Abstract
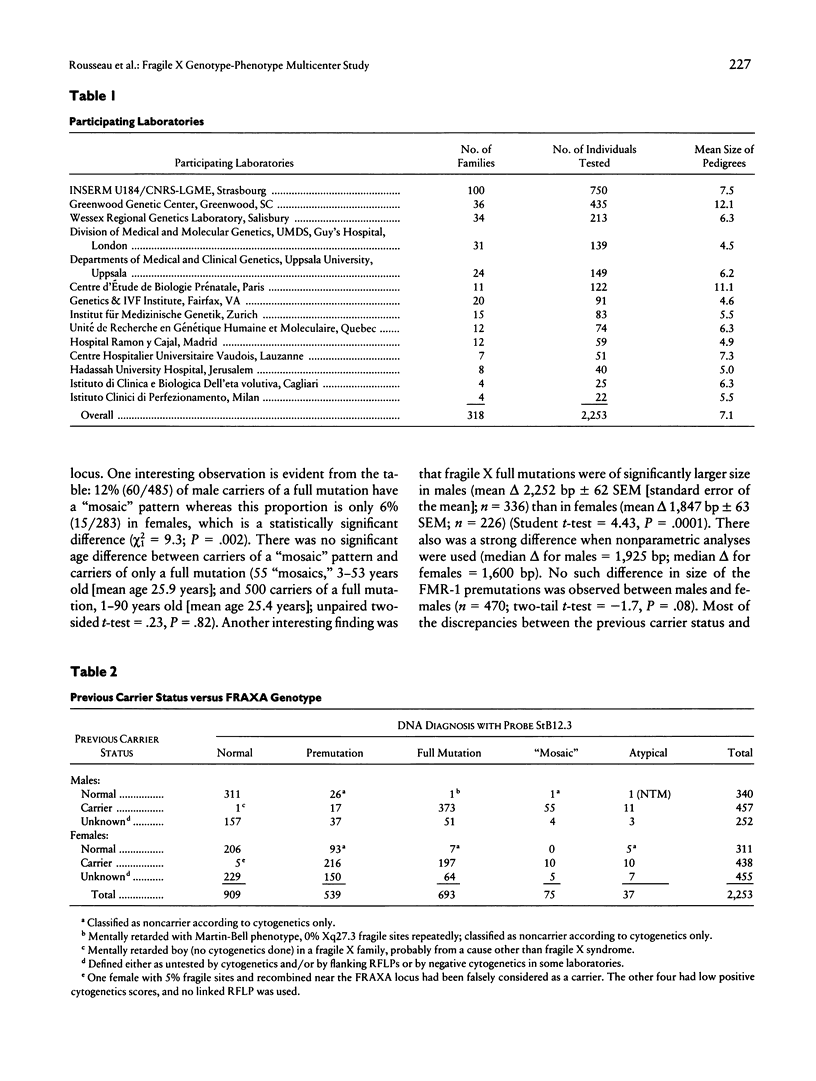
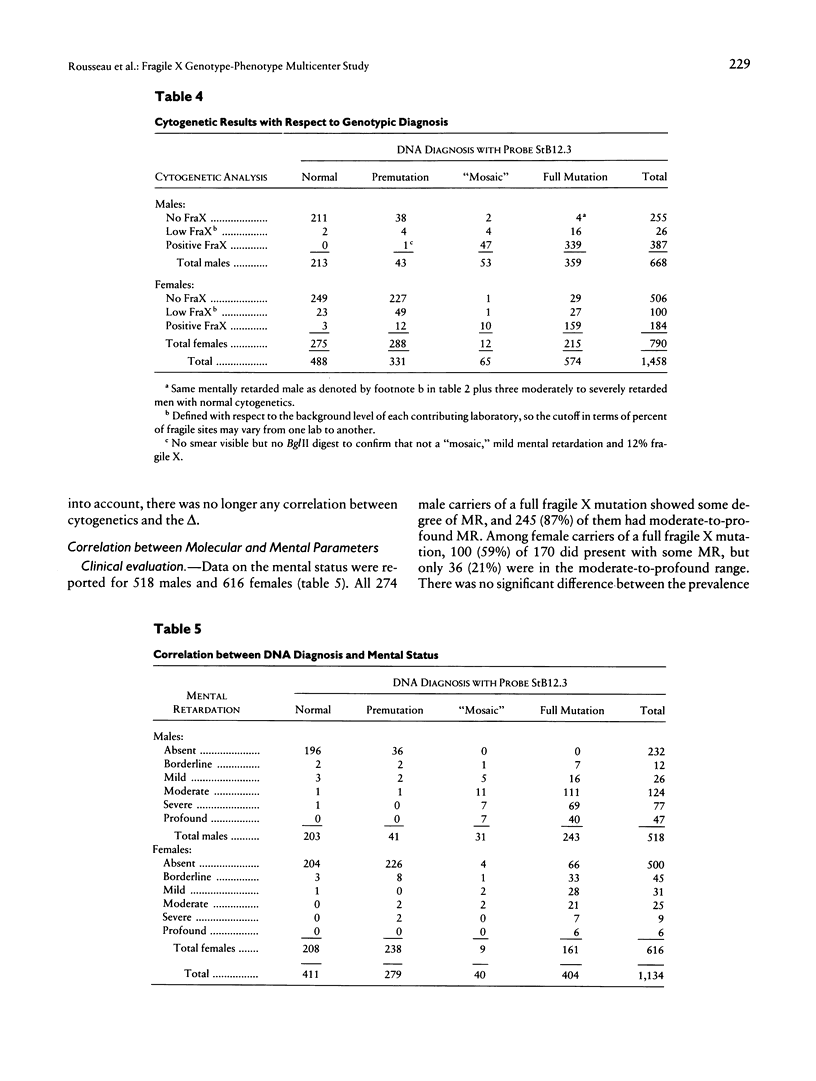
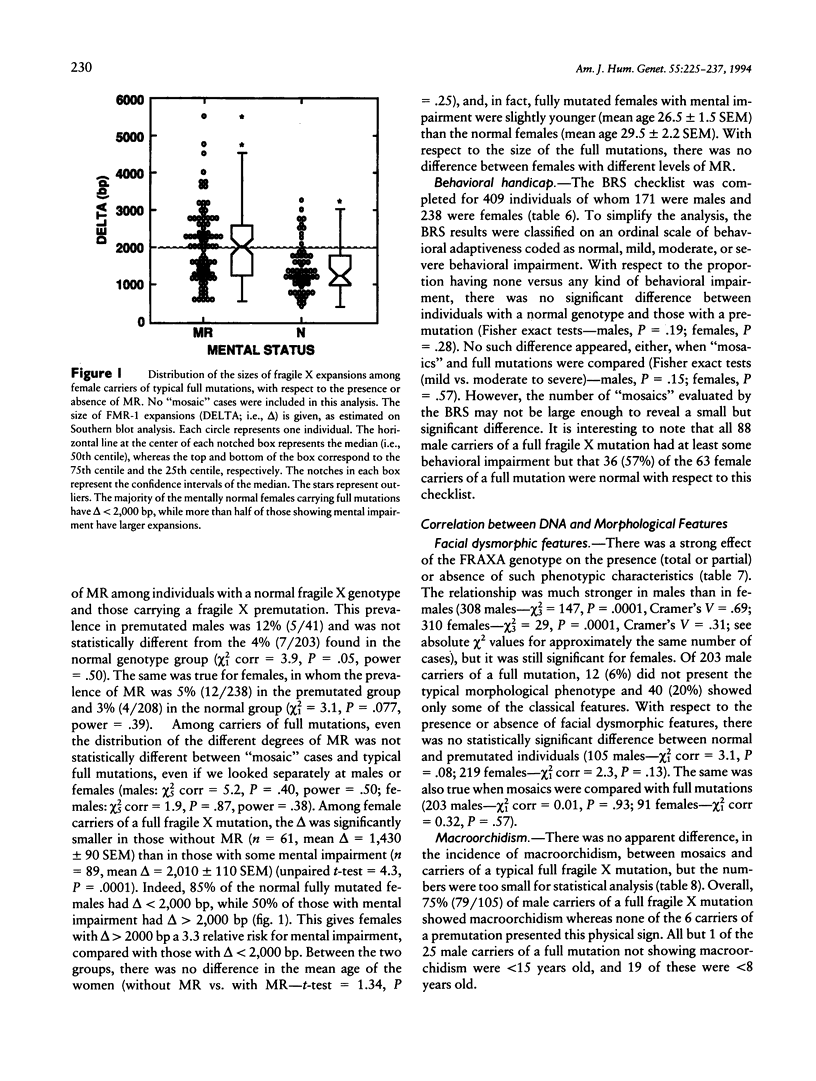
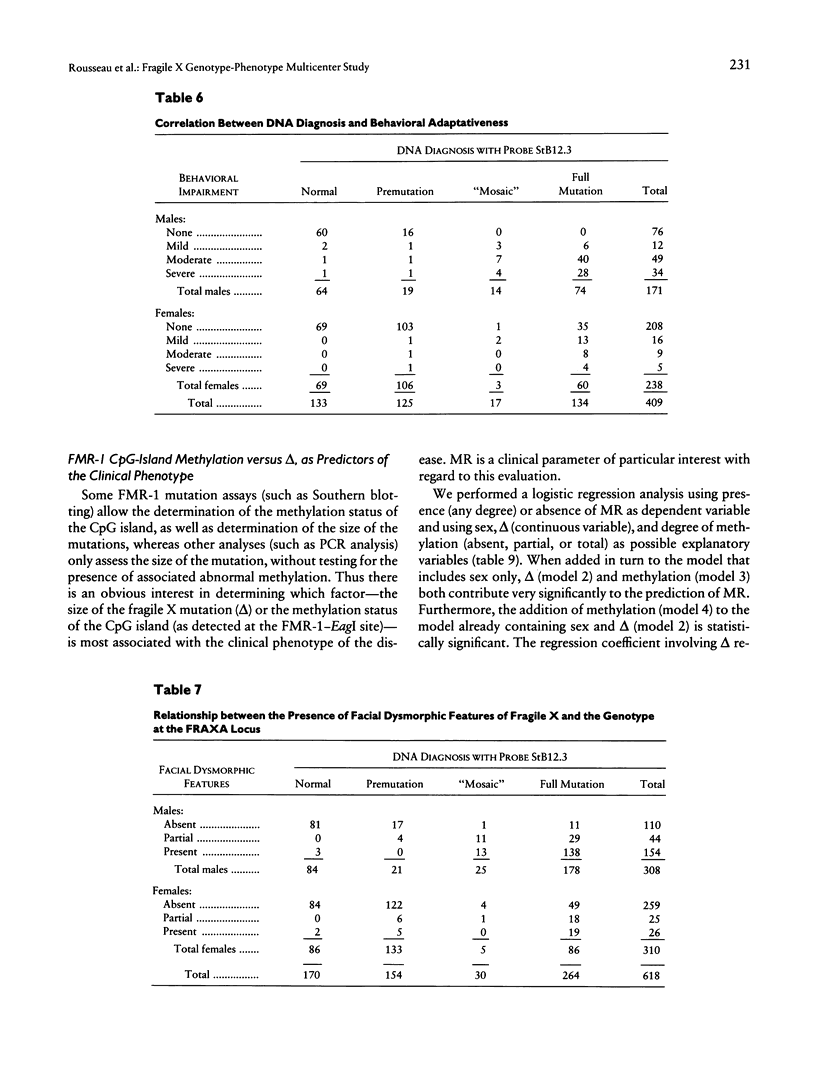
We report the results of a 14-center collaborative study of genotype-phenotype correlations in 318 fragile X families; these families comprised 2,253 individuals, 1,344 of whom carried a fragile X mutation and 693 of whom had a typical full fragile X mutation. This study demonstrates that direct DNA diagnosis establishes the genotype at the FRAXA-FMR-1 locus. There was a significantly higher prevalence of “mosaic” cases among males who carry a full mutation (12%) than among females who carry a full mutation (6%); the mosaic males had a larger expansion than did the mosaic females. Mental status of premutated individuals did not differ from that of those with a normal genotype. Both the abnormal methylation of the FMR-1–EagI site and the size of the expansion were highly correlated with cytogenetics, facial dysmorphism, macroorchidism, and mental retardation (MR). Among female carriers of a full mutation, those with MR had significantly larger expansion than did those without MR. Among 164 independent couples, 3 unrelated husbands carried a premutation that suggests that the prevalence of fragile X premutations in the general population is ∼0.9% of the X chromosomes. Our data validate the use of direct DNA testing for fragile X diagnosis as well as for carrier identification and support and complete the established relationships among the DNA results and the cytogenetic, physical, and psychological aspects of the disease.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Boulle K., Verkerk A. J., Reyniers E., Vits L., Hendrickx J., Van Roy B., Van den Bos F., de Graaff E., Oostra B. A., Willems P. J. A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nat Genet. 1993 Jan;3(1):31–35. doi: 10.1038/ng0193-31. [DOI] [PubMed] [Google Scholar]
