Abstract
Foreign body multinucleate giant cells were produced by the implantation of a strip of Melenex™ in the subcutaneous tissues of mice. The implants were removed at various intervals, and the proportion of multinucleate giant cells as well as the number of nuclei they contained were counted and statistically assessed. The greatest proportion of giant cells was reached 4 weeks after implantation, when 25% of the attached cells were multinucleated. The mean nuclear content however was greatest approximately 2 weeks after implantation and rapidly fell over the ensuing weeks. The fusion potential however, remained almost unaltered for the remainder of the experimental period. Transplantation of 7-day Melenex implants from normal donors into lethally irradiated recipients demonstrated that the halflife of the giant cells is only a few days. Treatment with carrageenin, species-specific antisera, actinomycin D and cortisone inhibited, while puromycin enhanced, multinucleate cell formation. Calcium gluconate, EDTA and irradiation had no significant effect. The possible interpretation of some of these findings is discussed.
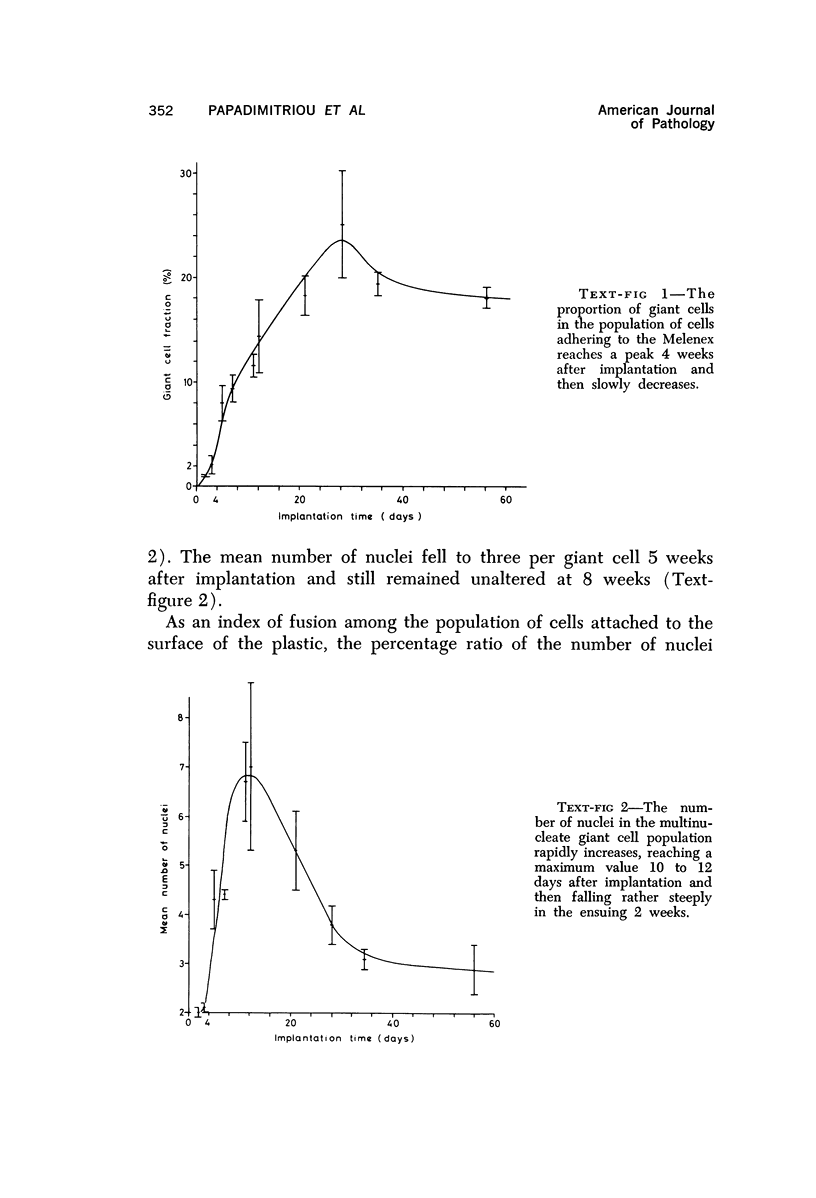
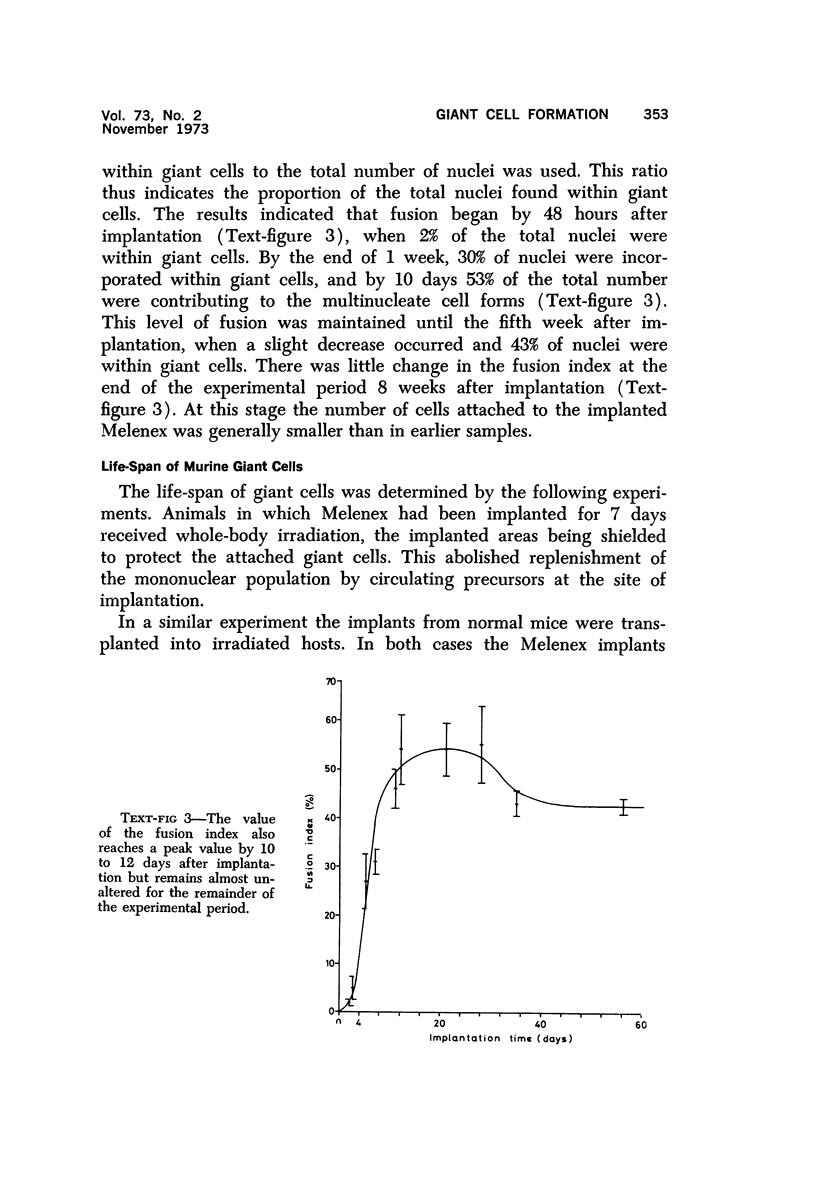
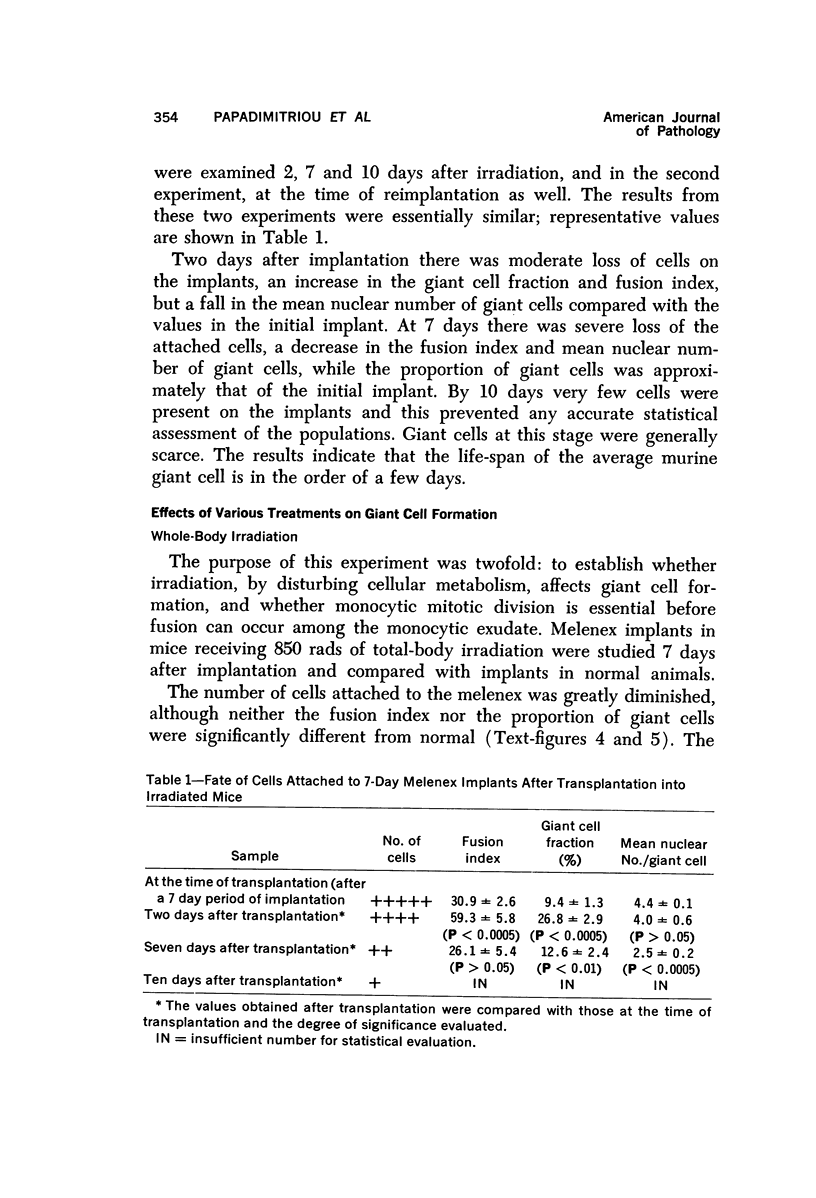
Full text
PDF


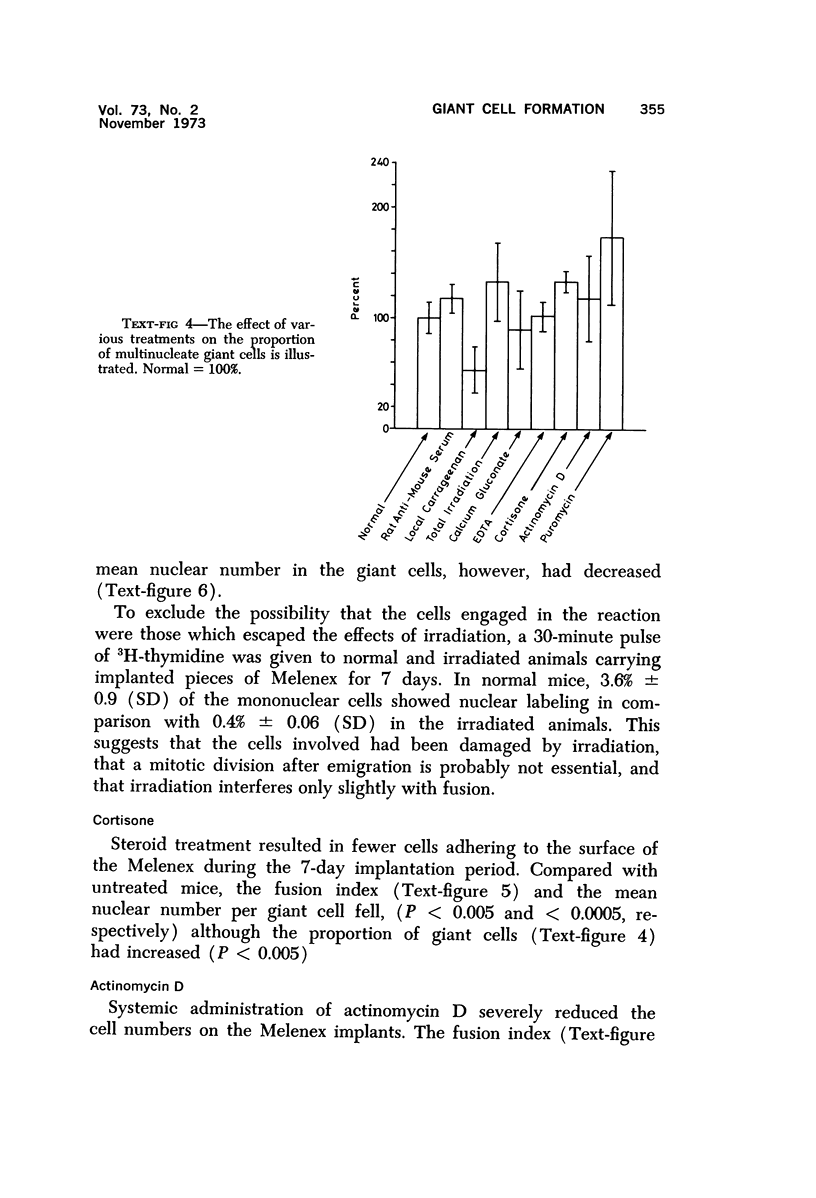
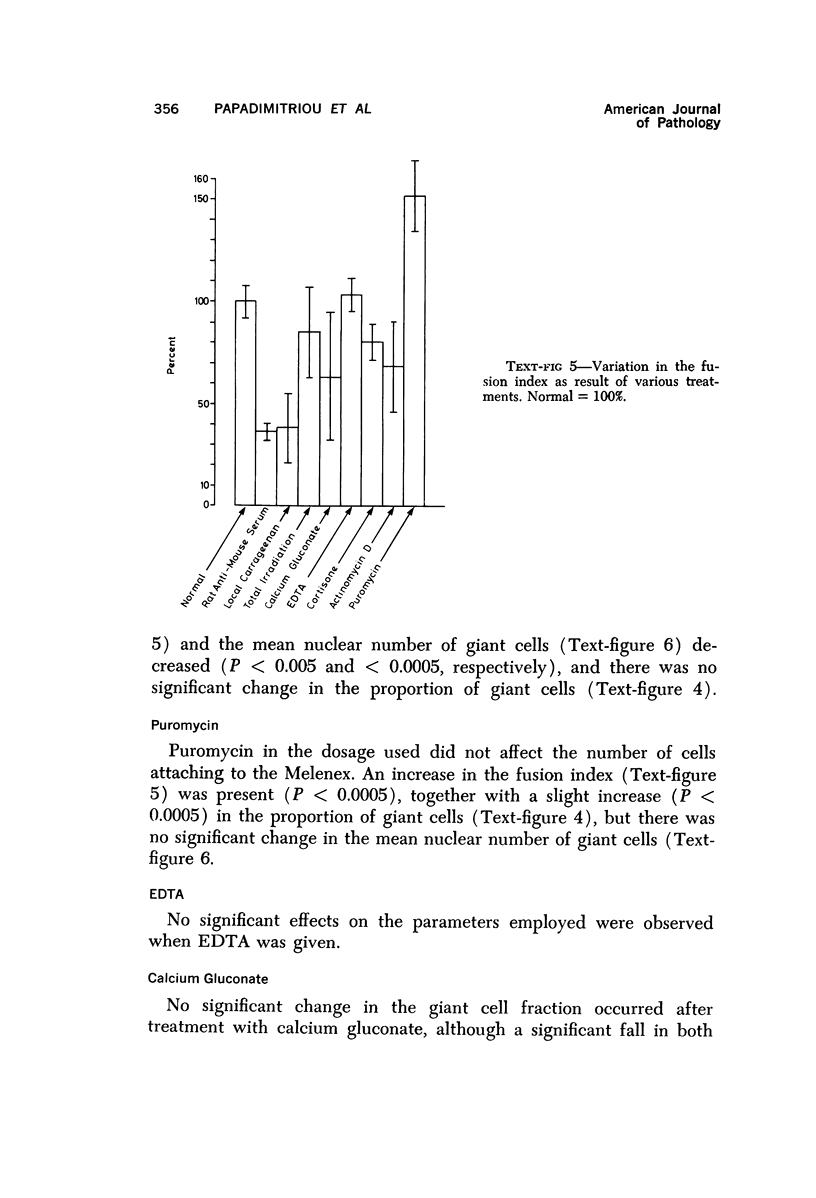
