Abstract
A Cre/lox-conditional mouse line was generated to evaluate the role of ATR in checkpoint responses to ionizing radiation (IR) and stalled DNA replication. We demonstrate that after IR treatment, ATR and ATM each contribute to early delay in M-phase entry but that ATR regulates a majority of the late phase (2–9 h post-IR). Double deletion of ATR and ATM eliminates nearly all IR-induced delay, indicating that ATR and ATM cooperate in the IR-induced G2/M-phase checkpoint. In contrast to the IR-induced checkpoint, checkpoint delay in response to stalled DNA replication is intact in ATR knockout cells and ATR/ATM and ATR/p53 double-knockout cells. The DNA replication checkpoint remains intact in ATR knockout cells even though the checkpoint-stimulated inhibitory phosphorylation of Cdc2 on T14/Y15 and activating phosphorylation of the Chk1 kinase no longer occur. Thus, incomplete DNA replication in mammalian cells can prevent M-phase entry independently of ATR and inhibitory phosphorylation of Cdc2. When DNA replication inhibitors are removed, ATR knockout cells proceed to mitosis but do so with chromosome breaks, indicating that ATR provides a key genome maintenance function in S phase.
Keywords: ATR, ATM, checkpoints, Chk1, Cdc2, chromosome breaks
DNA damage checkpoints are feedback mechanisms that sense the physical state of the genome. Should the integrity of the genome be compromised, checkpoint proteins signal these events and thereby prevent initiation of the next cell cycle phase. To do so, DNA damage checkpoint proteins interface with and inhibit the Cdk/cyclin machinery, the normal function of which is to activate and coordinate genome duplication and partitioning (Zhou and Elledge 2000; Nyberg et al. 2002). In mammalian cells, two important regulators of DNA damage checkpoints are ATR and ATM, unconventional protein kinases that phosphorylate and activate signal transduction pathways that ultimately interface with the Cdk/Cyclin machinery.
ATR and ATM are structurally and functionally similar to two checkpoint genes in yeast, MEC1 (Saccharomyces cerevisiae) and RAD3 (Schizosaccharomyces pombe), which regulate analogous pathways to control the cell cycle machinery. However, whereas Mec1 and Rad3 regulate nearly all responses to damage or inhibited DNA replication in their respective organisms, checkpoint regulation in mammals appears to be unevenly divided between ATR and ATM. Based mostly on studies of signal transduction pathways activated upon DNA damage and stalled DNA replication, ATR has been shown to regulate responses to a broad range of damage, including pyrimidine dimers, stalled replication, and double-stand breaks (DSBs). ATM, on the other hand, seems to be more specifically involved in responses to DSBs (Zhou and Elledge 2000; Nyberg et al. 2002). For example, through dominant-negative overexpression studies, ATR has been implicated in the regulation of IR- and ultraviolet light (UV)-induced phosphorylation of p53 on serine 15 (S15) and UV- and aphidicolin-induced phosphorylation of the Chk1 protein kinase on S345 (Tibbetts et al. 1999; Liu et al. 2000; Zhao and Piwnica-Worms 2001). In addition, a recently generated Cre/lox-conditional cell line has been used to demonstrate that ATR is required for phosphorylation of Rad17 in response to UV (Zou et al. 2002). In contrast to ATR deficiency, ATM loss predominantly affects responses to DSBs, such as IR-induced phosphorylation of the Chk2 protein kinase, S15 p53, NBS1, and other proteins (Matsuoka et al. 1998; Kastan and Lim 2000).
Although the effects of ATR dominant-negative overexpression and ATM loss on signaling pathways have led to a relatively simple model of checkpoint regulation, the comparative requirements for ATR and ATM in ultimately preventing cell cycle progression has remained less clear. For example, although both ATR and ATM have each been shown to play a role in preventing mitotic entry in response to IR (Cliby et al. 1998; Cortez et al. 2001; Xu et al. 2002), it is unknown whether ATR and ATM regulate this response in an additive or epistatic manner or whether one protein may play a more important role than the other. Moreover, ATR's involvement in preventing mitotic entry in response to stalled DNA replication has remained particularly obscure. Although in vitro experiments using Xenopus extracts have shown an important role for ATR in preventing nuclear envelope breakdown upon inhibition of DNA synthesis (Guo et al. 2000; Hekmat-Nejad et al. 2000), systems using dominant-negative ATR overexpression have led to contradictory results in mammalian cells (Cliby et al. 1998; Nghiem et al. 2001, 2002). At the present time, it is not clear whether these contradictory results may be caused by differences in the degree of dominant-negative overexpression or in the undefined genetic background of the tumor cell lines used. Adding to the complexity of ATR's role in the DNA replication checkpoint are recent studies in yeast that imply a role for ATR in preventing DSBs in response to stalled replication (Lopes et al. 2001; Cha and Kleckner 2002). Because one would then expect stalled replication forks to be converted into DSBs in the absence of ATR, it is difficult to predict if the DNA replication checkpoint would be eliminated by ATR deficiency. Loss of genome stability in ATR mutants could contribute to cell cycle inhibition upon replication arrest and do so in an ATR-independent manner.
To compare the role of ATR and ATM in cell cycle checkpoint control, a mouse line expressing a Cre/lox-conditional allele of ATR was generated. This system allowed comparison of checkpoint requirements for ATR and ATM in cells that are untransformed and of an isogenic background. Using ATRflox/−, ATM−/−, and ATRflox/− ATM−/− murine embryonic fibroblasts (MEFs), we have assessed the roles of ATR and ATM in checkpoint responses to IR and stalled DNA replication. In response to IR, we show that ATR contributes to preventing mitotic entry in a time-dependent manner, regulating the late phase of the response and cooperating with ATM in the early phase. Because little or no delay is observed in IR-treated ATR/ATM double knockouts, these results indicate that together these genes regulate a majority of the response to IR that inhibits mitotic entry. However, in contrast to IR-induced checkpoint responses, we have found that delayed mitotic entry in response to stalled DNA replication occurs even when ATR and ATM are deleted. This delay is evident despite the fact that ATR is required both for phosphorylation of Chk1 and for inhibitory phosphorylation of Cdc2 in response to either IR or stalled replication. Furthermore, although we find that DSBs are, indeed, generated specifically in ATR knockout cells upon DNA replication stalling, these breaks themselves do not prevent mitotic entry once DNA replication inhibitors are removed. These data show that both IR- and aphidicolin-induced checkpoints use ATR for signaling events that ultimately lead to inhibitory phosphorylation of Cdc2; however, in response to stalled replication, at least one additional mechanism must be at work in preventing mitotic entry.