- Devys D., Biancalana V., Rousseau F., Boué J., Mandel J. L., Oberlé I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Fryns J. P., Kleczkowska A., Kubień E., Petit P., Van den Berghe H. Inactivation pattern of the fragile X in heterozygous carriers. Hum Genet. 1984;65(4):400–401. doi: 10.1007/BF00291567. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Gedeon A. K., Baker E., Robinson H., Partington M. W., Gross B., Manca A., Korn B., Poustka A., Yu S., Sutherland G. R. Fragile X syndrome without CCG amplification has an FMR1 deletion. Nat Genet. 1992 Aug;1(5):341–344. doi: 10.1038/ng0892-341. [DOI] [PubMed] [Google Scholar]
- Hansen R. S., Gartler S. M., Scott C. R., Chen S. H., Laird C. D. Methylation analysis of CGG sites in the CpG island of the human FMR1 gene. Hum Mol Genet. 1992 Nov;1(8):571–578. doi: 10.1093/hmg/1.8.571. [DOI] [PubMed] [Google Scholar]
- Heitz D., Devys D., Imbert G., Kretz C., Mandel J. L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992 Nov;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst M. C., Nakahori Y., Knight S. J., Schwartz C., Thibodeau S. N., Roche A., Flint T. J., Connor J. M., Fryns J. P., Davies K. E. Genotype prediction in the fragile X syndrome. J Med Genet. 1991 Dec;28(12):824–829. doi: 10.1136/jmg.28.12.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer E. J., Pritchard M., Lynch M., Yu S., Holman K., Baker E., Warren S. T., Schlessinger D., Sutherland G. R., Richards R. I. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991 Jun 21;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H., Ledbetter S. A., Nussbaum R. L. Implications of fragile X expression in normal males for the nature of the mutation. Nature. 