Abstract
Phosphatidylserine exposed on the surface of apoptotic mammalian cells is considered an “eat-me” signal that attracts phagocytes. The generality of using phosphatidylserine as a clearance signal for apoptotic cells in animals and the regulation of this event remain uncertain. Using ectopically expressed mouse MFG-E8, a secreted phosphatidylserine-binding protein, we detected specific exposure of phosphatidylserine on the surface of apoptotic cells in Caenorhabditis elegans. Masking the surface phosphatidylserine inhibits apoptotic cell engulfment. CED-7, an ATP-binding cassette (ABC) transporter, is necessary for the efficient exposure of phosphatidylserine on apoptotic somatic cells, and for the recognition of these cells by phagocytic receptor CED-1. Alternatively, phosphatidylserine exposure on apoptotic germ cells is not CED-7 dependent, but instead requires phospholipid scramblase PLSC-1, a homologue of mammalian phospholipid scramblases. Moreover, deleting plsc-1 results in the accumulation of apoptotic germ cells but not apoptotic somatic cells. These observations suggest that phosphatidylserine might be recognized by CED-1 and act as a conserved eat-me signal from nematodes to mammals. Furthermore, the two different biochemical activities used in somatic cells (ABC transporter) and germ cells (phospholipid scramblase) suggest an increased complexity in the regulation of phosphatidylserine presentation in response to apoptotic signals in different tissues and during different developmental stages.
INTRODUCTION
In metazoans, cells undergoing apoptosis are recognized and internalized by living cells via phagocytosis, and they are degraded inside phagocytes. This process removes unwanted cells before they release potentially harmful contents, and it is important for immunological tolerance, suppression of inflammatory responses, and tissue remodeling (Savill and Fadok, 2000). Phagocytes use cell surface receptors to recognize surface features of apoptotic cells. Several changes have been detected on the surface of mammalian cells undergoing apoptosis, including the exposure of phosphatidylserine (PS) to the outer leaflet of the plasma membrane, and changes in cell surface carbohydrates and ionic charges (Savill and Fadok, 2000).
PS is a component of cellular membranes, making up 2–10% of total phospholipids (for review, see Vance and Steenbergen, 2005). In living cells, PS is almost exclusively localized to the inner leaflet of plasma membrane, at least partially due to an ATP-dependent aminophospholipid translocase activity that selectively returns PS and phosphatidylethanolamine (PE) from the outer to the inner leaflet (for review, see Vance and Steenbergen, 2005). The exact identity of the protein(s) responsible for this activity in vivo remains to be revealed. During the early stage of apoptosis, PS is detected on the outer leaflet, as observed in mammals, Drosophila, Xenopus, and chick (Fadok et al., 1992; van den Eijnde et al., 1998), suggesting a process of transbilayer redistribution. Not much is known about the exact mechanism that triggers the exposure of PS in response to apoptotic stimuli in animals. Phospholipid scramblases (PLSCs), by catalyzing the random, bidirectional “flip-flop” of phospholipids across the membrane bilayer, could potentially counter the aminophospholipid translocase activity (Sims and Wiedmer, 2001). Four homologous phospholipid scramblases have been identified in humans, however, the in vivo roles of these scramblases in the redistribution of PS have not been established (Sims and Wiedmer, 2001). In addition, ABCA1, the prototype of the A subclass of mammalian ATP-binding cassette (ABC) transporter 1, has been implicated in the translocation of PS from inner to the outer leaflet (Hamon et al., 2000). ABCA1 facilitates the engulfment of apoptotic cells (Hamon et al., 2000). The inactivation of aminophospholipid translocase and the activation of phospholipid scramblases or ABC transporters together might lead to the exposure of PS on the surface of apoptotic cells. However, it is unclear whether and how apoptotic cells of a particular cell type choose to activate either the ABC transporter or the phospholipid scramblase or both to promote PS exposure.
PS on the surface of apoptotic mammalian cells could act as one of the “eat-me” signals and be recognized both directly by certain phagocytic receptors that have PS-binding activity, or indirectly by others that associate with “bridging” molecules that bind to PS (for review, see Wu et al., 2006). One such bridging molecule is mammalian milk fat globule-EGF-factor 8 (MFG-E8) (Hanayama et al., 2002). This glycoprotein, when secreted from macrophages, which are professional phagocytes, interacts with apoptotic cells via its high-affinity, Ca2+-independent PS binding activity and with macrophages via its association with integrin αvβ5, a prominent phagocytic receptor (Hanayama et al., 2002). The apoptotic cell–MFG-E8–integrin αvβ5 interaction results in the activation of Rac1 GTPase-mediated cytoskeleton reorganization and phagocytosis (Akakura et al., 2004).
One hundred and thirty-one of the 1090 somatic cells developed in the nematode Caenorhabditis elegans hermaphrodite undergo apoptosis, among those 113 die during embryogenesis (Sulston and Horvitz, 1977; Sulston et al., 1983). In addition, >300 germ cells undergo apoptosis during germline development in adult hermaphrodite gonads (Gumienny et al., 1999). In living animals, apoptotic cells, or “cell corpses,” can be distinguished from living cells by using Nomarski differential interference contrast (DIC) microscopy by their highly refractile, button-like appearance (Sulston and Horvitz, 1977; Sulston et al., 1983). In C. elegans, cell corpses are swiftly engulfed and digested by their neighboring cells (for review, see Zhou et al., 2004). Genetic screens have identified eight genes acting within two parallel and partially redundant pathways to regulate the engulfment of apoptotic cells, cell corpse-engulfment defective (ced)-1, -6, -7, and dyn-1 in one pathway, and ced-2, -5, -10, and -12 in the other pathway (Zhou et al., 2004; Mangahas and Zhou, 2005; Yu et al., 2006). ced-10 may also convey certain activities for the ced-1 pathway (Kinchen et al., 2005). Mutations of each of these genes result in the accumulation of cell corpses in the body. The protein products of ced-6 (PTB-containing adaptor), dyn-1 (dynamin), ced-2 (CrkII homologue), ced-5 (Dock180 homologue), ced-10 (Rac GTPase), and ced-12 (ELMO1 homologue), all act exclusively inside engulfing cells to control engulfment (Liu and Hengartner, 1998; Wu and Horvitz, 1998a; Reddien and Horvitz, 2000; Zhou et al., 2001a; Gumienny et al., 2001; Wu et al., 2001; Yu et al., 2006). CED-1, a single-pass transmembrane protein, in contrast, is localized to the surface of engulfing cells and acts as a phagocytic receptor to recognize cell corpses and initiate their engulfment (Zhou et al., 2001b). The exact ligand(s) that CED-1 recognizes on the surface of cell corpses is not known; however, the function of CED-7, a homologue of mouse ABCA1, is essential for the recognition of cell corpses by CED-1 (Zhou et al., 2001b). Among the eight known engulfment genes, ced-7 is the only gene that plays a role in dying cells (Wu and Horvitz, 1998b). It is hypothesized that CED-7 promotes the exposure of a particular signaling molecule to the surface of apoptotic cells to attract CED-1 (Zhou et al., 2001b).
In the past, it was unknown whether PS is exposed on the surface of apoptotic cells in C. elegans, and if so, whether PS plays any role in cell corpse engulfment. In addition, the identity of the eat-me signal presented by CED-7 remains elusive. In this study, we investigated these issues. We established that PS acts as an eat-me signal and a CED-1 ligand in C. elegans, and we further identified the roles of two potential PS exposure activities during C. elegans development.
MATERIALS AND METHODS
C. elegans Strains
C. elegans strains were grown at 20°C as described previously (Brenner, 1974). The N2 Bristol strain was used as the reference wild-type strain. Mutations are described by Riddle et al. (1997), except where noted otherwise: LGI, ced-1(e1735), ced-12(n3261), plsc-2(tm820) (this study), plsc-3(ok1313) (this study); LGIII, ced-6(n2095), ced-7(n1996); LGIV, ced-5(n1812), ced-10(n1993); LGV, unc-76(e911), plsc-1(ok1178) (this study). plsc-1(ok1178) and plsc-3(ok1313) were provided by the C. elegans Gene Knockout Consortium in strains RB1149 and RB1249, respectively. plsc-1(ok1178) was outcrossed twice with the wild-type strain. plsc-2(tm820) was provided by Shohei Mitani (Tokyo Women's College, Tokyo, Japan). The strain UL809, which carries the reporter Pplsc-1NLS-gfp on an extrachromosomal array (Mounsey et al., 2002), was provided by Ian Hope (University of Leeds, Leeds, United Kingdom). Transgenic lines were generated by microinjection (Jin, 1999). Plasmids were coinjected with p76-18B (Bloom and Horvitz, 1997) into unc-76(e911) mutants, and non-Unc progeny were identified as transgenic animals. The enIs7[Pced-1ced-1::gfp] and enIs18[Plim-7mfg-e8::gfp] transgenes were both integrated on chromosome X. Double mutants between plsc-1(ok1178) and ced-1(e1735) and between plsc-1(ok1178) and plsc-2(tm820) were generated by standard genetic crosses, and the presence of homozygous ok1178 and tm820 alleles was verified by polymerase chain reaction (PCR) (Supplemental Figure S2). Because plsc-2 and plsc-3 are both on chromosome I, instead of generating plsc-1(ok1178); plsc-2(tm820) plsc-3(ok1313) triple mutant strain, we chose to inactivate plsc-3 by using RNAi in the plsc-1(ok1178); plsc-2(tm820) double mutant strain.