Results
Generation of a lox-conditional allele of ATR
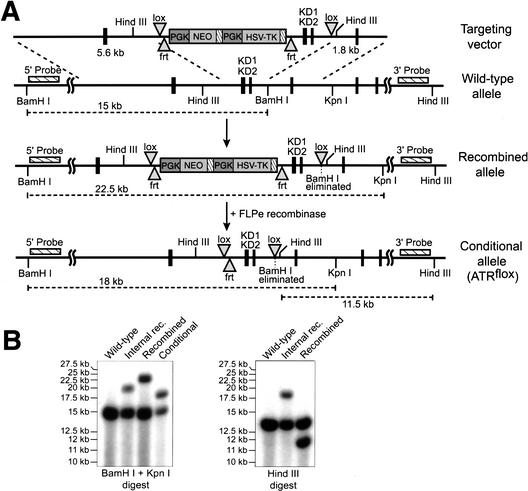
Previous ATR knockout studies demonstrated that ATR is required for genomic stability in the early embryo and that its loss leads to early embryonic lethality (Brown and Baltimore 2000). To further explore the cellular functions of ATR in cell cycle regulation and genome maintenance, a Cre/lox-conditional allele of ATR was generated in mice. Murine genomic clones of the 3′ end of the ATR locus were mapped and sequenced to reveal two exons encoding essential components of the ATR kinase domain (KD1 and KD2), including the catalytic residues conserved in human ATR, D2475 and D2494 (Fig. 1A). lox sites were placed on each side of a 1.2-kb region that includes KD1 and KD2 in the ES cell targeting vector shown (Fig. 1A). Upon lox recombination of this allele, KD1 and KD2 deletion and a subsequent frameshift in 3′ exons is predicted, thereby truncating the ATR gene product 5′ of KD1 (amino acid 2398). Following transfection and selection of D3 ES cells, recombination into the ATR locus was confirmed by Southern blot hybridization to two probes generated from sequences 5′ and 3′ of the targeted region; detection of a 22.5-kb BamHI–KpnI (5′-probe) and an 11.5-kb HindIII (3′-probe) band indicated appropriate recombination (Fig. 1A,B, Recombined). Note that when recombinatorial crossover occurs within the 1.2-kb KD1/KD2 region rather than outside of it, then 19.5-kb BamHI–KpnI and 18-kb HindIII bands are generated (Fig. 1A,B, Internal rec.). Appropriately targeted ES cells were then transfected with a vector expressing FLP recombinase (Buchholz et al. 1998) to remove the selection cassette by frt recombination (Fig. 1A). Removal of the selection cassette resulted in an allele in which KD1 and KD2 are flanked by lox sites and only a single frt site remains (Fig. 1A). This final configuration was detected by a 4.5-kb decrease in the BamHI–KpnI fragment length to 18 kb (Fig. 1A,B, Conditional). An ES cell clone with this configuration was subsequently used to generate chimeric mice, 3 in 10 of which transmitted the lox-conditional ATR allele through the germ line. In the studies described below, this conditional allele is abbreviated as ATRflox, and the following lox recombination is referred to as ATRΔ.
Figure 1.
Generation of a floxed conditional allele of ATR (ATRflox). (A) Schematic representations of the targeting vector, wild-type ATR locus, recombined locus, and the final conditional allele of ATR (ATRflox) are shown. Following confirmation by Southern blot, the ES cells with a recombined ATR allele were transfected with the pCAGGS-FLPe expression vector (Buchholz et al. 1998) to remove the selection cassette. Removal of the selection cassette results in the lox-conditional (ATRflox) allele shown. (B) Confirmation of the ATRflox allele by Southern blot. DNA prepared from ES cell clones was digested with BamHI + KpnI or HindIII alone and Southern-blotted for hybridization with the 5′ and 3′ probes indicated in A, respectively. DNA fragments representing wild-type, internally recombined, recombined, and ATRflox alleles are discussed in the text and indicated in A.
ATRΔ/− cells divide normally 1–2 times after ATR loss and then exit the cell cycle
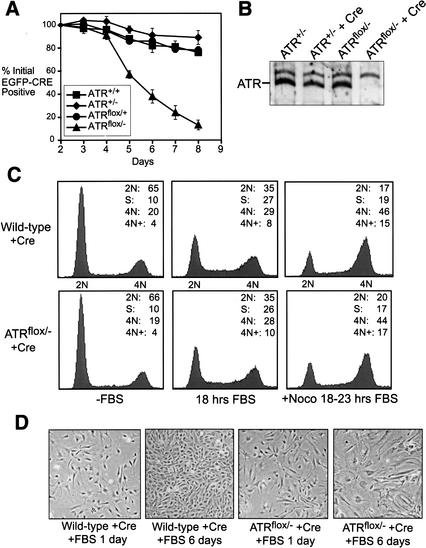
To study the effect of ATR loss in primary cell proliferation, embryonic day 14.5 (E14.5) embryos isolated from parental crosses of ATRflox/+ with ATR+/− (Brown and Baltimore 2000) mice were used to generate wild-type, ATR+/−, ATRflox/+, and ATRflox/− murine embryonic fibroblasts (MEFs). Previously, we have shown that ATR is required for proliferation of early embryonic cells in culture (Brown and Baltimore 2000). To test if E14.5 MEFs also require ATR, the ATRflox/− and control cells described above were infected with a lentivirus that expresses EGFP fused to a nuclear-localized form of Cre recombinase (NLS-Cre). Cells initially expressing the EGFP–Cre fusion protein were then followed for continued representation in proliferating cultures by flow cytometry over the course of 8 d (Fig. 2A). A selective loss of ATRflox/− cells expressing EGFP–Cre was observed 5 to 8 d after infection. However, because lox recombination was typically complete within 36 h of Cre expression (data not shown), these results indicated that ATR-depleted cells may divide normally soon after ATR depletion, but ultimately exit the cell cycle.
Figure 2.
ATRΔ/− cells divide and then exit the cell cycle. (A) Asychronously expanding ATRflox/− MEFs expressing EGFP–Cre are selectively lost from culture. The cells that continue to express EGFP–Cre over the course of 8 d of growth were quantitated as a percentage of their initial representation 1 d after infection with lenti-EGFP–Cre. ▪, wild type; ♦, ATR+/−; ●, ATRflox/+; ▴, ATRflox/−. (B) Western blot quantitation of ATR protein levels in ATRflox/− and ATR+/− MEFs 60 h after NLS-Cre expression (lenti-Cre). (C) Cell cycle analysis of lenti-Cre-infected wild-type and ATRflox/− MEFs. DNA content was determined by propidium iodide staining and flow cytometric analysis. (D) Exit of ATRΔ/− cells from the cell cycle. Cells treated as in C are shown after 1 and 6 d of FBS stimulation. Cells were split 1:4 at day 3.
To test this possibility, ATRflox/− and wild-type cells were synchronized in G0 by culturing in reduced serum (0.5% FBS) for 1 d and were then infected with Cre-expressing lentivirus at near saturation levels (>90% infected). These wild-type and ATRΔ/− cultures were then maintained in reduced serum for an additional 2 d and subsequently stimulated with 10% FBS for 18 to 24 h. Whereas ATR was depleted within 60 h of lenti-Cre infection (Fig. 2B), little difference was observed in the ability of lenti-Cre-infected ATRflox/− and wild-type cells to enter S and G2/M phase within 24 h of FBS stimulation (66–72 h after lenti-Cre infection; Fig. 2C). However, 2–6 d of continued culture in growth media resulted in a loss of proliferation accompanied by the appearance of large flattened cells (Fig. 2D) and a small increase in Annexin V staining (less than twofold over wild type). Thus, although ATR is required for long-term proliferation, ATR-depleted cells exhibit a relatively normal cell division cycle soon after ATR loss.
ATR and ATM cooperate in preventing mitotic entry in response to IR
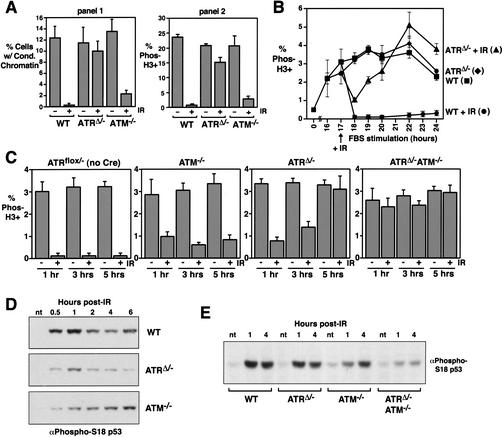
The synchronization, infection, and serum-restimulation procedure described above and in Figure 2B and C, provided a system to analyze ATR's role in cell cycle checkpoint regulation. Because previous studies have attributed IR-induced checkpoint responses to both ATR and ATM (Cliby et al. 1998; Cortez et al. 2001; Xu et al. 2002), we first wished to clarify the roles of ATR and ATM in this response using a defined genetic background and in the context of a single cell type (MEFs). To compare the roles of ATR and ATM in this checkpoint response, serum-starved wild-type, ATRflox/−, and ATM−/− MEFs were infected with Cre-expressing lentivirus and restimulated with serum as described above (Fig. 2B,C). After 17 h of FBS stimulation, ATRΔ/− and control cells began to enter mitosis (Fig. 3B). Cells were exposed to 20 Gy of γ-radiation at this time and were subsequently treated with nocodazole (0.5 μM) for 5 h to allow accumulation in mitosis. Entry into mitosis was then analyzed in two ways. In the first, mitotic spreads were prepared, and the ratio of mitotic and interphase cells was counted (Fig. 3A, panel 1). In the second, cells were stained for phosphohistone H3, a mitotic marker, and analyzed by flow cytometry (Fig. 3A, panel 2). Using either form of quantitation, these data demonstrated that ATR plays an important role in preventing cells from entering mitosis after IR exposure and by this analysis appeared to play a more important role than ATM (Fig. 3A). Using a 10-Gy dose, a similar level of ATR dependence for checkpoint delay was observed (data not shown). It is important to point out that nocodazole “pile up” nullifies temporal differences in mitotic entry by collecting mitotic cells over a set course of time, in this case 5 h. Because complete loss of checkpoint function was not observed in either single knockout, we thought these genes might cooperate in the IR response, perhaps in a temporal manner. Evidence in support of ATM contributing to early delays in M phase entry in response to IR has been reported (Xu et al. 2002).