1986 Nov 13;324(6093):161–163. doi: 10.1038/324161a0. [DOI] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R., Hay D. A., Gedeon A. K., Mulley J. C., Sutherland G. R. Genotype-phenotype relationships in fragile X syndrome: a family study. Am J Hum Genet. 1993 Nov;53(5):1064–1073. [PMC free article] [PubMed] [Google Scholar]
- Macpherson J., Harvey J., Curtis G., Webb T., Heitz D., Rousseau F., Jacobs P. A reinvestigation of thirty three fragile(X) families using probe StB12.3. Am J Med Genet. 1992 Jul 15;43(5):905–912. doi: 10.1002/ajmg.1320430535. [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A., Lachiewicz A. M., Spiridigliozzi G. A., Tarleton J., Schoenwald S., Phelan M. C., Goonewardena P., Ding X., Brown W. T. Evidence that methylation of the FMR-I locus is responsible for variable phenotypic expression of the fragile X syndrome. Am J Hum Genet. 1993 Oct;53(4):800–809. [PMC free article] [PubMed] [Google Scholar]
- Nelson D. L., Warren S. T. Trinucleotide repeat instability: when and where? Nat Genet. 1993 Jun;4(2):107–108. doi: 10.1038/ng0693-107. [DOI] [PubMed] [Google Scholar]
- Nielsen K. B., Tommerup N., Poulsen H., Jacobsen P., Beck B., Mikkelsen M. Carrier detection and X-inactivation studies in the fragile X syndrome. Cytogenetic studies in 63 obligate and potential carriers of the fragile X. Hum Genet. 1983;64(3):240–245. doi: 10.1007/BF00279401. [DOI] [PubMed] [Google Scholar]
- Oberlé I., Rousseau F., Heitz D., Kretz C., Devys D., Hanauer A., Boué J., Bertheas M. F., Mandel J. L. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991 May 24;252(5009):1097–1102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- Oudet C., Mornet E., Serre J. L., Thomas F., Lentes-Zengerling S., Kretz C., Deluchat C., Tejada I., Boué J., Boué A. Linkage disequilibrium between the fragile X mutation and two closely linked CA repeats suggests that fragile X chromosomes are derived from a small number of founder chromosomes. Am J Hum Genet. 