Abstract
Cytomegalovirus (CMV) infection is endemic in Gambian infants, with 62% infected by 3 months and 85% by 12 months of age. We studied the CD8 T-cell responses of infants to CMV following primary infection. CMV-specific CD8 T cells, identified with tetramers, showed a fully differentiated phenotype (CD28− CD62L− CD95+ perforin+ granzyme A+ Bcl-2low). Strikingly, the overall CD8 T-cell population developed a similar phenotype following CMV infection, which persisted for at least 12 months. In contrast, primary infection was accompanied by up-regulation of markers of activation (CD45R0 and HLA-D) on both CMV-specific cells and the overall CD8 T-cell population and division (Ki-67) of specific cells, but neither pattern persisted. At 12 months of age, the CD8 T-cell population of CMV-infected infants was more differentiated than that of uninfected infants. Although the subpopulation of CMV-specific cells remained constant, the CMV peptide-specific gamma interferon response was lower in younger infants and increased with age. As the CD8 T-cell phenotype induced by CMV is indicative of immune dysfunction in the elderly, the existence of a similar phenotype in large numbers of Gambian infants raises the question of whether CMV induces a similarly deleterious effect.
Human cytomegalovirus (CMV) is endemic throughout the world, with infection typically occurring earlier in life in low-income countries than in the industrialized nations where it has been most intensively studied (29). Congenital infection is often associated with pathology, while early postnatal infection, which is usually transmitted by breast-feeding, is normally asymptomatic (17).
The CD8 T-cell response has been associated with protection from CMV disease following some organ transplants (5, 6, 44). However, a vigorous CD8 T-cell response alone fails to control primary CMV infection transmitted during kidney transplantation.
The phenotype of CMV-specific CD8 T cells in chronic infection is well documented and shows characteristics distinct from cells specific for other viruses. The vast majority lose expression of the B7 ligand CD28, and a large number lose expression of the costimulatory molecule CD27 (1) and express the RA isoform of CD45 during chronic infection (7, 50). Many CMV-specific CD8 T cells express Ki-67 in the early stages of infection (10), indicating that they are in the process of mitotic division (12), and the number of dividing cells wanes over time.
Chronic infection with CMV typically leads to large expansions of CMV-specific T cells. An average of around 4% of CD8 T cells can produce gamma interferon (IFN-γ) in response to CMV (39). This probably underestimates the size of the CMV-specific CD8 population, as clones of a similar magnitude that are specific for single peptides have been detected by tetramer staining in children and adults (8, 24). The profound effect that CMV has on the overall CD8 T-cell population is evident from the substantially higher proportion of differentiated CD28− cells present in CMV-infected compared to non-CMV-infected adults (26, 28, 48).
Considerably fewer studies have been carried out on children than adults, although the accumulation of differentiated CD28− CD8 T cells has been shown in children as young as 18 months (8) and in umbilical cord blood samples from congenitally infected infants (27). In the latter study, the CD8 T cells showed cytolytic activity and were able to produce IFN-γ, implying that their mature phenotype was matched by some degree of functionality. However, studies on other viral infections have shown that infant CD8 T cells are less able to produce IFN-γ (19, 25, 35) in response to antigenic stimulation than those of adults, implying that infant CD8 T cells are not as functionally mature as their phenotype suggests.
More than half of infants in The Gambia are infected with CMV by 6 months of age (2). Consequently, CMV is one of the first challenges that their immune systems encounter, and investigation of the response of infant T cells to CMV may offer insights into how infants respond to natural infections.
We recruited subjects from a birth cohort established in Sukuta, The Gambia, in order to determine the onset of infection in infants and to evaluate the subsequent CD8 T-cell response.
MATERIALS AND METHODS
Subjects.
Subjects were recruited at birth from the maternity ward of Sukuta Health Centre as part of an ongoing birth cohort. Informed consent was obtained from their mothers and documented by signature or thumb print. Recruitment was restricted to healthy singleton infants, defined by a birth weight of at least 1.95 kg and no congenital abnormalities. The catchment area of Sukuta Health Centre is periurban and characterized by low income and crowded living conditions. The human immunodeficiency virus statuses of the study subjects were unknown, but adult prevalence in the region was 1 to 4% at the time of the study (National AIDS Control Program, unpublished data) and so was not thought to be a significant confounder of this study.
Mononuclear cells, plasma, and buffy coats were separated from umbilical cord blood and stored. Limited molecular human leukocyte antigen (HLA) typing was carried out on the buffy coats (4) in order to identify subjects with HLA-A2, -B7, -B8, and -B35, for which known epitope peptides were available.
In total, 258 infants were followed up for 12 months to establish the age at CMV infection. A subset of 116 infants was selected for studies on the development of the CD8 T-cell response to CMV, which was biased toward HLA types for which peptides were available.
The prevalence of CMV in the adult population was established by serological detection of CMV-specific immunoglobulin G (IgG) in a subsample of 194 mothers, using ELICYTOK-G enzyme-linked immunosorbent assay (Diasorin). All samples were seropositive.
The study was approved by the Medical Research Council-Gambian Government Ethics Committee.
Diagnosis of CMV infection and blood-sampling schedule.
To determine the onset of CMV infection, urine samples were collected at birth, fortnightly for the first 3 months of life, and monthly until the age of 12 months and tested for the presence of CMV DNA by PCR. If it was detected, a second sample was taken immediately to confirm infection, and urine sampling was discontinued. Excretion in the urine is usually continuous for at least 6 months following infection (D. J. C. Miles and S. Kaye, unpublished data). Congenital infection was defined by CMV detection in a urine sample collected in the first 2 weeks of life.
Following CMV diagnosis, a blood sample was taken as soon as possible and follow-up samples were scheduled for 4 and 12 months after diagnosis. Samples were collected in heparin and transported to the laboratory within 4 h. If an infant reached 12 months of age without CMV DNA being detected, a blood sample was collected at that time.
