Abstract
The Ca2+-selective TRPV6 as well as the L-type Ca2+ channel are regulated by the Ca2+-binding protein calmodulin (CaM). Here, we investigated the interaction of CaM with rat (r)TRPV6 in response to alterations of intracellular Ca2+, employing Ca2+-imaging and patch-clamp techniques. Additionally, confocal Förster resonance energy transfer (FRET) microscopy on living cells was utilized as a key method to visualize in vivo protein–protein interactions essential for CaM regulation of rTRPV6 activity. The effects of overexpressed CaM or its Ca2+-insensitive mutant (CaMMUT) was probed on various rTRPV6 mutants and fragments in an attempt to elucidate the molecular mechanism of Ca2+/CaM-dependent regulation and to pinpoint the physiologically relevant rTRPV6–CaM interaction site. A significant reduction of rTRPV6 activity, as well as an increase in current inactivation, were observed when CaM was overexpressed in addition to endogenous CaM. The Ca2+-insensitive CaMMUT, however, failed to affect rTRPV6-derived currents. Accordingly, live cell confocal FRET microscopy revealed a robust interaction for CaM but not CaMMUT with rTRPV6, suggesting a strict Ca2+ dependence for their association. Indeed, interaction of rTRPV6 or its C terminus with CaM increased with rising intracellular Ca2+ levels, as observed by dynamic FRET measurements. An rTRPV6Δ695–727 mutant with the very C-terminal end deleted, yielded Ca2+ currents with a markedly reduced inactivation in accordance with a lack of CaM interaction as substantiated by FRET microscopy. These results, in contrast with those for CaM-dependent L-type Ca2+ channel inactivation, demonstrate a dynamic association of CaM with the very C-terminal end of rTRPV6 (aa 695–727), and this enables acceleration of the rate of rTRPV6 current inactivation with increasing intracellular CaM concentrations.
TRPV6, a member of the vanilloid transient receptor potential (TRPV) family, plays an important role in Ca2+ transport across cell membranes. It is expressed in bone, as well as in intestine (Nijenhuis et al. 2003) and human lymph node prostate cancer (LNCaP) cells (Vanden Abeele et al. 2003). This highly Ca2+-selective channel is known to be regulated by Ca2+ and the Ca2+-binding protein calmodulin (CaM) (Niemeyer et al. 2001; Hirnet et al. 2003; Lambers et al. 2004).
Ca2+/CaM-dependent channel gating is well known from various other channels (Saimi & Kung, 2002). In voltage-gated Ca2+ channels, such as L-type channels (a member of Cav1.2 channels), Ca2+-free CaM (apoCaM) is constitutively (i.e. permanently) associated with the C-terminus of the α1c subunit, and Ca2+ influx triggers Ca2+/CaM-dependent channel inactivation (Erickson et al. 2003; Halling et al. 2005). A similar regulatory mechanism where CaM is preassociated is supposed for canonical TRP (TRPC) channels. TRPC3 in particular, but also other TRPCs (Ordaz et al. 2005; Zhu, 2005), are assumed to be regulated by a competitive interaction of the inositol trisphosphate receptor (IP3R) and CaM at the same site on the TRPC C-terminus. CaM is suggested to keep the channel in the inactivated state, whereas its displacement by the IP3R activates it (Tang et al. 2001; Zhang et al. 2001).
Various biochemical and electrophysiological studies on the TRPV6 channel suggest that Ca2+/CaM binding to TRPV6 plays an important role in channel regulation (Niemeyer et al. 2001; Wissenbach et al. 2001). Flockerzi's group (Niemeyer et al. 2001) identified a 1-5-10 CaM binding motif on the C-terminus of human (h)TRPV6 (698–707 aa, accession no. Q9H1D0) to which CaM binds in the presence but not absence of Ca2+, similar to the mouse (m)TRPV6 (CaM-binding site: 700–710 aa, accession no. Q91WD2) (Hirnet et al. 2003). Recently, Lambers et al. (2004) identified several CaM-binding sites on the mTRPV6 protein by the use of in vitro binding assays. These sites were located at the N-terminus (aa 88–97, 1-5-10 motif), the transmembrane region (aa 327–577) and the C-terminus (aa 643–656, 1-8-14 motif), where the corresponding N- and C-terminal CaM-binding sites are preserved in rat (r)TRPV6. This C-terminally located CaM-binding motif (aa 643–656), as reported by Lambers et al. (2004), is clearly distinct from those determined for the mouse (aa 700–710) and human (aa 698–707) TRPV6 (Niemeyer et al. 2001). Furthermore, biochemical experiments have suggested that CaM may be constitutively bound to mTRPV6 at resting-cell Ca2+ concentrations (Lambers et al. 2004), in contrast to previous studies on hTRPV6 (Niemeyer et al. 2001).
To clarify the in vivo relevance of the proposed TRPV6/CaM inactivation sites that have been identified mainly from in vitro experiments, this study aimed at the visualization and characterization of the dynamic interaction of CaM with rTRPV6 in living cells, and its dependence on the intracellular Ca2+ concentration; results were correlated with rTRPV6 current inactivation. These results led to a model that opposes Ca2+/CaM-dependent inactivation of the voltage-gated L-type with that of rTRPV6 Ca2+ channels.
Methods
Cell culture and molecular cloning
Experiments were performed on HEK 293 cells cultured according to Schindl et al. (2002). HEK 293 cells were grown in an incubator (Forma Scientific) under a humidified (95%) atmosphere containing 5% CO2, and a temperature of 37°C. In all experiments the rat (r)TRPV6 construct (accession no. AF160798, kindly provided by M. Hediger, University of Berne, Switzerland) was used. The coding region of rTRPV6 was cleaved from pTracer-CMV2 (Invitrogen) and transferred to the plasmid of pEYFP-C1 (Clontech, Germany). For subcloning of rTRPV6 the coding region was cleaved with the restriction enzymes NaeI and XbaI, and the purified fragment was ligated with SmaI- and XbaI-digested pEYFP-C1. This resulted in N-terminally tagged EYFP-constructs. The C-terminally truncated rTRPV6 channel (or C-deletion mutant, aa 1–586) was cloned to the plasmid pTracer-CMV2 using the restriction enzymes KpnI and BsrBI (1763 bp). Further subcloning in pEYFP-C1 was carried out with the restriction enzymes NaeI and XbaI. For preparation of the N154-rTRPV6 fragment, pTracer-CMV2-rTRPV6 was digested with KpnI and StuI. The construct was subcloned in pTracer-CMV2, which was digested with KpnI and EcoRV. From this new pTracer-CMV2-N154-rTRPV6, the coding region of N154-rTRPV6 was cleaved (NaeI and XbaI) and subcloned into the pEYFP-C1 vector (SmaI and XbaI). The C-terminal fragment (aa 601–727) of rTRPV6 was cloned to the plasmid pTracer-CMV2 using the restriction enzymes BsrBI and XbaI. An ORF (open reading frame) was verified by applying the ORF finder from PubMed (http://www.ncbi.nlm.nih.gov). Further subcloning to plasmids pEYFP-C1 was made with the restriction enzymes EcoRI and XbaI. The integrity of the rTRPV6-containing plasmids was confirmed by qualitative restriction digests and sequence analysis (VBC Genomics, Bioscience Research GmBH, Vienna, Austria).