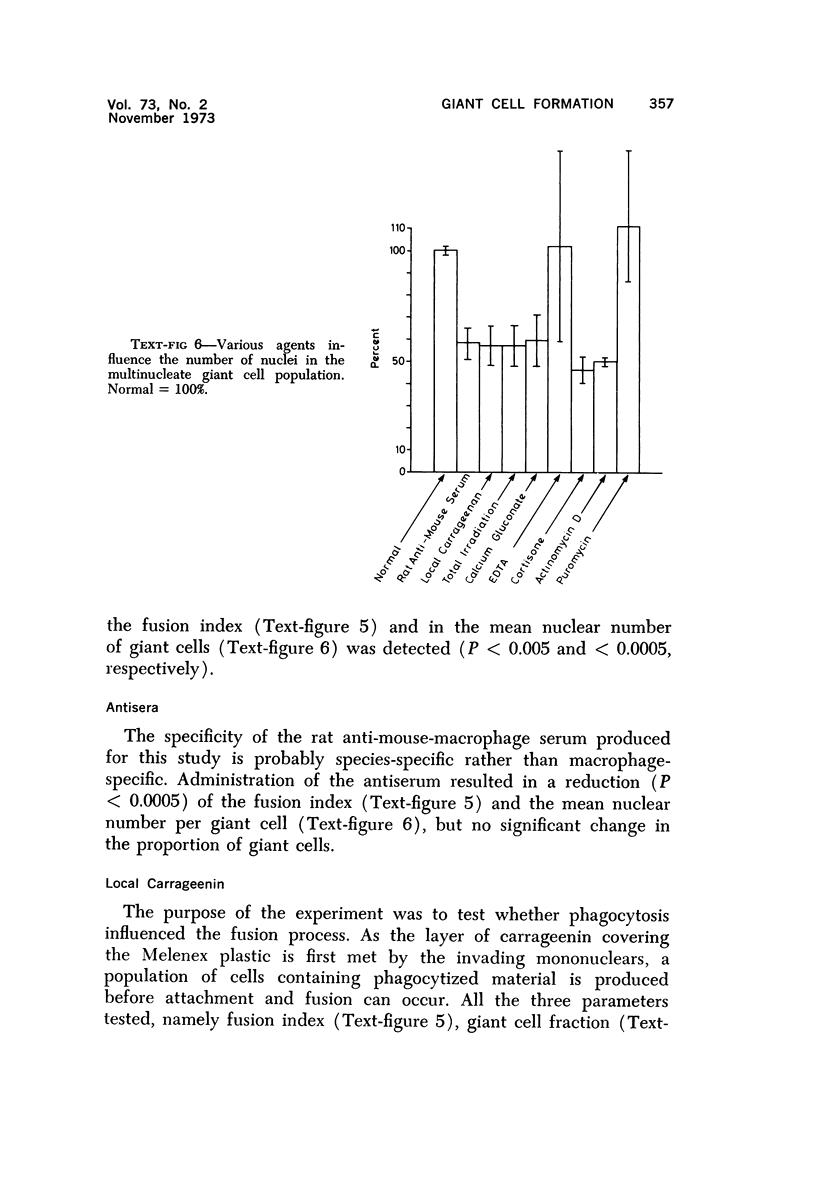






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gillman T., Wright L. J. Probable in vivo origin of multi-nucleated giant cells from circulating mononuclears. Nature. 1966 Jan 15;209(5020):263–265. doi: 10.1038/209263a0. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M. Direct embedding in epoxy resin of cells attached to cellophane. Exp Cell Res. 1972 Feb;70(2):449–452. doi: 10.1016/0014-4827(72)90161-9. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Finlay-Jones J. M., Walters M. N. Surface characteristics of macrophages, epithelioid and giant cells using scanning electron