Abstract
The actin-based foot processes of kidney podocytes and the interposed slit diaphragm form the final barrier to proteinuria. Mutations affecting several podocyte proteins cause disruption of the filtration barrier and rearrangement of the highly dynamic podocyte actin cytoskeleton. Proteins regulating the plasticity of the podocyte actin cytoskeleton are therefore of critical importance for sustained kidney barrier function. Synaptopodin is an actin-associated protein essential for the integrity of the podocyte actin cytoskeleton because synaptopodin-deficient mice display impaired recovery from protamine sulfate-induced foot process effacement and lipopolysaccharide-induced nephrotic syndrome. Moreover, bigenic heterozygosity for synaptopodin and CD2AP is sufficient to induce spontaneous proteinuria and focal segmental glomerulosclerosis-like glomerular damage in mice. Mechanistically, synaptopodin induces stress fibers by blocking the proteasomal degradation of RhoA. Here we show that synaptopodin directly binds to IRSp53 and suppresses Cdc42:IRSp53:Mena-initiated filopodia formation by blocking the binding of Cdc42 and Mena to IRSp53. The Mena inhibitor FP4-Mito suppresses aberrant filopodia formation in synaptopodin knockdown podocytes, and when delivered into mice protects against lipopolysaccharide-induced proteinuria. The identification of synaptopodin as an inhibitor of Cdc42:IRSp53:Mena signaling defines a novel antiproteinuric signaling pathway and offers new targets for the development of antiproteinuric therapeutic modalities.
The kidney podocyte is a unique cell with a complex cellular organization consisting of cell body, major processes, and foot processes (FPs). Podocyte FPs form a characteristic interdigitating pattern with FPs of neighboring podocytes leaving in between the filtration slits that are bridged by the slit diaphragm (SD),1 thereby establishing the final barrier to urinary protein loss.2 Podocyte FPs harbor an actin-based contractile apparatus that is comparable to that of smooth muscle cells or pericytes.3 Disruption of the SD complex and effacement of FPs is associated with the development of proteinuria and, if not reversed in a certain time, with permanent deterioration of the glomerular filter.4,5 Mutations affecting several podocyte proteins, including nephrin, podocin, and α-actinin-4, lead to renal disease owing to disruption of the filtration barrier and rearrangement of the actin cytoskeleton,5,6,7 but the underlying molecular mechanisms remain primarily elusive. However, there is mounting evidence that proteins regulating the plasticity of the podocyte actin cytoskeleton such as Rho GDIα,8 podocalyxin,9 α-actinin-4,10 FAT1,11,12 and Nck13,14 are of critical importance for sustained function of the glomerular filtration barrier.
Synaptopodin is a proline-rich actin-associated protein expressed in highly dynamic cell compartments, such as podocyte FPs and dendritic spines in the brain.15 Synaptopodin-deficient mice show deficits in activity-dependent synaptic plasticity.16 During kidney development, synaptopodin is absent from the early stages of glomerular development in which the presumptive podocytes display a cortical actin cytoskeleton characteristic of epithelial cells.1 Synaptopodin expression commences during the subsequent capillary loop stage when podocytes start developing FPs harboring their characteristic actin-based contractile system.17,18 Gene silencing of synaptopodin suppresses stress fiber formation,19 implying that synaptopodin is critically involved in the development and maintenance of the podocyte contractile apparatus. Consistent with this hypothesis, synaptopodin-deficient mice display impaired recovery from protamine sulfate (PS)-induced FP effacement and lipopolysaccharide (LPS)-induced nephrotic syndrome.19 Synaptopodin directly binds to the SD protein CD2AP and bigenic heterozygosity for synaptopodin and CD2AP leads to spontaneous proteinuria and focal segmental glomerulosclerosis-like glomerular damage in mice.20 Mechanistically, synaptopodin synchronizes podocyte actin dynamics and cell migration by blocking Smurf-1-mediated ubiquitination of RhoA. This in turn protects RhoA against proteasomal degradation and promotes the formation of stress fibers.21
The Rho family GTPase Cdc42 initiates filopodia formation by interacting with distinct downstream effector proteins22 including insulin receptor substrate 53 (IRSp53). IRSp53 harbors an N-terminal Rac binding (RCB) domain, a partial Cdc42 and Rac1-interactive binding (CRIB) motif, an SH3 domain, and a C-terminal PDZ-binding motif.23 The partial CRIB motif of IRSp53 binds activated Cdc42 and can induce filopodia through binding of its SH3 domain to Mena,24 which can be enhanced by binding of Eps8 to IRSp53.25 Here we describe the first detailed analysis of a novel antiproteinuric signaling pathway in kidney podocytes that is controlled by synaptopodin. We show that synaptopodin acts as inhibitor of IRSp53:Mena signaling by blocking the interaction of IRSp53 with Mena and Cdc42. We further show that this novel pathway is physiologically relevant because the Mena inhibitor FP4-Mito26 suppresses aberrant filopodia formation in synaptopodin-knockdown podocytes and protects mice against the development of proteinuria. Our data support the emerging concept that the onset of podocyte FP effacement and proteinuria is a migratory event.27 The suppression of podocyte motility by synaptopodin stabilizes the glomerular filter by blocking the rearrangement of the podocyte actin cytoskeleton into a migratory phenotype.
Materials and Methods
Plasmid Constructs
Polymerase chain reaction (PCR) products were ligated into pEGFP-C1 (BD Biosciences Clontech, Palo Alto, CA), pFLAG6c, or pFLAG5a (Sigma, St. Louis, MO), respec-tively. Truncated synaptopodin cDNA constructs SP1-SP10 have been described before.19 Truncated cDNA constructs encoding N-terminal (amino acids 1 to 249), C-terminal (amino acids 250 to 521), and SH3 domain deleted C-terminal (amino acids 250 to 363, 425 to 521) human IRSp53 fragments were generated by PCR. IRSp53-binding site deleted Synpo-TΔSHB was amplified by PCR. Dominant active Cdc42(G12V) was provided by John Ci-Jiang He (Mount Sinai School of Medicine, New York, NY). GFP-FP4-Mito and GFP-AP4-Mito, kindly provided by Frank Gertler (Massachusetts Institute of Technology, Cambridge, MA), were subcloned into pFLAG. All constructs were verified by DNA sequencing.
