3-{3-[4-ethylbenzoylamino]benzyloxy}aspartate
- GLAST
L-glutamate/L-aspartate transporter
- GLT-1
glutamate transporter 1
- Glu
L-glutamate
- GTRAP
glutamate transporter-associated protein
- 4MG
(2S,4R)-4-methylglutamate
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl-D-aspartate
- SLC1
solute carrier family 1
- TBOA
L-threo-β-benzyloxyaspartate
- TNF
tumor necrosis factor
Conflict of interest
The authors' research was supported in part by Annovis Inc. (USA) and Schering AG (Germany).
References
- Abe K, Misawa M. Astrocyte stellation induced by Rho kinase inhibitors in culture. Dev Brain Res. 2003;143:99–104. doi: 10.1016/s0165-3806(03)00096-8. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Deitch JS, Seeburger JL, Del Valle L, Heiman-Patterson TD. Elevated cortical extracellular fluid glutamate in transgenic mice expressing human mutant (G93A) Cu/Zn superoxide dismutase. J Neurochem. 2000;74:1666–1673. doi: 10.1046/j.1471-4159.2000.0741666.x. [DOI] [PubMed] [Google Scholar]
- Amara SG, Fontana ACK. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Ambati J, Chalam KV, Chawla DK, D'Angio CT, Guillet EG, Rose SJ, et al. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997;115:1161–1166. doi: 10.1001/archopht.1997.01100160331011. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Apricò K, Beart PM, Lawrence AJ, Crawford D, O'Shea RD. [3H]-(2S, 4R)-4-methylglutamate: a novel ligand for the characterisation of glutamate transporters. J Neurochem. 2001;77:1218–1225. doi: 10.1046/j.1471-4159.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, et al. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci. 2003;17:2106–2118. doi: 10.1046/j.1460-9568.2003.02657.x. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzberger T, Krampfl K, Leimgruber S, Weindl A. Changes of NMDA receptor subunit (NR1, NR2B) and glutamate transporter (GLT1) mRNA expression in Huntington's disease – an in situ hybridization study. J Neuropathol Exp Neurol. 1997;56:440–454. doi: 10.1097/00005072-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Balcar VJ. Molecular pharmacology of the Na+-dependent transport of acidic amino acids in the mammalian central nervous system. Biol Pharmaceut Bull. 2002;25:291–301. doi: 10.1248/bpb.25.291. [DOI] [PubMed] [Google Scholar]
- Balcar VJ, Johnston GAR. The structural specificity of the high affinity uptake of L-glutamate and L-aspartate by rat brain slices. J Neurochem. 1972;19:2657–2666. doi: 10.1111/j.1471-4159.1972.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Barnett NL, Pow DV, Bull ND. Differential perturbation of neuronal and glial glutamate transport systems in retinal ischaemia. Neurochem Int. 2001;39:291–299. doi: 10.1016/s0197-0186(01)00033-x. [DOI] [PubMed] [Google Scholar]
- Beart PM. The autoradiographic localization of L-[3H] glutamate in synaptosomal preparations. Brain Res. 1976;103:350–355. doi: 10.1016/0006-8993(76)90804-0. [DOI] [PubMed] [Google Scholar]
- Beart PM, O'Shea RD, Apricò K, Lawrence AJ, Maccecchini M-L.Screen for glutamate reuptake inhibitors, stimulators, and modulators 2002. World Intellectual Property Organization, WO0218941
- Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate–glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125:1908–1922. doi: 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- Boehmer C, Palmada M, Rajamanickam J, Schniepp R, Amara S, Lang F. Post-translational regulation of EAAT2 function by co-expressed ubiquitin ligase Nedd4-2 is impacted by SGK kinases. J Neurochem. 2006;97:911–921. doi: 10.1111/j.1471-4159.2006.03629.x. [DOI] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Esslinger CS. The excitatory amino acid transporters: pharmacological insights on substrate and inhibitor specificity of the EAAT subtypes. Pharmacol Ther. 2005;107:271–285. doi: 10.1016/j.pharmthera.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bridges RJ, Kavanaugh MP, Chamberlin AR. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr Pharm Des. 1999;5:363–379. [PubMed] [Google Scholar]
- Butchbach MER, Guo H, Lin C-LG. Methyl-beta-cyclodextrin but not retinoic acid reduces EAAT3-mediated glutamate uptake and increases GTRAP3-18 expression. J Neurochem. 2003;84:891–894. doi: 10.1046/j.1471-4159.2003.01588.x. [DOI] [PubMed] [Google Scholar]