Whole blood was used for flow cytometry, and peripheral blood mononuclear cells (PBMCs) were separated on lymphoprep (Axis-Shield PoC AS) columns for use in enzyme-linked immunospot (ELISpot) assays.
Flow cytometry.
All flow cytometry reagents were from Becton Dickinson. The CD8 population was identified using peridinin chlorophyll-conjugated anti-CD8 antibody. Preliminary experiments demonstrated that it was possible to identify a pure population of CD3+ CD8high T cells and that cells binding tetramers expressed CD8 at the same high level. Further phenotyping for surface markers was carried out using allophycocyanate-conjugated antibodies to CD27, CD45R0, and CD62L and fluorescein isothiocyanate-conjugated antibodies to CD28, CD45RA, CD95, and the class II HLA-D molecules DR, DP, and DQ. Intracellular markers were identified using fluorescein isothiocyanate-conjugated antibodies to Bcl-2, Ki-67, perforin, and granzyme A. All antibodies were titrated prior to use to ensure that they were used at their optimal concentrations. Where possible, CMV-specific cells were identified with phycoerythrin-conjugated B7-TPRVGGGAM and A2-NLVPMVATV tetramers, synthesized as previously described (13, 27). New batches of tetramer were initially used in parallel with older batches to ensure consistent staining. Red blood cells were lysed using FACSlyse solution, and lymphocytes were permeabilized using FACSperm II.
Statistical analysis of flow cytometry data.
As the study involved the collection of over 1,800 flow cytometry samples over a prolonged period of time, and the study design involved both cross-sectional and longitudinal data, objectively defining subpopulations of interest was a major challenge. In order to eliminate the inconsistencies and possible bias inherent in a human operator repetitively analyzing large amounts of data, automatic algorithms were used to define clusters for various combinations of markers using MATLAB 6.1 (The Mathworks Inc). For each marker set, the same algorithm was applied to each subject to maintain objectivity and consistency of the flow cytometry analysis. As no objective criteria exist for the definition of the subpopulations described in the present study, we made the assumption that the algorithms were valid if they recognized the same subpopulations as an experienced flow cytometry operator.
Lymphocyte populations were identified using a combination of contouring and morphological operators on the pixels of the front versus side scatter plot. After gating this population, similar algorithms were used to identify CD8 T-cell populations and tetramer+ cells. This part of the process was tested on a small number of samples stained for CD3 and CD8, and it was established that while there was a substantial overlap between the expression of CD8 on CD3− and CD3+ cells, >90% of the CD8+ cells identified were CD3+ T cells, and this population included an average of 72% of all CD8+ CD3+ cells (data not shown).
Cells stained with CD27 and CD28, CD27 and perforin, and CD27 and granzyme A were divided into three subpopulations, and cells stained with CD95 and CD62L were divided into two subpopulations using a fuzzy c-means clustering algorithm. The extremities of the population region were defined by the furthest outliers.
Cells expressing Ki-67 and cells expressing either high or low levels of Bcl-2 were identified using morphological operators to locate a region of highest event density. Where this was not possible, a weighted average of the events was used to separate the two regions.
For cells stained with HLA-D and CD45R0, an area of highest event density was identified where possible using algorithms similar to those used for Ki-67 and Bcl2, and the populations were then classified using a cross-hair placed with horizontal and vertical positions defined by the extreme borders of the densest region.
For cells stained with CD45RA and CD45R0, a breakpoint regression through the smoothed trend of CD45RA expression was used to identify at which point only CD45RA was expressed, thus defining a CD45RAbright CD45R0− population. To further explore the phenotype of the cells as defined by the expression of CD45 isoforms, a line of regression was plotted through the population of cells expressing both CD45RA and CD45R0, and the midpoint was calculated. A “CD45 ratio” was calculated by dividing the distance from the midpoint of the regression line to the border with the CD45RAbright CD45R0− subpopulation by the distance from the midpoint to the event with the lowest expression of CD45RA as defined by fluorescence intensity. A relatively high value for the CD45 ratio indicated a high level of expression of CD45R0 and a low level of expression of CD45RA.
A conservative approach was used for clustering definition, and for each algorithm limits were set to define a successful and meaningful cluster/region definition. Of the 1,886 samples scanned, 130 (6.9%) were discarded because they could not be characterized after an iterative process in which scans were manually checked to identify obvious algorithm failure.
For each set of markers, appropriate region statistics were defined, and depending on their distribution, they were summarized using means and medians. The within-subject repeated measurements were accounted for using panel-averaged models (fitted using the generalized estimating equation function in Stata 8). Where necessary, the region statistics were appropriately transformed to improve the normality and constant variance assumptions. Due to the small number of time points, the most appropriate correlation structure was the exchangeable model, which acknowledges the correlation but assumes it is the same between all time points. The significance of differences over time was quantified by a Wald test, and if significant at the 5% level, appropriate contrasts were calculated and P values were adjusted using a step-down Bonferroni method.
Diagnosis of EBV infection.
A subset of plasma samples from 12-month-old infants was tested for Epstein-Barr virus (EBV) seropositivity. To allow for the persistence of maternal IgG, an infant was regarded as infected if he/she expressed IgM to the viral capsid antigen (VCA) at 12 months or a higher level of IgG to the VCA antigen than was present in umbilical cord plasma collected. The tests were carried out using the ETI-VCA-G and ETI-EBV-M enzyme-linked immunosorbent assays (Diasorin).
ELISpot.
A kit for the detection of IFN-γ (Mabtech) was used in ELISpot assays. The peptides used were the A2-restricted MLNIPSINV, NLVPMVATV, VLGPISGHV, YILEETSVM, and IAGNSAYEYV; the B7-restricted RPHERNGFTV, TPRVTGGGAM, and CRVLCCYVL; the B35-restricted IPSINVHHY and VFPTKDVAL; and the B8-restricted DANDIYRIF (11, 22, 33, 38, 42, 47, 49).