Yeast Two-Hybrid Screening
A Synpo-alt-GAL4 DNA binding domain fusion protein was used to screen a pretransformed human kidney Matchmaker cDNA library (Clontech) in yeast as described before.19
Cell Culture and Transient Transfection
Wild-type podocytes were cultured as described before.18 The generation and characterization of synaptopodin knockdown cells have been described before.19 Stable gene silencing of IRSp53 in podocytes was done as previously reported for the knockdown of synaptopodin.19
The IRSp53-specific insert was a 19-nucleotide sequence (5′-GCACTGAAGAAATACCAAA-3′) corresponding to nucleotides 256 to 274 of the mouse IRSp53 ORF, which is separated by a nine-nucleotide noncomplementary spacer (5′-TTCAAGAGA-3′) from the reverse complement of the same 19-nucleotide sequence. A control vector pSUPER-control was produced as described before.19 Podocytes were transfected with 1 μg of siRNA plasmid using FuGene 6 transfection reagent (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Seventy-two hours after transfection, stable transfectants were selected with 400 μg/ml zeocin (Invitrogen, Carlsbad, CA). Clonal cell lines were generated and tested for IRSp53 expression by Western blotting. Transient transfection of podocytes, human embryonic kidney (HEK) 293, or COS-7 cells was performed using FuGene 6 at a 1:3 or 2:3 DNA/FuGene 6 ratio as described before.21 GFP-fusion proteins were analyzed by direct fluorescence microscopy in living cells or after fixation and double-labeling immunofluorescence microscopy as reported before.19
Immunochemistry
Immunofluorescence microscopy for synaptopodin and rhodamine-labeled phalloidin (Molecular Probes, Eugene, OR) was done as described before.19 Monoclonal anti-Mena (kindly provided by Frank Gertler, Massachussetts Institute of Technology, Cambridge, MA) was used at 1:100, anti-IRSp53 (Abcam, Cambridge, MA) at 1:200, anti-FLAG (Sigma) at 1:2000. Images were captured using epifluorescence on a DMI 6000 B microscope (Leica, Wetzlar, Germany) and evaluated by deconvolution software (Leica) as recently described.21 For the quantification of filopodia formation, FLAG-tagged IRSp53, IRSp53-N-terminal fragment,28 IRSp53ΔSH3,24 or GFP-Cdc42(G12V) were transfected into undifferentiated wild-type podocytes for 48 hours, fixed, and immunolabeled as previously described.19 For each construct, pictures of more than 35 transfected cells were captured by immunofluorescence microscopy under a ×100 oil immersion objective on a DMI 6000 B microscope (Leica) and scored positive if they showed at least 15 filopodia per cell.28 The data represent the mean ± SD of five independent experiments.
Bradykinin (Bk)-Induced Filopodia Formation
GFP-tagged Synpo-T, Synpo-TΔSH3, FP4-Mito, AP4-Mito, IRSp53, or IRSp53ΔSH3 were transfected into undifferentiated wild-type or IRSp53 knockdown podocytes for 48 hours and treated with 100 or 1000 ng/ml Bk (Calbiochem, La Jolla, CA) for 5 minutes29 before fixation, and immunolabeled. Pictures of more than 30 transfected cells were captured under a ×100 oil immersion objective on a DMI 6000 B microscope (Leica) and scored positive if each cell showed at least 15 filopodia.28 The data represent the mean ± SD of three independent experiments.
Western Blotting and Immunoprecipitation
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, coimmunoprecipitation from transfected HEK or COS-7 cells, as well as endogenous coimmunoprecipitation experiments using extracts from differentiated wild-type podocytes were done as recently described.19 Synaptopodin NT15 and anti-Synpo-long/-Synpo-alt19 antibodies were used at 1:300, anti-Cdc42 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200, anti-GAPDH (Abcam) at 1:5000, anti-Mena (BD Transduction Laboratories, Franklin Lakes, NJ) at 1:250 and anti-IRSp53 (Abcam) at 1:167. Antibodies against FLAG (Sigma) and GFP (BD Transduction Laboratories) were used at 1:10,000 and 1:300, respectively.21 Horseradish peroxidase-conjugated secondary antibodies (Promega, Madison, WI) were used at 1:10,000.21
GST Binding Assays with Purified Recombinant Proteins
The competitive binding of Synpo-T and Mena or Synpo-T and Cdc42 to IRSp53 was analyzed in GST pull down studies with purified recombinant proteins according to previously described protocols.21,30 FLAG-tagged proteins were expressed in HEK cells and purified as described before.30 One μg of GST-IRSp53 (full-length or C-terminal fragment amino acids 364 to 521) was immobilized on GSH-agarose beads. The beads were washed five times in 1% Triton X-100 in phosphate-buffered saline (PBS) and 1 μg of purified FLAG-Mena or FLAG-Cdc42(G12V) in 500 μl of PBS were added to the beads. For competition studies 0.0, 0.1, 0.5, or 1.0 μg of purified FLAG-Synpo-T or FLAG-Synpo-long were added. Reactions were incubated under rotation for 2 hours at 4°C, and beads were washed five times in PBS. Proteins were eluted in 100 μl of sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting.21,30
Migration Assays
Transwell migration assays were conducted exactly as recently reported.21 The data presented represent the mean ± SD of six independent experiments. Wound-healing assays were conducted as previously reported,21 with some modifications. Differentiated wild-type podocytes and IRSp53 knockdown podocytes (5 × 105) were seeded in six-well plates and wounded with a 200-μl pipette tip. Wounded monolayers were washed with PBS and incubated in RPMI 1640 medium. Time-lapse images were taken with a ×10 phase-contrast objective on a DMI 6000 B microscope (Leica) at 0, 12, and 24 hours. At the indicated time points, the monolayers were photographed using the grid as a marker,31 and the wound width (μm) was measured at each time point using Leica FW4000 software. Migratory rates were calculated as (A − B)/A × 100% or (A − C)/A × 100%, with A, B, and C reflecting the width of the wound at 0, 12, or 24 hours, respectively. The data represent the mean ± SD of eight independent experiments.
Puromycin Aminonucleoside (PAN) Nephrosis
The induction of PAN nephrosis in male adult Sprague-Dawley rats has been previously reported.27,32 The ratios of urinary total protein (mg/dl)/urine Cr (mg/dl) ± SD (n = 4) were as follows: day 0, 0.223 ± 0.087; day 4, 73.405 ± 13.987; day 14, 84.809 ± 44.171; and day 28, 9.473 ± 4.278. We analyzed kidneys that had been harvested on day 4 (development of proteinuria), day 8 (peak of proteinuria), and day 28 (return of proteinuria to baseline levels) after injection of PAN. Kidneys from the PBS-injected rats served as control.
In Vivo Gene Delivery
In vivo hydrodynamic gene delivery was achieved by tail vein injection.33,34 Baseline urinary protein excretion of 8-week-old female wild-type mice was analyzed by urine dipstick (Albustix; Bayer, Pittsburgh, PA) as previously reported.19,35 The mice (n = 9 per group) were injected with 50 μg of FLAG-FP4-Mito or FLAG-AP4-Mito cDNA and 15 μg of luciferase cDNA (provided by Makiko Yasuda, Mount Sinai School of Medicine, New York, NY) in 2.0 ml of 0.9% NaCl. In control experiments, mice were injected with 15 μg of luciferase cDNA in 2.0 ml of 0.9% NaCl. The mice were then injected (intraperitoneally) with 300 μg of ultrapure LPS (LPS-EK ULT Escherichia coli K12; Invivogen, San Diego, CA) at 1 hour and 24 hours after gene delivery. Thirty-six hours after gene delivery, proteinuria was assessed by dipstick as previously reported.19,35 The mice were sacrificed and the efficacy of the gene transfer was verified in liver homogenates by luciferase reporter assays33 and Western blot analysis of FP4-Mito or AP4-Mito. Results are expressed as mean ± SD.