1993 Feb;52(2):297–304. [PMC free article] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Reiss A. L., Freund L., Abrams M. T., Boehm C., Kazazian H. Neurobehavioral effects of the fragile X premutation in adult women: a controlled study. Am J Hum Genet. 1993 May;52(5):884–894. [PMC free article] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Friend K., Kremer E., Hillen D., Staples A., Brown W. T., Goonewardena P., Tarleton J., Schwartz C. Evidence of founder chromosomes in fragile X syndrome. Nat Genet. 1992 Jul;1(4):257–260. doi: 10.1038/ng0792-257. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Oberlé I., Mandel J. L. Selection in blood cells from female carriers of the fragile X syndrome: inverse correlation between age and proportion of active X chromosomes carrying the full mutation. J Med Genet. 1991 Dec;28(12):830–836. doi: 10.1136/jmg.28.12.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. S., Nelson D. L., Zhang F., Pieretti M., Caskey C. T., Saxe D., Warren S. T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992 Sep;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- Sutherland G. R., Gedeon A., Kornman L., Donnelly A., Byard R. W., Mulley J. C., Kremer E., Lynch M., Pritchard M., Yu S. Prenatal diagnosis of fragile X syndrome by direct detection of the unstable DNA sequence. N Engl J Med. 1991 Dec 12;325(24):1720–1722. doi: 10.1056/NEJM199112123252407. [DOI] [PubMed] [Google Scholar]