A detectable response was defined as a mean treatment spot count that was more than 10 spots 105 cells−1 greater than twice the mean negative control. These criteria have previously been found to provide high specificity while allowing reasonable sensitivity in the detection of peptide-specific responses (18). Responses were measured as specific spot-forming units, calculated as the difference between the mean treatment spot count and the mean negative control spot count. Data were analyzed using Stata 8 and MINITAB 14.
RESULTS
Most infants are CMV infected by 12 months of age.
Congenital infection, defined as excretion of CMV in the urine within the first 2 weeks of life, was detected in 4.1% of the infants. Prevalence increased most rapidly between 6 and 12 weeks of age, by which time 62% were infected. Thereafter, prevalence continued to rise steadily until sampling concluded at 12 months, by which time 85% were infected (unpublished data).
Sampling for CD8 T-cell studies.
Longitudinal studies on the CD8 T-cell response to CMV infection were carried out on 98 infants. From these subjects, 64 samples were taken immediately after diagnosis, 92 4 months later, and 79 12 months later, so that 235 of the 294 scheduled samples were collected successfully. A further 18 samples were collected from infants who had reached 12 months of age without being infected with CMV, so the total cohort numbered 116 infants.
Frequencies of peptide-specific CD8 T cells were very variable.
Of 67 subjects who were identified as expressing HLA-A2, at least one sample containing CD8 T cells that bound the A2-NLVP tetramer was collected from 20 (30%). Of the 37 subjects who expressed HLA-B7, at least one sample that bound the B7-TPRV tetramer was collected from 16 (43%). The mean frequency of CD8 T cells that bound either tetramer was 3.4%, but there was considerable variability, with frequencies ranging from 0.05% to as high as 32%.
The age of CMV infection had no influence on the frequency of tetramer+ cells, and there were no significant differences in frequency between the samples collected immediately after diagnosis and those collected 4 or 12 months later (data not shown).
The phenotypes of tetramer+ CD8 T cells followed the same trends as the overall population.
The statistical interaction between the phenotypes of tetramer+ cells and the overall population was assessed to establish whether the time elapsed since diagnosis had different effects on the phenotypes of tetramer+ cells and the overall population. The only significant interaction found was in the case of the proportion of cells expressing Ki-67 (P = 0.015), which is discussed below. For all other parameters measured, changes in the phenotype of the tetramer+ cells were reflected by changes in the phenotype of the overall CD8 T-cell population, although the actual proportions of cells in different subpopulations at any given sampling time were often very different.
Age at time of diagnosis had no effect on the magnitudes of either the tetramer+ subpopulations or any of the subpopulations defined by phenotype (data not shown).
Activation was highest in the early stages of infection in tetramer+ cells and the overall CD8 T-cell population.
Cells were stained for CD45RA and CD45R0, and a breakpoint regression was used to divide them into a CD45RAbright CD45R0− subpopulation and a subpopulation expressing approximately reciprocal proportions of both isoforms. Nearly all tetramer+ cells expressed CD45R0 (P < 0.0001), which is typically expressed by functional memory or effector cells (37, 43), considerably more than the overall CD8 T-cell population (Table 1 and Fig. 1A and B). However, the CD45 ratio among the subpopulation that expressed both isoforms was not different between tetramer+ cells and the overall CD8 T-cell population (Table 1 and Fig. 1C), indicating that while a higher proportion of the tetramer+ subpopulation expressed CD45R0 than the overall CD8 T-cell population, the level of CD45R0 expression did not differ between these populations.
TABLE 1.
Relative sizes of subpopulations of interest within the CD8 T-cell population
| Subpopulation | CD8 T-cell population | Size
|
|||
|---|---|---|---|---|---|
| Infected (time postdiagnosis)a
|
Uninfected at 12 mo | ||||
| Immediately | 4 mo | 12 mo | |||
| CD45RAbright CD45R0− | Tetramer+ | 10.3 ± 8.1Ab | 14.5 ± 7.6Bb | 18.8 ± 10.5Bb | |
| Overall | 33.0 ± 11.1A | 37.7 ± 10.9B | 40.9 ± 10.8B | 41.3 ± 19.3 | |
| HLA-D+ | Tetramer+ | 67.5 ± 24.4Ab | 55.1 ± 26.1Bb | 56.6 ± 23.9Bb | |