Results
Synaptopodin Is a Novel IRSp53-Interacting Protein
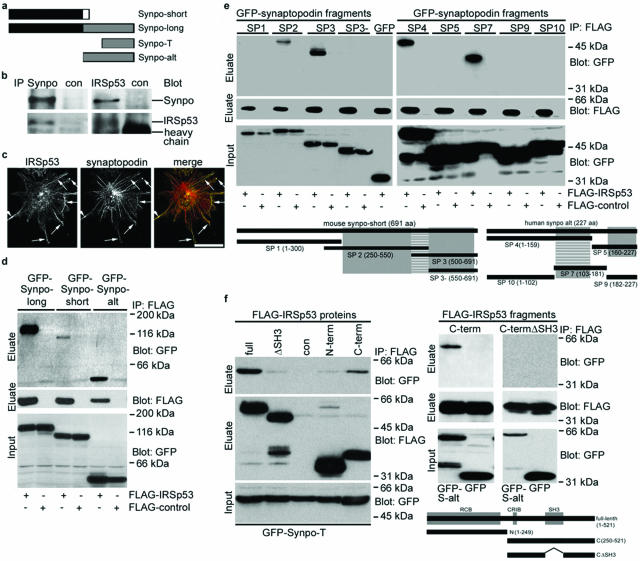
Synaptopodin knockdown podocytes develop aberrant Mena-containing filopodia,21 suggesting that synaptopodin suppresses the Mena-dependent formation of filopodia. To identify effector proteins that link synaptopodin to filopodia dynamics, we conducted a two-hybrid screen using Synpo-alt, the C-terminal fragment of Synpo-long (Figure 1a), as bait. We identified IRSp53, a signaling intermediate in Rac1- and Cdc42-induced actin dynamics,36 which can promote filopodia by forming signaling complexes with Mena and Cdc42,24 as novel synaptopodin interacting protein. The interaction between synaptopodin and IRSp53 was confirmed by endogenous coimmunoprecipitation studies using podocyte extracts. Anti-Synpo-long19 precipitated Synpo-long and also coprecipitated IRSp53 (Figure 1b). Conversely, anti-IRSp53 precipitated IRSp53 and coprecipitated Synpo-long. No interaction was found with an irrelevant control antibody (Figure 1b). Consistent with the biochemical interaction, IRSp53 and synaptopodin partially colocalize along stress fibers in differentiated wild-type podocytes (Figure 1c). Next, FLAG-IRSp53 was coexpressed in HEK cells with GFP-Synpo-long, GFP-Synpo-short, or GFP-Synpo-alt, respectively. All three synaptopodin variants but not GFP alone specifically interacted with FLAG-IRSp53 (Figure 1d). To map the IRSp53 binding site(s) in synap-topodin, various truncated GFP-Synpo-short and GFP-Synpo-alt constructs19 were tested for their ability to coprecipitate with FLAG-IRSp53 (Figure 1e). Two IRSp53 binding sites were found, one in Synpo-short (amino acids 500 to 550) and one in Synpo-alt/Synpo-T (amino acids 103 to 159) (Figure 1e, striped boxes). To map the synaptopodin binding site(s) in IRSp53, several FLAG-IRSp53 fragments were tested for their ability to coprecipitate with GFP-Synpo-alt (Figure 1f) as well as Synpo-short and Synpo-T (data not shown). Two synaptopodin binding sites were found, a weaker one in the N terminus overlapping with the RCB domain, and a stronger one being identical with the SH3 domain of IRSp53 (Figure 1f).
Figure 1.
Synaptopodin is a novel IRSp53-interacting protein. a: Schematic of synaptopodin isoforms. The Synpo-alt fragment of Synpo-long was used as bait construct in the two-hybrid screen and identified IRSp53 as synaptopodin-interacting protein. b: Coimmunoprecipitation experiments showing that endogenous synaptopodin interacts with endogenous IRSp53 in podocytes. Left: IP with anti-synaptopodin (Synpo). Right: IP with anti-IRSp53. No interaction is found with a control antibody against GFP (con). c: Deconvolution microscopy shows that IRSp53 and synaptopodin partially colocalize (arrows) in differentiated wild-type podocytes. d: GFP-tagged Synpo-long, Synpo-short, and Synpo-alt but not GFP alone coprecipitate with FLAG-IRSp53 from co-transfected HEK cells. e: Coimmunoprecipitation studies of FLAG-IRSp53 and GFP-tagged synaptopodin fragments SP1-SP10. Left: Synpo-short contains one interaction site for IRSp53 (striped box; amino acids 500 to 550) located between the α-actinin binding sites (gray boxes). SP1 and GFP alone do not interact with IRSp53. Right: Synpo-alt contains an independent IRSp53 binding site (striped box, amino acids 103 to 159), which overlaps with the first α-actinin binding site (gray boxes) of Synpo-alt.19 f: Left: Cotransfection of GFP-Synpo-T and various FLAG-tagged IRSp53 proteins. The strongest binding is seen with full-length IRSp53 (full) followed by the C-terminal fragment (C-term). A significantly weaker binding is found with IRSp53 lacking the SH3 domain (ΔSH3) or with an N-terminal fragment (N-term). No binding is seen with the FLAG control (con). Right: Cotransfection of GFP-Synpo-alt (S-alt) or GFP and FLAG-tagged IRSp53 fragments C-term or C-termΔSH3. Synaptopodin does not bind to C-termΔSH3. Altogether, IRSp53 contains two independent synaptopodin-binding sites. The first, weaker site overlaps with the RCB domain, the second, stronger site is the SH3 domain of IRSp53. Scale bar = 25 μm.
Synaptopodin Inhibits IRSp53:Mena-Induced Filopodia in Podocytes
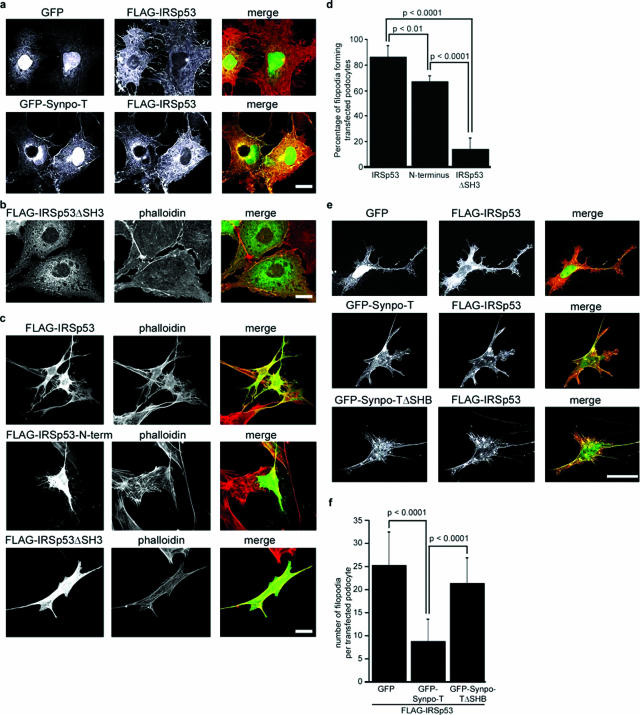
The knockdown of synaptopodin induces Mena-containing filopodia,21 suggesting a role for synaptopodin as inhibitor of IRSp53:Mena-induced filopodia.24,25 To test this hypothesis, we analyzed the actin cytoskeleton of COS-7 cells that are devoid of filopodia and express virtually no endogenous synaptopodin. As expected from previous studies,24,25 the co-transfection of FLAG-IRSp53 with GFP induced abundant filopodia (Figure 2a, top). In contrast, GFP-Synpo-T (as well as GFP-Synpo-short, Synpo-alt, or Synpo-long; data not shown) suppressed IRSp53-induced filopodia (Figure 2a, bottom). When COS-7 cells were transfected with an IRSp53 mutant lacking the SH3 domain required for Mena binding,24 filopodia were almost completely absent (Figure 2b). Next, the relevance of the heterologous results for podocyte biology was assessed in wild-type podocytes maintained under permissive conditions. Under these conditions, podocytes display a cortical actin cytoskeleton and lack synaptopodin.18 Single transfection of FLAG-IRSp53 induced phalloidin-positive filopodia (Figure 2c, top). The FLAG-tagged N-terminal fragment of IRSp53, which when expressed alone exhibits filopodia forming activity,25,28,37 also induced filopodia albeit to a lesser degree (Figure 2c, middle). In contrast, only very few filopodia were seen in podocytes expressing FLAG-IRSp53ΔSH3 (Figure 2c, bottom). To confirm these results in a quantitative manner, we determined the percentage of transfected podocytes displaying more than 15 filopodia per cell as previously reported by others.28 We found that 86.0 ± 8.944% of full-length FLAG-IRSp53-expressing podocytes displayed filopodia versus 66.67 ± 2.36% for the N-terminal fragment and 13.75 ± 4.73% for FLAG-IRSp53ΔSH3 (n = 5, P < 0.0001; Figure 2d). We then conducted co-transfection studies in podocytes and found that, similar to COS-7 cells (Figure 2a), GFP-Synpo-T but not GFP-Synpo-TΔSHB, lacking the SH3 domain-binding motif, or GFP suppressed IRSp53-induced filopodia (Figure 2e). GFP-Synpo-T significantly reduced the average number of filopodia when compared to GFP-Synpo-TΔSHB or GFP (n = 20; GFP-Synpo-T, 8.75 ± 4.84; GFP-Synpo-TΔSHB, 21.35 ± 5.56; GFP, 25.25 ± 7.42; P < 0.0001 (Figure 2f). Together, these data show that synaptopodin blocks IRSp53-induced filopodia formation in podocytes.