Figure 3.
ATR and ATM cooperate in checkpoint responses to γ-irradiation. (A) Serum-deprived and lenti-Cre-infected wild-type, ATRflox/−, and ATM−/− MEFs were γ-irradiated (20 Gy) after 17 h of FBS stimulation and then treated with 0.5 μM nocodazole for 5 h (corresponding to 18–23 h of FBS stimulation). The percentage of cells exhibiting chromosome condensation (panel 1) and phosphorylated histone H3 (panel 2) were quantitated by chromosome spread preparation and immunostaining, respectively. Differences between the total percent of cells with condensed chromatin (panel 1) versus phosphohistone H3 staining (panel 2) is consistent with the phosphorylation of histone H3 occurring prior to condensation and remaining phosphorylated throughout M phase. (B) ATR is required for late-phase mitotic delay in response to IR. Entry into mitosis (without nocodazole treatment) was quantitated by phosphohistone H3 staining before and after treatment with 20 Gy of γ-radiation at 17 h of FBS stimulation. ▪, wild-type; ♦, ATRΔ/−; ●, wild-type + IR; ▴, ATRΔ/− + IR. (C) ATR and ATM cooperate in the cell cycle delay elicited by IR. Mitotic entry was assessed after IR exposure as in B to compare mitotic entry of ATRflox/− (no lenti-Cre infection), ATM−/−, and lenti-Cre-infected ATRflox/− and ATRflox/− ATM−/− (ATRΔ/− and ATRΔ/− ATM−/−) MEFs. ATRflox/− and ATRflox/− ATM−/− MEFs were derived from embryonic littermates. (D) ATR and ATM cooperate in p53 S18 phosphorylation. Lysates generated from lenti-Cre-infected wild-type, ATRflox/−, and ATM−/− cells at various times after IR treatment were Western-blotted and detected with a phospho-specific antibody to phospho-S18 p53. (E) Cells treated as described in C were analyzed for p53 S18 phosphorylation by Western blot detection as described in D. Error bars represent the standard deviation (S.D.) from the mean of values obtained in these experiments. nt, not treated with IR.
To test if ATR plays a time-dependent role in IR-induced responses, ATR-depleted and wild-type MEFs were assayed for the rate of entry into mitosis after exposure to IR at 17 h of FBS stimulation (Fig. 3B). Nocodazole treatment was not used in these experiments; therefore, the percentage of mitotic cells represents normal entry into mitosis following FBS stimulation. As shown in Figure 3B, ATR is required for the late phase of the response, beginning ∼3–4 h after IR treatment, but it is less important for the checkpoint response soon after IR exposure (e.g., 1 h after IR). To test if ATM plays a role in the early phase of the response in the context of this system, the ATRflox allele was bred into an ATM knockout background, and the same assay was performed (Fig. 3C). Whereas ATR and ATM individually played only partial and time-dependent roles in this checkpoint response, ATR/ATM double-knockout cells entered mitosis similarly to unirradiated cells. Because significant delay was not observed either early or late in the response, these data indicate that a majority, if not all, of IR-induced cell cycle delay is governed cooperatively by ATR and ATM (Fig. 3C).
To further analyze the time-dependent requirement for ATR and ATM, p53 S18 phosphorylation was analyzed for its kinetic dependence on ATR and ATM. S18 phosphorylation has been shown to be important for p53's checkpoint activity in the mouse (Chao et al. 2000), and phosphorylation of the sequence-context equivalent in human cells (S15) has previously been suggested to be ATR-dependent in the late phase based on dominant-negative ATR overexpression (Tibbetts et al. 1999). Figure 3D and E shows that consistent with the assay for mitotic entry, p53 phosphorylation appears to require both ATR and ATM in a time-dependent manner and that both ATR and ATM must be deleted to prevent phosphorylation over the long term.
In response to stalled DNA replication, cell cycle delay is intact in ATR and ATR/ATM double-knockout cells
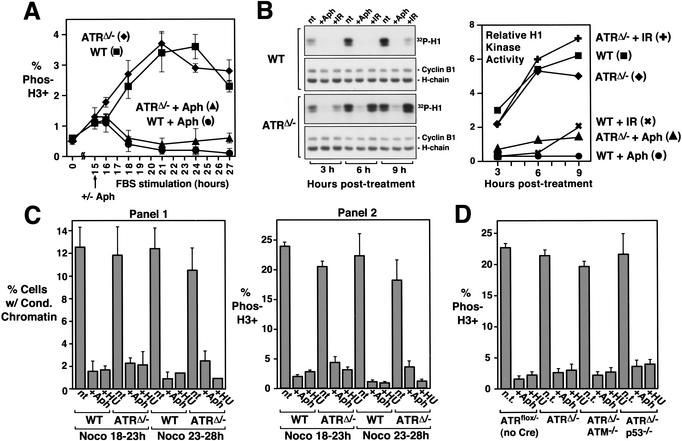
After assessing ATR's role in IR-induced checkpoint regulation, we set out to determine if ATR was required in a similar manner for other checkpoint responses, such as the cell cycle delay resulting from stalled DNA replication. Three assays were used to quantitate mitotic entry: histone H3 phosphorylation, Cdc2/Cyclin B1 activation, and chromosome condensation. First, in a time-course assay similar to that used for IR-induced delay, wild-type and ATRΔ/− cells were treated or left untreated with aphidicolin (5 μM) as cells began to pass into S phase, 15 h after FBS stimulation; cells subsequently entering mitosis were quantitated by phosphohistone H3 staining. Surprisingly, aphidicolin treatment potently inhibited both wild-type and ATRΔ/− cells from entering mitosis (Fig. 4A). To further examine this inhibition, cyclin B1-associated H1 kinase activity was assayed 3–9 h after aphidicolin or IR treatment (Fig. 4B). Consistent with the loss of IR-induced mitotic delay in ATRΔ/− cells (Fig. 3), cyclin B1-associated H1 kinase activity was not significantly inhibited by IR exposure (Fig. 4); however, H1 kinase activity was inhibited by aphidicolin treatment of either wild-type or ATRΔ/− cells (Fig. 4). Finally, ATR's role in the DNA replication checkpoint was examined by quantitating the percentage of cells with condensed chromatin (Fig. 4C). These results also showed that ATR is dispensable for preventing mitotic entry in response to stalled DNA replication (Fig. 4C).
Figure 4.