| Overall | 28.3 ± 16.6A | 23.6 ± 11.8B | 24.0 ± 13.6B | 22.4 ± 20.1 | |
| CD45RO+ HLA-D+ | Tetramer+ | 42.0 ± 26.0Ab | 30.8 ± 20.1Bb | 34.3 ± 20.1Bb | |
| Overall | 14.3 ± 11.6A | 10.7 ± 7.6B | 9.1 ± 6.9B | 9.9 ± 11.8 | |
| Ki-67+ | Tetramer+ | 31.3 ± 22.3Ab | 14.5 ± 14.5B | 10.4 ± 9.9B | |
| Overall | 12.5 ± 9.7 | 8.4 ± 5.2A | 8.5 ± 5.5A | 13.4 ± 14.7 | |
| CD62L+ | Tetramer+ | 16.6 ± 13.0Ab | 10.3 ± 8.9Ab | 11.9 ± 16.3Ab | |
| Overall | 64.8 ± 12.4A | 62.6 ± 14.9A | 64.8 ± 14.7A | 73.2 ± 13.4c | |
| Bcl-2low | Tetramer+ | 97.1 ± 3.2Ab | 89.5 ± 15.5Ab | 84.3 ± 23.6Ab | |
| Overall | 56.0 ± 19.1A | 59.3 ± 19.7A | 55.4 ± 22.9A | 40.2 ± 18.7 | |
| CD27+ CD28+ | Tetramer+ | 26.7 ± 26.9Ab | 15.6 ± 18.6Ab | 14.4 ± 13.0Ab | |
| Overall | 59.3 ± 19.4A | 60.7 ± 17.9A | 62.8 ± 19.8A | 86.9 ± 20.4c | |
| CD27+ CD28− | Tetramer+ | 61.4 ± 23.9Ab | 60.8 ± 18.4ABb | 58.3 ± 16.4Bb | |
| Overall | 28.4 ± 14.4A | 22.9 ± 10.7AB | 20.0 ± 9.9A | 10.6 ± 16.7c | |
| CD27− CD28− | Tetramer+ | 10.8 ± 5.8Ab | 22.2 ± 14.6Bb | 26.6 ± 14.5Bb | |
| Overall | 12.3 ± 8.1A | 16.5 ± 10.2B | 17.3 ± 12.4B | 2.5 ± 3.8c | |
| CD27+ perforin− | Tetramer+ | 31.7 ± 28.0Ab | 33.4 ± 28.0Ab | 29.9 ± 27.1Ab | |
| Overall | 63.5 ± 20.0A | 60.8 ± 18.5A | 65.1 ± 19.7A | 83.8 ± 21.3c | |
| CD27+ perforin+ | Tetramer+ | 53.7 ± 25.6Ab | 44.1 ± 20.9Ab | 46.4 ± 20.4Ab | |
| Overall | 23.8 ± 13.8A | 21.5 ± 10.0A | 20.0 ± 13.8A | 12.2 ± 16.1c | |
| CD27− perforin+ | Tetramer+ | 14.1 ± 8.6Ab | 21.8 ± 16.0Bb | 22.8 ± 14.9Bb | |
| Overall | 12.6 ± 8.4A | 17.7 ± 10.2B | 14.9 ± 10.2B | 4.0 ± 5.3c | |
| CD27+ granzyme A− | Tetramer+ | 6.7 ± 9.8Ab | 6.4 ± 12.8Ab | 2.7 ± 2.7Ab | |
| Overall | 51.8 ± 20.0A | 51.5 ± 18.4A | 52.7 ± 20.6A | 69.3 ± 22.8c | |
| CD27+ granzyme A+ | Tetramer+ | 72.7 ± 20.7Ab | 66.7 ± 16.8Bb | 67.0 ± 15.1Bb | |
| Overall | 33.6 ± 15.5A | 27.8 ± 11.8B | 26.6 ± 11.8B | 24.6 ± 18.9c | |
| CD27− granzyme A+ | Tetramer+ | 17.6 ± 10.3Ab | 26.1 ± 16.5Bb | 29.1 ± 15.9Bb | |
| Overall | 14.7 ± 8.7A | 20.8 ± 10.8B | 20.7 ± 13.5B | 6.1 ± 5.1c | |
Represented as mean percentages ± standard deviations. Different uppercase letters are placed next to subpopulations that are significantly different between sample times.
Significant differences in the relative size of the relevant subpopulation between the tetramer+ and the overall CD8 T-cell populations.
Significant differences between the relative sizes of the relevant population between infants uninfected with CMV at 12 months and infected infants of the same age.
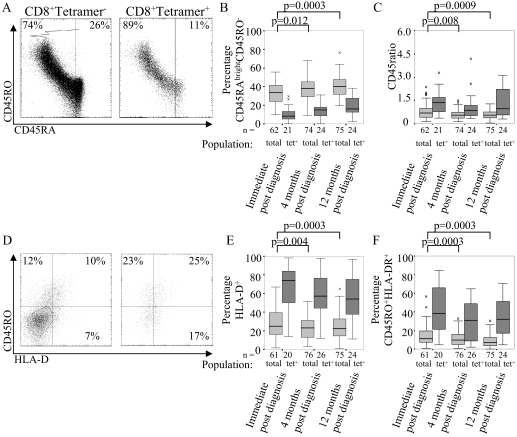
FIG. 1.
CMV-specific CD8 T cells are more activated than the overall CD8 T-cell population. (A) Expression of CD45RA and CD45R0 by tetramer+ and tetramer− CD8 T cells, showing the line of best fit and the cutoff used to define the CD45RAbright CD45R0− population. (B) Mean percentage of CD45RAbright CD45R0− CD8 T cells in tetramer+ and total populations for each sampling time. (C) Mean CD45 ratios of tetramer+ and total CD8 T cells at each sample time. (D) Expression of CD45R0 and HLA-DR on tetramer+ and tetramer− CD8 T cells, showing the cutoffs used to define the CD45R0+ and HLA-DR+ populations. (E) Mean percentages of HLA-D+ and (F) CD45R0+ HLA-DR+ CD8 T cells among tetramer+ and total populations at each sample time. The percentages in the scatter plots refer to cells within that quadrant. The boxes indicate medians and interquartile ranges.
The proportion of cells expressing both isoforms was at its highest immediately postdiagnosis and was substantially lower in the samples taken 4 and 12 months later (P = 0.012) (Table 1 and Fig. 1B). The CD45 ratio showed the same pattern (P = 0.008), indicating that not only the proportion of cells that expressed CD45R0 but also the level of CD45R0 expression was higher immediately after diagnosis than in the following months.
Cells were also stained with CD45R0 and HLA-D, and the cells that expressed either or both were identified. As with CD45R0, the proportion of cells that expressed HLA-D was greater among tetramer+ cells than among the whole CD8 T-cell population (P < 0.0001), and expression was higher immediately after diagnosis than 4 or 12 months later (P = 0.004) (Table 1 and Fig. 1D and E). The proportion of cells expressing both CD45R0 and HLA-D molecules followed the same pattern (Table 1 and Fig. 1F).
The proportion of CD8 T cells in the mitotic cycle was highest immediately after infection.
The age at the time of diagnosis did not affect the proportion of cells expressing Ki-67 (i.e., in the mitotic cycle) (12) at any of the sampling times (data not shown).