Plasmid Construction
mfg-e8 cDNA was PCR amplified from a Mus musculus breast cDNA library by using primers designed based on M. musculus mgf-e8 mRNA (GenBank accession no. AB021130). The splice variant that lacks the Pro/Thr-rich domain (amino acids 111–146) (mfg-e8ΔP/T), also known as mfg-e8-S (Hanayama et al., 2002), was first isolated. To construct a full-length mfg-e8 clone, the IMAGE clone 6333582 (GenBank accession no. BU147625) was digested using StuI and BamHI, a 440-base pair (bp) fragment containing the 111-bp Pro/Thr domain was inserted to replace the StuI–BamHI fragment in the mfg-e8ΔP/T clone. To construct mfg-e8ΔC2, nucleotides 1–918 were PCR amplified from mfg-e8 cDNA. The three mfg-e8 isoforms were fused with green fluorescent protein (GFP) at their C termini and placed behind the following promoters: Pced-1, which is expressed in all types of engulfing cells for somatic and germ cell corpses (Zhou et al., 2001b); Pdyn-1, which is ubiquitously expressed in embryos (Yu et al., 2006); and Plim-7, which is specifically expressed in gonadal sheath cells (Hall et al., 1999).
RNA Interference (RNAi)
The RNAi feeding constructs for dyn-1, plsc-1, plsc-2, and plsc-3 were obtained from a C. elegans RNAi library (Kamath et al., 2003), and animals of particular genotypes were treated with RNAi as described previously (Fraser et al., 2000). To score germ cell corpses, mid-L4–stage animals were transferred to RNAi plates, and then they were transferred to a new set of RNAi plates after 24 h. The numbers of germ cell corpses per gonad arm were scored under DIC microscope 48 h later. The progeny of these adults as L1 larvae hatched within an hour were used to score the number of somatic cell corpses in the head.
Annexin V Staining of Apoptotic Germ Cells
The anterior gonadal arms of adult hermaphrodites were dissected from the body in a glass slide by using established protocol (Rose et al., 1997). The gonad suspension was incubated at 20°C in the dark with 25 μl of ApoAlert binding buffer and 7 μl of ApoAlert fluorescein isothiocyanate (FITC)-labeled Annexin V (BD Biosciences, San Jose, CA) for 30 min according to the manufacturer's protocol. Dissected gonads were analyzed using fluorescence microscopy.
Quantification of Number of Cell Corpses by Using DIC Microscopy
Cell corpses were recognized under the Nomarski DIC microscope, and the numbers of cell corpses were scored using previously established methods (Yu et al., 2006). Somatic cell corpses were scored in embryos at different stages or in the head of young L1 larvae hatched within an hour. Germ cell corpses were scored in the gonad of adult hermaphrodites 48 h post-L4 stage unless otherwise stated.
Irradiation and Time-Lapse Recording of Duration of Germ Cell Corpses
Time-lapse analysis was performed on a DeltaVision system with a Olympus IX70 microscope (Applied Precision) by using the following protocol (Yu and Zhou, unpublished data). Adult hermaphrodites 24 h post-L4 stage were irradiated with a 137Cs source (Gammacell 1000, Atomic Energy of Canada Ltd., Kanata, ON, Canada; 8.33 Gy/min) at 180 Gy. Two hours after irradiation, samples were transferred onto NGM plates containing 1 mM Aldicarb; after 20 min, samples were mounted on a slide immersed in 0.5 mM Aldicarb solution, and then they were observed under 60× DIC optics at 20°C. Thirty serial z-sections, at 1.0 μm/section, of the gonadal region were recorded every 3 min. Recording began at 3 h after irradiation, and the period of duration of cell corpses that show up during the first 2 h were scored, because in the germline of wild-type hermaphrodites, the number of cell corpses reaches its peak 4 h after irradiation (Gartner et al., 2000). Animals were closely monitored for signs of viability during recording.
Fluorescence Microscopy
Animals were anesthetized in 30 mM sodium azide and placed on a glass slide on a 4% Agar pad. DIC and GFP images from serial z-sections were used to score the number of cell corpses and GFP-labeled cell corpses. Olympus IX70-Applied Precision DeltaVision microscope equipped with CoolSNAP digital camera (Photometrics, Tucson, AZ) and Softworx software (Applied Precision) was used to acquire serial Z-stacks of fluorescence images at 0.5-μm intervals and to deconvolve these images of embryos, newly hatched L1 larvae, and hermaphrodite gonads. Hermaphrodite gonads were sometimes analyzed using an Axioplan 2 compound microscope (Carl Zeiss, Thornwood, NY) with Nomarski DIC accessories, AxioCam camera, and AxioVision version 4.3 imaging software. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify GFP signal intensity in the gonads. The “Freehand” tool was used to trace GFP(+) circles around germ cell corpses and on the surface of adjacent living germ cells, the intensity of GFP signal was measured in the chosen areas, and relative ratio of GFP signal intensity was calculated.
RESULTS
Phosphatidylserine Is Detected on the Surface of Apoptotic Germ Cells
Mouse MFG-E8 has an N-terminal signal sequence but no putative membrane-spanning region, and it was reported to be a secreted protein (Figure 1A) (Hanayama et al., 2002). We generated a construct that allows the expression of a mfg-e8::gfp fusion cDNA (GFP was fused to the C terminus of MFG-E8) under the control of Plim-7, a promoter specifically active in gonadal sheath cells (Hall et al., 1999) that wrap around the germline and are responsible for the engulfment and degradation of germ cell corpses that die in the adult hermaphrodite gonad (Gumienny et al., 1999). We anticipated that MFG-E8::GFP, once secreted from gonadal sheath cells, would label the surface of nearby germ cell corpses if PS was present on the surface of these cells (Figure 1A).
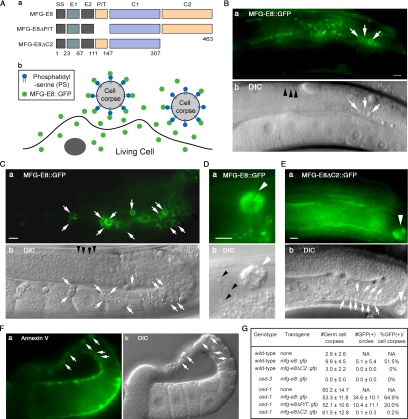
Figure 1.
PS is specifically exposed on the surface of germ cell corpses. (A) a, domain structures of mouse MFG-E8 and two deletion constructs. SS, signal sequence; E1 and E2, epidermal growth factor (EGF) domains; C1 and C2, Factor VIII-homologous domains. Numbers indicate amino acid positions. b, diagram depicting that MFG-E8::GFP secreted from living cells specifically associates with PS exposed on the surface of apoptotic cells and thus labels apoptotic cells. (B–F) All transgenes are expressed under the control of Plim-7. Dorsal is to the top. Midbody is to the left. Bars, 5 μm. (B) Epifluorescence (a) and corresponding DIC (b) images of MFG-E8::GFP in the gonad of a wild-type adult hermaphrodite. Arrows indicate three germ cell corpses labeled with MFG-E8::GFP. Black arrowheads in b indicate several living germ cells. (C) Epifluorescence (a) and corresponding DIC (b) images of MFG-E8::GFP in the gonad of a ced-1(e1735) adult hermaphrodite. Arrows indicate germ cell corpses labeled with MFG-E8::GFP. Black arrowheads in b indicate several living germ cells that lack GFP signal on their surfaces. (D) Epifluorescence (a) and corresponding DIC (b) images of MFG-E8::GFP observed in a coelomocyte (white arrowheads) near a gonad arm in a ced-1(e1735) adult hermaphrodite. Black arrowheads mark several living germ cells. (E) Epifluorescence (a) and corresponding DIC (b) images of MFG-E8ΔC2::GFP in the gonad of a ced-1(e1735) mutant adult hermaphrodite. Arrowheads indicate GFP signal in a nearby coelomocyte. Arrows mark 10 germ cell corpses that lack GFP signal on their surfaces. (F) Epifluorescence (a) and corresponding DIC (b) images of Annexin V staining of a gonad arm dissected out of a ced-1(e1735) adult hermaphrodite. Arrows indicate Annexin V-labeled germ cell corpses. (G) The numbers of total (DIC optics) and GFP-labeled (fluorescence microscopy) germ cell corpses expressing MFG-E8::GFP and its truncated forms. For each transgenic construct, at least two independent lines were scored and at least 15 animals were scored for each line, and similar results were obtained. The results of one representative line are reported. Data are presented as mean ± SD. NA, not applicable.