ATR loss does not eliminate the DNA replication checkpoint. (A) Both wild-type and ATRΔ/− cells are prevented from entering mitosis by aphidicolin (Aph) treatment. Cre-expressing wild-type and ATRflox/− cells were treated with aphidicolin upon S-phase entry following FBS stimulation (15 h post-FBS), and the percentage of cells entering mitosis was quantitated by phosphohistone H3 staining. ▪, wild-type; ♦, ATRΔ/−; ●, wild-type + Aph; ▴, ATRΔ/− + Aph. (B) Cyclin B1-associated H1 kinase activity in wild-type and ATRΔ/− cells. After 15 h of FBS stimulation, Cre-expressing wild-type and ATRflox/− cells were treated with aphidicolin (Aph), exposed to 20 Gy of γ-radiation (IR), or were left untreated (nt). Cells were then harvested 3, 6, and 9 h later, and immunoprecipitated cyclin B1-associated H1 kinase activity was assayed. Autoradiographs and PhosphorImager-quantitation (graph) of 32P-labeled H1 following SDS-PAGE are shown. Immunoprecipitates were also Western-blotted and detected for cyclin B1 to control for equivalent cyclin B1 recovery (Western: cyclin B1). ▪, wild-type; ♦, ATRΔ/−; ✖, wild-type + IR; ✚, ATRΔ/− + IR; ●, wild-type + Aph; ▴, ATRΔ/− + Aph. (C) Chromosome condensation is prevented by DNA replication inhibitors in lenti-Cre-infected wild-type and ATRflox/− cells. Aphidicolin (5 μM) or hydroxyurea (0.5 mM) was added 16 h after FBS stimulation. Following a 2-h incubation to allow cells already in G2 and M phase at the time of treatment to pass through to G1, cells were treated with nocodazole (0.5 μM) for 5 h (18–23 and 23–28 h of FBS stimulation). Mitotic cells were quantitated by chromosome condensation (panel 1) and histone H3 phosphorylation (panel 2). Note that quantitation by chromosome condensation includes mitotic cells with normal or fragmented (PCC) chromosome structure. (D) The DNA replication checkpoint is intact in ATRΔ/− ATM−/− and ATRΔ/− p53−/− cells. Mitotic entry was analyzed by phosphohistone H3 staining cells as described in C with nocodazole treatment for 5 h, from 18 to 23 h of FBS stimulation. Error bars represent the standard deviation (S.D.) from the mean of values obtained in these experiments.
Because ATM cooperates with ATR in IR-induced responses (Fig. 3), we wondered if ATM may be compensating in the DNA replication checkpoint for ATR's loss. It was also possible that p53 may cooperate with ATR in this checkpoint, as suggested by ATR dominant-negative studies (Nghiem et al. 2002). To test these possibilities, the checkpoint function of ATRΔ/− cells was compared with that of ATRΔ/− ATM−/− and ATRΔ/− p53−/− double-knockout MEFs (Fig. 4D). In each case, the DNA replication checkpoint function remained intact. These results demonstrate that ATR and ATM are dispensable for preventing mitotic entry upon stalled DNA replication.
Roles of ATR and ATM in Chk1 and Chk2 regulation
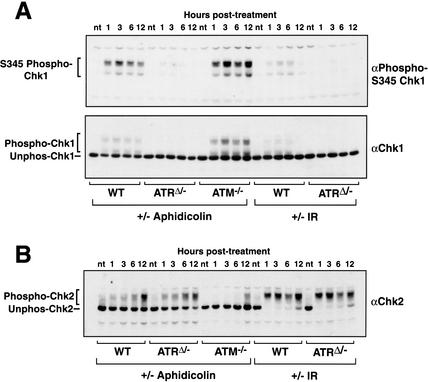
The ability of ATR knockout cells to arrest normally in the face of stalled DNA replication is surprising given numerous reports of ATR's role in regulating downstream signaling events, such as the phosphorylation of Chk1 (Guo et al. 2000; Hekmat-Nejad et al. 2000; Liu et al. 2000; Zhao and Piwnica-Worms 2001). To confirm ATR's role in regulating downstream signaling events using the ATR knockout system described herein, wild-type and ATRΔ/− cells were treated with aphidicolin (5 μM) or exposed to 20 Gy of γ-radiation, and cells were harvested at various times posttreatment for Western blot analysis (Fig. 5A). Chk1 phosphorylation was then evaluated by both detection with phospho-specific antibodies to serine 345 (Liu et al. 2000) and by mobility shift (Sanchez et al. 1997; Kumagai et al. 1998). In wild-type cells, Chk1 phosphorylation was strongly increased in response to aphidicolin treatment and moderately to weakly increased following exposure to IR. Note that multiple forms of decreased electrophoretic mobility were detected, similar to those observed for Xenopus Chk1 upon in vitro phosphorylation (Kumagai et al. 1998; Guo et al. 2000; Hekmat-Nejad et al. 2000). In ATRΔ/− cells, both aphidicolin- and IR-induced phosphorylation of Chk1 was inhibited, confirming an important role for ATR in regulating Chk1.
Figure 5.
Regulation of Chk1 and Chk2 by ATR and ATM, respectively. (A) Chk1 phosphorylation in response to either aphidicolin or IR requires ATR. After 15 h of FBS stimulation, Cre-expressing wild-type and ATRflox/− MEFs were treated with aphidicolin or IR. Lysates were prepared at various times posttreatment, and Western blots were detected for phospho-S345 Chk1 (upper panel) or total Chk1 (lower panel). (B) Chk2 phosphorylation in response to aphidicolin and IR is not dependent on ATR. Samples generated as in A were Western-blotted and detected for Chk2. The slower-migrating phosphorylated and faster-migrating unphosphorylated forms are indicated.
We then analyzed phosphorylation of Chk2 to determine its requirements for ATR and ATM in response to stalled replication. Whereas ATR deletion had no inhibitory effect on either the potent IR-induced phosphorylation of Chk2 or the mild phosphorylation resulting from aphidicolin treatment (Fig. 5B), ATM deletion prevented most of the mild aphidicolin-induced phosphorylation (Fig. 5B), consistent with previous studies using IR (Matsuoka et al. 1998). Therefore, because the DNA replication checkpoint remains intact even in ATRΔ/− ATM−/− cells (Fig. 4D), mitotic delay in response to stalled replication cannot be solely through Chk2 phosphorylation. It is also important to point out that the late phase of IR-induced Chk2 phosphorylation in ATRΔ/− cells (Fig. 5B) is not sufficient to prevent mitotic entry (Fig. 3B), indicating that moderate to high levels of Chk2 phosphorylation are incapable of generating delay on their own. Thus, in regard to the checkpoint response to stalled replication, our studies indicate that the ATR–Chk1 and ATM–Chk2 pathways are dispensable for the mitotic delay induced by DNA replication inhibitors.
Checkpoint-induced inhibitory phosphorylation of Cdc2 on T14 and Y15 is ATR-dependent
Although ATR and ATM are required for cell cycle delay in response to IR, the results described above indicate that ATR and ATM and the activation of Chk1 and Chk2 are dispensable for the ultimate cell cycle arrest that occurs in response to stalled DNA replication. Chk1 and Chk2 are upstream regulators of several signaling pathways that ultimately control phosphorylation of Cdc2 on threonine 14 (T14) and tyrosine 15 (Y15), thereby inhibiting Cdc2's catalytic activity (Norbury and Nurse 1992; Nyberg et al. 2002). Given that inhibitory phosphorylation of Cdc2 on T14 and Y15 is an important mode of cell cycle regulation in metazoans and fission yeast (Norbury and Nurse 1992; Nyberg et al. 2002), it was conceivable that stalled DNA replication may somehow bypass Chk1 and Chk2 to control Cdc2 activity through T14/Y15 phosphorylation. The phosphorylation state of Cdc2 on T14 and Y15 was therefore assessed in aphidicolin-treated wild-type and ATRΔ/− cells and compared with that of IR-treated cells.
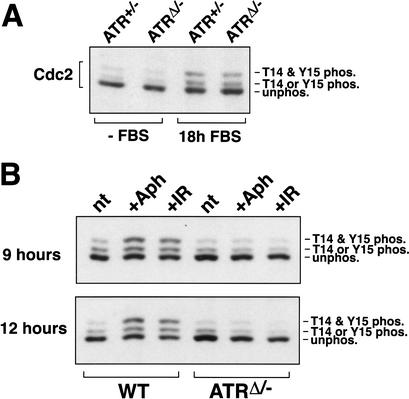
Cdc2 is initially phosphorylated in S phase by the Wee1 and Myt1 kinases (Norbury and Nurse 1992; Nyberg et al. 2002). This phosphorylation is maintained until the early stages of mitosis, at which time these phosphates are removed by changes in the rates of phosphorylation and dephosphorylation. As indicated by slowed electrophoretic mobility, the well-characterized phosphorylation of Cdc2 was observed in both ATRΔ/− and control cells upon serum-stimulated entry into S and G2 phase 18 h after FBS stimulation (Fig. 6A). Therefore, ATR is not required for the initial phosphorylation of Cdc2 that occurs upon S-phase entry. To examine the ability of DNA damage or stalled replication to maintain Cdc2 phosphorylation, wild-type and ATRΔ/− cells were treated with aphidicolin or IR for 9 and 12 h, and the accumulation of the T14/Y15-phosphorylated form of Cdc2 was assessed by Western blot (Fig. 6B). In wild-type cells, this accumulation occurred as expected over the course of treatment. In ATRΔ/− cells, however, increased phosphorylation of Cdc2 was not observed following treatment with either aphidicolin or IR (Fig. 6B). Together these data indicate that both IR- and aphidicolin-induced checkpoints use ATR for signaling events that ultimately lead to inhibitory phosphorylation of Cdc2; however, an additional mechanism to prevent cell cycle progression must be at work when DNA replication is incomplete.