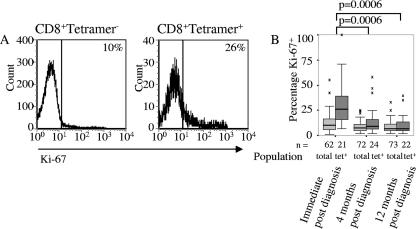
Mitosis was the only variable for which the development of the tetramer+ population followed a different pattern than the overall CD8 T-cell population. Immediately after diagnosis, the tetramer+ cells exhibited significantly more mitosis than in either of the two subsequent samples (P = 0.0006) (Table 1 and Fig. 2A and B), and more tetramer+ cells were undergoing mitosis than in the overall CD8 T-cell population (P < 0.0001). However, the proportion of tetramer+ cells in mitosis halved from 31% immediately after diagnosis to 15% 4 months later and ceased to be different from the overall CD8 T-cell population.
FIG. 2.
CMV-specific CD8 T cells divide more than the overall CD8 T-cell population during the early stages of infection. (A) Expression of Ki-67 by tetramer+ and tetramer− CD8 T cells in a representative sample collected immediately postdiagnosis. (B) The expression of Ki-67 for all subjects where tetramer staining was carried out, with differences in expression of Ki-67 between tetramer+ cells indicated. There were no differences between the overall populations. The boxes indicate medians and interquartile ranges.
Among the overall CD8 T-cell population, the highest proportion of CD8 T cells in mitosis never exceeded 12%, and there were no significant differences between the proportion of cells expressing Ki-67 at different sampling times (Table 1 and Fig. 2B).
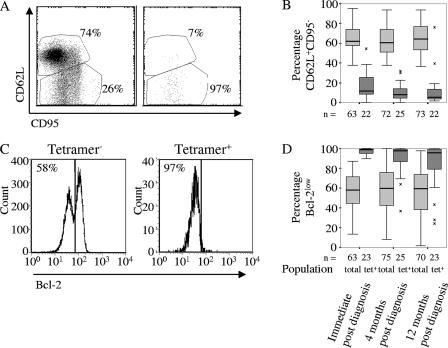
Expression of CD62L, CD95, and Bcl-2 remained constant in the year following infection with CMV.
The CD8 T-cell population was divided into an undifferentiated CD62L+ subpopulation (20, 43), expressing various levels of CD95 (Fas), and a differentiated CD62L− subpopulation, in which nearly all cells expressed CD95 (Fig. 3A and B). Nearly all of the tetramer+ cells were in the latter subpopulation (P < 0.0001). Differentiation status could also be defined by high and low levels of Bcl-2 expression, and nearly all tetramer+ cells were in the more differentiated Bcl-2low subpopulation (P < 0.0001) (23) (Table 1 and Fig. 3C and D).
FIG. 3.
CMV-specific CD8 T cells have a more apoptosis-prone phenotype than the overall CD8 T-cell population. (A) Expression of CD62L and CD95 on tetramer+ and tetramer− CD8 T cells, showing the definition of the CD62L+ and CD95+ CD62L+ populations. (B) Mean percentages of CD62L+ CD8 T cells among tetramer+ and tetramer− populations at each sample time. (C) Expression of Bcl-2 on tetramer+ and tetramer− CD8 T cells, showing the definition of the Bcl-2high and Bcl-2low populations. The percentages refer to proportions of Bcl-2low cells. (D) Mean percentages of Bcl-2low CD8 T cells among tetramer+ and tetramer− populations at each sample time. Differences between phenotypes of the total CD8 T-cell population at different sampling times are indicated. The boxes indicate medians and interquartile ranges. The percentages in the scatter plots refer to adjacent regions.
In contrast to CD45 isoforms, HLA-D, and Ki-67, the relative sizes of the subpopulations defined by CD62L and Bcl-2 remained constant for 12 months following diagnosis in both the tetramer+ cells and the overall CD8 T-cell population.
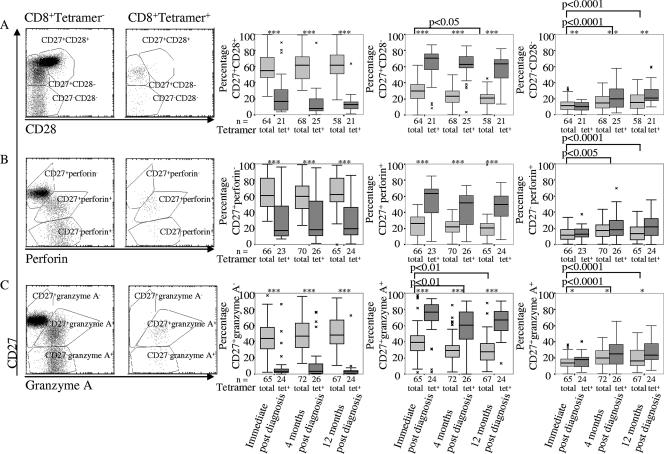
The proportion of differentiated CD8 T cells is constant in the year following CMV infection, but CD27 is lost progressively.
Cells stained for the costimulatory molecules CD27 and CD28 were divided into naïve (CD27+ CD28+), intermediate (CD27+ CD28−), and fully differentiated (CD27− CD28−) subpopulations (1, 16, 43). Few tetramer+ cells expressed any CD28, so they nearly all fell into the last two subpopulations (Table 1 and Fig. 4A). Among the CD8 T-cell population as a whole, cells in the intermediate and fully differentiated populations nearly all expressed perforin (Table 1 and Fig. 4B) and granzyme A (data not shown).
FIG. 4.