- Butchbach MER, Tian G, Guo H, Lin C-LG. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function. J Biol Chem. 2004;279:34388–34396. doi: 10.1074/jbc.M403938200. [DOI] [PubMed] [Google Scholar]
- Campiani G, De Angelis M, Armaroli S, Fattorusso C, Catalanotti B, Ramunno A, et al. A rational approach to the design of selective substrates and potent nontransportable inhibitors of the excitatory amino acid transporter EAAC1 (EAAT3). New glutamate and aspartate analogues as potential neuroprotective agents. J Med Chem. 2001;44:2507–2510. doi: 10.1021/jm015509z. [DOI] [PubMed] [Google Scholar]
- Campiani G, Fattorusso C, De Angelis M, Catalanotti B, Butini S, Fattorusso R, et al. Neuronal high-affinity sodium-dependent glutamate transporters (EAATs): Targets for the development of novel therapeutics against neurodegenerative diseases. Curr Pharm Des. 2003;9:599–625. doi: 10.2174/1381612033391261. [DOI] [PubMed] [Google Scholar]
- Chamberlin AR, Koch HP, Bridges RJ. Design and synthesis of conformationally constrained inhibitors of high-affinity, sodium-dependent glutamate transporters. Methods Enzymol. 1998;296:175–189. doi: 10.1016/s0076-6879(98)96014-1. [DOI] [PubMed] [Google Scholar]
- Chen S, Diamond JS. Synaptically released glutamate activates extrasynaptic NMDA receptors on cells in the ganglion cell layer of rat retina. J Neurosci. 2002;22:2165–2173. doi: 10.1523/JNEUROSCI.22-06-02165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, et al. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, Desilva T, Tanaka K, et al. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Glover G, Banker G, Amara SG. A novel sorting motif in the glutamate transporter excitatory amino acid transporter 3 directs its targeting in Madin–Darby canine kidney cells and hippocampal neurons. J Neurosci. 2002;22:10643–10652. doi: 10.1523/JNEUROSCI.22-24-10643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien F, Vallat-Decouvelaere AV, Bossuet C, Rimaniol AC, Le Grand R, Le Pavec G, et al. Expression of excitatory amino acid transporter-2 (EAAT-2) and glutamine synthetase (GS) in brain macrophages and microglia of SIVmac251-infected macaques. Neuropathol Appl Neurobiol. 2002;28:410–417. doi: 10.1046/j.1365-2990.2002.00426.x. [DOI] [PubMed] [Google Scholar]
- Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- Cimarosti H, Jones NM, O'Shea RD, Pow DV, Salbego C, Beart PM. Hypoxic preconditioning in neonatal rat brain involves regulation of excitatory amino acid transporter 2 and estrogen receptor alpha. Neurosci Lett. 2005;385:52–57. doi: 10.1016/j.neulet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Coon TR, Li B, Nottebaum LM, Williams JP, Petroski RE, Naeve GS, et al. Design and synthesis of inhibitors of the neuronal glutamate transporter EAAT3 Abstract Viewer/Itinerary Planner 2004Society for Neuroscience: Washington, DC; Program No. 168.10 [Google Scholar]
- Cross AJ, Slater P, Reynolds GP. Reduced high-affinity glutamate uptake sites in the brains of patients with Huntington's disease. Neurosci Lett. 1986;67:198–202. doi: 10.1016/0304-3940(86)90397-6. [DOI] [PubMed] [Google Scholar]
- Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM-Y, et al. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26:644–654. doi: 10.1523/JNEUROSCI.3861-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dehnes Y, Chaudhry FA, Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: a glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18:3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA. Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol. 1996;114:299–305. doi: 10.1001/archopht.1996.01100130295012. [DOI] [PubMed] [Google Scholar]
- Droge W, Murthy KK, Stahl-Hennig C, Hartung S, Plesker R, Rouse S, et al. Plasma amino acid dysregulation after lentiviral infection. AIDS Res Hum Retroviruses. 1993;9:807–809. doi: 10.1089/aid.1993.9.807. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Eliasof S, Stack G, McIlvain HB, Greenfield A, Kowal D, et al. WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake. Br J Pharmacol. 2003;140:839–846. doi: 10.1038/sj.bjp.0705509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop J, McIlvain HB, Carrick TA, Jow B, Lu Q, Kowal D, et al. Characterization of novel aryl-ether, biaryl, and fluorene aspartic acid and diaminopropionic acid analogs as potent inhibitors of the high-affinity glutamate transporter EAAT2. Mol Pharmacol. 2005;68:974–982. doi: 10.1124/mol.105.012005. [DOI] [PubMed] [Google Scholar]