In wild-type animals, MFG-E8::GFP was specifically detected on the surface of dying germ cells but not on living germ cells (Figure 1B). In the gonad of ced-1 null mutant adult hermaphrodites, where a large number of unengulfed germ cell corpses reside, ∼65% of germ cell corpses identified by DIC microscopy were labeled with MFG- E8::GFP on the surface (Figure 1, C and G). Similar to wild type, no GFP signal was detected on the surface of living germ cells (Figure 1C). Consistently, no MFG-E8::GFP circle was observed on the surface of any germ cells in ced-3(n717) mutant animals, where programmed cell death was abolished due to a loss-of-function mutation in the C. elegans caspase CED-3 (Figure 1G) (Yuan et al., 1993). We further observed a strong GFP signal in coelomocytes, scavenger cells that nonspecifically internalize fluid from the pseudocoelom (body cavity) via endocytosis and micropinocytosis (Fares and Greenwald, 2001) (Figure 1D). Because Plim-7 is not active in coelomocytes (Supplemental Figure S1), this observation suggests that MFG-E8::GFP is secreted from gonadal sheath cells as expected and subsequently internalized by coelomocytes from the extracellular space.
Previous studies indicate that C1 and C2, the two factor-VIII homologous domains in MFG-E8 (Figure 1A), are essential for the PS binding activity (Hanayama et al., 2002). We found that a truncated form of MFG-E8 lacking the C2 domain (MFG-E8ΔC2::GFP) expressed under the control of Plim-7 failed to label germ cell corpses (Figure 1E). Meanwhile, strong MFG-E8ΔC2::GFP signal was detected in coelomocytes near the gonad (Figure 1E), indicating that the production and secretion of this truncated protein were normal. In addition, MFG-E8ΔP/T::GFP, another truncated form lacking the proline/threonine-rich (P/T) domain (Figure 1A) and reported to display decreased PS binding activity in vitro (Hanayama et al., 2002), was detected on merely 20% of germ cell corpses (Figure 1G). Together, these results indicate that MFG-E8 is recruited to the surface of apoptotic cells through its specific binding to PS.
To further confirm the above-mentioned results using a different PS detector, we stained dissected adult hermaphrodite gonads with FITC-conjugated Annexin V, a known PS-binding protein (see Materials and Methods) (van Engeland et al., 1998). Annexin V was detected specifically on the surface of germ cell corpses but not on that of living germ cells in gonads dissected from ced-1 mutants (Figure 1F), indicating that PS is indeed specifically presented on the surface of apoptotic cells. Together, our observations indicate that MFG-E8::GFP is a specific marker for detecting PS exposed on the surface of apoptotic germ cells in living C. elegans.
PS Is Specifically Presented on the Surface of Apoptotic Somatic Cells
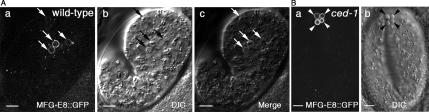
During embryogenesis, the execution of apoptosis in somatic cells, like that in germ cells, requires the functions of cell death genes ced-4 and ced-3 (Gumienny et al., 1999). However, whether the downstream events regulated by the caspase CED-3 are identical remains unclear. To examine whether apoptotic somatic cells expose PS on their surfaces, we expressed mfg-e8::gfp under the control of Pdyn-1, the promoter of dyn-1 that is ubiquitously expressed in embryos (Yu et al., 2006). In this scenario, we anticipated that the secreted MGF-E8::GFP would label the outer surface of any cells that exposed PS. In wild-type embryos, GFP was detected on the surface of transiently present cell corpses; in contrast, no GFP signal was detected on the surface of living somatic cells (Figure 2A). In ced-1 null mutant embryos, GFP was detected on the surface of cell corpses inside embryos as well as on unengulfed cell corpses that are subsequently extruded to the embryonic cavity (Figure 2B). In ced-1 L1 larvae expressing Pdyn-1 mfg-e8::gfp, 92% of the persistent somatic cell corpses are labeled with a GFP ring; however, in ced-1 L1 larvae expressing MFG-E8ΔC2::GFP, only 5.9% somatic cell corpses are labeled with GFP (Figure 3A). Together, these results indicate that, like germ cell corpses, somatic cell corpses specifically expose PS on their outer surfaces.
Figure 2.
Somatic cell corpses are specifically labeled with MFG-E8::GFP. Epifluorescence and corresponding DIC images of embryonic cell corpses labeled with MFG-E8::GFP in a wild-type (A) and a ced-1(e1735) embryo. MFG-E8::GFP is expressed under the control of Pdyn-1. Anterior is to the top. Ventral is to the left. Bars, 5 μm. Arrows in A indicate cell corpses inside embryos that are labeled with MFG-E8::GFP. Arrowheads in B indicate four cell corpses extruded into the embryonic fluid and labeled with bright GFP signal.
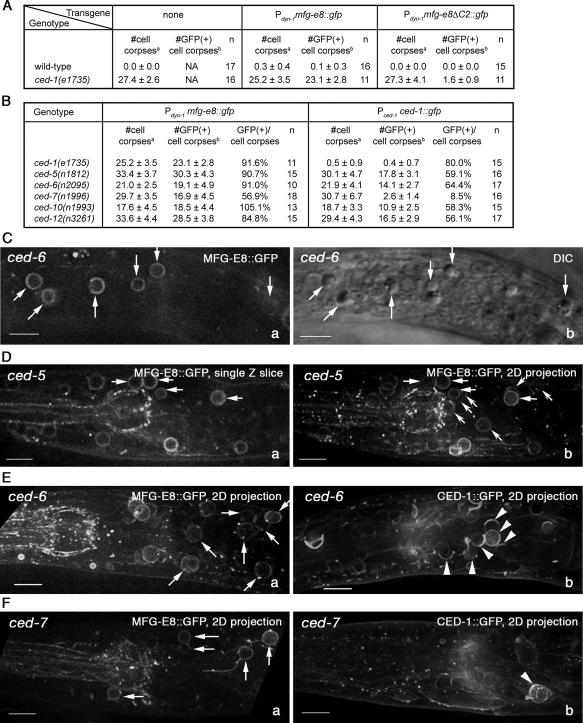
Figure 3.
PS is exposed on the surface of somatic cell corpses in a CED-7-dependent manner. CED-1's recognition of somatic cell corpses is also dependent on CED-7. (A) The numbers of total (DIC optics) and GFP-labeled (fluorescence microscopy) somatic cell corpses in different genotypes expressing MFG-E8::GFP and its truncated form. For each reporter construct, at least 15 animals were scored. Data are presented as mean ± SD. NA, not applicable. (B) The numbers of somatic cell corpsesa (DIC) and GFP-labeled somatic cell corpsesb (fluorescence microscopy) in different engulfment mutants expressing MFG-E8::GFP or CED-1::GFP were scored in the heads of L1 larvae hatched within 1 h. a,bData are presented as mean ± SD. n, number of animals scored. (C–F) Genotype and reporter constructs are indicated inside each image. MFG-E8::GFP and CED-1::GFP are expressed under the control of Pdyn-1 and Pced-1, respectively. All images show part of the head region of L1 larvae. Anterior is to the left. Bars, 5 μm. Arrows indicate several cell corpses labeled with MFG-E8::GFP. (C) b, DIC image of a. (D) A single focal plane (a) and a two-dimensional (2D) projection of 20 serial z-sections (including a) that cover the entire depth of one head (b). A comparison of a and b reveals many additional GFP-labeled cell corpses (concaved arrows) in b. (E and F) Each image is a 2D projection of 20 serial z-sections that cover the entire depth of an L1 head expressing MFG-E8::GFP (a) or CED-1::GFP (b). Arrowheads in b indicate full or partial CED-1::GFP circles around cell corpses.
The Association of MFG-E8 with the Surface of Apoptotic Cells Interferes with Their Engulfment
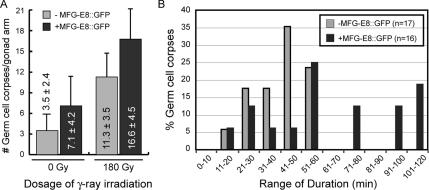
In the gonad of wild-type hermaphrodites expressing Plim-7 mfg-e8::gfp, we detected a 2.4-fold increase in the number of germ cell corpses over the wild-type animals not expressing any transgene or expressing Plim-7 mfg-e8ΔC2::gfp (Figure 1G). To further determine whether the ectopic expression of MFG-E8 affected cell corpse removal, we monitored the duration of germ cell corpses induced by γ-ray irradiation, which occur rather synchronously, by using time-lapse recording (see Materials and Methods). Four hours after irradiation, >2-fold increase in the numbers of germ cell corpses were observed in wild-type animals both expressing and not expressing Plim-7mfg-e8::gfp compared with their corresponding nonirradiated controls (Figure 4A), indicating that irradiation was effective in inducing apoptosis. Moreover, a significantly larger number of irradiation-induced cell corpses were observed in animals expressing than those not expressing Plim-7 mfg-e8::gfp (Figure 4A), suggesting that the overexpressed MFG-E8 may affect the removal of these cell corpses or the irradiation-induced apoptosis. In adult hermaphrodites not carrying any transgene, all germ cells undergoing apoptosis disappear within 1 h of its first appearance (Figure 4B); however, in animals expressing Plim-7mfg-e8::gfp, 44% of germ cell corpses analyzed persisted for a significantly longer period (Figure 4B). These results indicate that MFG-E8 delays the removal of cell corpses.