Figure 6.
Cdc2 phosphorylation on T14 and Y15. (A) Cdc2 is phosphorylated normally as cells enter S phase. After 18 h of FBS stimulation, lysates from lenti-Cre-infected ATRflox/− cells were generated and subjected to SDS-PAGE and Western blot detection of Cdc2. The unphosphorylated (fastest-migrating), singly phosphorylated (phospho-T14 or phospho-Y15), and doubly phosphorylated (slowest-migrating, phospho-T14 and phospho-Y15) forms of Cdc2 are indicated. (B) Accumulation of phospho-T14/Y15 Cdc2 following aphidicolin or IR does not occur in ATRΔ/− cells. After 15 h of FBS stimulation, Cre-expressing wild-type and ATRflox/− cells were either left untreated (nt) or treated with either aphidicolin (Aph) or 20 Gy of γ-radiation (IR). Cells were then harvested 9 and 12 h after treatment, and lysates were Western-blotted and detected for Cdc2 as in A.
ATR prevents generation of double-strand breaks in the event of stalled DNA replication
Recent studies in yeast have suggested that ATR may play a role in stabilizing stalled DNA replication forks. For example, stalled replication in Rad53 mutants, a conventional protein kinase that lies downstream of the ATR ortholog in S. cerevisiae Mec1, results in replication fork collapse and DSB formation (Lopes et al. 2001). More recently, Mec1 has been shown to prevent generation of DSBs at slowly replicating regions of the genome (Cha and Kleckner 2002). These results have suggested a role for Mec1-dependent checkpoint signaling to stabilize stalled replication forks and prevent the generation of DSBs (Carr 2002). We therefore reasoned that generation of such breaks in aphidicolin-treated ATR knockout cells may play a role in preventing cell cycle progression in a manner that is independent of ATR's cell cycle-inhibitory functions.
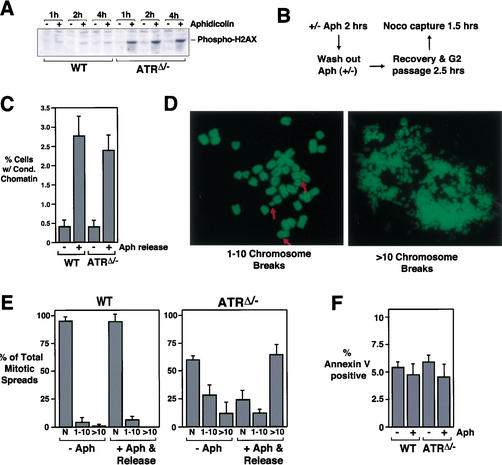
To test if ATR is required to prevent generation of DSBs in the event of stalled DNA replication, we first used H2AX phosphorylation as a marker of DSB formation in wild-type and ATRΔ/− MEFs. H2AX is phosphorylated on serine 139 in response to IR-induced DSBs and in the course of VDJ recombination (Rogakou et al. 1998; Chen et al. 2000). Other forms of DNA damage cause only mild phosphorylation of H2AX (Burma et al. 2001), indicating that phosphorylation may be DSB-specific. Finally, recent studies indicate that phosphorylation of H2AX is predominantly regulated by DNA-PK and ATM (Paull et al. 2000; Burma et al. 2001), and consistent with this analysis we have seen no effect of ATR loss on H2AX phosphorylation in response to IR (data not shown). Therefore, if DSBs are generated in ATRΔ/− upon stalled DNA replication, then aphidicolin treatment should cause a strong increase in H2AX phosphorylation specifically in ATR knockout cells and little increase in wild-type cells. To assay for increased H2AX phosphorylation, lysates were prepared from wild-type and ATRΔ/− cells that were treated or left untreated with aphidicolin for various times. Western blots of these lysates were then incubated with phospho-specific H2AX antibodies. As shown in Figure 7A, little increase in H2AX phosphorylation occurred in wild-type cells upon aphidicolin treatment, consistent with previous studies (Burma et al. 2001). In contrast, a robust increase in H2AX phosphorylation was observed in ATRΔ/− cells after 1 h of aphidicolin treatment and beyond (Fig. 7A). These experiments support a model in which ATR is required to prevent the formation of DSBs upon stalled DNA replication.
Figure 7.
Aphidicolin causes DSBs in ATR-depleted cells, but DSBs do not prevent mitotic entry upon aphidicolin release. (A) Aphidicolin causes phosphorylation of H2AX in ATRΔ/− cells. At 15 h of FBS stimulation, lenti-Cre-infected wild-type and ATRflox/− cells were left untreated or treated with aphidicolin. Cells were harvested 1, 2, and 4 h later. Lysates were Western-blotted and detected for phosphohistone H2AX. (B) Schematic representation of an assay to quantitate the effect of DSBs on preventing mitotic entry and detect increased chromosome breakage upon aphidicolin treatment. (C) Mitotic entry of aphidicolin-treated wild-type and ATRΔ/− cells after aphidicolin release. After a 2-h aphidicolin treatment, Cre-expressing wild-type and ATRflox/− MEFs were washed four times with 10% FBS DMEM to remove aphidicolin. Two and a half hours later, cells were collected in mitosis by nocodazole treatment (0.5 μM) for 1.5 h. Chromosome spreads were prepared to quantitate the percentage of cells entering mitosis and to assess chromosome breakage as described in D and E below. Cells were released from or maintained in aphidicolin as indicated. (D) Mitotic spreads with differing degrees of chromosome breakage were observed and quantitated using the assay described in B and C. Examples of spreads with 1–10 breaks and >10 breaks are shown. (E) Aphidicolin induces breaks specifically in ATRΔ/− cells. Cre-expressing wild-type and ATRflox/− MEFs released from aphidicolin treatment as described in B and C (+Aph & Release) were analyzed for the frequency of each category of mitotic chromosome spread shown in D. The chromosome breakage observed in cells left untreated with aphidicolin is also shown (−Aph). N, normal chromosome spread. (F) Aphidicolin treatment does not cause apoptosis in ATRΔ/− cells. Cre-expressing wild-type and ATRflox/− MEFs left untreated, treated with aphidicolin, or treated with and released from aphidicolin were stained for Annexin V and quantitated by flow cytometry.
If breaks were induced by stalled DNA replication specifically in ATR-depleted cells, then it was conceivable that these breaks might somehow prevent mitotic entry in an ATR/ATM-independent manner. To test this possibility and to further analyze ATR's involvement in preventing the appearance of DSBs, a chromosome spread-based assay was developed. In this assay (Fig. 7B), short-term treatment with aphidicolin was used to cause replication fork stalling, but this treatment was followed by removal of aphidicolin to allow cells to proceed into mitosis. Then, 2.5 h after removal of aphidicolin, ATRΔ/− and control cells began to enter and accumulate in mitosis over the course of a 1.5-h nocodazole pile-up assay. Both mitotic spread quantitation (Fig. 7C) and phosphohistone H3 assays (data not shown) indicated that upon aphidicolin release, ATRΔ/− cells were only slightly inhibited from entering mitosis in comparison with control cells. Importantly, these results indicate that aphidicolin-treated ATRΔ/− cells have the ability to enter mitosis once inhibition of DNA replication is alleviated.