The C-terminal deletion mutant TRPV6Δ695–727 was constructed by the internal restriction enzyme MfeI (2084 bp) and transferred to the plasmids pTracer-CMV-II and pECFP-C1.
Preparation of pcDNA3-CFP/YFP-CaM and pcDNA3-CFP/YFP-CaMMUT, encoding CaM and CaMMUT, respectively, was performed as described in Poteser et al. (2003). The CaM mutant (CaMMUT) has mutations introduced on all four EF hands, rendering it insensitive to Ca2+.
pcDNA3-77, encoding the voltage-gated L-type Ca2+ channel α1C,77 subunit, has been described in Romanin et al. (2000). pcDNA3-β2a and pcDNA3-α2-δ, encoding subunits of the L-type ion channel, were kindly provided by Franz Hofmann (Institute of Pharmacology, Munich).
Transfection
Transfection of HEK cells (Schindl et al. 2002) was performed using either SuperFect (Qiagen, Germany) or TransFectin (Biorad, Germany), with the corresponding plasmids. Measurements were carried out 24–72 h following transfection.
Electrophysiology
Electrophysiological experiments were performed (Schindl et al. 2002) at 20–24°C using the patch-clamp technique (Hamill et al. 1981) in the whole-cell recording configuration. For TRPV6 current measurements, voltage ramps were usually applied every 5 s from a holding potential of +70 mV, covering a range of −90 to 90 mV over 200 ms. Voltage steps to −80 mV (850 ms) were applied from a holding potential of +70 mV every 5 s. The internal pipette solution contained (mm) 3.5 MgCl2, 145 caesium methane sulphonate, 8 NaCl, 10 Hepes and 10 EGTA, pH 7.2. The extracellular solution consisted of (mm) 145 NaCl, 5 CsCl, 1 MgCl2, 10 Hepes, 10 glucose, and 10 CaCl2 or 10 BaCl2, pH 7.4. For experiments with the L-type Ca2+ channel, voltage steps to +30 mV were applied from a holding potential of −70 mV. The same external and internal solutions as for TRPV6 measurements were used, while 3 mm MgATP was included in the latter (pH adjusted to 7.2 by CsOH). The internal solution with 10 mm BAPTA is identical to that described in Lambers et al. (2004). The CaM antagonist calmidazolium (CMZ), and the Ca2+ ionophore ionomycin, were solubilized at 10 mm in DMSO, and the applied concentrations were 1 and 10 μm, respectively.
Confocal Förster resonance energy transfer (FRET) fluorescence microscopy
Tranfected HEK cells grown on coverslips for 1–2 days were transferred to an extracellular solution consisting of (mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose and 10 Hepes, pH 7.4 (NaOH). The Ca2+-free extracellular solution was the same as that described above, but omitting Ca2+. The 1 mm EGTA extracellular solution contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 1 EGTA, 10 glucose and 10 Hepes, pH 7.4 (NaOH).
A QLC100 Real-Time Confocal System (VisiTech International Ltd, Sunderland, UK) was used for recording fluorescence images, and was connected to two Photometrics CoolSNAPHQ monochrome cameras (Roper Scientific, USA). This system was attached to an Axiovert 200M microscope (Zeiss, Germany) in conjunction with an argon ion multiwavelength (457, 488, 514 nm) laser (Laser Physics, Inc., Salt Lake City, UT, USA). The wavelengths were selected by an Acousto Optical Tuneable Filter (VisiTech). The dual port adapter contains a 505lp dichroic, a 485/30 cyan emission filter and a 535/50 yellow emission filter (Chroma Technology Corp., Rockingham, VT, USA). MetaMorph 6.0 software (Universal Imaging Corp., West Chester, USA) was used to acquire images and to control the confocal system. Illumination times for CFP/FRET and YFP images recorded consecutively with a minimum delay were about 900 ms. The FRET image had to be corrected due to a cross-talk from one channel to the other. For this, the corrected FRET image (NFRET) was calculated after background subtraction and threshold determination using custom-made software (Kahr et al. 2004) integrated in MatLab 6 according to the following equation (Xia & Liu, 2001):
IFRET denotes the background corrected intensity of the FRET image (excitation 457 nm, emission 535 nm), IYFP is the background-corrected intensity of the YFP image (excitation 514 nm, emission 535 nm), and ICFP is the background-corrected intensity of the CFP image (excitation 457 nm, emission 485 nm). Prior to the calculation, the images were treated as follows. A threshold was determined after background subtraction, and it was set to 17.5% of the maximum intensity of the respective images. Excluding lower intensity values substantially eliminates false positive/negative FRET values from the calculation. The CalCFP factor and the CalYFP factor were obtained for each experiment from separate samples where only CFP- or YFP-tagged protein was expressed. The local ratio between CFP and YFP might vary due to different localizations of diverse protein constructs, which could lead to the calculation of false FRET values (Berney & Danuser, 2003). Accordingly, the analysis was limited to pixels with a CFP:YFP ratio between 1:10 and 10:1 (Berney & Danuser, 2003) to yield reliable results. Expression levels of rTRPV6 proteins compared with their fragments varied on average by a factor of 2. Fluorescence intensity measurements of CFP/YFP-tagged CaM and CaMMUT exhibited comparable single-cell expression levels with a slightly lower amount (∼0.45, n = 9–15 cells) of fluorescent-tagged CaM compared with CaMMUT. Consistent results were found by semiquantitative Western blot measurements employing an anti-GFP antibody (Roche Applied Science, catalogue no. 118144600001).
Ca2+ fluorescence measurements
HEK cells were grown on coverslips for 1 day and loaded with fura-2/AM (1 μm) for 20 min at 20°C in Dulbecco's modified Eagle's Medium, washed for three times, and dyes were allowed to de-esterify for 15 min at 20°C. Coverslips were transferred to an extracellular solution without Ca2+ and mounted at an inverted Axiovert 100 TV microscope (Zeiss, Germany). Excitation of Fura-2 was performed at 340 and 380 nm, and Ca2+ measurements are shown as 340/380 ratios of HEK cells. Absolute Ca2+ concentrations were calculated according to Grynkiewicz et al. (1985), with an effective KD as determined for living cells in Negulescu & Machen (1990).
Statistics
Results are presented as means ± s.e.m. calculated for the indicated number of experiments. Student's two-tailed t test was used for statistical comparison considering differences statistically significant at P < 0.05.
Results
Overexpression of CaM reduces rTRPV6 activity
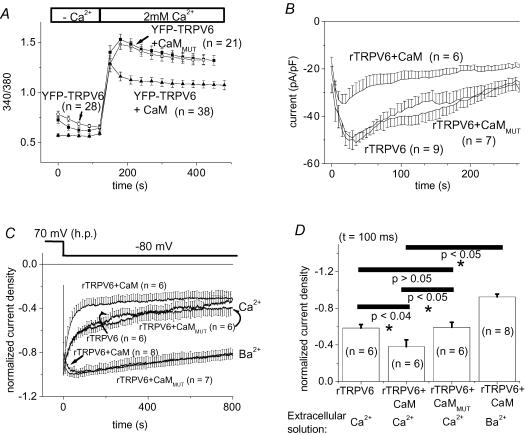
To evaluate the overall effect of CaM on rTRPV6 activity, we compared Ca2+ entry, as well as membrane currents, from HEK 293 cells containing endogenous CaM, with those overexpressing CaM. In the latter, as monitored by Fura-2 fluorescence microscopy, the rTRPV6-derived Ca2+ plateau reached after addition of Ca2+ was significantly reduced compared with cells containing endogenous CaM only (Fig. 1A). Expression of CaMMUT, defective in Ca2+ binding, yielded an rTRPV6-derived Ca2+ influx that was not significantly different to that observed in the presence of endogenous CaM.