Figure 4.
The overexpression of MFG-E8::GFP in the gonad reduces the efficiency for the removal of germ cell corpses. (A) Significantly more germ cell corpses were observed in the gonad of adult hermaphrodites overexpressing MFG-E8::GFP in the absence or presence of γ-ray irradiation. The numbers of germ cell corpses per gonad arm were scored under DIC optics in wild-type adult hermaphrodites not carrying (light gray bars) or carrying (dark bars) the integrated Plim-7mfg-e8::gfp transgene 28 h post-L4 stage, 4 h after γ-ray irradiation. Data are presented as mean ± SD. (B) Histogram indicating the distribution of the duration of germ cell corpses after γ-ray irradiation (see Materials and Methods). Germ cell corpses occur within 3–4 h of γ-ray irradiation were recorded by DIC time-lapse imaging. The y-axis indicates the percentage of germ cell corpses that last for the period of time indicated in the x-axis. n, number of cell corpses analyzed.
In wild-type animals, somatic embryonic apoptotic cells are swiftly engulfed and degraded during embryogenesis; thus, no cell corpse is detected in the head of newly hatched L1 larvae (Figure 3A). We thus scored the number of persistent cell corpses in the head of newly hatched L1 larvae to measure the degree of engulfment defect during embryogenesis. We observed that the expression of MFG-E8::GFP, but not that of MFG-E8ΔC2::GFP in wild-type embryos, resulted in a weak yet still significant (p < 0.05, Student's t test) inhibitory effect on the removal of somatic cell corpses (Figure 3A).
The above-mentioned observations suggest that the association of secreted MFG-E8 with the surface of cell corpses may mask PS or certain adjacent molecules from being recognized by engulfing cells. This notion is consistent with the hypothesis that PS acts as an eat-me signal to activate phagocytic receptor(s) on the surface of engulfing cells.
CED-7, a Member of the ABC Transporter Family, Is Involved in Exposure of PS on the Surface of Apoptotic Somatic Cells but Not Germ Cells
To examine whether the functions of any of the engulfment ced genes are required for the exposure of PS on the surface of somatic apoptotic cells, the Pdyn-1 mfg-e8::gfp transgene was expressed in mutant alleles of each of the seven genes, and the number of cell corpses as well as that of MFG-E8::GFP rings around cell corpses was scored in the heads of newly hatched L1 larvae (see Materials and Methods). Any persistent cell corpses observed in the head of newly hatched L1 larvae should have been generated during embryogenesis (Sulston and Horvitz, 1977; Sulston et al., 1983). ced-1(e1735), ced-5(n1812), and ced-7(n1996) are null mutants of each corresponding gene, whereas ced-6(n2095), ced-10(n1993), and ced-12(n3261) represent strong loss-of-function mutants of the corresponding genes (Zhou et al., 2004). We found that in ced-1, ced-5, ced-6, ced-10, and ced-12 mutants, at least 85% of somatic cell corpses were labeled with MFG-E8::GFP circles (Figure 3, B–E). However, in ced-7 mutants, only on average 57% of cell corpses were labeled with MFG-E8::GFP circles (Figure 3, B and F). These results indicate that ced-7, unlike ced-1, ced-5, ced-6, ced-10, or ced-12, is required for the efficient association of MFG-E8 with cell corpses. A likely interpretation is that CED-7 contributes to the presentation of PS on the surface of apoptotic cells.
We also quantified whether mutations in engulfment genes in the ced-1 pathway affect the association of MFG-E8::GFP on the surface of persistent germ cell corpses. Contrary to what we observed with somatic cell corpses, the percentage of germ cell corpses labeled with MFG-E8::GFP in ced-7 mutant hermaphrodites seemed similar to that obtained from ced-1 or ced-6 mutant hermaphrodites or hermaphrodites that lost dyn-1 function due to RNAi treatment (Table 1) (see Materials and Methods) (Yu et al., 2006), suggesting that inactivating ced-7 or other known ced-1 pathway components does not affect the exposure of PS on the surface of germ cell corpses.
Table 1.
The clustering of both MFG-E8::GFP and CED-1::GFP around germ cell corpses is relatively normal in ced-7 mutants
| Genotype | Plim-7mfg-e8::gfp |
Pced-1ced-1ΔC::gfp |
||||||
|---|---|---|---|---|---|---|---|---|
| No. cell corpses | No. GFP(+) cell corpses | %GFP(+)/total corpsesa | n | No. cell corpses | No. GFP(+) cell corpses | %GFP(+)/total corpsesa | n | |
| ced-1 (e1735) | 59.1 ± 15.8 | 32.6 ± 10.8 | 56.8 ± 17.2 | 30 | 65.3 ± 15.3 | 37.1 ± 13.1 | 57.1 ± 15.4 | 30 |
| ced-6 (n2095) | 62.6 ± 11.3 | 32.5 ± 8.9 | 51.8 ± 11.1 | 30 | 68.8 ± 15.2 | 33.3 ± 9.0 | 50.2 ± 16.7 | 29 |
| ced-7 (n1996) | 66.0 ± 15.2 | 31.9 ± 11.9 | 48.4 ± 15.5 | 30 | 74.7 ± 10.7 | 41.6 ± 15.2 | 56.6 ± 20.2 | 26 |
| dyn-1(RNAi)b | 21.5 ± 7.0 | 11.2 ± 3.3 | 55.1 ± 13.1 | 13 | 22.0 ± 9.1 | 11.3 ± 7.1 | 52.2 ± 28.3 | 21 |
The numbers of total germ cell corpses (DIC optics) and GFP(+) germ cell corpses (fluorescence microscopy) were scored in one gonad arm of each adult hermaphrodite 48 h post-mid-L4 stage. Data are presented as mean ± SD. n, number of animals scored.
a The percentage of GFP(+) cell corpses were calculated for each animal and mean and SD were deduced.
b Mid-L4-stage wild-type animals carrying the indicated transgenes were transferred to dyn-1(RNAi) plates 48 h before scoring.
PS and Perhaps an Additional Ligand(s) Both Contribute to the Recognition of Cell Corpses by Phagocytic Receptor CED-1
The phagocytic receptor CED-1 recognizes cell corpses and clusters in a high concentration on the side of an engulfing cell in contact with a cell corpse. A CED-1::GFP reporter, which retains full CED-1 activity, forms bright green circles around transiently existing apoptotic cells in wild-type embryos, and primarily partial circles around persistent cell corpses in engulfment mutant embryos and larvae (Zhou et al., 2001b). Previously, we reported that in ced-7 mutants, the clustering of ced-1 around cell corpses was severely defective. In this study, we scored the number of CED-1::GFP circles around persistent cell corpses using the DeltaVision deconvolution microscope (see Materials and Methods). In ced-5, ced-6, ced-10, and ced-12 mutant L1 larvae, we identified a larger percentage of persistent somatic cell corpses labeled with CED-1::GFP circles or partial circles than previously reported (∼60 vs. ∼30%) (Zhou et al., 2001b; Figure 3, B–E). This increase is primarily due to the increased sensitivity in detecting fluorescence signals by the DeltaVision deconvolution microscope compared with the fluorescence microscope we used previously. Strikingly, in ced-7 mutant L1 larvae, merely 8.5% of cell corpses were labeled with CED-1::GFP circles or partial circles (Figure 3, B and F), consistent with our previous observation (Zhou et al., 2001b) and confirming that the function of ced-7 is essential for CED-1 to recognize and cluster around cell corpses. Thus, in ced-7 mutants, the efficiency of PS exposure on somatic cell corpses and the efficiency of CED-1's recognition of cell corpses both decrease.
The loss of ced-7 function seems to affect CED-1's recognition of somatic cell corpses to a much larger extent than affecting the exposure of PS. Compared with ced-6 mutants that are defective in the engulfment events downstream of CED-1's recognition of cell corpses (Zhou et al., 2001b), in ced-7 L1 larvae, the percentage of cell corpses presenting PS is decreased from 91 to 57%, yet the percentage of cell corpses recognized by CED-1 is dramatically decreased from 64 to 8.5% (Figure 3B). A comparison with ced-5, ced-10, or ced-12 mutants that are defective in cytoskeleton reorganization during engulfment generates a similar conclusion (Figure 3B). These differential effects suggest that PS exposure alone is necessary but might not be sufficient for cell corpses to be recognized by CED-1. Among multiple possibilities, perhaps an additional signaling molecule is exposed on the surface of apoptotic cells in a CED-7-dependent manner and acts together with PS to attract CED-1 (see Discussion).