Mitotic spreads prepared from cells treated as above (Fig. 7C) were then analyzed for the percentage of mitotic cells exhibiting DSBs. The degree of chromosome breakage was categorized into three groups: normal (N), 1–10 breaks, and >10 breaks per mitotic spread (Fig. 7D). Few wild-type mitotic spreads had chromosome breaks either with or without aphidicolin treatment (Fig. 7E). A similar lack of aphidicolin-induced chromosome breaks was observed in ATM−/− cells (data not shown). In contrast, ∼40% of ATRΔ/− mitotic cells exhibited some form of chromosome breakage even in the absence of aphidicolin treatment, most with <10 chromatid breaks per cell. These results are consistent with the chromosome breakage observed in previous ATR knockout studies (Brown and Baltimore 2000). However, when ATR-depleted cells were treated with aphidicolin for 2 h and then released into mitosis, an increase both in the degree of breakage per cell and in the percentage of cells exhibiting breaks was observed (Fig. 7E). The most dramatic difference was an increase in spreads exhibiting an extreme form of breakage, such as that shown in Figure 7D (>10 breaks). Because no differences in Annexin V staining were observed between wild-type and ATR knockout cells, either treated or left untreated with aphidicolin (Fig. 7F), the chromosome breakage apparent in aphidicolin-treated ATRΔ/− cells is unlikely to be the result of an apoptotic program. Thus, ATRΔ/− cells can enter mitosis after aphidicolin release (Fig. 7C) and do so despite the presence of extensive chromosome breakage (Fig. 7E).
These experiments demonstrate that although ATR is required for genome stability in the absence of exogenous treatment, it is particularly required in the event of stalled DNA replication. Moreover, the DSBs generated in aphidicolin-treated ATRΔ/− cells themselves do not prevent mitotic entry because entry with breaks is observed once DNA replication is allowed to proceed (Fig. 7C). The ability of aphidicolin-released ATRΔ/− cells to proceed into mitosis with breaks is consistent with the IR checkpoint studies described here, because IR also generates DSBs and ATRΔ/− cells continue to enter mitosis despite IR treatment. The implications of these results for our understanding of checkpoint control and for ATR's cell cycle regulatory and genome maintenance functions are discussed below.
Discussion
Using a Cre/lox-conditional system to study the effect of ATR loss, we have shown that ATR is an important regulator of checkpoint signaling pathways that are induced by IR and stalled DNA replication and lead to inhibitory phosphorylation of Cdc2. Despite this similarity, IR and stalled replication cause cell cycle delay in ways that differ in their requirements for this ATR-dependent pathway. Whereas loss of ATR eliminates IR-induced cell cycle delay, it has little effect on the delay induced by stalled DNA replication. It is clear that ATR regulates important arms of both the IR and DNA replication checkpoints through Cdc2 phosphorylation; however, these data also indicate that stalled DNA replication can prevent mitotic entry in an ATR-independent manner as well. These data are consistent with the existence of multiple independent mechanisms to prevent premature mitotic entry when DNA replication remains incomplete.
ATR and ATM involvement in IR-induced cell cycle delay
Our studies demonstrate that ATR and ATM cooperate in preventing mitotic entry in response to IR. This response involves a complex network of checkpoint signaling molecules with differing requirements for ATR and ATM. For example, our results indicate that ATR is the chief regulator of Chk1 phosphorylation but has no involvement in Chk2 phosphorylation in response to either IR or stalled replication. Because Chk2 phosphorylation has been previously shown to mostly require ATM (Matsuoka et al. 1998), these results imply that there is little if any overlap in Chk1 and Chk2 regulation by ATR and ATM, respectively. In contrast, direct phosphorylation of p53 on serine 18 is codependent on ATR and ATM in a time-dependent manner (ATM involved in the early phase, ATR in the late). The importance of these differences in signaling partnerships and kinetic dependence remains unclear, but it may be related to the manner in which DSBs are initially sensed and later processed for repair as discussed below.
We have shown that ATR assists in the early phase of mitotic delay in response to IR and is particularly required for the late phase. In contrast, ATM appears to be more noticeably important for the early phase, as shown in previous studies (Xu et al. 2002). There are at least two conceivable models to explain the time-dependent requirements for ATR and ATM. One explanation is that the initial form of DNA breakage caused by IR is best recognized by ATM, whereas the repair intermediates that result from later processing of these initial breaks are better recognized by ATR. Thus, in this model ATR and ATM are specialized to elicit checkpoint responses to DSBs that are in various stages of processing, and until repair is complete these checkpoint responses remain active. Another model involves the cell cycle phase in which damage is sensed; for example, ATR may be particularly apt at sensing damage during DNA synthesis, but not afterwards in the G2 phase. Nevertheless, according to either model the differing activation of signaling pathways, such as Chk1 and Chk2, by ATR and ATM may reflect specific downstream cellular responses to distinct stages of DNA metabolism. Because ATR, ATM, Chk1, and Chk2 have been shown to differentially phosphorylate various genes involved in DNA repair, such as BRCA1, NBS1, Blm1, and others (Kastan and Lim 2000; Tibbetts et al. 2000; Zhou and Elledge 2000), it is tempting to speculate that downstream processes may include influencing the appropriate type of repair. The differing roles of ATR–Chk1 and ATM–Chk2 pathways in such a repair capacity, however, have not been formally tested.
The DNA replication checkpoint
We show that ATR regulates an important arm of the DNA replication checkpoint as indicated by ATR's requirement for activating phosphorylation of Chk1 and inhibitory phosphorylation of Cdc2 on T14/Y15. Based on decades of research on Cdc2 regulation, there is little doubt that phosphorylation of Cdc2 on T14/Y15 can be an important part of cell cycle checkpoint regulation (Norbury and Nurse 1992; Weinert 1997; Nyberg et al. 2002). However, given that cell cycle delay still occurs even in the absence of ATR, at least one additional mechanism to prevent premature mitotic entry must be at work in MEFs. It is formally possible that this additional mechanism may function using levels of ATR protein that are far below those required for regulation of Cdc2 T14/Y15 phosphorylation; however, there is no evidence at the present time to assume this is the case. The simplest explanation of our data is that the Cdc2 T14/15-independent mechanism is also independent of ATR. The ATR-independent mechanism of inhibition is not the result of a general loss of cellular function because ATRΔ/− cells remain capable of entering mitosis once DNA replication inhibitors have been removed. Therefore, some aspect of inhibited DNA replication itself must be responsible for the ATR-independent mitotic delay. It is important to point out that deletion of the ATR orthologs MEC1 and RAD3 in budding and fission yeast, respectively, eliminate the DNA replication checkpoint (Al-Khodairy and Carr 1992; Enoch et al. 1992; Weinert et al. 1994; Boddy et al. 1998; Nyberg et al. 2002). Thus, our results indicate that incompletely replicated DNA in mammalian cells prevents mitotic entry differently than in yeast.
Our studies indicate that Cdc2/cyclin B itself may be the target of the ATR-independent mechanism of inhibition. This interpretation is based on previous studies implying that histone H3 phosphorylation is downstream of Cdc2/cyclin B activation (De Souza et al. 2000) and on our studies showing that aphidicolin-treatment of ATRΔ/− cells prevents activation of cyclin B1-associated kinase activity (Fig. 4B). There are many possible mechanisms by which Cdc2/cyclin B1 can be inhibited other than Cdc2 T14/Y15 phosphorylation (Weinert 1997). Along these lines, it is important to point out that a titratable inhibitor of Cdc2/cyclin B kinase activity that acts independently of Cdc2 T14/Y15 phosphorylation has been previously observed in aphidicolin-treated Xenopus extracts (Kumagai and Dunphy 1995). It is possible that an equivalent inhibitor is at work in mammalian cells, one that acts independently of ATR and prevents Cdc2/cyclin B activation when DNA replication is left incomplete.