Figure 1. Effect of calmodulin (CaM) on rTRPV6 activity and rTRPV6 current inactivation.
In addition to rTRPV6 protein, CaM or the Ca2+-insensitive CaMMUT were coexpressed in HEK 293 cells. A, intracellular [Ca2+], represented by the ratio of 340/380 of Fura-2-loaded HEK cells, was monitored initially in a nominally Ca2+-free extracellular solution, followed by a solution including 2 mm Ca2+ under each condition as stated above. B, time courses of constitutively active whole-cell Ca2+ currents measured from repetitive voltage ramps at −74 mV were compared for rTRPV6-expressing cells when CFP, CFP-CaM or CFP-CaMMUT were coexpressed. C, normalized Ca2+- and Ba2+-current traces (10 mm Ca2+/Ba2+) of cells expressing rTRPV6 and CaM or CaMMUT in response to a voltage step to −80 mV from a holding potential of +70 mV. D, statistical comparison of normalized currents at t = 100 ms from data depicted in C. With Ba2+ as the charge carrier, the currents of rTRPV6 + CaM and rTRPV6 + CaMMUT (not shown in figure) were not significantly different at t = 100 ms. YFP-labelled rTRPV6 and CFP-labelled CaM/CaMMUT yielded results that were identical to the unlabelled forms.
Complementary electrophysiological studies were carried out to analyse rTRPV6 activity under analogous conditions, as in the preceding Ca2+-imaging experiments (Fig. 1B). The time course of the constitutively active rTRPV6 Ca2+ currents, as determined from repetitive voltage-ramps at −74 mV, exhibited a mono-phasic inactivation behaviour after an initial transient increase in current density in control cells expressing only endogenous CaM. We have previously shown that this biphasic behaviour is dependent on the holding potential applied (Schindl et al. 2002). Overexpression of wild-type CaM revealed a similar activation/inactivation profile, but current densities were at all time points significantly lower than in the control. Overexpression of CaMMUT led to substantially higher current densities than those obtained with cells overexpressing CaM, reaching levels that were not significantly different to those of control cells (Fig. 1B), contrary to the results of Lambers et al. (2004). These results indicate that an increase in intracellular CaM concentration decreases the overall level of rTRPV6 activity, whereas CaMMUT does not seem to interfere in terms of a competitive interaction with the endogenous CaM.
In a further analysis of this Ca2+/CaM-dependent effect, rapid inactivation of rTRPV6 currents was examined during a hyperpolarizing test pulse to −80 mV. rTRPV6 current inactivation within the 850 ms test pulse occurred significantly faster, particularly during the first 100 ms when CaM was overexpressed compared with CaMMUT or endogenous CaM (Fig 1C and D). The pronounced inactivation of currents from rTRPV6-, rTRPV6 + CaM- and rTRPV6 + CaMMUT-expressing cells reached after 800 ms was almost abolished when Ba2+, which is also permeable through rTRPV6 channels, was substituted for Ca2+ in the extracellular solution (Fig 1C and D). These findings of modulation of rTRPV6 current inactivation by CaM but not CaMMUT expression levels suggest a Ca2+-dependent interaction of CaM with rTRPV6, thereby promoting its inactivation. This lack of effect of CaMMUT, which is contrary to its inhibitory effect on Ca2+-dependent inactivation of L-type Ca2+ channels (Romanin et al. 2000; Erickson et al. 2001, 2003), further implied a distinct mechanism for rTRPV6 inactivation.
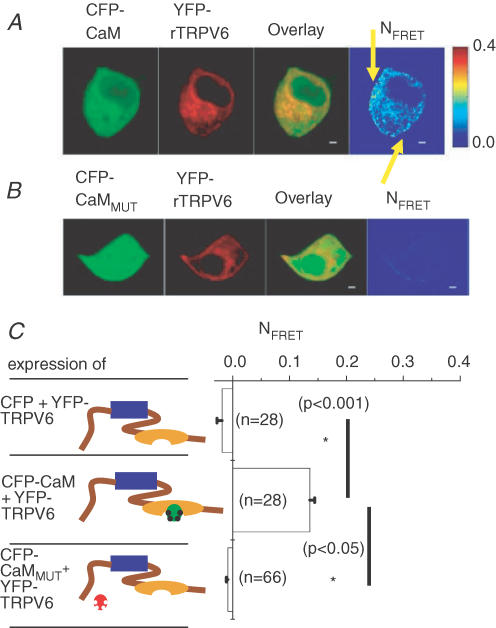
Hence, to relate these functional electrophysiological effects to an interaction of rTRPV6 with CaM, confocal FRET microscopy of living cells was employed to visualize this association, and consequently to localize the in vivo relevant interaction site within the rTRPV6 protein. Experiments with CFP-CaM as the donor and YFP-rTRPV6 as the acceptor were initially carried out in a Ca2+-containing extracellular solution that enables constitutive Ca2+ entry through rTRPV6 channels, as also known from patch-clamp studies (Schindl et al. 2002). As an interaction of CaMMUT with mTRPV6 has been recently reported (Lambers et al. 2004), we examined for a potential association despite a lack of effect of CaMMUT on rTRPV6 activity as shown above.
Both CaM and its mutant form CaMMUT, each fluorescent-labelled, showed comparable expression levels (see also Methods) as well as uniform distribution when coexpressed with rTRPV6 (Fig 2A and B). Semi-quantitative Western blots normalized both to protein content and transfection efficiency yielded a CaM:CaMMUT ratio of ∼0.7 (n = 3, data not shown). In both CaM or CaMMUT overexpressing cells, rTRPV6 seemed to colocalize with the respective CaMs in the cytosol and plasma membrane. However, a substantial FRET signal (Fig. 2C) was obtained only from cells expressing rTRPV6 and CaM, while those expressing CaMMUT exhibited a similarly low FRET value as control cells (YFP-rTRPV6 + CFP). It seems that TRPV6–CaM interaction not only occurred at the plasma membrane, but could also take place on TRPV6 localized in the cytoplasmic space promoted by increased intracellular Ca2+ levels due to constitutive TRPV6-derived Ca2+ entry. Thus, the FRET values given include both that of the plasma membrane as well as the cytoplasmic space. These results suggest that in living cells CaM but not CaMMUT binds in a Ca2+-dependent fashion onto rTRPV6 which provides constitutive Ca2+ entry, in line with Ca2+-imaging and electrophysiological experiments presented in Fig. 1.
Figure 2. rTRPV6 interaction with CaM or CaMMUT.