A Null Mutation in ced-7 Does Not Affect CED-1's Recognition of Germ Cell Corpses
To determine the correlation between PS exposure and CED-1 clustering in the adult hermaphrodite gonad, we examined whether CED-1 clusters around germ cell corpses in engulfment mutants. We used a CED-1ΔC::GFP reporter, in which the intracellular domain of CED-1 was replaced with GFP, for this assay. CED-1ΔC::GFP on the surface of engulfing cells retains the ability to cluster around cell corpses yet loses the ability to initiate engulfment and thus failed to rescue ced-1 mutant phenotypes (Zhou et al., 2001b). Interestingly, unlike in the soma in which a ced-7 null mutation blocks CED-1 clustering, similar percentages of persistent germ cell corpses are labeled with CED-1ΔC::GFP rings in ced-1, ced-6, and ced-7 mutants and dyn-1(RNAi) animals (Table 1). This observation correlates well with the normal exposure of PS on the surface of germ cell corpses observed in ced-1, ced-6, and ced-7 mutants and dyn-1(RNAi) animals (Table 1), and further suggests that somatic and germ cell corpses may use different mechanisms to regulate the exposure of PS and/or another unidentified eat-me signal(s). Kinchen et al. (2005) also observed the clustering of CED-1 around germ cell corpses in ced-7 mutants.
Mutations of Phospholipid Scramblase Homologues PLSC-1, PLSC-2, and PLSC-3 Do Not Cause Obvious Defect in the Engulfment of Somatic Cell Corpses
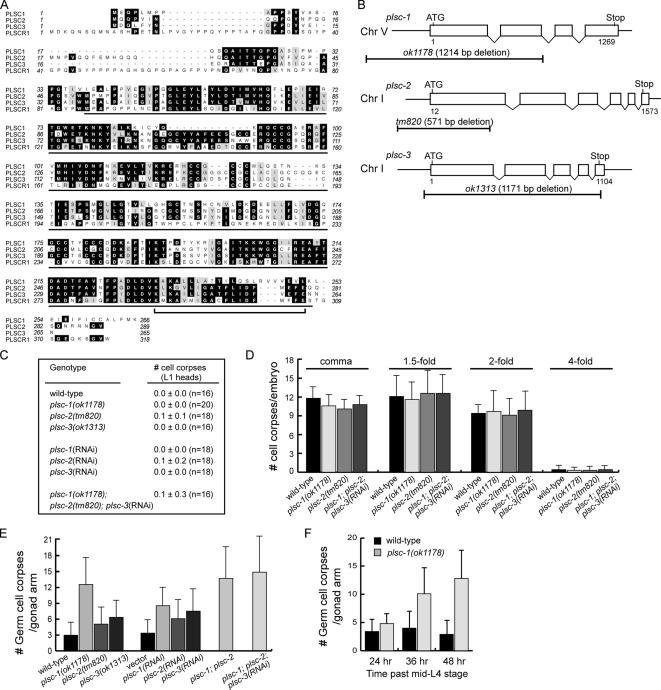
Human phospholipid scramblase PLSCR1 was reported to induce the random movement of phospholipids between plasma membrane leaflets in response to the increase of intracellular Ca2+ and perhaps other signals (Basse et al., 1996; Zhou et al., 1997). This activity could potentially promote the exposure of phospholipids that are usually kept in the inner leaflet of the plasma membrane, such as PS and PE, to the outer surface. In the C. elegans genome, we identified four predicted polypeptides, C04E12.7, T22H2.5, ZK1053.5, and F11A6.2, that display extensive homology to PLSCR1 throughout their entire length (Figure 5A). The genes encoding C04E12.7, T22H2.5, ZK1053.5, and F11A6.2 are named plsc-1, plsc-2, plsc-3, and plsc-4, respectively (www.wormbase.org). F11A6.2 lacks a part of the scramblase domain at the N-terminal region and is not studied in this report. We analyzed whether PLSC-1, -2, and -3, which possess the intact scramblase domain, are required for the exposure of PS and for cell corpse engulfment by using deletion alleles of each gene (see Materials and Methods).
Figure 5.
The C. elegans homologues of human PLSCR1 contain persistent germ cell corpses. (A) The alignment of human PLSCR1 with its three C. elegans homologues. The amino acid residues identical or similar among at least three proteins are shaded in black or gray, respectively. One black line underlines the scramblase domain in human PLSCR1; the bracket under PLSCR1 sequence indicates predicted transmembrane domain (Zhou et al., 1997). (B) The gene structure (adopted from www.wormbase.org) and regions of the deletions in plsc-1, plsc-2, and plsc-3. Open boxes indicate exons and the lines in between indicate introns. The line under each deletion allele indicates the regions of the deleted genomic DNA. Chr, chromosome. (C–F) The complete genotype of the plsc triple mutants scored in C–F is plsc-1(ok1178); plsc-2(tm820); plsc-3(RNAi). Data are presented as mean ± SD. (C) In single and triple plsc mutants, the numbers of somatic cell corpses were scored in the head of L1 larvae hatched within 1 h of scoring. n, number of animals scored. (D) Bar graph of the numbers of somatic cell corpses at different stages of embryonic development in wild-type and plsc mutant embryos. Comma-, 1.5-fold, 2-fold, and fourfold stages correspond to ∼380, ∼420, ∼460, and 650–800 min post-first-cleavage, respectively. At least 15 animals were scored for each data point. (E) Bar graph of the numbers of germ cell corpses scored in the gonadal arms of adults 48 h post-mid-L4 stage wild-type and plsc single, double, and triple mutants and RNAi-treated adults. At least 20 animals were scored for each data point. (F) Bar graph of the numbers of germ cell corpses at different stages of gonad development in adult hermaphrodites of wild-type and plsc-1(ok1178) animals. At least 20 animals were scored for each data point.
In plsc-1(ok1178) deletion mutants, we detected a 1240-base pair deletion that removed exons 1 and 2 and intron 1 of the predicted plsc-1 gene as well as 444 base pairs 5′ of the translational start site (Figure 5B and Supplemental Figure S2). Such a deletion eliminates N-terminal 2/3 of the open reading frame and the 5′ promoter sequence for plsc-1 transcription, and it should result in a null allele. In the plsc-3(ok1313) allele, we detected a 1171-base pair deletion that removed the entire coding region of plsc-3 as well as 67 base pairs upstream of the ATG start codon and that should create a null allele (Figure 5B and Supplemental Figure S2). The plsc-2(tm820) allele bears a 571-base pair deletion that removes most of exon 1 of plsc-2 and 230 base pairs upstream of the ATG start codon (www.wormbase.org), again likely to result in a null allele (Figure 5B and Supplemental Figure S2). We further confirmed that in each of the homozygous mutant strains, the wild-type allele of the corresponding genes was absent from the genome (Supplemental Figure S2), excluding the possibility of the presence of duplicated wild-type alleles elsewhere in the genome.
All three mutant alleles are homozygous viable and lack obvious developmental defects. In the head of L1 larvae, we did not observe persistent somatic cell corpses in any single mutant background, or in animals subjected to RNAi treatment of each of the three genes (Figure 5C). In plsc-1(ok1178) or plsc-2(tm820) single mutant embryos, or in plsc-1(ok1178); plsc-2(tm820); plsc-3(RNAi) triple mutant embryos, the numbers of somatic cell corpses scored at four different stages of embryogenesis are similar to that observed in wild-type embryos (p > 0.05, Student's t tests) (Figure 5D). These results seem to indicate a lack of obvious defect in the engulfment of somatic cell corpses or in the execution of apoptosis.