ATR prevents the appearance of DSBs upon stalled DNA replication
Previous work in yeast has indicated important roles for the ATR ortholog Mec1 and Mec1-dependent signaling pathways (Rad53) in preventing DSB upon slowing or stalled DNA replication (Lopes et al. 2001; Cha and Kleckner 2002). The studies described herein confirm an important role for ATR in preventing DSBs during stalled DNA replication in mammalian cells. Because ATR also regulates cell cycle checkpoint signaling pathways, such as Chk1 phosphorylation and Cdc2 inhibition, our studies indicate that ATR affects both cell cycle regulation and genome maintenance in the event of stalled replication. It is interesting to note that, as a mammalian ortholog of Rad53, Chk1 may be the common regulator of both the checkpoint and genome maintenance functions downstream of ATR. Chk1 knockout mice die early in development, similar to ATR knockout mice (Liu et al. 2000). Although it is not known whether Chk1 knockout cells exhibit a form of genomic instability similar to that observed in ATR knockouts (Brown and Baltimore 2000), dominant-negative Chk1 overexpression has been reported to affect genome stability (Nghiem et al. 2001). The mechanism by which ATR, and possibly Chk1, maintains stability upon stalled replication is unknown but might involve the correct processing of regressed replication forks (Carr 2002) or stabilization of stalled forks by translesion bypass (Kai and Wang 2003), a process that is not inhibited by aphidicolin.
Processive PIK-related kinase activation?
Our data suggest a model in which ATR, ATM, and DNA-PK act in multiple layers to preserve genomic integrity. For example, we show that ATR is important to prevent DSB formation in the event of stalled DNA replication and that H2AX is phosphorylated when these breaks form in ATR's absence (Fig. 7A). H2AX has previously been shown to be phosphorylated by ATM and DNA-PK at the sites of DSB formation (Rogakou et al. 1999; Burma et al. 2001). Thus, if ATR is unavailable to prevent DSB formation in the event of stalled replication, then these breaks are likely sensed by DNA-PK and/or ATM, which then go on to phosphorylate H2AX. It is interesting to point out that H2AX has recently been shown to be required for efficient homologous recombination (Celeste et al. 2002); therefore, it is possible that phosphorylation of H2AX may be part of a process to repair these breaks or reinitiate replication fork progression (Cox et al. 2000). Although purely speculative at this time, our studies are consistent with the possibility that the multiple pathways in checkpoint regulation not only respond differently to the initial forms of DNA damage, but may also act in layers of activation that processively safeguard genomic integrity should an upstream system fail.
Materials and methods
Generation of mice with a lox-conditional allele of ATR and MEFs preparation
D3 ES cells were transfected with the targeting vector shown (Fig. 1A), selected in neomycin, and cloned as described (Brown and Baltimore 2000). To remove the neomycin cassette, pCAGGS-FLPe (Buchholz et al. 1998) was transfected into recombined ES cells, and these cells were transiently selected in puromycin (2 μg/mL) for 2 d, expanded for 5 d, and then selected in gancylovir (1.5 μM) for 4 d. ES cell clone DNA was analyzed by Southern blot as described (Brown and Baltimore 2000). To produce ATRflox/− and control MEFs, ATR+/− (Brown and Baltimore 2000) and ATRflox/+ mice were mated and E14.5 embryos were isolated. MEFs prepared from these embryos were grown in 10% FBS DMEM, genotyped, and infected with lenti-Cre virus within 2 or 3 doublings after isolation, as described below. ATRflox/− ATM−/− and ATRflox/− p53−/− MEFs were isolated similarly using the following parental crosses: ATRflox/flox ATM+/− × ATR+/− ATM+/− mice and ATRflox/flox p53+/− × ATR+/− p53+/− mice. These parental genotypes were produced by breeding ATRflox/+ and ATR+/− (Brown and Baltimore 2000) mice with ATM+/− (Xu and Baltimore 1996) and p53+/− (Jacks et al. 1994) mice; progeny were subsequently intercrossed.
Lenti-Cre constructs and infection of MEFs
Cre-expressing lentiviruses were produced using retroviral expression vectors (Lois et al. 2002) and a packaging system previously described (Dull et al. 1998). Concentrated lenti-Cre preparations were titered using a lox-recombination reporter cell line [NIH3T3-lox-Lac Z, provided by Carlos Lois (Massachusetts Institute of Techology, Cambridge, MA) and Dan Van Antwerp (California Institute of Technology, Pasadena, CA)]. To recombine the ATRflox allele, MEFs were trypsinized and resuspended at 1 × 106 cells/mL in 0.5% FBS DMEM and replated in the same media containing concentrated lenti-Cre virus (1–3 × 108 TU/mL) at a multiplicity of infection (m.o.i.) of 5–10. This m.o.i. consistently resulted in expression of Cre in >90% of the cells, as expected by Poisson distribution. At 18–24 h after the start of infection, virus-containing media was removed and fresh 0.5% FBS DMEM was applied. The studies described herein were replicated using two forms of Cre recombinase, NLS-Cre (provided by J. Jacob, Emery University) and Cre-ERT2 (Feil et al. 1997), both of which recombined the ATRflox allele with similar efficiency and produced similar results.
Propidium iodide, phosphohistone H3, and Annexin V staining
MEFs were tyrpsinized, washed in PBS, stained as described below, and analyzed using a Becton Dickinson FACScan flow cytometer (488 nm excitation laser). For propidium iodide staining, cells were resuspended in a solution containing 50 μg/mL propidium iodide, 0.1% Triton X-100, 50 μg/mL RNAse A, and 5 mM EDTA at room temperature for 1 h. This solution was then diluted 1:1 in 2% FBS PBS for cytometric analysis. For Annexin V staining, PBS-washed cells were resuspended in 2% FBS PBS containing 1 mM CaCl2 and a 1:100 dilution of Annexin V-PE (BD Pharmingen), incubated on ice for 15–30 min, and analyzed for PE emission by flow cytometry. Phosphohistone H3 staining was performed by a procedure similar to that previously described (Xu et al. 2002). Briefly, cells were fixed in ice cold 70% ethanol for 1 h, followed by fixation in ice cold 95% ethanol/5% acetic acid for 5 min. Cells were washed twice with PBS and then permeabilized in 1% FBS/0.1% Triton X-100 PBS (FTP) for 30 min, followed by staining in 2 μg/mL anti-phosphohistone H3 antibody (Upstate Biotechnology) in FTP at room temperature (RT) for 1.5 h. Cells were then washed twice in FTP and incubated with FITC-conjugated anti-rabbit F(ab‘)2 antibody (Jackson ImmunoResearch) in FTP at RT for 1.5 h. After two additional washes in FTP, phosphohistone H3-positive cells were quantitated by flow cytometric detection of FITC emission.
Mitotic spread preparation and quantitation
Mitotic spreads were prepared from MEFs by methods described previously (Brown and Baltimore 2000). Slides of chromosome spreads were stained and mounted in SYBR green (Molecular Probes) diluted 1:10,000 in 85% glycerol/15% PBS at pH 7.9. To determine the percentage of cells in mitosis, the total number of mitotic spreads (normal + fragmented) were counted and divided by the total number of cells (interphase + mitotic). For measuring mitotic entry, >500 cells were counted for each replicate. Classification of chromosome fragmentation state (Fig. 7E) was performed by analyzing >50 mitotic spreads for each of four experiments per condition.
Cyclin B1-associated H1 kinase assays
Cells were washed with 4°C PBS and then lysed at 4°C in 1 mL of PIP buffer (Brown et al. 1994) containing 150 mM NaCl and no DTT for 30 min. Lysates were then clarified by centrifugation, and 1 μg of anti-Cyclin B1 monoclonal antibody (CB-169, Upstate Biotechnology) was added to supernatants. After 2 h of gentle agitation, 5 μL of (packed) protein A/G beads (Oncogene Sciences) was added and incubated with extract for an additional 45 min. Beads were then washed four times with lysis buffer and once with kinase reaction buffer (50 mM HEPES at pH 7.5, 10 mM MgCl2, 50 mM 2-glycerophosphate, 0.1% Triton X-100, and 1 mM DTT). Kinase reactions were initiated by the addition of 30 μL of kinase reaction buffer containing 5 μg of histone H1 (Roche) and 50 μM 32P-γ-ATP (1.67 Ci/mmole) and incubated at 37°C for 10 min. Reactions were stopped by addition of 40 μL of 2× Laemmli sample buffer and boiling for 5 min. Phosphorylation of H1 was quantitated following SDS-PAGE by autoradiography (Fig. 4B) and by a Molecular Dynamics Storm 860 PhosphorImager (Fig. 4B, graph).