- Dunlop J, Zaleska MM, Eliasof S, Moyer JA. Excitatory amino acid transporters as emerging targets for central nervous system therapeutics. Emerg Ther Targets. 1999;3:543–570. [Google Scholar]
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. doi: 10.1016/0140-6736(93)90754-5. [DOI] [PubMed] [Google Scholar]
- Dutuit M, Touret M, Szymocha R, Nehlig A, Belin MF, Didier-Bazes M. Decreased expression of glutamate transporters in genetic absence epilepsy rats before seizure occurrence. J Neurochem. 2002;80:1029–1038. doi: 10.1046/j.0022-3042.2002.00768.x. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, et al. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26:5978–5989. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Kreman M, Kavanaugh MP, Wright EM, Zampighi GA. Pentameric assembly of a neuronal glutamate transporter. Proc Natl Acad Sci USA. 2000;97:8641–8646. doi: 10.1073/pnas.97.15.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass J, Gehler S, Sarmiere P, Letourneau P, Bamburg JR. Regulating filopodial dynamics through actin-depolymerizing factor/cofilin. Anat Sci Int. 2004;79:173–183. doi: 10.1111/j.1447-073x.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- Figiel M, Maucher T, Rozyczka J, Bayatti N, Engele J. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol. 2003;183:124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]
- Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, et al. Tumor necrosis factor α inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Van Der Brug M, Hwang JW, Gochee PA, Matsumoto I, Niwa S-I, et al. Alcohol-responsive genes in the frontal cortex and nucleus accumbens of human alcoholics. J Neurochem. 2005;93:359–370. doi: 10.1111/j.1471-4159.2004.03021.x. [DOI] [PubMed] [Google Scholar]
- Flowers JM, Powell JF, Leigh PN, Andersen P, Shaw CE. Intron 7 retention and exon 9 skipping EAAT2 mRNA variants are not associated with amyotrophic lateral sclerosis. Ann Neurol. 2001;49:643–649. [PubMed] [Google Scholar]
- Fontana ACK, Guizzo R, Beleboni RO, Meirelles E, Silva AR, Coimbra NC, et al. Purification of a neuroprotective component of Parawixiabistriata spider venom that enhances glutamate uptake. Br J Pharmacol. 2003;139:1297–1309. doi: 10.1038/sj.bjp.0705352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau J, Robert T, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ganel R, Ho T, Maragakis NJ, Jackson M, Steiner JP, Rothstein JD. Selective up-regulation of the glial Na+-dependent glutamate transporter GLT1 by a neuroimmunophilin ligand results in neuroprotection. Neurobiol Dis. 2006;21:556–567. doi: 10.1016/j.nbd.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Ge S, Pachter JS. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine monocyte chemoattractant protein-1 by human astrocytes. J Biol Chem. 2004;279:6688–6695. doi: 10.1074/jbc.M311769200. [DOI] [PubMed] [Google Scholar]
- Gendreau S, Voswinkel S, Torres-Salazar D, Lang N, Heidtmann H, Detro-Dassen S, et al. A trimeric quaternary structure is conserved in bacterial and human glutamate transporters. J Biol Chem. 2004;279:39505–39512. doi: 10.1074/jbc.M408038200. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Nagiel A, Lin AJ, Golan DE, Michel T. Small interfering RNA-mediated down-regulation of caveolin-1 differentially modulates signaling pathways in endothelial cells. J Biol Chem. 2004;279:40659–40669. doi: 10.1074/jbc.M407051200. [DOI] [PubMed] [Google Scholar]
- González MI, Bannerman PG, Robinson MB. Phorbol myristate acetate-dependent interaction of protein kinase Cα and the neuronal glutamate transporter EAAC1. J Neurosci. 2003;23:5589–5593. doi: 10.1523/JNEUROSCI.23-13-05589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry. 2005;44:11913–11923. doi: 10.1021/bi050987n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol. 2005;203:1–20. doi: 10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Lai L, Butchbach MER, Lin C-LG. Human glioma cells and undifferentiated primary astrocytes that express aberrant EAAT2 mRNA inhibit normal EAAT2 protein expression and prevent cell death. Mol Cell Neurosci. 2002;21:546–560. doi: 10.1006/mcne.2002.1198. [DOI] [PubMed] [Google Scholar]