CMV-specific CD8 T cells are more differentiated and show higher cytotoxic potential than the remainder of the CD8 T-cell population. (A) Scatter plots of representative samples of tetramer+ and tetramer− CD8 T cells stained for CD27 and CD28 with the indicated populations and the percentage of cells within each population, box plots of all samples indicating medians and interquartile ranges, and differences between the distributions of all populations between sample times indicated. (B) The same data for cells stained for CD27 and perforin. (C) The same data for cells stained for CD27 and granzyme A. The populations are identified, and the percentages indicate the proportion of cells in each of them.
Age at diagnosis did not affect the relative distribution of cells between the three populations or their subsequent changes in phenotype. Similarly, the proportion of cells expressing the naïve phenotype remained constant in the 12 months following diagnosis. However, there was a progressive, albeit nonsignificant, reduction of the intermediate population and a more pronounced increase in the fully differentiated population among both the tetramer+ cells and the overall CD8 T-cell population (P = 0.0003), indicating loss of CD27 expression from the differentiated CD8 T-cell population (Table 1 and Fig. 4A).
The changes that were apparent when the populations were defined by CD27 and CD28 expression remained when the stain for CD28 was replaced with stains for perforin (Table 1 and Fig. 4B) or granzyme A (data not shown).
Production of IFN-γ by peptide-specific cells is impaired in younger infants.
The IFN-γ production of the PBMCs of samples that responded to tetramers was assessed by ELISpot assay, using the peptide incorporated into the tetramer as the stimulant. The proportion of subjects in which a response was detected was higher in the samples collected 12 months after diagnosis (16/19) than in those collected immediately (6/11) or 4 months (2/11) after diagnosis, though the only significant difference was between the 4- and 12-month postdiagnosis responses (Fisher's exact probability test; P = 0.0012). As frequencies of CMV-specific cells remained constant with time since diagnosis, the increased number of IFN-γ-producing PBMCs indicated an increase in the proportion of functional CD8 T cells. Among the samples in which responses were detected, there were no effects of either age at time of sampling or time since diagnosis on the magnitude of the response (data not shown).
To establish whether the same trend was apparent in a larger data set, the ELISpot response to peptides with the appropriate HLA restriction was evaluated using PBMCs from 18 of the 50 infants assessed immediately postdiagnosis who had a detectable response to at least one peptide, while 17 of 34 (50%) responded at 4 months postdiagnosis and 29 of 65 (44%) responded at 12 months postdiagnosis, indicating no increase in the number of detectable responses over time since diagnosis.
However, when the magnitudes of the response in those cases where the response was detectable at all were considered, the age at diagnosis correlated with the IFN-γ response at 12 months postdiagnosis (Spearman's r = 0.46; n = 29; P = 0.012), although not in the samples collected immediately and 4 months postdiagnosis (Fig. 5A, B, and C).
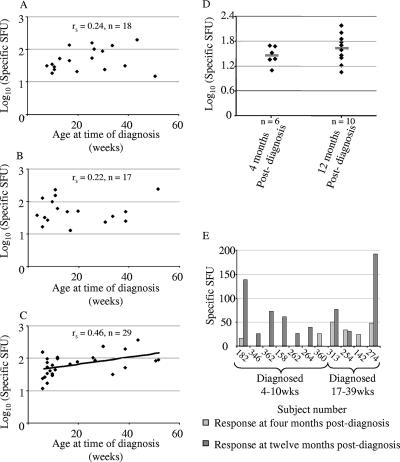
FIG. 5.
The magnitude of IFN-γ responses was greater in older infants. There was no correlation between age at the time of diagnosis and the magnitude of IFN-γ response in those cases where a peptide induced a peptide-specific response (A) immediately after diagnosis or (B) 4 months later, but (C) there was a positive correlation by 12 months following diagnosis. (D) Samples collected from subjects between 40 and 60 weeks of age who had been infected 4 months previously had an IFN-γ response similar to that of infants who had been infected 12 months previously. The bars indicate means. (E) Twelve-month samples were collected from infants diagnosed with CMV at 4 to 10 weeks when they reached 40 to 60 weeks of age, and 4-month samples were collected from infants diagnosed at 17 to 39 weeks when they reached the same age. When the 4- and 12-month samples from subjects who fell into either of these two categories were compared, a greater IFN-γ response was elicited from the 12-month sample in nearly all cases. The absence of a bar indicates that the sample was collected but no response was detected.
As the age at which the 12-month-postdiagnosis sample was taken depended on the age at diagnosis, it was necessary to establish whether the correlation was a direct consequence of the age of infection or simply due to IFN-γ production increasing as the infants aged. To eliminate the effect of age at sampling on the magnitude of the IFN-γ response, a subset of infants who were all the same age of 40 to 60 weeks was identified, but it included infants that had been infected either 4 or 12 months previously. The infants who were sampled 4 months postdiagnosis had been infected at 17 to 39 weeks of age, and those sampled 12 months postdiagnosis had been infected at 4 to 10 weeks of age. There was no difference in the magnitude of response by Mann-Whitney U test (Fig. 5D), indicating that age at diagnosis was not a determinant of the response in itself.
In order to establish whether there was an effect of age at sampling, the magnitudes of response at 4 and 12 months postdiagnosis were compared longitudinally among the subset described above. Nearly all showed a higher magnitude of response at 12 months postdiagnosis than at 4 months postdiagnosis (Wilcoxon statistic = 59; n = 11; P = 0.023) (Fig. 6E). As the increase in the magnitude of the response increased with age at sampling but was not related to the age at diagnosis, the results indicate that the function of the CD8 T cells was low in early infancy and increased with age.
FIG. 6.