Figure 2.
Synaptopodin inhibits IRSp53:Mena-induced filopodia. a: Top: Cotransfection of FLAG-IRSp53 and GFP induces filopodia in COS-7 cells. Bottom: GFP-Synpo-T (as well as Synpo-short, Synpo-long, or Synpo-alt; data not shown) suppresses IRSp53-induced filopodia. b: Overexpression of IRSp53ΔSH3 does not induce filopodia in transfected COS-7 cells. c: Induction of filopodia in transfected undifferentiated wild-type podocytes by FLAG-IRSp53 (top) and the truncated FLAG-N-terminal fragment (middle). Virtually no filopodia are seen with IRSp53ΔSH3 (bottom). d: Quantitative analysis of filopodia formation in transfected undifferentiated wild-type podocytes. See Results for details. e: Top: cotransfection of FLAG-IRSp53 and GFP induces filopodia in undifferentiated podocytes. Middle: GFP-Synpo-T suppresses IRSp53-induced filopodia. Bottom: GFP-Synpo-TΔSHB does not suppress IRSp53 filopodia. f: Quantitative analysis of synaptopodin-mediated inhibition of IRSp53-induced filopodia formation in podocytes. See Results for details. Scale bars = 25 μm.
FP4-Mito Suppresses IRSp53-Induced Filopodia in Podocytes
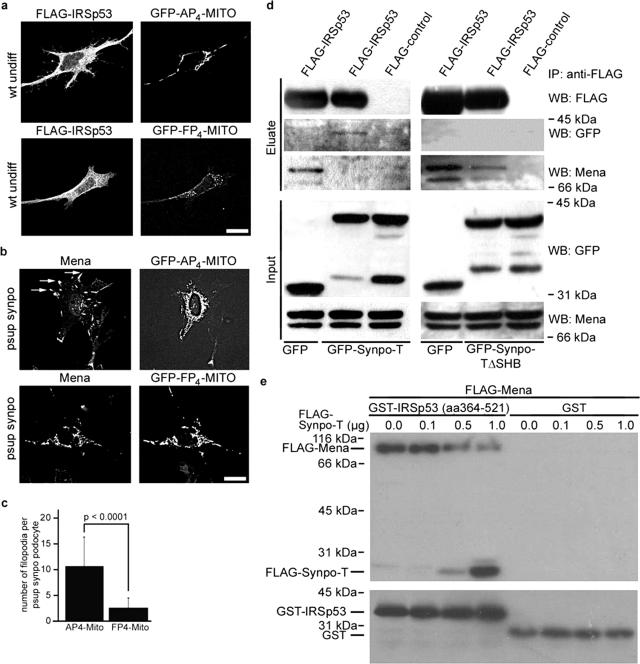
IRSp53:Mena signaling complexes can initiate actin filament assembly into filopodia.24 We therefore tested whether the observed aberrant Mena-containing filopodia in synaptopodin knockdown podocytes21 resulted from the ablation of synaptopodin-mediated inhibition of IRSp53:Mena signaling. Toward this, we co-transfected undifferentiated podocytes with FLAG-IRSp53 and GFP-FP4-Mito, which ablates Ena-Mena-VASP protein function by sequestering them to mitochondria.26 We detected the formation of filopodia in podocytes co-transfected with the GFP-AP4-Mito control, but not GFP-FP4-Mito (n = 20; AP4-Mito, 22.381 ± 6.399; FP4-mito, 9.050 ± 3.546; P < 0.0001) (Figure 3a). Next we transfected synaptopodin knockdown podocytes with GFP-FP4-Mito or GFP-AP4-Mito and found that GFP-AP4-Mito-expressing cells displayed aberrant Mena-containing filopodia (Figure 3b, top) similar to those found in nontransfected synaptopodin knockdown podocytes.21 In contrast, GFP-FP4-Mito sequestered Mena on the mitochondrial surface and suppressed filopodia formation (Figure 3b, bottom). To confirm these data in a quantitative manner, we counted the number of Mena-positive filopodia in synaptopodin knockdown podocytes. GFP-FP4-Mito but not AP4-Mito significantly reduced the average number of filopodia per podocyte (n = 10; AP4-Mito, 10.50 ± 5.82 versus FP4-Mito, 2.50 ± 2.01; P < 0.0001) (Figure 3c).
Figure 3.
Synaptopodin suppresses filopodia formation by disrupting IRSp53:Mena signaling complexes. a: Cotransfection with FP4-Mito but not AP4-Mito suppresses IRSp53-induced filopodia in undifferentiated wild-type (wt undiff) podocytes. b: In GFP-AP4-Mito-transfected, synaptopodin-knockdown (psup synpo) podocytes, Mena is targeted to aberrant filopodia (arrows). In contrast, GFP-FP4-Mito sequesters Mena to the mitochondrial surface and suppresses filopodia formation. c: Quantitative analysis of FP4-Mito-mediated inhibition of filopodia formation in synaptopodin knockdown podocytes. See Results for details. d: Left: Coimmunoprecipitation of endogenous Mena with FLAG-IRSp53 from COS-7 cells transfected with FLAG-IRSp53 and GFP. Cotransfection with GFP-Synpo-T blocks the binding of Mena to FLAG-IRSp53. Instead, GFP-Synpo-T coprecipitates with FLAG-IRSp53. No binding is found with a FLAG control. Right: Restored coimmunoprecipitation of endogenous Mena with FLAG-IRSp53 from COS-7 cells cotransfected with FLAG-IRSp53 and the GFP-Synpo-T mutant lacking the IRSp53 binding site (GFP-Synpo-TΔSHB). e: Immobilized GST-IRSp53 (amino acids 364 to 521) but not GST alone directly binds to purified FLAG-Mena. In the presence of increasing amounts of FLAG-Synpo-T, the binding of IRSp53 to Mena is gradually lost, whereas increased binding of Synpo-T to IRSp53 can be detected.