A, image series depicting CFP-CaM, YFP-rTRPV6, overlay and pixelwise-calculated NFRET for a representative cell. The yellow arrows denote plasma membrane localization. B, a similar image series shows a representative cell coexpressing CFP-CaMMUT and YFP-rTRPV6. C, mean NFRET determined from the averages of whole-cell areas for the respective number of cells expressing the depicted constructs; blue box, transmembrane region; brown line, N-/C-terminus; orange ellipse, CaM-binding domain on the C-terminus; green symbol, CaMWT without bound Ca2+; green symbol with black dots, CaMWT with bound Ca2+; red symbol, CaMMUT without bound Ca2+. FRET experiments were carried out in an extracellular solution containing 2 mm Ca2+.
At this point, it remains unclear by which site within the rTRPV6 protein CaM exerts its functional effect. In the following section, confocal FRET microscopy was employed under conditions that enabled us to resolve dynamic CaM/rTRPV6 interaction and its dependence on intracellular Ca2+ concentrations, with the aim of localizing the relevant CaM-binding site in living cells.
Interaction of rTRPV6 C-terminus and CaMs
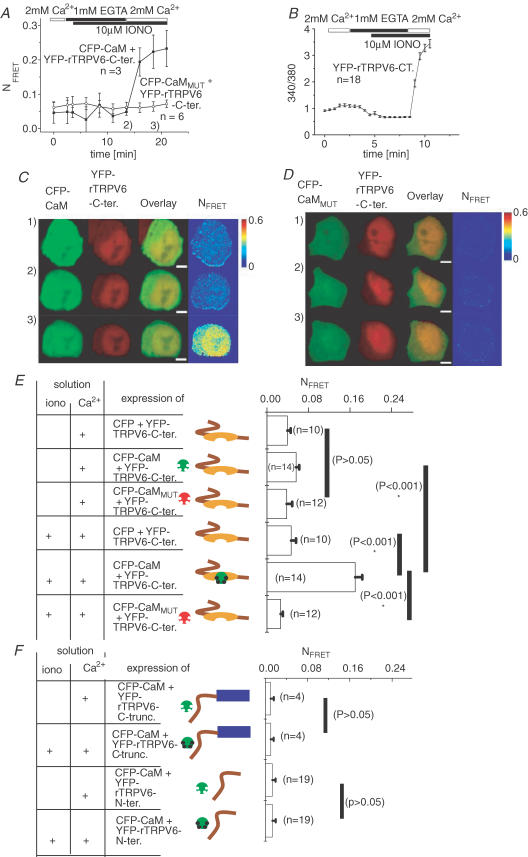
The following set of experiments, as illustrated in Fig. 3, focused on resolving the association of CaM with rTRPV6 C-terminus, which represents a favourite candidate based on previous studies from other groups (Niemeyer et al. 2001; Lambers et al. 2004). As expression of the rTRPV6 C-terminus alone does not provide the elevated intracellular Ca2+ levels observed with the whole rTRPV6 protein, ionomycin was used as a surrogate to trigger Ca2+ entry; this allowed the monitoring of the interaction of CaM with the rTRPV6 C-terminus, with increasing intracellular Ca2+ levels. To additionally evaluate the extent to which CaM was already prebound to rTRPV6 at resting-cell Ca2+ concentrations, intracellular Ca2+ levels were initially decreased below resting cell levels by the presence of both ionomycin and EGTA, as shown in Fig. 3A. Using the same protocol in Ca2+-imaging experiments (Fig. 3B) revealed a reduction in intracellular Ca2+ level clearly below its resting cell level, from ∼100 nm in 2 mm extracellular Ca2+ to <25 nm Ca2+ in the presence of EGTA and ionomycin. The concomitant live cell FRET image series (Fig. 3A and C), which enabled us to monitor CaM interaction with the rTRPV6 C-terminus, exhibited no significant decrease in FRET when lowering intracellular Ca2+ concentrations below resting cell Ca2+. The subsequent increase in intracellular Ca2+ was accompanied by a rapid increase in FRET, reflecting the profound CaM association with the rTRPV6 C-terminus in a similar way to that observed with the whole rTRPV6 (compare Fig. 3B with Fig. 2A). In contrast, the CaMMUT together with rTRPV6 C-terminus, under either condition, did not show a substantial alteration in FRET (Fig. 3A and D). Besides cytosolic expression, the rTRPV6 C-terminus represents nuclear localization to some extent, a phenomenon that might deserve further attention as it is reminiscent to a potentially important nuclear signalling mechanism reported for the C-terminus of the L-type Ca2+ channel (Dolmetsch, 2003). Nevertheless, Ca2+-dependent binding of CaM to the rTRPV6 C-terminal fragment was clearly visible in the cytosolic compartment, suggesting that its conformation is similar in the whole TRPV6 protein.
Figure 3. rTRPV6 C-terminus and CaM/CaMMUT interaction and its dependence on intracellular Ca2+ concentration.
A, time course of mean NFRET from HEK 293 cells coexpressing YFP-rTRPV6 C-terminus with either CFP-CaM or CFP-CaMMUT, monitored initially in 2 mm Ca2+-containing solution, followed by the addition of ionomycin (10 μm) in a solution containing 1 mm EGTA or 2 mm Ca2+ solution. B, intracellular [Ca2+]i represented by the ratio 340/380 of FURA-2-loaded HEK cells expressing YFP-rTRPV6 C-terminus was monitored during manipulations of intracellular Ca2+, as described in A. C and D, corresponding time courses of a live cell image series depicting representative cells coexpressing CFP-CaM (C) or CFP-CaMMUT (D) together with YFP-rTRPV6 C-terminus. Time points (1, 2 and 3) when images were taken corresponding to the conditions indicated in A. The white scale bar represents 4.1 μm. E and F, mean NFRET determined from the averages of whole-cell areas for the respective number of cells expressing the depicted constructs at resting cell (Ca2+: +) and elevated (iono: +; Ca2+: +) intracellular Ca2+ concentrations. The symbols are as described for Fig. 2.
Figure 3E summarizes the Ca2+-dependent alterations in FRET as a measure of the interaction between the rTRPV6 C-terminal fragment and CaM, as well as CaMMUT, and for the negative control experiments with free CFP. At resting cellular Ca2+ concentrations, the FRET values determined for the rTRPV6 C-terminus with CaM or CaMMUT were not significantly different to each other, and or compared with the negative control with CFP, suggesting a lack of interaction of CaM or CaMMUT with the rTRPV6 C-terminus. A further decrease of intracellular Ca2+ concentrations beyond resting cell levels did not significantly alter the FRET signal, consistent with a lack of TRPV6–CaM interaction at these low Ca2+ concentrations. With increasing intracellular Ca2+ levels, a significant increase in FRET was observed for CaM but not CaMMUT coexpressed with the rTRPV6 C-terminus, indicating the latter as an important structure providing the relevant CaM-binding domain for the strictly Ca2+-dependent interaction with CaM.
As an additional control, we tested for a CaM interaction of an rTRPV6 C-terminal deletion mutant (rTRPV6ΔC-terminus), in which the whole C-terminus (aa 587–727) was truncated. As this rTRPV6ΔC-terminus mutant formed no functional channel (data not shown), intracellular Ca2+ levels were similarly increased with ionomycin. Co-expression of rTRPV6ΔC-terminus with CaM yielded only a very weak FRET signal at basal intracellular Ca2+ concentrations, and this was not significantly changed after ionomycin-induced elevation of intracellular Ca2+ levels (Fig. 3F), confirming the role of the rTRPV6 C-terminus in the Ca2+-dependent interaction with CaM.