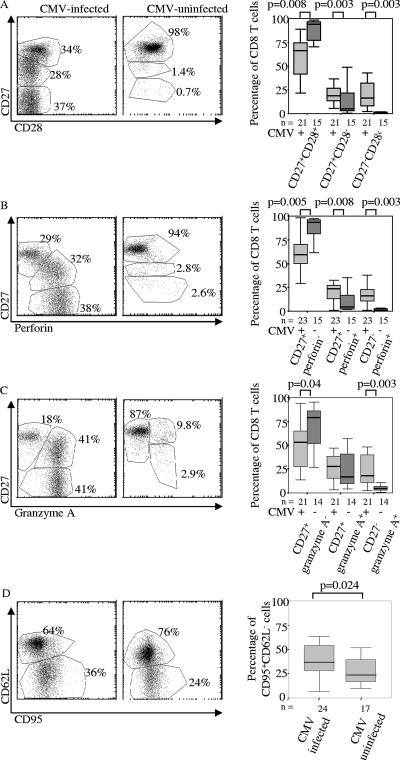
The CD8 T-cell population of CMV-infected infants is more activated and differentiated than that of uninfected infants. (A) Expression of CD27 and CD28 in representative samples and all infants sampled, drawn from the groups of CMV-infected and non-CMV-infected infants at 12 months of age. (B) Expression of CD27 and perforin in representative samples and all infants sampled, drawn from the groups of CMV-infected and non-CMV-infected infants at 12 months of age. (C) Expression of CD27 and granzyme A in representative samples and all infants sampled, drawn from the groups of CMV-infected and non-CMV-infected infants at 12 months of age. (D) Expression of CD62L and CD95 in representative samples and all infants sampled, drawn from the groups of CMV-infected and non-CMV-infected infants at 12 months of age. The percentages in the scatter plots refer to cells within the adjacent region. The boxes indicate medians and interquartile ranges.
There was no effect of time since diagnosis on the magnitudes.
The range of peptides recognized does not alter with age.
The number of subjects with the appropriate HLA type that responded to any given peptide at any given time point varied from 0 to 59%, with an average of 18.5%. The PBMC samples collected immediately after diagnosis responded on average to 16% of the peptides with the appropriate restriction, but samples collected 4 months later responded to only 12%. A broader response was apparent 12 months later, as PBMCs responded to an average of 28% of peptides tested (data not shown). These differences were not significant by the Kruskal-Wallis test.
Infection with CMV drives differentiation of the CD8 T-cell subset over the first year of life.
It was not possible to ascertain whether the changes in the CD8 T-cell subpopulations were a consequence of CMV infection or of the ontogeny of the CD8 T-cell subset by considering only changes within the CD8 T-cell populations of infected infants. Consequently, the overall CD8 T-cell phenotypes of 51- to 60-week-old uninfected infants were compared with those of infants of a similar age who had been diagnosed at least 43 weeks previously.
Over 90% of the CD8 T cells of 10 of 16 (63%) uninfected infants from whom data on CD27 and CD28 were collected fell into the naïve (CD27+ CD28+) population, which was a higher proportion of naïve cells than was observed in any CMV-infected infant (P = 0.003) (Table 1 and Fig. 6A). Similar results were found when the stain for CD28 was replaced with a stain for perforin or granzyme A (Fig. 6B and C).
The more differentiated phenotype of the CD8 T cells of infants infected with CMV was also indicated by the greater expression of CD95 and loss of expression of CD62L (P = 0.024) (Table 1 and Fig. 6D). No differences in the relative expression of CD45 isoforms, Bcl-2, HLA-D, or Ki-67 were detected.
The possibility remained that the differences between infants infected or uninfected with CMV were not a direct result of CMV infection but were due to exposure to a wider range of immune challenges than those that remained uninfected. If CMV status is indeed a correlate of exposure, the same is probably true of EBV. The EBV status of the subset of infants that were included in the analyses of the effect of CMV on the CD8 T-cell phenotype was determined, and 29 out of 46 (63%) were found to be infected at the time of sampling. All of the phenotypes that were different between CMV-infected and uninfected infants were tested to establish whether there were similar differences according to EBV status, but none were found (data not shown).
DISCUSSION
The interactions between the CD8 T-cell population of adults and CMV are well documented (1, 10, 13, 26, 39). Considerably less information is available on the response of the CD8 T-cell population of infants. We studied the development of the CD8 T-cell response of infants in a prospective cohort. A major technical challenge that we encountered was the analysis of the very large volume of flow cytometry data produced. There were a number of technical sources of uncertainty over nearly 3 years of data collection, as well as the subjectivity inherent in the usual method of fitting regions according to the opinion of the operator. To address these issues, we used a set of algorithms that defined subpopulations by applying repeatable criteria. When developing the algorithms, we regarded them as valid if they were in agreement with the opinions of experienced T-cell biologists, who examined every plot produced. The algorithms had the advantage of being able to rapidly and consistently apply the same criteria for the definition of a given subpopulation to the entire data set, which was particularly important given the longitudinal nature of the data collection.
Gambian infants are typically infected with CMV within the first year of life, so CMV is one of the first immunological insults that they encounter. Studies on neonates infected congenitally have shown a mature CD8 T-cell response to CMV (27), and the suggestion that the infant CD8 T-cell response develops in a similar way to that of adults is supported by the observation that there was no effect of age on the frequency of tetramer+ cells. Further, there was no effect of age at diagnosis on any phenotypic marker.
Cells specific for CMV showed higher levels of HLA-D and CD45R0 than the overall CD8 T-cell population, indicating a relatively high state of activation. Activation markers declined after the first few months of infection, implying a transition from the acute to the chronic phase of infection. It is remarkable that the pattern of change with time after diagnosis that was seen in the phenotypes of the tetramer+ cells was reflected in the overall CD8 T-cell population.
A limitation of the tetramers is that they could identify only the subpopulation of CMV-specific cells that responded to the peptide they incorporated, so it was impossible to determine what proportion of the CMV-specific population was represented in each individual. As CMV is a large virus composed of over 200 proteins, that proportion probably varied widely between individuals. Both the proportion of CD8 T cells that respond to chronic CMV infection and the range of available epitopes are typically considerable (39, 45, 46, 49). In the present study, the proportion of CMV-specific CD8 T cells was probably similarly high, as the median proportion of tetramer+ cells was 3.4% and several individuals sustained tetramer+ populations of over 10% of the total CD8 T-cell population throughout the year following infection.