Synaptopodin Disrupts IRSp53:Mena Signaling Complexes
Next we tested the hypothesis that synaptopodin suppresses filopodia by competing with Mena for binding to the SH3 domain of IRSp53, thereby disrupting IRSp53:Mena signaling complexes.24 In support of this in COS-7 cells, endogenous Mena coprecipitated with FLAG-IRSp53 in the presence of GFP (Figure 3d, left). In contrast, when FLAG-IRSp53 was co-transfected with GFP-Synpo-T, the interaction between Mena and IRSp53 was abrogated. Instead, GFP-Synpo-T coprecipitated with FLAG-IRSp53 (Figure 3d, left). Transfection with Synpo-TΔSHB did not block the interaction of Mena with FLAG-IRSp53 (Figure 3d, right). These results provide a biochemical explanation for the observation that Synpo-TΔSHB cannot suppress IRSp53-induced filopodia (Figure 2, e and f). To demonstrate further that synaptopodin can directly bind to IRSp53 and compete with Mena for IRSp53 binding, we conducted in vitro reconstitution studies with purified recombinant proteins according to our previously published protocols.21,30 In the absence of Synpo-T, Mena did bind to GST-IRSp53 (amino acids 364 to 521) but not GST alone (Figure 3e). In contrast, the binding of Synpo-T to GST-IRSp53 decreased the binding of FLAG-Mena to IRSp53 in a concentration-dependent manner (Figure 3e).
Synaptopodin Suppresses the Formation of Cdc42-Induced Filopodia
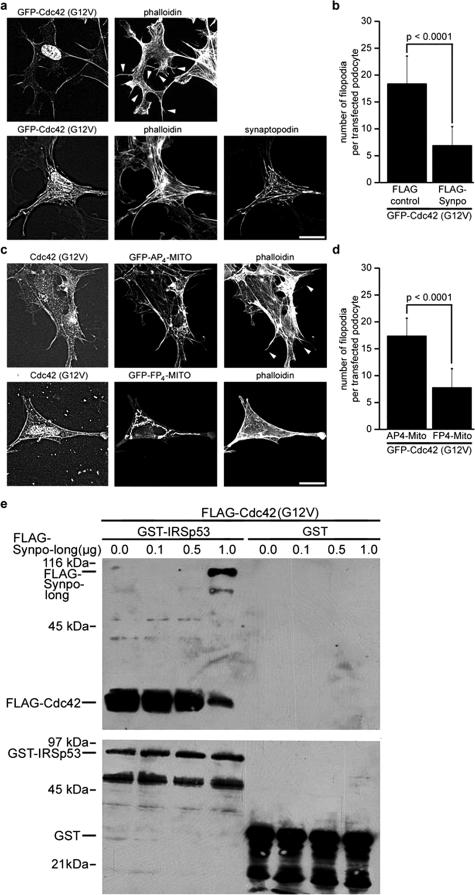
The initiation of actin filament assembly into filopodia by IRSp53:Mena complexes is promoted by the interaction of activated Cdc42 with IRSp53, which relieves an intramolecular, autoinhibitory interaction with the N-terminus of IRSp53, allowing the recruitment of Mena to the SH3 domain of IRSp53.24 To explore the relevance of this pathway in podocytes, we transfected undifferentiated wild-type podocytes with GFP-tagged dominant active Cdc42(G12V). We detected the formation of filopodia in podocytes expressing GFP-Cdc42(G12V) (Figure 4a, top), whereas the co-transfection with FLAG-Synpo-long suppressed Cdc42(G12V)-induced filopodia (Figure 4a, bottom). The quantitative analysis showed that podocytes co-transfected with a FLAG-control displayed significantly more filopodia when compared to FLAG-Synpo-long (n = 20; FLAG-control, 18.4 ± 5.12; versus FLAG-Synpo-long, 6.9 ± 3.54; P < 0.001) (Figure 4b). To demonstrate that Cdc42 induces filopodia in podocytes in a Mena-dependent manner, we co-transfected undifferentiated podocytes with FLAG-Cdc42(G12V) and GFP-FP4-Mito or the GFP-AP4-Mito control. Cdc42(G12V) induced filopodia in podocytes co-transfected with GFP-AP4-Mito, but not GFP-FP4-Mito (Figure 4c). Cells co-transfected with GFP-AP4-Mito contained on average 17.2 ± 3.33 filopodia versus 7.8 ± 3.43 per cell co-transfected with GFP-FP4-Mito (n = 20, P < 0.0001; Figure 4d).
Figure 4.
Synaptopodin blocks Cdc42:Mena-dependent filopodia formation by disrupting Cdc42:IRSp53 signaling complexes. a: Cotransfection with synaptopodin (bottom) suppresses the formation of Cdc42-induced filopodia (arrowheads) in undifferentiated wild-type podocytes (top). b: Quantitative analysis of synaptopodin-mediated inhibition of Cdc42-induced filopodia formation in undifferentiated wild-type podocytes. See Results for details. c: Cotransfection with FP4-Mito but not AP4-Mito suppresses Cdc42-induced filopodia (arrowheads) in undifferentiated wild-type podocytes. d: Quantitative analysis of FP4-Mito-mediated inhibition of Cdc42-induced filopodia formation in undifferentiated wild-type podocytes. See Results for details. e: Immobilized GST-IRSp53 (full-length) but not GST alone directly binds to purified FLAG-Cdc42. In the presence of increasing amounts of FLAG-Synpo-long, the binding of IRSp53 to Cdc42 is gradually reduced, whereas increasing binding of Synpo-long to IRSp53 can be observed. Scale bars = 25 μm.
Synaptopodin Disrupts Cdc42:IRSp53 Complexes
To test whether synaptopodin blocks Cdc42-induced filopodia not only by preventing the binding of Mena to IRSp53 (Figure 3) but also by blocking the binding of Cdc42 to IRSp53, we conducted in vitro reconstitution studies with purified FLAG-Cdc42(G12V), FLAG-Synpo-long, and GST-IRSp53 according to our previously published protocols.21,30 In the absence of synaptopodin, Cdc42 bound to GST-IRSp53 but not to the GST control (Figure 4e). In contrast, the addition of Synpo-long decreased the binding of FLAG-Cdc42 to IRSp53 in a concentration-dependent manner (Figure 4e). Altogether, the data presented thus far show that synaptopodin can inhibit IRSp53-induced filopodia by blocking the formation of Cdc42:IRSp53:Mena signaling complexes. The remainder of our studies is focused on determining the physiological relevance of this novel inhibitory signaling pathway.