- Had-Aissouni L, Ré DB, Nieoullon A, Kerkerian-Le Goff L. Importance of astrocytic inactivation of synaptically released glutamate for cell survival in the central nervous system – are astrocytes vulnerable to low intracellular glutamate concentrations. J Physiol (Paris) 2002;96:317–322. doi: 10.1016/s0928-4257(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Lu C-C. GAT1 (GABA:Na+:Cl−) cotransport function: database reconstruction with an alternating access model. J Gen Physiol. 1999;114:459–476. doi: 10.1085/jgp.114.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisano S. Vesicular glutamate transporters in the brain. Anat Sci Int. 2003;78:191–204. doi: 10.1046/j.0022-7722.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Honig LS, Chambliss DD, Bigio EH, Carroll SL, Elliott JL. Glutamate transporter EAAT2 splice variants occur not only in ALS, but also in AD and controls. Neurology. 2000;55:1082–1088. doi: 10.1212/wnl.55.8.1082. [DOI] [PubMed] [Google Scholar]
- Hoogland G, van Oort RJ, Proper EA, Jansen GH, van Rijen PC, van Veelen CW, et al. Alternative splicing of glutamate transporter EAAT2 RNA in neocortex and hippocampus of temporal lobe epilepsy patients. Epilepsy Res. 2004;59:75–82. doi: 10.1016/j.eplepsyres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Huang YH, Sinha SR, Tanaka K, Rothstein JD, Bergles DE. Astrocyte glutamate transporters regulate metabotropic glutamate receptor-mediated excitation of hippocampal interneurons. J Neurosci. 2004;24:4551–4559. doi: 10.1523/JNEUROSCI.5217-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Gimenez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59:394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- Huggett J, Vaughan-Thomas A, Mason D. The open reading frame of the Na(+)-dependent glutamate transporter GLAST-1 is expressed in bone and a splice variant of this molecule is expressed in bone and brain. FEBS Lett. 2000;485:13–18. doi: 10.1016/s0014-5793(00)02175-x. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Maguire JL, McMinn MT, Scholz RE, Sutherland ML. Loss of glial fibrillary acidic protein results in decreased glutamate transport and inhibition of PKA-induced EAAT2 cell surface trafficking. Mol Brain Res. 2004;124:114–123. doi: 10.1016/j.molbrainres.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Dick KA, Weatherspoon MR, Gincel D, Armbrust KR, Dalton JC, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–190. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- Inage YW, Itoh M, Wada K, Takashima S. Expression of two glutamate transporters, GLAST and EAAT4, in the human cerebellum – their correlation in development and neonatal hypoxic–ischemic damage. J Neuropathol Exp Neurol. 1998;57:554–562. doi: 10.1097/00005072-199806000-00003. [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu M-Y, Jin L, Dykes-Hoberg M, Lin CL, et al. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- Janjua NA, Itano T, Kugoh T, Hosokawa K, Nakano M, Matsui H, et al. Familial increase in plasma glutamic acid in epilepsy. Epilepsy Res. 1992;11:37–44. doi: 10.1016/0920-1211(92)90019-p. [DOI] [PubMed] [Google Scholar]
- Jin XP, Peng JB, Huang F, Zhu YN, Fei J, Guo LH. A mRNA molecule encoding truncated excitatory amino acid carrier 1 (EAAC1) protein (EAAC2) is transcribed from an independent promoter but not an alternative splicing event. Cell Res. 2002;12:257–262. doi: 10.1038/sj.cr.7290132. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479:237–247. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- Kanner BI, Borre L. The dual-function glutamate transporters: structure and molecular characterisation of the substrate-binding sites. Biochim Biophys Acta. 2002;1555:92–95. doi: 10.1016/s0005-2728(02)00260-8. [DOI] [PubMed] [Google Scholar]
- Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, et al. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Koch HP, Kavanaugh MP, Esslinger CS, Zerangue N, Humphrey JM, Amara SG, et al. Differentiation of substrate and nonsubstrate inhibitors of the high-affinity, sodium-dependent glutamate transporters. Mol Pharmacol. 1999;56:1095–1104. doi: 10.1124/mol.56.6.1095. [DOI] [PubMed] [Google Scholar]