Interaction of rTRPV6 N-terminus and CaM
Since Lambers et al. (2004) have reported an additional N-terminal CaM-binding site (aa 88–97) on mTRPV6, as revealed by an in vitro GST-pulldown assay, we examined this proposed interaction in living cells by analysing FRET of the relevant N-terminal fragment (N154-rTRPV6) with CaM, both at resting and following ionomycin-induced elevation of intracellular Ca2+ concentrations. The N154-rTRPV6 fragment and CaM were colocalized within the cytoplasmic space, enabling a potential interaction. However, we failed to detect an alteration in FRET upon increasing intracellular Ca2+ levels (Fig. 3F), suggesting a minor role of the in vitro characterized (Lambers et al. 2004) N-terminally located CaM-binding site in living cells.
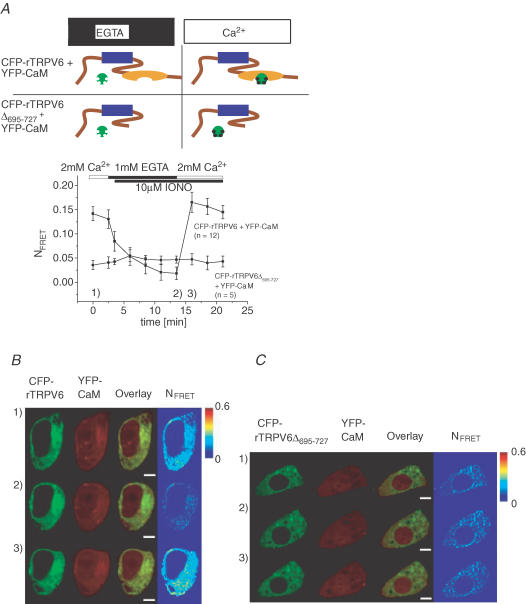
Localization of the in vivo functional CaM interaction site on the rTRPV6
Since our experiments in living cells showed a Ca2+-dependent CaM binding predominantly to the C-terminal fragment of rTRPV6, a more physiological rTRPV6 deletion mutant, missing the final 695–727 aa in the C terminus (rTRPV6Δ695–727), based on a report on hTRPV6 (Niemeyer et al. 2001; Hirnet et al. 2003), was examined for its potential interaction with CaM (Fig. 4). To elucidate whether CaM is able to associate in a Ca2+-dependent manner with rTRPV6Δ695–727 in comparison with rTRPV6, ionomycin together with EGTA were used to dynamically manipulate intracellular Ca2+ concentrations independent of the constitutive activity (see below Fig. 5) of both rTRPV6 channels. Reduction of the intracellular Ca2+ levels by ionomycin + EGTA led to a substantial decrease of FRET between rTRPV6 and CaM, followed by a strong increase in FRET when ionomycin + Ca2+ was applied (Fig. 4A and B). Here we used a YFP-CaM that showed uniform distribution when coexpressed with either CFP-rTRPV6 (Fig. 4B) or CFP-rTRPV6Δ695–727 (Fig. 4C). In contrast, these manipulations of the intracellular Ca2+ levels failed to affect FRET between rTRPV6Δ695–727 and CaM (Fig. 4A and C), suggesting that the deleted portion of aa 695–727 at the very end of rTRPV6 is functionally essential for CaM interaction at increased Ca2+. Consistently manipulating intracellular Ca2+ levels in an identical way when rTRPV6Δ695–727 was coexpressed with CaMMUT similarly failed to significantly change the FRET value (data not shown) in line with our experiments shown in Fig. 2.
Figure 4. Dynamic CaM association onto rTRPV6 or rTRPV6Δ695–727 and its dependence on intracellular Ca2+ concentration.
A, time course of mean NFRET recorded from HEK 293 cells that coexpressed YFP-CaM with either CFP-rTRPV6 or CFP-rTRPV6Δ695–727, where intracellular Ca2+ was manipulated by ionomycin in the absence (EGTA) and presence of extracellular Ca2+ as described in Fig. 3. B and C, corresponding time courses of a live cell image series depicting representative cells coexpressing CFP-rTRPV6 (B) or CFP-rTRPV6Δ695–727 (C) together with YFP-CaM. Time points (1, 2 and 3) when images were taken corresponding to conditions indicated in A. The white scale bars in B and C represent 4.1 μm. The symbols are as described for Fig. 2.
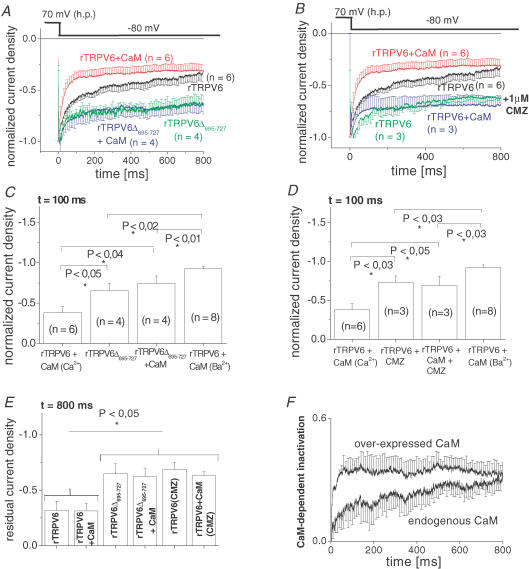
Figure 5. Comparison of current inactivation of rTRPV6Δ695–727 and rTRPV6 in the presence of CMZ.
A, inactivation profile of current traces from rTRPV6- or rTRPV6Δ695–727-expressing HEK 293 cells, with or without coexpression of CaM, recorded in response to a voltage step to −80 mV from a holding potential of +70 mV. B, inactivation profile of current traces from rTRPV6-expressing cells, with or without CaM coexpressed in the absence and presence of calmidazolium (CMZ; 1 μm). C and D, significance of inactivation at t = 100 ms determined for traces as depicted in A and B, respectively. E, normalized residual currents at t = 800 ms determined for the various conditions in A and B. F, time courses of relative CaM-dependent inactivation of rTRPV6 currents, without and with CAM overexpressed. The respective inactivation profile was obtained by subtracting current trace of rTRPV6Δ695–727 mutant from rTRPV6 either without or with CaM overexpressed. Respective error bars were calculated by error propagation.
In a complementary set of electrophysiological experiments, rTRPV6Δ695–727 currents were analysed for their Ca2+/CaM-dependent inactivation in the presence of endogenous or overexpressed CaM, as well as CaMMUT. Inactivation of these rTRPV6Δ695–727-derived currents during a 850 ms test pulse to −80 mV was substantially reduced compared with wild-type rTRPV6 (Fig. 5A and C) and reached a similar level in endogenous or overexpressed CaM levels (Fig. 5C). This profound reduction in Ca2+/CaM-dependent inactivation suggested the final rTRPV6 C-terminal stretch (aa 695–727) to be a functionally significant CaM-binding domain.