Synaptopodin Suppresses Bradykinin-Induced IRSp53:Mena-Dependent Filopodia
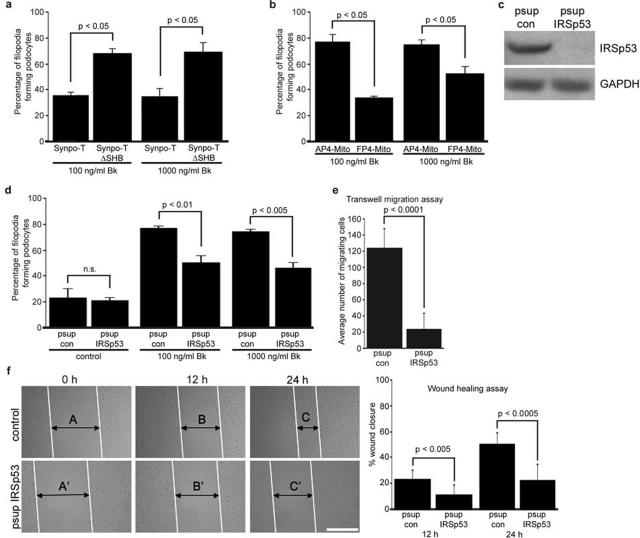
Bk induces filopodia through a well-characterized physiological pathway that signals through Cdc4229 and podocytes respond to Bk by changes in intracellular calcium concentration.18 Therefore, we exposed podo-cytes transfected with GFP-Synpo-T or GFP-Synpo-TΔSHB to 100 or 1000 ng/ml bradykinin29 for 5 minutes and counted filopodia as described above. Synpo-T but not Synpo-TΔSHB, which cannot disrupt IRSp53:Mena signaling complexes, significantly reduced Bk-induced filopodia both at 100 and 1000 ng/ml of Bk treatment (n = 3; GFP-Synpo-T, 35.00 and 34.44% versus GFP-Synpo-TΔSHB, 67.50 and 68.89%; P < 0.05 each) (Figure 5a). Next, we repeated the Bk treatment with podocytes expressing FP4-Mito or AP4-Mito. Similar to Synpo-T, FP4-Mito, which blocks Mena-dependent actin assembly,26 but not the AP4-Mito control, significantly reduced Bk-induced filopodia (n = 3; AP4-Mito, 76.11% and 73.94% versus FP4-Mito, 33.39 and 51.84%; P < 0.005) (Figure 5b). To strengthen these results further, we generated stable IRSp53 knockdown podocytes that showed an almost complete loss of IRSp53 protein expression (Figure 5c). In the absence of Bk we found no significant difference in baseline filopodia content between control and IRSp53 knockdown podocytes. In contrast, the absence of IRSp53 significantly reduced Bk-induced filopodia formation (n = 3; psup con, 76.43 and 73.86% versus psup IRSp53, 49.87 and 45.71%; P < 0.05 each) (Figure 5d). These results show that synaptopodin decreases Bk-induced filopodia formation in podocytes by preventing IRSp53:Mena signaling.
Figure 5.
Synaptopodin inhibits Bk-induced, IRSp53:Mena-mediated filopodia formation in podocytes. a: Synpo-T but not Synpo-TΔSHB, which cannot block the binding of Mena to IRSp53, reduces Bk-induced filopodia formation in transfected undifferentiated wild-type podocytes. See Results for details. b: FP4-Mito, but not the AP4-Mito control, reduces Bk-induced filopodia formation in undifferentiated wild-type podocytes. See Results for details. c: Western blot analysis of IRSp53 after gene silencing by stable expression of an IRSp53-specific siRNA (psup IRSp53). psup IRSp53 but not a control siRNA construct (psup con) suppresses IRSp53 protein expression in podocytes. d: Knockdown of IRSp53 significantly impairs Bk-induced filopodia formation in podocytes. e: Compared with wild-type podocytes, IRSp53 knockdown podocytes display significantly impaired motility in Transwell migration experiments. f: Scrape wound assay. In the absence of IRSp53, wound closure is significantly delayed at 12 and 24 hours. Compared with wild-type cells, IRSp53 knockdown podocytes display significantly impaired directed cell migration. See Results for details. Scale bar = 200 μm.
Gene Silencing of IRSp53 Impairs Podocyte Migration
In migrating cells, Cdc42 signaling regulates the direction of migration.38 To explore the role of IRSp53 signaling in podocyte migration, we compared the migration of control and IRSp53 knockdown podocytes. First, using a Transwell filter system, we found that IRSp53 knockdown podocytes are significantly less motile than control podocytes (n = 6; psup con, 123.33 ± 24.05 versus psup IRSp53, 23.33 ± 20.09; P = 0.0001) (Figure 5e). To confirm that IRSp53 deficiency also impairs directed podocyte migration, we performed wound-healing assays as previously reported.21 We created a scrape wound to separate two layers of differentiated podocytes and examined directional cell movement at 12 and 24 hours after wound formation (Figure 5f). The knockdown of IRSp53 significantly inhibited directional podocyte migration at 12 hours (n = 8; psup con, 23.31 ± 6.73 versus psup IRSp53, 10.88 ± 7.50; P = 0.0036) and 24 hours (n = 8; psup con, 50.27 ± 8.62 versus psup IRSp53, 22.50 ± 12.24; P = 0.0001) (Figure 5f).
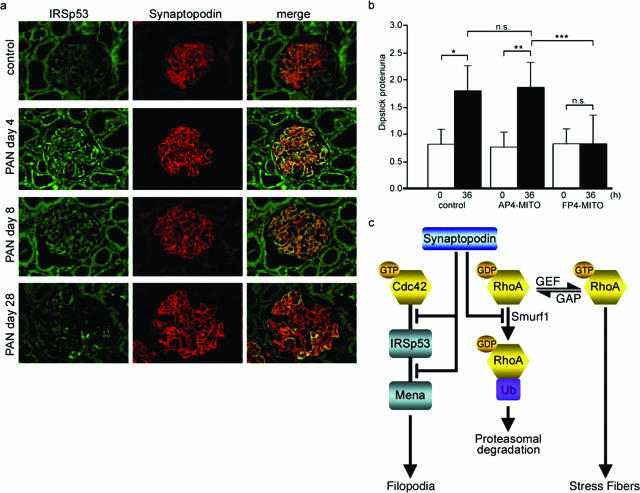
Up-Regulation of IRSp53 in Podocyte Precedes FP Effacement and Proteinuria in Experimental Nephrotic Syndrome
Next, we performed double-labeling immunofluorescence microscopy of IRSp53 with synaptopodin on rat kidney sections before and after PAN induced reversible podocyte FP effacement and proteinuria.27 IRSp53 was weakly expressed in podocytes of PBS-treated control rats (Figure 6a). In contrast, on day 4 after PAN injection, when FP process effacement and proteinuria develop,32 IRSp53 expression was significantly increased in podocytes as shown by co-localization with synaptopodin. On day 8 after PAN injection, at the peak of proteinuria, podocyte IRSp53 expression started to decline and returned to near baseline levels at day 28 when proteinuria had returned to baseline levels.32
Figure 6.
FP4-Mito ameliorates proteinuria by blocking IRSp53:Mena signaling. a: Transient up-regulation of IRSp53 protein expression in podocytes during PAN nephrosis. In PBS controls, only weak glomerular IRSp53 labeling is observed. On day 4 after PAN injection, IRSp53 expression is significantly increased in podocytes as shown by co-localization with synaptopodin. IRSp53 immunostaining in podocytes starts to decline on day 8 and returns to control levels on day 28. b: In vivo gene transfer of FP4-Mito but not AP4-Mito or luciferase (control) protects against LPS-induced proteinuria. *P = 0.000047, **P = 0.000014, ***P = 0.00039; n.s., not significant. c: A model for the antagonistic regulation of Cdc42 and RhoA signaling by synaptopodin. Synaptopodin suppresses IRSp53:Mena-mediated filopodia by blocking the binding of Cdc42 and Mena to IRSp53. In addition, synaptopodin induces stress fibers by competitive blocking of Smurf-1-mediated ubiquitination of RhoA, thereby preventing the targeting of RhoA for proteasomal degradation.