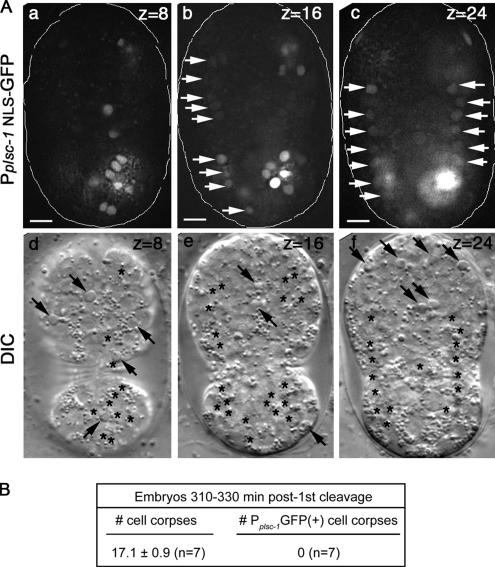
We further analyzed the embryonic expression pattern of plsc-1. A GFP reporter containing a nuclear localization signal (NLS) fused to its N terminus and expressed under the control of a 6.4-kb plsc-1 promoter (Pplsc-1NLS-gfp) (Mounsey et al., 2002) was reported to express predominantly in neurons and body wall muscles in larvae and adults (www.wormbase.org). Using this reporter, we observed that in embryos, plsc-1 was predominantly expressed in body wall muscle precursor cells and certain yet-to-be identified cells that could be of neuronal lineages (Figure 6A). We further observed that throughout embryogenesis, none of the cell corpses expressed any GFP signal. In particular, in embryos 310–330 min post-first cleavage, 100% of the 17 cell corpses are GFP− (Figure 6, A and B). These observations, together with the above-mentioned observation indicating that the deletion of plsc-1 does not affect the removal of somatic cell corpses, suggest that plsc-1 is unlikely to be involved in the exposure of the eat-me signal in somatic apoptotic cells.
Figure 6.
plsc-1 is not expressed in apoptotic cells during embryogenesis. (A) Epifluorescence (a–c) and corresponding DIC (d–f, respectively) images of three focal planes of one embryos ∼330 min post-first cleavage that expresses NLS-GFP under the control of the plsc-1 promoter (Pplsc-1NLS-GFP). Anterior is to the top, ventral side faces readers. Bar, 5 μm. White arrows in b and c indicate body wall muscle cells. Black arrows in d and f indicate cell corpses. Asterisks indicate cells that express Pplsc-1GFP. (B) Quantitative assay results indicating that Pplsc-1NLS-GFP is not expressed in apoptotic cells. The numbers of cell corpses were scored using DIC microscopy. Data are presented as mean ± SD. n, number of embryos scored.
The Inactivation of PLSC-1, PLSC-2, and PLSC-3 Affects the Removal of Germ Cell Corpses to Different Extents
In the gonad of adult hermaphrodites of plsc-1, -2, and -3 single mutants, in contrast, we observed significantly larger numbers of germ cell corpses than in wild-type animals (p < 0.05, Student's t tests) (Figure 5E). This Ced phenotype was also reproduced by RNAi treatment of individual plsc genes, indicating that it is caused by the deletion of plsc-1, -2, or -3, and not by any unrelated background mutations (Figure 5E). The plsc-1(ok1178) allele that has been outcrossed twice displays a same level of Ced phenotype observed in the original mutant strain (Supplemental Figure S2E), further confirmed the above-mentioned notion. plsc-1(ok1178) animals display a much stronger phenotype than plsc-2 or plsc-3 deletion animals—the number of germ cell corpses scored in plsc-1(ok1178) animals is at least twofold of that scored in plsc-2(tm820) or plsc-3(ok1313) animals (Figure 5E). plsc-1(ok1178); plsc-2(tm820) double mutant animals possess a slightly larger number of germ cell corpses than plsc-1(ok1178) animals (13.5 vs. 12.5/gonadal arm); a further inactivation of plsc-3 via RNAi in the plsc-1(ok1178); plsc-2(tm820) double mutant animals leads to a further slight increase of germ cell corpse number to 14.8/gonadal arm (Figure 5E). These observations seem to suggest that among the plsc genes, plsc-1 plays a major role, and that any minor contributions of plsc-2 and -3 to the removal of germ cell corpses are likely either conducted through plsc-1, or partially redundant to that of plsc-1. In addition, we observed that in a period of 24 h of observation, the numbers of germ cell corpses continue to increase over time in plsc-1(ok1178) adult hermaphrodites, suggesting an accumulation of germ cell corpses as a consequence of the defect in their removal (Figure 5F). We thus further characterized the function of plsc-1 in the exposure of PS in the germline.
PLSC-1 Promotes the Exposure of PS on the Surface of Apoptotic Germ Cells
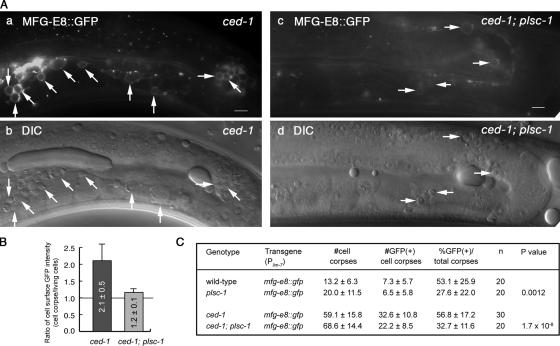
We observed two closely related defects regarding the exposure of PS on the surface of germ cells corpses in homozygous plsc-1(ok1178) mutants carrying the Plim-7 mfg-e8::gfp reporter construct. First, the MFG-E8::GFP signal is significantly weaker on the surface of germ cell corpses than in wild-type animals. The reduction of GFP signal intensity was observed in the plsc-1(ok1178) single mutants (data not shown) as well as in ced-1(e1735); plsc-1(ok1178) double mutants (Figure 7, A and B). The overall GFP signal intensity in the gonad remains normal (Figure 7A); in addition, bright GFP signals were observed in coelomocytes near the gonad (Supplemental Figure S3), indicating that the deletion of plsc-1 does not affect the production or the secretion of MFG-E8::GFP from gonadal sheath cells. Second, in plsc-1 mutants, the number of germ cell corpses labeled with MFG-E8::GFP is significantly lower than in wild-type animals (Figure 7C). Similarly, the number of persistent germ cell corpses labeled with MFG-E8::GFP in the ced-1; plsc-1 double mutants is significantly lower than that in ced-1 mutants (Figure 7, A and C). Together, the reductions of GFP signal intensity on the surface of germ cell corpses and the percentage of germ cell corpses labeled with MFG-E8::GFP strongly suggest that PLSC-1 plays an important role in promoting the presentation of PS on the surface of germ cell corpses. The plsc-1(ok1178) mutants expressing MFG-E8::GFP display a further enhanced Ced phenotype—containing more germ cell corpses (20.2) than plsc-1(ok1178) mutants alone (12.5) or wild-type animals expressing MFG-E8::GFP (13.2) (Figures 5E and 7C), suggesting an additive effect of two independent forces that impair the function of PS as a signal to attract engulfing cells: decreased efficiency of PS exposure in plsc-1 mutant background, and further sequestration of PS from phagocytic receptor(s) by MFG-E8. Due to the frequently occurring germline-specific repression of the expression of transgenes in the extrachromosomal array (Kelly et al., 1997), Pplsc-1NLS-gfp is not a suitable reporter for examining whether plsc-1 is expressed in the germline. The expression pattern of plsc-1 in the germline remains to be determined.
Figure 7.
plsc-1 mutants are defective in the exposure of PS on the surface of germ cell corpses. (A) Epifluorescence (a and c) and corresponding DIC (b and d, respectively) images of adult hermaphrodite gonads that express Plim-7mfg-e8::gfp. Midbody is to the left. Dorsal is to the top. Bars, 5 μm. (a) and (c) were captured under the same exposure time and were processed identically. Arrows indicate germ cell corpses labeled with MFG-E8::GFP. (B) Bar graph indicating the ratio of GFP intensity on the surface of germ cell corpses versus on the surface of adjacent living germ cells. The ratio was obtains for the 10 cell corpses marked by arrows in Aa and four cell corpses marked by arrows in Ac. Data are presented as mean ± SD. (C) The numbers of germ cell corpses (DIC) and MFG-E8::GFP–labeled germ cell corpses in adult hermaphrodites 48 h post-mid-L4 stage. Data are presented as mean ± SD. n, the number of animals scored. P values of comparisons between wild-type and plsc-1 animals and between ced-1 and ced-1; plsc-1 mutant animals were deduced using Student's t test assuming unequal variances.
DISCUSSION
The Exposure of PS Is a Conserved Feature of Apoptotic Cells from Nematodes to Human
The cuticle that covers larvae and adults and the chitin-based eggshell, which are impermeable to most staining reagents without fixation, made it difficult to determine the surface features of apoptotic cells in living C. elegans. In this study, we detected PS exposure on the surface of apoptotic cells by using ectopically expressed, secreted MFG-E8::GFP in living animals, and by using Annexin V staining in surgically dissected gonads. In ced-1 mutants that contain many unengulfed cell corpses, strong PS accumulation was detected on the surface of >90% of somatic cell corpses and ∼60% of germ cell corpses, and during embryogenesis, larval development, and adulthood, suggesting that the exposure of PS is a common feature for apoptotic cell, regardless of tissue types or developmental stages. Recently, using Annexin V staining, Wang et al. (2007) also reported PS exposure on the surface of apoptotic germ cells in C. elegans.