- Koch HP, Larsson HP. Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J Neurosci. 2005;25:1730–1736. doi: 10.1523/JNEUROSCI.4138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrande C, Velly L, Canolle B, Guillet B, Masmejean F, Nieoullon A, et al. Neuroprotective effects of tacrolimus (FK506) in a model of ischemic cortical cell cultures: role of glutamate uptake and FK506 binding protein 12 kDa. Neuroscience. 2006;137:231–239. doi: 10.1016/j.neuroscience.2005.08.080. [DOI] [PubMed] [Google Scholar]
- Larsson HP, Tzingounis AV, Koch HP, Kavanaugh MP. Fluorometric measurements of conformational changes in glutamate transporters. Proc Natl Acad Sci USA. 2004;101:3951–3956. doi: 10.1073/pnas.0306737101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderback CM, Harris-White ME, Wang YN, Pedigo NW, Carney JM, Butterfield DA. Amyloid beta-peptide inhibits Na+-dependent glutamate uptake. Life Sci. 1999;65:1977–1981. doi: 10.1016/s0024-3205(99)00459-2. [DOI] [PubMed] [Google Scholar]
- Legay V, Deleage C, Beaulieux F, Giraudon P, Aymard M, Lina B. Impaired glutamate uptake and EAAT2 downregulation in an enterovirus chronically infected human glial cell line. Eur J Neurosci. 2003;17:1820–1828. doi: 10.1046/j.1460-9568.2003.02621.x. [DOI] [PubMed] [Google Scholar]
- Li L-B, Toan SV, Zelenaia O, Watson DJ, Wolfe JH, Rothstein JD, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- Li S, Mallory M, Alford M, Tanaka S, Masliah E. Glutamate transporter alterations in Alzheimer disease are possibly associated with abnormal APP expression. J Neuropathol Exp Neurol. 1997;56:901–911. doi: 10.1097/00005072-199708000-00008. [DOI] [PubMed] [Google Scholar]
- Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Stys PK. Na+–K+–ATPase inhibition and depolarization induce glutamate release via reverse Na+-dependent transport in spinal cord white matter. Neuroscience. 2001;107:675–683. doi: 10.1016/s0306-4522(01)00385-2. [DOI] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. J Neurochem. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Liévens J-C, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, et al. Impaired glutamate uptake in the R6 Huntington's disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Lin CL, Orlov I, Ruggiero AM, Dykes-Hoberg M, Lee A, Jackson M, et al. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3–18. Nature. 2001;410:84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- Mallolas J, Hurtado O, Castellanos M, Blanco M, Sobrino T, Serena J, et al. A polymorphism in the EAAT2 promoter is associated with higher glutamate concentrations and higher frequency of progressing stroke. J Exp Med. 2006;203:711–717. doi: 10.1084/jem.20051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–477. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Marie H, Billups D, Bedford FK, Dumoulin A, Goyal RK, Longmore GD, et al. The amino terminus of the glial glutamate transporter GLT-1 interacts with the LIM protein Ajuba. Mol Cell Neurosci. 2002;19:152–164. doi: 10.1006/mcne.2001.1066. [DOI] [PubMed] [Google Scholar]
- Markert JM, Fuller CM, Gillespie GY, Bubien JK, Mclean LA, Hong RL, et al. Differential gene expression profiling in human brain tumors. Physiol Genomics. 2001;5:21–33. doi: 10.1152/physiolgenomics.2001.5.1.21. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, et al. Hypoxia–ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Deteresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, Mallory M, Rockenstein E, Moechars D, Van Leuven F. Abnormal glutamate transport function in mutant amyloid precursor protein transgenic mice. Exp Neurol. 2000;163:381–387. doi: 10.1006/exnr.2000.7386. [DOI] [PubMed] [Google Scholar]
- Massie A, Vandesande F, Arckens L. Expression of the high-affinity glutamate transporter EAAT4 in mammalian cerebral cortex. Neuroreport. 2001;12:393–397. doi: 10.1097/00001756-200102120-00041. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Mendoza D, Lozada A, Pretorius JK, Dehnes Y, Danbolt NC, et al. Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology. 1999;52:453–472. doi: 10.1212/wnl.52.3.453. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Enomoto T, Masuko T. Identification of truncated human glutamate transporter. Tohoku J Exp Med. 1999;187:173–182. doi: 10.1620/tjem.187.173. [DOI] [PubMed] [Google Scholar]