In Vivo Gene Transfer of FP4-Mito Protects Against LPS-Induced Proteinuria
We previously reported that podocyte FP effacement leading to the development of proteinuria is a migratory event.27 These data raise the possibility that the observed up-regulation of IRSp53 in podocytes contributes to the development of proteinuria by promoting IRSp53:Mena-initiated actin filament assembly. If true, the inhibition of Mena with FP4-Mito should protect against proteinuria. To test this hypothesis, we induced proteinuria in mice with LPS19,35 after in vivo gene delivery33,34 of FLAG-FP4-Mito or FLAG-AP4-Mito together with luciferase cDNA to monitor the efficiency of the gene transfer.33 The gene delivery of luciferase alone served as negative control. Proteinuria was measured in all three groups before and 36 hours after gene delivery. At baseline, the levels of proteinuria were not significantly different between the three groups (Figure 6b). At 36 hours of observation, proteinuria was significantly higher in mice injected with the luciferase control (baseline, 0.78 ± 0.51 versus 36 hours, 1.72 ± 0.44; P = 0.000047) or AP4-Mito (baseline, 0.72 ± 0.26 versus 36 hours, 1.78 ± 0.44; P = 0.000014). In contrast, mice injected with FP4-Mito did not show a significant increase in proteinuria (baseline, 0.78 ± 0.26 versus 36 hours, 0.78 ± 0.51; not significant) (Figure 6b). These data demonstrate that the inhibition of IRSp53:Mena signaling by gene delivery of FP4-Mito can protect mice against LPS-induced proteinuria.
Discussion
The current study identified the actin-associated protein synaptopodin as the first inhibitor of Mena:IRSp53 signaling. Our results shed new light on the molecular mechanisms governing a fundamental cell biological process, the dynamic regulation of the actin cytoskeleton in health and disease. Our results further support the concept that the onset of proteinuria represents a migratory event27 involving the activation of actin assembly-promoting signaling pathways.13,14 In concert, synaptopodin orchestrates podocyte actin dynamics via activation of RhoA21 and simultaneous inhibition of Cdc42 signaling (Figure 6c). Synaptopodin contributes to the stabilization the glomerular filter by shifting the plasticity of the podocyte actin cytoskeleton from a motile to a contractile phenotype.
Podocytes are unique cells with a complex cellular organization. With respect to their cytoarchitecture, podocytes can be divided into three structurally and functionally different segments: cell body, major processes, and FPs.1 The function of podocytes is primarily based on their cytoarchitecture, in particular on the maintenance of the normal FP structure with their highly ordered parallel contractile actin filament bundles.3 The podocyte FPs are defined by three membrane domains: i) the apical membrane domain, ii) the SD protein complex, and iii) the basal membrane domain or sole plate.1,4 All three domains are linked to the FP actin cytoskeleton, making it the common denominator in podocyte function and dysfunction.4,39 The actin bundles are linked to the SD complex through several scaffolding proteins including α-actinin,3 CD2AP,40 densin,41 nephrin,42 Nck,13,14 and ZO-1.43 Interference with any of the three FP domains changes the actin cytoskeleton from parallel contractile bundles3 into a dense network with FP effacement and proteinuria.4
It has long been known that the podocyte FP actin cytoskeleton is highly dynamic, although the underlying mechanisms remained ill defined. The perfusion of rat kidneys with the polycation PS causes FP effacement and SD disruption within 15 minutes44 and tyrosine phosphorylation of nephrin.14 The reperfusion with heparin for another 15 minutes can reverse PS-induced FP effacement45 and nephrin phosphorylation.14 PS-induced FP effacement requires the active reorganization of actin filaments,9,46 and disruption of the actin cytoskeleton by cytochalasin can prevent PS-induced FP effacement.47 Altogether these data show that proteins regulating the plasticity of the contractile apparatus of podocytes are of critical importance for the maintenance of glomerular filter function. Consistent with this idea, mice lacking synaptopodin display impaired recovery from PS-induced FP effacement and LPS-induced nephrotic syndrome.19 Moreover, heterozygosity for synaptopodin is sufficient to induce spontaneous proteinuria and focal segmental glomerulosclerosis-like glomerular damage in CD2AP heterozygous mice,20 thereby underscoring the importance of synaptopodin for the sustained function of the kidney filter.
The experiments described here allowed us to explore several important questions. First, we show that synaptopodin is a novel suppressor of filopodia formation. In this context it is interesting to note that synaptopodin has antagonistic effects on Cdc42 and RhoA signaling (Figure 6c). We previously reported that synaptopodin promotes RhoA signaling by increasing the levels of active RhoA.21 Now we find an opposite effect on Cdc42 signaling in that synaptopodin acts as competitive inhibitor of Cdc42 and Mena binding to IRSp53. Cdc42 can induce filopodia via several downstream effectors, one of them being the activation of IRSp53:Mena signaling complexes.24 The latter study showed that the interaction of Cdc42 with the partial CRIB motif of IRSp53 relieves an intramolecular, autoinhibitory interaction with the N terminus, allowing the recruitment of Mena to the SH3 domain of IRSp53. This IRSp53:Mena complex then initiates actin filament assembly into filopodia,24 but the relevance of this pathway in a physiological context had been unclear. Our results suggest that this is a major Cdc42 downstream effector pathway in podocytes, because the knockdown of IRSp53 or the functional inhibition of Mena by FP4-Mito suppresses Bk- and Cdc42-induced filopodia in podocytes. Most significantly, the inactivation of Mena by FP4-Mito also protects mice against LPS-induced proteinuria. At present, it is not clear if the beneficial effect of FP4-Mito is attributable to Mena inhibition by FP4-Mito synthesized in podocytes or to circulating FP4-Mito taken up by podocytes. Future studies will be required to address this issue.
Finally, our results have also helped clarifying the possible role of the N-terminal fragment of IRSp53 (also termed IMD28,37) in the formation of filopodia. Consistent with previous results by others,28,37 a truncated N-terminal fragment of IRSp53 can induce filopodia when overexpressed in COS-7 cells or podocytes. In contrast, an IRSp53 protein containing the IMD domain but lacking the SH3 domain did not display any significant filopodia-inducing activity. Together, these data suggest that in the intact full-length IRSp53 protein, IMD has little or no filo-podia inducing activity, at least in podocytes. It is intriguing to speculate that under pathological conditions, IRSp53 may undergo proteolytic processing leading to the release of active IMD, thereby switching from SH3-dependent to SH3-independent filopodia formation.37 Cleary, future studies will be necessary to confirm or refute this hypothesis.
In summary, our studies define a novel comprehensive mechanism for the control of Rho family GTPases by synaptopodin, which promotes RhoA signaling and antagonizes Cdc42 signaling (Figure 6c). These findings show how ubiquitously expressed proteins such as Rho family GTPases can converge on an integrative regulator such as synaptopodin to orchestrate the actin cytoskeleton in a highly dynamic cell compartment such as podocyte FP. The inhibition of Cdc42:IRSp53:Mena signaling by synaptopodin defines a novel physiological pathway and identifies new targets for the development of antiproteinuric therapeutic modalities.