microscopy. Exp Cell Res. 1973 Feb;76(2):353–362. doi: 10.1016/0014-4827(73)90387-x. [DOI] [PubMed] [Google Scholar]
- Papadimitriou J. M., Spector W. G. The origin, properties and fate of epithelioid cells. J Pathol. 1971 Nov;105(3):187–203. doi: 10.1002/path.1711050305. [DOI] [PubMed] [Google Scholar]
- Poste G., Allison A. C. Membrane fusion reaction: a theory. J Theor Biol. 1971 Jul;32(1):165–184. doi: 10.1016/0022-5193(71)90144-5. [DOI] [PubMed] [Google Scholar]
- Poste G., Reeve P. Inhibition of cell fusion by local anaesthetics and tranquillizers. Exp Cell Res. 1972 Jun;72(2):556–560. doi: 10.1016/0014-4827(72)90029-8. [DOI] [PubMed] [Google Scholar]
- Ptak W., Porwit-Bòbr Z., Chlap Z. Transformation of hamster macrophages into giant cells with antimacrophage serum. Nature. 1970 Feb 14;225(5233):655–657. doi: 10.1038/225655a0. [DOI] [PubMed] [Google Scholar]
- Spector W. G., Lykke A. W. The cellular evolution of inflammatory granulomata. J Pathol Bacteriol. 1966 Jul;92(1):163–167. doi: 10.1002/path.1700920117. [DOI] [PubMed] [Google Scholar]
- Sutton J. S., Weiss L. Transformation of monocytes in tissue culture into macrophages, epithelioid cells, and multinucleated giant cells. An electron microscope study. J Cell Biol. 1966 Feb;28(2):303–332. doi: 10.1083/jcb.28.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]