- Matute C, Domercq M, Sánchez-Gómez M-V. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Meyer T, Fromm A, Munch C, Schwalenstocker B, Fray AE, Ince PG, et al. The RNA of the glutamate transporter EAAT2 is variably spliced in amyotrophic lateral sclerosis and normal individuals. J Neurol Sci. 1999;170:45–50. doi: 10.1016/s0022-510x(99)00196-3. [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Plesko F, Vandenberg RJ. Zn2+ inhibits the anion conductance of the glutamate transporter EAAT4. J Biol Chem. 2001;276:26071–26076. doi: 10.1074/jbc.M011318200. [DOI] [PubMed] [Google Scholar]
- Mortensen OV, Amara SG. Dynamic regulation of the dopamine transporter. Eur J Pharmacol. 2003;479:159–170. doi: 10.1016/j.ejphar.2003.08.066. [DOI] [PubMed] [Google Scholar]
- Münch C, Ebstein M, Seefried U, Zhu B, Stamm S, Landwehrmeyer GB, et al. Alternative splicing of the 5′-sequences of the mouse EAAT2 glutamate transporter and expression in a transgenic model for amyotrophic lateral sclerosis. J Neurochem. 2002;82:594–603. doi: 10.1046/j.1471-4159.2002.01012.x. [DOI] [PubMed] [Google Scholar]
- Münch C, Penndorf A, Schwalenstocker B, Troost D, Ludolph AC, Ince P, et al. Impaired RNA splicing of 5′-regulatory sequences of the astroglial glutamate transporter EAAT2 in human astrocytoma. J Neurol Neurosurg Psychiatry. 2001;71:675–678. doi: 10.1136/jnnp.71.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch C, Zhu BG, Leven A, Stamm S, Einkorn H, Schwalenstocker B, et al. Differential regulation of 5′ splice variants of the glutamate transporter EAAT2 in an in vivo model of chemical hypoxia induced by 3-nitropropionic acid. J Neurosci Res. 2003;71:819–825. doi: 10.1002/jnr.10536. [DOI] [PubMed] [Google Scholar]
- Nagai M, Abe K, Okamoto K, Itoyama Y. Identification of alternative splicing forms of GLT-1 mRNA in the spinal cord of amyotrophic lateral sclerosis patients. Neurosci Lett. 1998;244:165–168. doi: 10.1016/s0304-3940(98)00158-x. [DOI] [PubMed] [Google Scholar]
- Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest Ophthalmol Vis Sci. 2000;41:1940–1944. [PubMed] [Google Scholar]
- Navarro A, Anand-Apte B, Parat M-O. A role for caveolae in cell migration. FASEB J. 2004;18:1801–1811. doi: 10.1096/fj.04-2516rev. [DOI] [PubMed] [Google Scholar]
- O'Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 2002;29:1018–1023. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- O'Shea RD, Lau CL, Farso MC, Diwakarla S, Zagami CJ, Svendsen BB, et al. Effects of lipopolysaccharide on glial phenotype and activity of glutamate transporters: evidence for delayed up-regulation and redistribution of GLT-1. Neurochem Int. 2006;48:604–610. doi: 10.1016/j.neuint.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Otis TS, Brasnjo G, Dzubay JA, Pratap M. Interactions between glutamate transporters and metabotropic glutamate receptors at excitatory synapses in the cerebellar cortex. Neurochem Int. 2004;45:537–544. doi: 10.1016/j.neuint.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Parton RG. Caveolae – from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Persson M, Brantefjord M, Hansson E, Rönnbäck L. Lipopolysaccharide increases microglial GLT-1 expression and glutamate uptake capacity in vitro by a mechanism dependent on TNF-alpha. Glia. 2005;51:111–120. doi: 10.1002/glia.20191. [DOI] [PubMed] [Google Scholar]
- Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL. Developmental expression of excitatory amino acid transporter 5: a photoreceptor and bipolar cell glutamate transporter in rat retina. Neurosci Lett. 2000;280:21–24. doi: 10.1016/s0304-3940(99)00988-x. [DOI] [PubMed] [Google Scholar]
- Pow DV, Naidoo T, Lingwood BE, Healy GN, Williams SM, Sullivan RK, et al. Loss of glial glutamate transporters and induction of neuronal expression of GLT-1B in the hypoxic neonatal pig brain. Dev Brain Res. 2004;153:1–11. doi: 10.1016/j.devbrainres.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Rae C, Lawrance ML, Dias LS, Provis T, Bubb WA, Balcar VJ. Strategies for studies of neurotoxic mechanisms involving deficient transport of L-glutamate: antisense knockout in rat brain in vivo and changes in the neurotransmitter metabolism following inhibition of glutamate transport in guinea pig brain slices. Brain Res Bull. 2000;53:373–381. doi: 10.1016/s0361-9230(00)00372-5. [DOI] [PubMed] [Google Scholar]