- Turner G., Robinson H., Laing S., Purvis-Smith S. Preventive screening for the fragile X syndrome. N Engl J Med. 1986 Sep 4;315(10):607–609. doi: 10.1056/NEJM198609043151002. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Webb T., Jacobs P. A. Fragile Xq27.3 in female heterozygotes for the Martin-Bell syndrome. J Med Genet. 1990 Oct;27(10):627–631. doi: 10.1136/jmg.27.10.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhrle D., Hennig I., Vogel W., Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993 Jun;4(2):140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Hirst M. C., Kennerknecht I., Davies K. E., Steinbach P. Genotype mosaicism in fragile X fetal tissues. Hum Genet. 1992 Apr;89(1):114–116. doi: 10.1007/BF00207057. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Kotzot D., Hirst M. C., Manca A., Korn B., Schmidt A., Barbi G., Rott H. D., Poustka A., Davies K. E. A microdeletion of less than 250 kb, including the proximal part of the FMR-I gene and the fragile-X site, in a male with the clinical phenotype of fragile-X syndrome. Am J Hum Genet. 1992 Aug;51(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Mulley J., Loesch D., Turner G., Donnelly A., Gedeon A., Hillen D., Kremer E., Lynch M., Pritchard M. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet. 1992 May;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Pritchard M., Kremer E., Lynch M., Nancarrow J., Baker E., Holman K., Mulley J. C., Warren S. T., Schlessinger D. Fragile X genotype characterized by an unstable region of DNA. Science. 1991 May 24;252(5009):1179–1181. doi: 10.1126/science.252.5009.1179. [DOI] [PubMed] [Google Scholar]
- de Vries B. B., Wiegers A. M., de Graaff E., Verkerk A. J., Van Hemel J. O., Halley D. J., Fryns J. P., Curfs L. M., Niermeijer M. F., Oostra B. A. Mental status and fragile X expression in relation to FMR-1 gene mutation. Eur J Hum Genet. 1993;1(1):72–79. doi: 10.1159/000472389. [DOI] [PubMed] [Google Scholar]