In an alternative approach to interfere with the action of CaM on rTRPV6, the CaM-antagonist calmidazolium (CMZ) was employed (Tang et al. 2001; Zhang et al. 2001; Shi et al. 2004; Zhang & Barritt, 2004). Notably, CMZ appears to be ineffective if CaM is constitutively tethered to ion channels such as the L-type Ca2+ channel or the Ca2+-activated K+ channel (Xia et al. 1998; Zuhlke & Reuter, 1998; Zuhlke et al. 1999; Litjens et al. 2004). Application of CMZ led to a substantial reduction in the rTRPV6 Ca2+-dependent inactivation (Fig. 5B and D), resulting in an inactivation profile similar to that observed with the rTRPV6Δ695–727 mutant form that lacks the relevant CaM-binding site.
In addition to this CaM-dependent inactivation, a more rapid inactivation process that occurred independent of CaM (compare columns 2 and 3 in Fig. 5C and D) remained in both the rTRPV6Δ695–727 mutant and the CMZ-treated rTRPV6. This was apparently mediated by Ca2+, as evident from a comparison with rTRPV6 current inactivation with Ba2+ as charge carrier (compare Figs 1C and 5B and D). Calculation of the respective inactivation at 15 ms (Niemeyer et al. 2001) for each current trace, as shown in Fig. 5A and B, did not reveal a significant difference (data not shown), suggesting that this Ca2+-dependent inactivation occurred independently of the CaM-dependent process.
Quantitative evaluation of the CaM-dependent fraction of rTRPV6 inactivation
For a more quantitative delineation of the inactivation process dominated by CaM, quasi steady-state inactivation was determined as the residual current at 800 ms (Fig. 5E). These normalized residual current densities, as depicted in Fig. 5E for the various experimental conditions, clearly showed a similar residual current of rTRPV6 or rTRPV6 + CaM at 800 ms that was significantly smaller than those of rTRPV6Δ695–727 and CMZ-treated-TRPV6. The larger residual currents of the two latter, corresponding to a reduced inactivation, reflect the elimination of the CaM-dependent fraction of inactivation, while preserving the Ca2+-dependent fraction. This separation of the Ca2+- from the CaM-dependent process enabled us to estimate the time course of the CaM-dependent fraction of rTRPV6 inactivation, particularly its dependence on intracellular CaM concentration. For this, the current trace of rTRPV6Δ695–727 was subtracted from that of rTRPV6 or rTRPV6 + CaM, as shown in Fig. 5F. It was evident that the CaM-dependent fraction of inactivation, both in the presence of endogenous as well as overexpressed CaM, reached a similar level after 800 ms. However, on a shorter time scale (at ∼100 ms), inactivation showed an obvious dependence on intracellular CaM levels in a way that higher CaM levels, obtained by overexpression of CaM, substantially enhanced the rate by which steady-state inactivation was reached.
These observations underline the assumption of a dynamic but not constitutive CaM association with rTRPV6 that is strictly dependent on the intracellular Ca2+ concentration and is speeded up with increasing intracellular CaM levels.
Discussion
Our study demonstrates a dynamic but not constitutive (i.e. permanent) interaction of CaM with rTRPV6 in living cells. This regulatory binding process, which occurs when intracellular Ca2+ concentrations increase, involves the very end (aa 695–727) of rTRPV6 C-terminus and correlates with rTRPV6 current inactivation. Other potential interaction domains that have been delineated from in vitro experiments (Niemeyer et al. 2001; Lambers et al. 2004), such as the N-terminus or the transmembrane domain of rTRPV6, are not functionally relevant for Ca2+ current inactivation in vivo. A Ca2+-insensitive CaMMUT neither interacts with rTRPV6 in living cells nor functionally affects its currents. In parallel to this Ca2+/CaM-dependent inactivation, a Ca2+-induced CaM-independent inactivation of rTRPV6 activity also occurs.
So far, two studies of TRPV6 regulation by CaM have been presented: One study by Niemeyer et al. (2001) describes Ca2+/CaM-dependent inactivation of hTRPV6 that is regulated by protein kinase C (PKC)-dependent phosphorylation, while the other report by Lambers et al. (2004) proposes that CaM positively affects mTRPV6 activity in contrast with the Ca2+-insensitive CaMMUT, which inhibits TRPV6 activity. Additionally, the latter report suggests a constitutive tethering of CaM to the mTRPV6 channel complex at resting cell Ca2+ concentrations (60–100 nm).
With the present study, we provide, utilizing a combined approach of patch-clamp and live cell FRET imaging experiments, strong evidence of a dynamic, Ca2+-dependent interaction of rTRPV6 with CaM. This interaction is triggered by the Ca2+ influx through rTRPV6, thereby promoting its inactivation. Moreover, overexpression of CaM significantly produced inactivation of currents compared with that observed in the presence of endogenous CaM. In contrast to the report by Lambers et al. (2004), a CaMMUT lacking all four Ca2+-binding sites neither interacted with the rTRPV6 channel complex in vivo, nor altered inactivation of rTRPV6 currents or their current densities. As different intracellular solutions were used in that study (Lambers et al. 2004) and our study (10 mm BAPTA versus 10 mm EGTA, respectively), experiments were repeated in conditions identical to those by Lambers et al. (2004). Nevertheless, CaM-overexpressing cells inactivated significantly faster and showed lower current densities than those expressing CaMMUT (data not shown), leaving the discrepancy between these studies open.
CaM was reported to interact with a 1-5-10 (698–707 aa) motif at the very end of the C-terminus of hTRPV6 (Niemeyer et al. 2001), while for mTRPV6 an N-terminal, a transmembrane domain, and a 1-8-14 (643–656 aa)-binding domain in the C-terminus have been additionally characterized by in vitro biochemical experiments (Lambers et al. 2004). While the 1-5-10 motif is conserved between human, mouse and rat TRPV6, the 1-8-14 motif is only present in rTRPV6 and mTRPV6. To analyse the functional relevance of these domains for their possible interaction with CaM in living cells, we studied the N-terminal fragment N154-rTRPV6, the rTRPV6 deletion mutant that lacked the whole C-terminus, and the C-terminus of rTRPV6, in their response to cytosolic Ca2+ levels, monitoring CaM association by confocal FRET microscopy. Neither the N-terminal fragment nor the rTRPV6 protein lacking the whole C-terminus responded by an increase in the FRET value with increasing intracellular Ca2+ concentrations, suggesting their in vivo involvement as functionally relevant CaM-binding domains to be highly unlikely. One might argue that tagging of the much smaller N154-terminal rTRPV6 fragment with YFP could impede interaction with CaM due to steric hindrance or misfolding. However, both the N-terminus, as well as its tagged form, are equally effective as dominant-negative species in blocking rTRPV6 currents (Kahr et al. 2004), rendering conformational mismatches highly unlikely.