PS May Act As an Eat-Me Signal and a Ligand for CED-1 in C. elegans
Our research provided several lines of evidence to suggest that the exposure of PS may facilitate the engulfment of apoptotic cells in C. elegans. First, the ectopic expression of mouse MFG-E8::GFP but not its PS binding-deficient form (ΔC2) in gonadal sheath cells results in the accumulation of persistent germ cell corpses. MFG-E8 is likely to delay cell corpse engulfment, because germ cell corpses last significantly longer than in animals not expressing MFG-E8. No obvious defects in germline morphology or fertility that could be associated with excessive death of germ cells were observed. In addition, during the time-lapse recording experiment, we observed that in both animals expressing or not expressing MFG-E8, similar numbers of germ cells underwent apoptosis in a fixed period after irradiation, suggesting MFG-E8 did not induce excessive apoptosis in response to irradiation. These observations suggest that MFG-E8 is unlikely to cause a significant amount of excessive germ cell death. Rather, the binding of MFG-E8 to cell-surface PS may sequester PS and/or another adjacent signaling molecule from phagocytic receptor(s) or bridging molecules. A similar yet much weaker engulfment-inhibitory effect was detected when MFG-E8::GFP was ubiquitously expressed in embryos and larvae. Pdyn-1, the somatic cell-specific promoter, is rather weak (Zhou, unpublished observation). In addition, as a secreted protein, a significant amount of MFG-E8::GFP was observed in embryonic cavity, unavailable to associate with apoptotic cells (data not shown). These observations might explain why the effect of MFG-E8 in embryos is weak.
Second, defects in PS exposure, observed in somatic cell corpses in ced-7 mutants and in germ cell corpses in plsc-1 mutants, are closely associated with defects in cell corpse engulfment, suggesting that the exposure of PS is necessary to attract engulfing cells. Thus, the exposure of PS is not only a conserved feature of apoptotic cells in metazoans but also plays a conserved role in the removal of apoptotic cells from nematodes to mammals.
CED-1 was proposed to recognize PS as a ligand (Zhou et al., 2001b). We report that in ced-7 mutants, both PS exposure and CED-1's recognition of somatic cell corpses are impaired. In addition, masking PS and/or an unknown signaling molecule via MFG-E8 does not seem to further increase the number of germ cell corpses in ced-1 mutants (Figure 1G and Table 1), suggesting that MFG-E8 elicits its effect over engulfment through CED-1. These observations thus provide further support to the above hypothesis. Previously, PSR-1, a C. elegans homologue of mammalian phosphatidylserine receptor, was implicated in recognizing PS on the surface of apoptotic cells and activating the CED-5 pathway for engulfment (Wang et al., 2003). Both CED-1 and PSR-1 may recognize PS as a ligand and each activate a separate signaling pathway. psr-1 deletion mutants seem to display a rather weak and transient engulfment defect (Wang et al., 2003), suggesting that among the two receptors, CED-1 may play a major role in recognizing apoptotic cells.
In mammals, MFG-E8 acts to link apoptotic cell and phagocytes by its affinity for PS and for αvβ5 integrin, a phagocytic receptor (Hanayama et al., 2006). The C. elegans genome does not contain any known homologue of MFG-E8. In addition, none of the three known C. elegans integrin subunits are involved in cell corpse engulfment (Gumienny et al., 2001; Wu et al., 2001). Our observation that the ectopically expressed MFG-E8 impairs instead of promoting engulfment suggests that MFG-E8 is unlikely to act as a bridging molecule for a phagocytic receptor in C. elegans.
There May Be Two Alternative, Tissue-specific Mechanisms That Regulate PS Exposure
ABCA1, the closest mammalian homologue of CED-7, promotes the translocation of PS from the inner to the outer leaflet of the plasma membrane (Hamon et al., 2000). In addition, mammalian ABCA7 enhances phagocytosis of apoptotic cells by macrophages, and it was proposed to be involved in the translocation of phospholipids across the lipid bilayer (Jehle et al., 2006). However, the specific phospholipid-flipping function of ABCA1 or ABCA7 in apoptotic cells is yet to be demonstrated (Hamon et al., 2000; Jehle et al., 2006). Our identification of CED-7's function in promoting PS exposure on the surface of apoptotic cells provides this missing piece of the puzzle. Interestingly, CED-7's ability to promote PS exposure and to facilitate CED-1's recognition of cell corpses seems to be limited to somatic cells, suggesting a novel tissue-specific regulation. Consistent with its function, CED-7 is wildly expressed in almost all cells in embryos and is localized to plasma membrane (Wu and Horvitz, 1998b).
Although in vitro reconstitution assays and cell culture based assays indicate that mammalian PLSCR1 can mediate a Ca2+-activated bidirectional transport of phospholipids between the two membrane leaflets, whether PLSCR1 functions as a phospholipid scramblase in vivo remains controversial (for review, see Sims and Wiedmer, 2001). The multiple closely related phospholipid scramblases in mammals may act redundantly and prevent the disclosure of gene function by single gene deletion analysis. We identified PLSC-1, a C. elegans homologue of PLSCR1 that contributed significantly and specifically to the exposure of PS in apoptotic germ cells but not somatic cells. In plsc-1 deletion mutant animals, the accumulation of germ cell corpses and the reduced PS exposure together strongly suggest the involvement of a putative phospholipid scramblase in the exposure of PS. Recently, Wang et al. (2007) also reported that C. elegans SCRM-1 (another name for PLSC-2) is involved in the exposure of PS in response to apoptotic signal.
Mutants of plsc-2 and -3 display very minor Ced phenotype in the gonad. Furthermore, the plsc-1; plsc-2; plsc-3(RNAi) triple mutants only display very slight increase of the number of persistent germ cells, suggesting that plsc-2 and -3 might contribute to PS exposure either in a plsc-1–dependent manner or in a manner partially redundant to plsc-1 (Figure 8A).
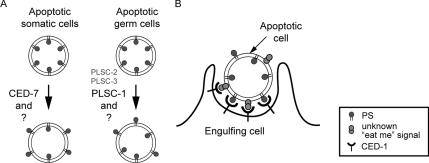
Figure 8.
Diagrams for the proposed mechanisms for the presentation and recognition of eat-me signals. (A) CED-7 and PLSC-1 regulate the exposure of PS on the surface of apoptotic cells in the soma or the germline, respectively. Smaller size and gray color are used to indicate that the effect of PLSC-2 and -3 might be minor. (B) PS and another unknown eat-me signal on the surface of apoptotic cells may be both recognized by phagocytic receptor CED-1.
We did not observe defects in the removal of somatic cell corpses in any of the plsc-1, -2, -3 single mutants or the plsc-1; plsc-2; plsc-3(RNAi) triple mutants. Although it was reported that the scrm-1/plsc-2 mutant animals have minor delays in the removal of cell corpses in embryos (Wang et al., 2007), because the Ced phenotype of ced-7 mutant embryos is much stronger than that reported for scrm-1 embryos, and because only ced-7 mutant animals display an obvious defect in the exposure of PS on apoptotic somatic cells (Figure 3), it seems that CED-7 makes a more significant contribution to the exposure of PS on somatic cell corpses than any of the PLSC proteins.
CED-7 and PLSC-1 may represent two alternative mechanisms that promote PS exposure in apoptotic cells of different tissue types (Figure 8A). Although germ cells and somatic cells in C. elegans both undergo apoptosis via the ced-3– and ced-4–mediated pathway, certain aspects of cell death are different. In particular, living germ cell nuclei are included in one germline syncytium and share a common cytoplasm and a common plasma membrane; when a germ cell nucleus dies, it undergoes a rapid cellularization process and is separated from the syncytium (Gumienny et al., 1999). Such a process does not occur to somatic apoptotic cells. In addition, cell corpses in the germline and soma are engulfed by different types of engulfing cells, one exclusively by gonadal sheath cells and the other by several cell types, including hypodermal cells, intestinal cells, and muscle cells (for review, see Zhou et al., 2004). The difference in the mechanism and dynamics of cell death and the types of engulfing cells may lead to the activation of different mechanisms that regulate PS exposure, hence the differential involvement of PLSC-1 and CED-7 in germ cell and somatic cell apoptosis. The existence of these alternative mechanisms may serve to increase the complexity for the regulation of eat-me signal exposure upon apoptotic stimuli during development. It would be of general interest to determine whether such differential mechanisms are also used in the mammalian system.
Why, then, in ced-7 mutant germline, are there still a large number of persistent germ cell corpses? Genetic mosaic analysis indicated that CED-7 functions are required in both germline and engulfing cells for efficient engulfment of germ cell corpses (Wu and Horvitz, 1998b). The defects observed in ced-7 mutant gonad might be primarily due to the loss of a major contribution of CED-7 activity from engulfing cells.