Acknowledgments
We thank Frank Gertler (MIT, Cambridge, MA) for anti-Mena antibody, FP4-Mito, and AP4-Mito cDNAs; John Ci-Jiang He (Mount Sinai School of Medicine, New York, NY) for Cdc42(G12V) cDNA; and Makiko Yasuda, Mount Sinai School of Medicine, for luciferase cDNA and advice on the hydrodynamic gene delivery experiments.
Footnotes
Address reprint requests to Peter Mundel, M.D., Division of Nephrology, Mount Sinai School of Medicine, One Gustave L. Levy Place, Box 1243, New York, NY 10029-6574. E-mail: peter.mundel@mssm.edu.
Supported by the National Institutes of Health (grants DA18886, DK57683, DK062472, and the George M. O’Brien Kidney Center DK064236 to P.M.), the National Kidney Foundation (to K.A.), the Mochida Memorial Foundation (to K.A.), the Naito Foundation (to K.A.), and the Ichiro Kanehara Foundation (to K.A.).
E.Y.-A., K.A., and K.K. contributed equally to this article.
References
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- Somlo S, Mundel P. Getting a foothold in nephrotic syndrome. Nat Genet. 2000;24:333–335. doi: 10.1038/74139. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Franke RP. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988;59:673–682. [PubMed] [Google Scholar]
- Kerjaschki D. Caught flat-footed: podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest. 2001;108:1583–1587. doi: 10.1172/JCI14629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Reiser J, Mundel P. Dynamic (re)organization of the podocyte actin cytoskeleton in the nephrotic syndrome. Pediatr Nephrol. 2004;19:130–137. doi: 10.1007/s00467-003-1367-y. [DOI] [PubMed] [Google Scholar]
- Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- Schmieder S, Nagai M, Orlando RA, Takeda T, Farquhar MG. Podocalyxin activates RhoA and induces actin reorganization through NHERF1 and Ezrin in MDCK cells. J Am Soc Nephrol. 2004;15:2289–2298. doi: 10.1097/01.ASN.0000135968.49899.E8. [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, Pollak MR. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- Ciani L, Patel A, Allen ND, French-Constant C. Mice lacking the giant protocadherin mFAT1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol Cell Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 2004;23:3769–3779. doi: 10.1038/sj.emboj.7600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T. Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature. 2006;440:818–823. doi: 10.1038/nature04662. [DOI] [PubMed] [Google Scholar]
- Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB. Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest. 2006;116:1346–1359. doi: 10.1172/JCI27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc Natl Acad Sci USA. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Gilbert P, Kriz W. Podocytes in glomerulus of rat kidney express a characteristic 44 KD protein. J Histochem Cytochem. 1991;39:1047–1056. doi: 10.1177/39.8.1856454. [DOI] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Borja AZ, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber TB, Kwoh C, Wu H, Asanuma K, Godel M, Hartleben B, Blumer KJ, Miner JH, Mundel P, Shaw AS. Bigenic mouse models of focal segmental glomerulosclerosis involving pairwise interaction of CD2AP, Fyn, and synaptopodin. J Clin Invest. 2006;116:1337–1345. doi: 10.1172/JCI27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Miki H, Yamaguchi H, Suetsugu S, Takenawa T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature. 2000;408:732–735. doi: 10.1038/35047107. [DOI] [PubMed] [Google Scholar]
- Krugmann S, Jordens I, Gevaert K, Driessens M, Vandekerckhove J, Hall A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr Biol. 2001;11:1645–1655. doi: 10.1016/s0960-9822(01)00506-1. [DOI] [PubMed] [Google Scholar]
- Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Fiore PP, Ciliberto A, Stradal TE, Scita G. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- Bear JE, Loureiro JJ, Libova I, Fassler R, Wehland J, Gertler FB. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell. 2000;101:717–728. doi: 10.1016/s0092-8674(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and α3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- Millard TH, Bompard G, Heung MY, Dafforn TR, Scott DJ, Machesky LM, Futterer K. Structural basis of filopodia formation induced by the IRSp53/MIM homology domain of human IRSp53. EMBO J. 2005;24:240–250. doi: 10.1038/sj.emboj.7600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul C, Huttelmaier S, Oh J, Hachet V, Singer RH, Mundel P. Promotion of importin {alpha}-mediated nuclear import by the phosphorylation-dependent binding of cargo protein to 14-3-3. J Cell Biol. 2005;169:415–424. doi: 10.1083/jcb.200411169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchange NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi S, Sakai T, Shirato I, Tomino Y, Koide H. Ultrastructural changes in glomerular epithelial cells in acute puromycin aminonucleoside nephrosis: a study by high-resolution scanning electron microscopy. Virchows Arch A Pathol Anat Histopathol. 1993;423:111–119. doi: 10.1007/BF01606585. [DOI] [PubMed] [Google Scholar]
- Al-Dosari MS, Knapp JE, Liu D. Hydrodynamic delivery. Adv Genet. 2005;54:65–82. doi: 10.1016/S0065-2660(05)54004-5. [DOI] [PubMed] [Google Scholar]
- Mayer G, Boileau G, Bendayan M. Furin interacts with proMT1-MMP and integrin alphaV at specialized domains of renal cell plasma membrane. J Cell Sci. 2003;116:1763–1773. doi: 10.1242/jcs.00394. [DOI] [PubMed] [Google Scholar]
- Reiser J, Von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Takenawa T. WAVE2 serves a functional partner of IRSp53 by regulating its interaction with Rac. Biochem Biophys Res Commun. 2002;293:93–99. doi: 10.1016/S0006-291X(02)00218-8. [DOI] [PubMed] [Google Scholar]
- Yamagishi A, Masuda M, Ohki T, Onishi H, Mochizuki N. A novel actin bundling/filopodium-forming domain conserved in insulin receptor tyrosine kinase substrate p53 and missing in metastasis protein. J Biol Chem. 2004;279:14929–14936. doi: 10.1074/jbc.M309408200. [DOI] [PubMed] [Google Scholar]
- Raftopoulou M, Hall A. Cell migration: Rho GTPases lead the way. Dev Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Zhao F, Lehtonen E. CD2-associated protein directly interacts with the actin cytoskeleton. Am J Physiol. 2002;283:F734–F743. doi: 10.1152/ajprenal.00312.2001. [DOI] [PubMed] [Google Scholar]
- Ahola H, Heikkila E, Astrom E, Inagaki M, Izawa I, Pavenstadt H, Kerjaschki D, Holthofer H. A novel protein, densin, expressed by glomerular podocytes. J Am Soc Nephrol. 2003;14:1731–1737. doi: 10.1097/01.asn.0000075553.33781.9f. [DOI] [PubMed] [Google Scholar]
- Yuan H, Takeuchi E, Salant DJ. Podocyte slit-diaphragm protein nephrin is linked to the actin cytoskeleton. Am J Physiol. 2002;282:F585–F591. doi: 10.1152/ajprenal.00290.2001. [DOI] [PubMed] [Google Scholar]
- Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–936. doi: 10.1016/S0002-9440(10)63354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189:390–393. doi: 10.1126/science.1145209. [DOI] [PubMed] [Google Scholar]
- Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36:48–61. [PubMed] [Google Scholar]
- Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat kidney glomeruli. The effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest. 1978;39:430–440. [PubMed] [Google Scholar]