The whole rTRPV6 protein, and correspondingly its C-terminus, were clearly found to associate with CaM when intracellular Ca2+ levels increased, indicating a dynamic Ca2+-dependent interaction. At resting cellular Ca2+ levels or even below, the very low FRET signals suggest that CaM is not preassociated with rTRPV6 C-terminus, consistent with a previous report (Niemeyer et al. 2001) in which no CaM binding was observed at resting cytosolic Ca2+ levels in in vitro binding studies. To pinpoint the in vivo functionally relevant CaM-binding site, a C-terminal rTRPV6 deletion mutant (TRPV6Δ695–727) was used that lacked the 1-5-10 motif, but not the 1-8-14 site, on the C-terminus. This deletion mutant failed to interact with CaM when Ca2+ was elevated, as revealed by FRET microscopy. In accordance, inactivation of rTRPV6Δ695–727 currents was largely reduced, substantiating that the 1-5-10 but not the 1-8-14 CaM-binding motif, which has been identified in vitro (Lambers et al. 2004), markedly contributed to rTRPV6 current inactivation in living cells. Moreover, a significant in vivo role of the latter, and the additional CaM-binding sites located in the TRPV6 N-terminus and the TRPV6 intramembrane portion, as recently found by in vitro techniques (Lambers et al. 2004), appears highly unlikely, as the inactivation profile obtained with rTRPV6Δ695–727 showed no dependence on intracellular CaM expression levels in contrast with wild-type rTRPV6, suggesting that the inactivation processes left did not involve CaM. It is important to note that our negative FRET experiments cannot completely rule out that a small amount of CaM may also interact in vivo with these sites. Nonetheless, in the context of the electrophysiological experiments, these sites are apparently not functionally important for rTRPV6 Ca2+ current inactivation.
rTRPV6 current inactivation has been proposed to consist of a Ca2+-dependent and a CaM-dependent phase (Nilius et al. 2000, 2001). Here, the CaM antagonist CMZ, as well as the rTRPV6Δ695–727 deletion mutant, were employed to eliminate the CaM-dependent inactivation. rTRPV6 currents in the presence of CMZ were inactivated to a similar extent as the rTRPV6Δ695–727 deletion mutant, substantiating the predominant role of the C-terminal stretch from aa 695–727 in functionally mediating CaM-dependent inactivation in accordance with Niemeyer et al. (2001). The initial inactivation phase that persisted with Ca2+ as the charge carrier was, however, effectively eliminated using Ba2+, suggesting an additional process that was Ca2+-dependent only (Niemeyer et al. 2001; Nilius et al. 2002, 2003)
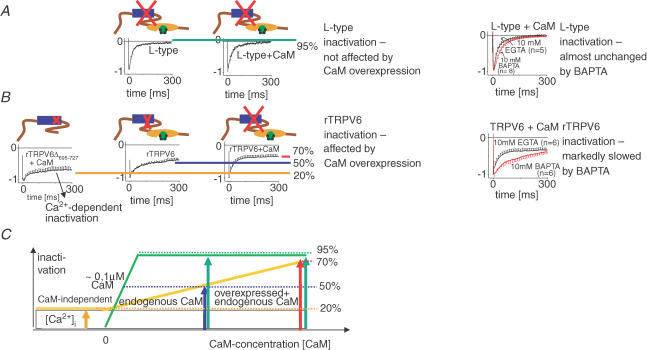
In the following, a model (Fig. 6) is presented that compares Ca2+/CaM-dependent inactivation of rTRPV6 with that of voltage-gated L-type Ca2+ channels. Manipulation of the intracellular CaM concentrations clearly revealed the difference in this Ca2+/CaM-dependent regulatory process. L-type Ca2+ channels showed a similar inactivation in the presence of endogenous CaM, as well as overexpressed CaM (Fig. 6A, left), while CaMMUT substantially reduced current inactivation (Romanin et al. 2000; Erickson et al. 2001). In sharp contrast, rTRPV6 currents inactivated similarly in the presence of endogenous CaM or overexpressed CaMMUT, whereas inactivation occurred to a larger extent when CaM was overexpressed (Fig. 6B, left). This different behaviour of rTRPV6 versus L-type Ca2+ channel inactivation when CaMMUT is overexpressed reflects the distinct mechanism of Ca2+/CaM-dependent inactivation of these two Ca2+ channel types, i.e. dynamic versus constitutive association of CaM. This is consistently evident from the effect on the inactivation profile of both Ca2+ channel types when EGTA, which we usually employed as intracellular Ca2+ buffer, was substituted by BAPTA (Fig. 6A and B, right). L-type channel inactivation remained almost unchanged, while rTRPV6 inactivation was markedly slowed in the presence of BAPTA. This might be explained by the concept of a subplasmalemmal Ca2+ microdomain that is formed upon Ca2+ entry through open Ca2+ channels, even in the presence of high Ca2+-chelator concentrations (Smith et al. 1996a). Calculations for a Ca2+ domain formed near a 0.1 pA source suggest (Smith et al. 1996a) increases to ∼1 μm Ca2+ at a distance of 200 nm in the presence of 10 mm EGTA. In the presence of the kinetically faster BAPTA, such a Ca2+ amount is reached only at a shorter distance of about 30 nm. The Ca2+ affinity of CaM, which is in the sub to low micromolar range of 5 × 10−7 to 5 × 10−6m (Chin & Means, 2000), fits with the aforementioned Ca2+ concentration reached in the microdomains. As apo-CaM is constitutively tethered to the C-terminus of the L-type α1C subunit (Erickson et al. 2001, 2003), and is thus very close to the channel mouth, sufficiently high Ca2+ concentrations for CaM-dependent L-type channel inactivation are obtained almost independent of the Ca2+ chelator used. In contrast, dynamic association of cytosolic CaM with rTRPV6 requires an increase in intracellular Ca2+ concentrations at distances further away from the channel. Such increases, as suggested by the previous calculations (Smith et al. 1996a), occur in the presence of EGTA and to a lesser extent with BAPTA, which is correspondingly seen by its inhibitory effect on rTRPV6 inactivation.
Figure 6. A quantitative model comparing Ca2+-dependent inactivation of L-type Ca2+channel with that of rTRPV6 channel.
A, right, inactivation profiles of L-type Ca2+ channel currents in response to a voltage step to +30 mV without (left) and with CaM (right) overexpressed. Left, L-type channel inactivation profile in the presence of 10 mm EGTA in comparison with 10 mm BAPTA. B, right, inactivation profiles of Ca2+ currents in response to a voltage step to −80 mV through rTRPV6Δ695–727 (left), rTRPV6 without (middle), and with (right) CaM overexpressed. The size of red ‘X’ corresponds to the extent of current inactivation. Left, TRPV6 channel inactivation profile in the presence of 10 mm EGTA in comparison with 10 mm BAPTA. C, the magnitude of rTRPV6 inactivation is composed of a fixed merely Ca2+-dependent proportion, and a variable CaM-dependent fraction that is enhanced with increasing intracellular CaM concentrations accelerating CaM/rTRPV6 association. The green line and green arrows correspond to the degree of inactivation of L-type currents. Likewise, the yellow line and the orange/blue/red/arrows represent the extent of inactivation of TRPV6 currents. The symbols are as described for Fig. 2.
At resting-cell Ca2+, CaM is apparently not preassociated with rTRPV6, suggesting a KD several fold higher than intracellular CaM concentrations determined as ∼1–10 μm (Saimi & Kung, 2002), in contrast to a KD in the 0.1 μm range calculated for CaM–L-type Ca2+ channel interaction (Erickson et al. 2003).
For both Ca2+ channels, inactivation during the first ∼15 ms is similarly rapid, requiring a mechanism for rTRPV6 beyond the slower Ca2+-driven CaM association. This initial phase is achieved by a potentially direct interaction with Ca2+ (Fig. 6B, left) that may involve transmembrane domains 2 and 3 of TRPV6 (Nilius et al. 2002).