The Evidence for a Novel Eat-Me Signal(s)
A loss-of-function mutation in ced-7 results in a much stronger defect in the clustering of CED-1 around cell corpses than in the exposure of PS. Multiple explanations may exist. First, the detection of PS on the surface of a cell corpse by CED-1 might be subject to a threshold. Second, CED-7 may play two distinct roles during engulfment: promoting the exposure of PS in apoptotic cells, and assisting CED-1 in recognizing eat-me signals on the surface of engulfing cells. Alternatively, a mutation in CED-7 may disrupt the presentation of a yet unknown eat-me signal recognized by CED-1 in addition to PS. Several molecules or structures have been implicated as eat-me signals besides PS. These molecules include cell surface carbohydrates, oxidized lipids, intercellular adhesion molecule-3, cell surface calreticulin (for review, see Gardai et al., 2006), and certain unidentified molecules sensitive to proteolytic cleavage (Guzik et al., 2007). Lysophosphatidylcholine secreted from apoptotic cells was also reported to attract phagocytes to the vicinity (Lauber et al., 2003). It is possible that a novel eat-me signal(s) is exposed to the surface of C. elegans cell corpses in a CED-7–dependent manner and acts cooperatively with PS as CED-1 ligands (Figure 8B).
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Mitani, Ian Hope, A. Fire (Stanford University School of Medicine, Palo Alto, CA), the C. elegans Gene Knockout Consortium, and the Caenorhabditis Genetics Center for reagents; X. He for the DeltaVision; X. Yu, R. Edlund, and C. Chuang for technical assistance; S. Nagata for helpful suggestions; and A. Kuspa, X. He, A. Antebi, K. Oommen, J. Roger, and members of the Zhou laboratory for helpful comments. Previous and current support for Z.Z. includes the NIH (GM067848), the Cancer Research Institute, the March of Dimes Foundation, and the Rita Allen Foundation. V. V. was partially supported by NIH IMSD GM56929.
Abbreviations used:
- ced
cell corpse-engulfment defective
- MFG-E8
milk-fat-globule EGF8
- PLSC
phospholipid scramblase
- PS
phosphatidylserine.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0138) on June 13, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Akakura S., Singh S., Spataro M., Akakura R., Kim J. I., Albert M. L., Birge R. B. The opsonin MFG-E8 is a ligand for the alphavbeta5 integrin and triggers DOCK180-dependent Rac1 activation for the phagocytosis of apoptotic cells. Exp. Cell Res. 2004;292:403–416. doi: 10.1016/j.yexcr.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Basse F., Stout J. G., Sims P. J., Wiedmer T. Isolation of an erythrocyte membrane protein that mediates Ca2+-dependent transbilayer movement of phospholipid. J. Biol. Chem. 1996;271:17205–17210. doi: 10.1074/jbc.271.29.17205. [DOI] [PubMed] [Google Scholar]
- Bloom L., Horvitz H. R. The Caenorhabditis elegans gene unc-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc. Natl. Acad. Sci. USA. 1997;94:3414–3419. doi: 10.1073/pnas.94.7.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fares H., Greenwald I. Genetic analysis of endocytosis in Caenorhabditis elegans: coelomocyte uptake defective mutants. Genetics. 2001;159:133–145. doi: 10.1093/genetics/159.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. G., Kamath R. S., Zipperlen P., Martinez-Campos M., Sohrmann M., Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Gardai S. J., Bratton D. L., Ogden C. A., Henson P. M. Recognition ligands on apoptotic cells: a perspective. J. Leukoc. Biol. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- Gartner A., Milstein S., Ahmed S., Hodgkin J., Hengartner M. O. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- Gumienny T. L., Lambie E., Hartwieg E., Horvitz H. R., Hengartner M. O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development. 1999;126:1011–1022. doi: 10.1242/dev.126.5.1011. [DOI] [PubMed] [Google Scholar]
- Gumienny T. L., et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Guzik K., Bzowska M., Smagur J., Krupa O., Sieprawska M., Travis J., Potempa J. A new insight into phagocytosis of apoptotic cells: proteolytic enzymes divert the recognition and clearance of polymorphonuclear leukocytes by macrophages. Cell Death Differ. 2007;14:171–182. doi: 10.1038/sj.cdd.4401927. [DOI] [PubMed] [Google Scholar]
- Hall D. H., Winfrey V. P., Blaeuer G., Hoffman L. H., Furuta T., Rose K. L., Hobert O., Greenstein D. Ultrastructural features of the adult hermaphrodite gonad of Caenorhabditis elegans: relations between the germ line and soma. Dev. Biol. 1999;212:101–123. doi: 10.1006/dbio.1999.9356. [DOI] [PubMed] [Google Scholar]
- Hamon Y., et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat. Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- Hanayama R., Tanaka M., Miwa K., Shinohara A., Iwamatsu A., Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R., Miyasaka K., Nakaya M., Nagata S. MFG-E8-dependent clearance of apoptotic cells, and autoimmunity caused by its failure. Curr. Dir. Autoimmun. 2006;9:162–172. doi: 10.1159/000090780. [DOI] [PubMed] [Google Scholar]
- Jehle A. W., et al. ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages. J. Cell Biol. 2006;174:547–556. doi: 10.1083/jcb.200601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. Transformation. In: Hope I. A., editor. C. elegans, A Practical Approach. Oxford, United Kingdom: Oxford, Oxford University Press; 1999. pp. 69–96. [Google Scholar]
- Kamath R. S., et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics. 1997;146:227–238. doi: 10.1093/genetics/146.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchen J. M., Cabello J., Klingele D., Wong K., Feichtinger R., Schnabel H., Schnabel R., Hengartner M. O. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- Lauber K., et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113:717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Liu Q. A., Hengartner M. O. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Mangahas P. M., Zhou Z. Clearance of apoptotic cells in Caenorhabditis elegans. Semin. Cell Dev. Biol. 2005;16:295–306. doi: 10.1016/j.semcdb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mounsey A., Bauer P., Hope I. A. Evidence suggesting that a fifth of annotated Caenorhabditis elegans genes may be pseudogenes. Genome Res. 2002;12:770–775. doi: 10.1101/gr208802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P. W., Horvitz H. R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2000;2:131–136. doi: 10.1038/35004000. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Blumenthal T., Meyer B. J., Priess J. R. C. elegans II. Plainview, NY: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- Rose K. L., Winfrey V. P., Hoffman L. H., Hall D. H., Furuta T., Greenstein D. The POU gene ceh-18 promotes gonadal sheath cell differentiation and function required for meiotic maturation and ovulation in Caenorhabditis elegans. Dev. Biol. 1997;192:59–77. doi: 10.1006/dbio.1997.8728. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb. Haemost. 2001;86:266–275. [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev. Biol. 1977;56:110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- van den Eijnde S. M., Boshart L., Baehrecke E. H., De Zeeuw C. I., Reutelingsperger C.P.M., Vermeij-Keers C. Cell surface exposure of phosphatidylserine during apoptosis is phylogenetically conserved. Apoptosis. 1998;3:9–16. doi: 10.1023/a:1009650917818. [DOI] [PubMed] [Google Scholar]
- van Engeland M., Nieland L. J., Ramaekers F. C., Schutte B., Reutelingsperger C. P. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Steenbergen R. Metabolism and functions of phosphatidylserine. Prog. Lipid Res. 2005;44:207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wang X., et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat. Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- Wang X., et al. Cell corpse engulfment mediated by C. elegans phosphatidylserine receptor through CED-5 and CED-12. Science. 2003;302:1563–1566. doi: 10.1126/science.1087641. [DOI] [PubMed] [Google Scholar]
- Wu Y., Horvitz H. R. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature. 1998a;392:501–504. doi: 10.1038/33163. [DOI] [PubMed] [Google Scholar]
- Wu Y., Horvitz H. R. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell. 1998b;93:951–960. doi: 10.1016/s0092-8674(00)81201-5. [DOI] [PubMed] [Google Scholar]
- Wu Y. C., Tsai M. C., Cheng L. C., Chou C. J., Weng N. Y. C. elegans CED-12 acts in the conserved crkII/DOCK180/Rac pathway to control cell migration and cell corpse engulfment. Dev. Cell. 2001;1:491–502. doi: 10.1016/s1534-5807(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Wu Y., Tibrewal N., Birge R. B. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yu X., Odera S., Chuang C. H., Lu N., Zhou Z. C. elegans Dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev. Cell. 2006;10:743–757. doi: 10.1016/j.devcel.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Yuan J., Shaham S., Ledoux S., Ellis H. M., Horvitz H. R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Zhao J., Stout J. G., Luhm R. A., Wiedmer T., Sims P. J. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J. Biol. Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Caron E., Hartwieg E., Hall A., Horvitz H. R. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev. Cell. 2001a;1:477–489. doi: 10.1016/s1534-5807(01)00058-2. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Hartwieg E., Horvitz H. R. CED-1 is a transmembrane receptor that mediates cell corpse engulfment in C. elegans. Cell. 2001b;104:43–56. doi: 10.1016/s0092-8674(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Mangahas P. M., Yu X. The genetics of hiding the corpse: engulfment and degradation of apoptotic cells in C. elegans and D. melanogaster. Curr. Top. Dev. Biol. 2004;63:91–143. doi: 10.1016/S0070-2153(04)63004-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.