Antibodies and Western blot detection
Whole-cell extracts were prepared by lysing cells in Laemmli sample buffer, separating proteins by SDS-PAGE, and blotting to either nitrocellulose or PVDF membranes (Immobilon P, Millipore). Blots were blocked in TBST containing 5% nonfat dried milk (NFDM) and incubated at RT for 1–2 h with primary antibodies as follows: ATR was detected with anti-human ATR (2381–2644) antibodies (Serotec) diluted in 1:5000 dilution in 5% NFDM TBST. Chk1 was detected with monoclonal antibodies to full-length Chk1 (sc-8408, Santa Cruz Biotechnology) diluted in 5% NFDM TBST and incubated with blots at RT for 1 h. Phosphoserine 18 of mouse p53 and phosphoserine 345 Chk1 were detected by incubation with commercially available antibodies (#9284 and #2341, Cell Signaling Technology) at RT for 1 h (P-S18 p53) or at 4°C for >16 h (P-S345 Chk1), diluted 1:1000 in 5% BSA TBST. Anti-Chk2 polyclonal antibodies [gift of Steve Elledge (Baylor College, Houston, TX); Matsuoka et al. 1998] were used at a 1:500 dilution in 5% BSA at RT for 2 h. Cdc2 and cyclin B1 were detected with 1 μg/mL dilutions of anti-Cdc2 (Ab-3, Calbiochem) and anti-cyclin B1 (GNS-1, BD Pharmingen) monoclonal antibodies in TBST. Finally, anti-phosphoserine 139 H2AX antibodies (Upstate Biotechnology) were used at a 1:1000 dilution in 3% NFDM TBST incubated with blots at 4°C for 6 h.
Acknowledgments
We especially thank Carlos Lois for assistance and training in lentivirus preparation, Shirley Pease for blastocyst injections, and Karlene Cimprich for critically reading the manuscript. We are also indebted to John Petrini, Steve Elledge, Chris Canman, and Joel Pomerantz for helpful advice; to Paul Nghiem and Thomas Glover for sharing unpublished results; and to Lilia Anonuevo, Shannon Witherow, and Bruce Kennedy for assistance in mouse care. Finally, we thank Steve Elledge, Francis Stewart, Didier Trono, Dan Van Antwerp, and Joshy Jacob for generously providing reagents. Funds for this research were provided by a grant from the NIH (2R01CA51462-14). E.J.B. was supported by a Breast Cancer Research Program postdoctoral fellowship.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
Corresponding authors.
Article published online ahead of print. Article and publication date are at http://www.genesdev.org/cgi/doi/10.1101/gad.1067403.
References
- Al-Khodairy F, Carr AM. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Furnari B, Mondesert O, Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. doi: 10.1126/science.280.5365.909. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes & Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Buchholz F, Angrand PO, Stewart AF. Improved properties of FLP recombinase evolved by cycling mutagenesis. Nat Biotechnol. 1998;16:657–662. doi: 10.1038/nbt0798-657. [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- Carr AM. Checking that replication breakdown is not terminal. Science. 2002;297:557–558. doi: 10.1126/science.1075456. [DOI] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha RS, Kleckner N. ATR homolog Mec1 promotes fork progression, thus averting breaks in replication slow zones. Science. 2002;297:602–606. doi: 10.1126/science.1071398. [DOI] [PubMed] [Google Scholar]
- Chao C, Saito S, Anderson CW, Appella E, Xu Y. Phosphorylation of murine p53 at ser-18 regulates the p53 responses to DNA damage. Proc Natl Acad Sci. 2000;97:11936–11941. doi: 10.1073/pnas.220252297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, Rogakou EP, Brotz TM, Bonner WM, Ried T, et al. Response to RAG-mediated VDJ cleavage by NBS1 and γ-H2AX. Science. 2000;290:1962–1965. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliby WA, Roberts CJ, Cimprich KA, Stringer CM, Lamb JR, Schreiber SL, Friend SH. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 1998;17:159–169. doi: 10.1093/emboj/17.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: Partners in checkpoint signaling. Science. 2001;294:1713–1716. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- Cox MM, Goodman MF, Kreuzer KN, Sherratt DJ, Sandler SJ, Marians KJ. The importance of repairing stalled replication forks. Nature. 2000;404:37–41. doi: 10.1038/35003501. [DOI] [PubMed] [Google Scholar]
- De Souza CP, Osmani AH, Wu LP, Spotts JL, Osmani SA. Mitotic histone H3 phosphorylation by the NIMA kinase in Aspergillus nidulans. Cell. 2000;102:293–302. doi: 10.1016/s0092-8674(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch T, Carr AM, Nurse P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes & Dev. 1992;6:2035–2046. doi: 10.1101/gad.6.11.2035. [DOI] [PubMed] [Google Scholar]
- Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237:752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kumagai A, Wang SX, Dunphy WG. Requirement for ATR in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes & Dev. 2000;14:2745–2756. doi: 10.1101/gad.842500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekmat-Nejad M, You Z, Yee M, Newport JW, Cimprich KA. Xenopus ATR is a replication-dependent chromatin-binding protein required for the DNA replication checkpoint. Curr Biol. 2000;10:1565–1573. doi: 10.1016/s0960-9822(00)00855-1. [DOI] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Kai M, Wang TS. Checkpoint activation regulates mutagenic translesion synthesis. Genes & Dev. 2003;17:64–76. doi: 10.1101/gad.1043203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Control of the Cdc2/cyclin B complex in Xenopus egg extracts arrested at a G2/M checkpoint with DNA synthesis inhibitors. Mol Biol Cell. 1995;6:199–213. doi: 10.1091/mbc.6.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Guo Z, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, et al. Chk1 is an essential kinase that is regulated by ATR and required for the G2/M DNA damage checkpoint. Genes & Dev. 2000;14:1448–1459. [PMC free article] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. doi: 10.1038/35087613. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc Natl Acad Sci. 2001;98:9092–9097. doi: 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem P, Park PK, Kim YS, Desai BN, Schreiber SL. ATR is not required for p53 activation but synergizes with p53 in the replication checkpoint. J Biol Chem. 2002;277:4428–4434. doi: 10.1074/jbc.M106113200. [DOI] [PubMed] [Google Scholar]
- Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Annu Rev Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–916. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, Elledge SJ. Conservation of the Chk1 checkpoint pathway in mammals: Linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. ; Comment, 277: 1450–1451. [DOI] [PubMed] [Google Scholar]
- Tibbetts RS, Brumbaugh KM, Williams JM, Sarkaria JN, Cliby WA, Shieh SY, Taya Y, Prives C, Abraham RT. A role for ATR in the DNA damage-induced phosphorylation of p53. Genes & Dev. 1999;13:152–157. doi: 10.1101/gad.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts RS, Cortez D, Brumbaugh KM, Scully R, Livingston D, Elledge SJ, Abraham RT. Functional interactions between BRCA1 and the checkpoint kinase ATR during genotoxic stress. Genes & Dev. 2000;14:2989–3002. doi: 10.1101/gad.851000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert T. A DNA damage checkpoint meets the cell cycle engine. Science. 1997;277:1450–1451. doi: 10.1126/science.277.5331.1450. [DOI] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes & Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Xu B, Kim ST, Lim DS, Kastan MB. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol Cell Biol. 2002;22:1049–1059. doi: 10.1128/MCB.22.4.1049-1059.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes & Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
- Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–4139. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: Putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zou L, Cortez D, Elledge SJ. Regulation of ATR substrate selection by Rad17-dependent loading of Rad9 complexes onto chromatin. Genes & Dev. 2002;16:198–208. doi: 10.1101/gad.950302. [DOI] [PMC free article] [PubMed] [Google Scholar]