In conclusion, two processes of Ca2+-dependent inactivation of rTRPV6 (Fig. 6C) that involve (i) merely Ca2+, and (ii) Ca2+/CaM, enable a similar inactivation profile as obtained for the L-type Ca2+ channel with CaM pre-tethered. While an increase in intracellular Ca2+ is directly translated into rTRPV6 inactivation, the intracellular CaM concentration exerts an additional modulatory impact, as higher levels could substantially increase the on-rate for Ca2+–CaM association with the rTRPV6 C-terminus, thereby further accelerating the rate of rTRPV6 current inactivation. Local enrichment of CaM concentrations has been recently observed with L-type Ca2+ channels (Mori et al. 2004). Alternatively, biological ageing, as well as oxidative stress, have been found to selectively oxidize CaM at sites that may critically regulate its function (Squier, 2001). These changes in effective CaM concentrations could lead to alterations in rTRPV6 current inactivation that may affect cellular Ca2+ homeostasis (Smith et al. 1996b).
Acknowledgments
We thank S. Buchegger and B. Kenda for excellent technical assistance. Isabella Derler is a graduate student with a scholarship from the Austrian Academy of Sciences. Martin Muik is a graduate student within the PhD Program W1201 ‘Molecular Bioanalytics’ from the Austrian Science Foundation (FWF). This work was supported by the Austrian Science Foundation (FWF): projects P14950 and P18280 to K.G., and projects P15387 and P16537, as well as subproject 11 within W1201, to C.R.
References
- Berney C, Danuser G. FRET or no FRET: a quantitative comparison. Biophys J. 2003;84:3992–4010. doi: 10.1016/S0006-3495(03)75126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- Dolmetsch R. Excitation-transcription coupling: signaling by ion channels to the nucleus. Sci STKE. 2003;2003:PE4. doi: 10.1126/stke.2003.166.pe4. [DOI] [PubMed] [Google Scholar]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE. 2005;2005:re15. doi: 10.1126/stke.3152005re15. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirnet D, Olausson J, Fecher-Trost C, Bodding M, Nastainczyk W, Wissenbach U, Flockerzi V, Freichel M. The TRPV6 gene, cDNA and protein. Cell Calcium. 2003;33:509–518. doi: 10.1016/s0143-4160(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Kahr H, Schindl R, Fritsch R, Heinze B, Hofbauer M, Hack ME, Mortelmaier MA, Groschner K, Peng JB, Takanaga H, Hediger MA, Romanin C. CaT1 knock-down strategies fail to affect CRAC channels in mucosal-type mast cells. J Physiol. 2004;557:121–132. doi: 10.1113/jphysiol.2004.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ. Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. J Biol Chem. 2004;279:28855–28861. doi: 10.1074/jbc.M313637200. [DOI] [PubMed] [Google Scholar]
- Litjens T, Harland ML, Roberts ML, Barritt GJ, Rychkov GY. Fast Ca2+-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J Physiol. 2004;558:85–97. doi: 10.1113/jphysiol.2004.065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- Negulescu PA, Machen TE. Lowering extracellular sodium or pH raises intracellular calcium in gastric cells. J Membr Biol. 1990;116:239–248. doi: 10.1007/BF01868463. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Bergs C, Wissenbach U, Flockerzi V, Trost C. Competitive regulation of CaT-like-mediated Ca2+ entry by protein kinase C and calmodulin. Proc Natl Acad Sci U S A. 2001;98:3600–3605. doi: 10.1073/pnas.051511398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijenhuis T, Hoenderop JG, Van Der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol. 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ. Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J Biol Chem. 2002;277:30852–30858. doi: 10.1074/jbc.M202418200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G. Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol. 2000;527:239–248. doi: 10.1111/j.1469-7793.2000.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ. The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem. 2001;276:1020–1025. doi: 10.1074/jbc.M006184200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Weidema F, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Droogmans G, Bindels RJ. The carboxyl terminus of the epithelial Ca2+ channel ECaC1 is involved in Ca2+-dependent inactivation. Pflugers Arch. 2003;445:584–588. doi: 10.1007/s00424-002-0923-9. [DOI] [PubMed] [Google Scholar]
- Ordaz B, Tang J, Xiao R, Salgado A, Sampieri A, Zhu MX, Vaca L. Calmodulin and calcium interplay in the modulation of TRPC5 channel activity: Identification of a novel C-terminal domain for calcium/calmodulin-mediated facilitation. J Biol Chem. 2005;280:30788–30796. doi: 10.1074/jbc.M504745200. [DOI] [PubMed] [Google Scholar]
- Poteser M, Wakabayashi I, Rosker C, Teubl M, Schindl R, Soldatov NM, Romanin C, Groschner K. Crosstalk between voltage-independent Ca2+ channels and L-type Ca2+ channels in A7r5 vascular smooth muscle cells at elevated intracellular pH: evidence for functional coupling between L-type Ca2+ channels and a 2-APB-sensitive cation channel. Circ Res. 2003;92:888–896. doi: 10.1161/01.RES.0000069216.80612.66. [DOI] [PubMed] [Google Scholar]
- Romanin C, Gamsjaeger R, Kahr H, Schaufler D, Carlson O, Abernethy DR, Soldatov NM. Ca2+ sensors of L-type Ca2+ channel. FEBS Lett. 2000;487:301–306. doi: 10.1016/s0014-5793(00)02361-9. [DOI] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Annu Rev Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Schindl R, Kahr H, Graz I, Groschner K, Romanin C. Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca2+-release-activated Ca2+ channel) channels. J Biol Chem. 2002;277:26950–26958. doi: 10.1074/jbc.M203700200. [DOI] [PubMed] [Google Scholar]
- Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK 293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer's. Nature. 1996b;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Smith GD, Wagner J, Keizer J. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys J. 1996a;70:2527–2539. doi: 10.1016/S0006-3495(96)79824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J Biol Chem. 2001;276:21303–21310. doi: 10.1074/jbc.M102316200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Roudbaraki M, Shuba Y, Skryma R, Prevarskaya N. Store-operated Ca2+ current in prostate cancer epithelial cells. Role of endogenous Ca2+ transporter type 1. J Biol Chem. 2003;278:15381–15389. doi: 10.1074/jbc.M212106200. [DOI] [PubMed] [Google Scholar]
- Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, Reus K, Meese E, Bonkhoff H, Flockerzi V. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- Xia Z, Liu Y. Reliable and global measurement of fluorescence resonance energy transfer using fluorescence microscopes. Biophys J. 2001;81:2395–2402. doi: 10.1016/S0006-3495(01)75886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Barritt GJ. Evidence that TRPM8 is an androgen-dependent Ca2+ channel required for the survival of prostate cancer cells. Cancer Res. 2004;64:8365–8373. doi: 10.1158/0008-5472.CAN-04-2146. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A. 2001;98:3168–3173. doi: 10.1073/pnas.051632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX. Multiple roles of calmodulin and other Ca2+-binding proteins in the functional regulation of TRP channels. Pflugers Arch. 2005;451:105–115. doi: 10.1007/s00424-005-1427-1. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Reuter H. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc Natl Acad Sci U S A. 1998;95:3287–3294. doi: 10.1073/pnas.95.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]