- Ré DB, Boucraut J, Samuel D, Birman S, Kerkerian-Le Goff L, Had-Aissouni L. Glutamate transport alteration triggers differentiation-state selective oxidative death of cultured astrocytes: a mechanism different from excitotoxicity depending on intracellular GSH contents. J Neurochem. 2003;85:1159–1170. doi: 10.1046/j.1471-4159.2003.01752.x. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Watkins JC. Structural requirements for the inhibition for L-glutamate uptake by glia and nerve endings. Brain Res. 1975;85:120–125. doi: 10.1016/0006-8993(75)91016-1. [DOI] [PubMed] [Google Scholar]
- Robinson MB. Regulated trafficking of neurotransmitter transporters: common notes but different melodies. J Neurochem. 2002;80:1–11. doi: 10.1046/j.0022-3042.2001.00698.x. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, et al. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Mitrovic AD, Vandenberg RJ. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem. 2004;279:20742–20751. doi: 10.1074/jbc.M304433200. [DOI] [PubMed] [Google Scholar]
- Sardar AM, Hutson PH, Reynolds GP. Deficits of NMDA receptors and glutamate uptake sites in the frontal cortex in AIDS. Neuroreport. 1999;10:3513–3515. doi: 10.1097/00001756-199911260-00009. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Lesch K-P, Kugler P. A splice variant of glutamate transporter GLT1/EAAT2 expressed in neurons: cloning and localization in rat nervous system. Neuroscience. 2002;109:45–61. doi: 10.1016/s0306-4522(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Zink M, Petroianu G, May B, Braus DF, Henn FA. Decreased gene expression of glial and neuronal glutamate transporters after chronic antipsychotic treatment in rat brain. Neurosci Lett. 2003;347:81–84. doi: 10.1016/s0304-3940(03)00653-0. [DOI] [PubMed] [Google Scholar]
- Scott HL, Pow DV, Tannenberg AEG, Dodd PR.Aberrant expression of the glutamate transporter excitatory amino acid transporter 1 (EAAT1) in Alzheimer's disease J Neurosci 200222RC206:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon AL, Gonzalez MI, Robinson MB. A carboxyl-terminal determinant of the neuronal glutamate transporter, EAAC1, is required for platelet-derived growth factor-dependent trafficking. J Biol Chem. 2006;281:4876–4886. doi: 10.1074/jbc.M504983200. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shimada F, Shiga Y, Morikawa M, Kawazura H, Morikawa O, Matsuoka T, et al. The neuroprotective agent MS-153 stimulates glutamate uptake. Eur J Pharmacol. 1999;386:263–270. doi: 10.1016/s0014-2999(99)00735-9. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, et al. DL-threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Otsubo Y, Shigeri Y, Yasuda-Kamatani Y, Satoh M, Kaneko S, et al. Characterization of the tritium labeled analog of L-threo-β-benzyloxyaspartate (TBOA) binding to glutamate transporters Mol Pharmacol 2006. doi:10.1124/mol.106.027250 [DOI] [PubMed]
- Shimamoto K, Sakai R, Takaoka K, Yumoto N, Nakajima T, Amara SG, et al. Characterization of novel L-threo-β-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol Pharmacol. 2004;65:1008–1015. doi: 10.1124/mol.65.4.1008. [DOI] [PubMed] [Google Scholar]
- Shouffani A, Kanner B. Cholesterol is required for the reconstruction of the sodium- and chloride-coupled, gamma-aminobutyric acid transporter from rat brain. J Biol Chem. 1990;265:6002–6008. [PubMed] [Google Scholar]
- Slotboom DJ, Konings WN, Lolkema JS. The structure of glutamate transporters shows channel-like features. FEBS Lett. 2001;492:183–186. doi: 10.1016/s0014-5793(01)02223-2. [DOI] [PubMed] [Google Scholar]
- Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry. 2001;158:1393–1399. doi: 10.1176/appi.ajp.158.9.1393. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64:1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs caveolae. Proc Natl Acad Sci USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Iversen LL. Uptake of [3H]glutamic acid in excitatory nerve endings: light and electronmicroscopic observations in the hippocampal formation of the rat. Neuroscience. 1979;4:1237–1243. doi: 10.1016/0306-4522(79)90154-4. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Rauen T, Fischer F, Wiessner M, Grewer C, Bicho A, et al. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45:155–169. doi: 10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Tatsu Y, Yumoto N, Nakajima T, Shimamoto K. Synthesis of carbamate-type caged derivatives of a novel glutamate transporter blocker. Bioorg Med Chem. 2004;12:3687–3694. doi: 10.1016/j.bmc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Shimamoto K, Tanaka K, Ozawa S. Glial glutamate transporters maintain one-to-one relationship at the climbing fiber–Purkinje cell synapse by preventing glutamate spillover. J Neurosci. 2006;26:6563–6572. doi: 10.1523/JNEUROSCI.5342-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Thai DR. Excitatory amino acid transporter EAAT-2 in tangle-bearing neurons in Alzheimer's disease. Brain Pathol. 2002;12:405–411. doi: 10.1111/j.1750-3639.2002.tb00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2:427–433. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- Umemura K, Gemba T, Mizuno A, Nakashima M. Inhibitory effect of MS-153 on elevated brain glutamate level induced by rat middle cerebral artery occlusion. Stroke. 1996;27:1624–1628. doi: 10.1161/01.str.27.9.1624. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416:312–316. doi: 10.1016/s0014-5793(97)01232-5. [DOI] [PubMed] [Google Scholar]
- Vallat-Decouvelaere AV, Chretien F, Gras G, Le Pavec G, Dormont D, Gray F. Expression of excitatory amino acid transporter-1 in brain macrophages and microglia of HIV-infected patients. A neuroprotective role for activated microglia. J Neuropathol Exp Neurol. 2003;62:475–485. doi: 10.1093/jnen/62.5.475. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Matute C. A novel alternative splicing form of excitatory amino acid transporter 1 is a negative regulator of glutamate uptake is a negative regulator of glutamate uptake. J Neurochem. 2005a;95:341–348. doi: 10.1111/j.1471-4159.2005.03370.x. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21:154–164. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Torres-Ramos M, Melone M, Conti F, Matute C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia. 2005b;50:276–279. doi: 10.1002/glia.20172. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Mitrovic AD, Chebib M, Balcar VJ, Johnston GAR. Contrasting modes of action of methylglutamate derivatives on the excitatory amino acid transporters, EAAT1 and EAAT2. Mol Pharmacol. 1997;51:809–815. doi: 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Mills CD, Ye Z, Fullwood SD, McAdoo DJ, Hulsebosch CE, et al. Rapid changes in expression of glutamate transporters after spinal cord injury. Brain Res. 2002;927:104–110. doi: 10.1016/s0006-8993(01)03329-7. [DOI] [PubMed] [Google Scholar]
- Vermeiren C, Najimi M, Vanhoutte N, Tilleux S, De Hemptinne I, Maloteaux J-M, et al. Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J Neurochem. 2005;94:405–416. doi: 10.1111/j.1471-4159.2005.03216.x. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, et al. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Yagihashi T, Kezuka J, Muramatsu D, Usui M, Iwasaki T. Glutamate levels in aqueous humor of patients with retinal artery occlusion. Retina. 2006;26:432–436. doi: 10.1097/00006982-200604000-00009. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, et al. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Westall FC, Hawkins A, Ellison GW, Myers LW. Abnormal glutamic acid metabolism in multiple sclerosis. J Neurol Sci. 1980;47:353–364. doi: 10.1016/0022-510x(80)90088-x. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Khabazian I, Pow DV, Craig UK, Shaw CA. Decrease in glial glutamate transporter variants and excitatory amino acid receptor down-regulation in a murine model of ALS-PDC. Neuromolecular Med. 2003;3:105–118. doi: 10.1385/NMM:3:2:105. [DOI] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine–glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- Yi JH, Pow DV, Hazell AS. Early loss of the glutamate transporter splice-variant GLT-1v in rat cerebral cortex following lateral fluid-percussion injury. Glia. 2005;49:121–133. doi: 10.1002/glia.20099. [DOI] [PubMed] [Google Scholar]
- Yoles E, Schwartz M. Elevation of intraocular glutamate levels in rats with partial lesion of the optic nerve. Arch Ophthalmol. 1998;116:906–910. doi: 10.1001/archopht.116.7.906. [DOI] [PubMed] [Google Scholar]
- Zagami CJ, O'Shea RD, Lau CL, Cheema SS, Beart PM. Regulation of glutamate transporters in astrocytes: evidence for a relationship between transporter expression and astrocytic phenotype. Neurotoxicol Res. 2005;7:143–149. doi: 10.1007/BF03033783. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]