The same trends were seen in the overall populations as in the tetramer+ populations in all characteristics that were studied except for division. It is possible that there was a high enough proportion of CMV-specific cells within the overall population to dominate the trends shown by that population, although it would be impossible to be certain without a method for identifying all CMV-specific CD8 T cells. However, it is also possible that CMV infection generated a cytokine environment that nonspecifically affected the activity and differentiation of T cells. Such bystander activation has been demonstrated in a number of contexts (21, 30, 40).
Tetramer+ CD8 T cells took on a differentiated phenotype, as defined by loss of CD27, CD28, and CD62L (1, 34, 37, 43), and in contrast to the transient expression of markers of activation, such as HLA-D, the proportion of differentiated cells remained stable for the year following infection. This observation supports the implication of the consistent CMV-specific subpopulations that a population of CMV-specific CD8 T cells is produced very soon after infection and maintained thereafter.
The observation that the relative size of the differentiated CD8 T-cell population was much smaller in uninfected 12-month-old infants than in infected infants suggests that the accumulation of differentiated CD8 T cells is largely driven by CMV itself. Studies on adults have shown that while older people tend to have more CD28− CD8 T cells than younger people, CMV-infected individuals tend to have a much larger population of CD28− CD8 T cells than their uninfected peers (26, 28, 48). Our findings show that such a difference begins with primary CMV infection, even when this occurs very early in life.
Differentiated CD8 T cells, defined by the loss of CD28, nearly all expressed perforin and granzyme A, indicating that the differentiated cells have the potential to function as cytotoxic effector cells (27). Further, the proportion of cells expressing CD95 or low levels of Bcl-2 remained constant for the year following diagnosis. Although both of these markers are associated with activation-induced cell death (3), these subpopulations remained constant while the proportion of dividing cells did not, so it is unlikely that apoptosis took place at a rate as constant as the expression of CD95 and Bcl-2 would suggest.
In the light of the stability of the differentiated CD28− population, the reduction in the proportion of cells expressing CD45R0 indicated that a substantial proportion of the differentiated cells must be CD28− CD45RAbright CD45R0− by 12 months after infection. It has been shown that a high proportion of CMV-specific CD8 T cells express CD45RA during chronic infection (7), and it seems that this population appeared gradually over the course of the year following infection among both the tetramer binding subpopulations and the overall CD8 T-cell population.
In spite of the emergence of a population of differentiated cells immediately after infection, the IFN-γ responses were impaired in early infancy, even when the presence of a constant population of cells specific for a given peptide was detected by tetramer staining. The difference was particularly evident in the sample collected 12 months following infection, and the lack of difference between children infected for different periods of time but sampled at the same age indicated that the difference was due to the age of the infants when sampled rather than the age at infection in itself.
Although an IFN-γ response to CMV peptides has been detected in the umbilical cord blood of congenitally infected infants (27), the ability of the T cells of infants to produce IFN-γ in response to antigenic stimuli is impaired by the poor interleukin-12 (14) and CD40 (9) expression of infant dendritic cells, and indeed, a reduced IFN-γ response to human immunodeficiency virus has been shown in infants below 1 year of age (25, 35). In spite of the phenotypic maturity of the CD8+ T-cell responses of some infants, it is evident that the cells were functionally impaired.
The expansion of the overall CD28− CD8 T-cell subpopulation observed in infants under 12 months of age raises some concerns. A subpopulation of a similar size is characteristic of Americans of approximately 70 years of age (15) or 40- to 60-year-old Swedes infected with CMV (28). Further, the presence of expanded CD28− CD45RA+ CD8 T cells is indicative of failure to respond to influenza vaccination in elderly people (36). Our findings indicate that CMV infection and the presence of a large population of CD28− CD45RAbright CD8 T cells are so commonplace as to be normal in Gambian infants. This is probably the case throughout sub-Saharan Africa, as our observations are consistent with the finding that large proportions of the CD8 T-cell population of young Ethiopian children have lost CD27 expression (41). As CMV infection in our study commonly occurred before the age of 12 weeks and immediately induced a CD28− CD8 T-cell population, most infants exhibited indicators for impaired immune function (31) while receiving childhood vaccinations and before their first exposure to endemic diseases of infancy, such as malaria, pneumonia, and rotavirus. Whether CMV infection interferes with vaccine responses is not known, although it has been suggested that there is a finite amount of immunological “space” and that the large clonal expansions of T cells that CMV induces may occupy so much of it that there is little room for the expansion of clones to newly encountered antigens (32). It is also possible that the presence of a large proportion of differentiated CD8 T cells and vaccine failure are unrelated consequences of events at a more fundamental level of the immune system.
Although a causal link between CMV infection and impaired vaccine responses has not been formally demonstrated and studies of infants in the United States (8) and Japan (24) have not associated postnatal CMV infection with poor health, the ubiquity of CMV infection in the African context makes it important to establish whether the effect of CMV on CD8 T-cell phenotypes is matched by the effect that studies on the elderly imply.
In summary, we found that the CD8 T-cell populations of CMV-infected infants exhibited high but transient levels of activation and that large subpopulations of differentiated cells emerged rapidly after infection and remained stable for at least a year following infection. Consequently, CMV-infected infants showed considerably more differentiated CD8 T cells than uninfected infants of the same age. The phenotypes of the CMV-specific CD8 T cells of infants appeared essentially similar to those reported in adults, and neither their phenotype nor their subsequent development was affected by the age of infection. In spite of the mature phenotype of CMV-specific CD8 T cells, considerably fewer of the cells of younger infants were able to produce IFN-γ in response to CMV-derived peptides, indicating that phenotypic maturity was not synonymous with functional maturity.
Acknowledgments
We thank the staff of the Sukuta birth cohort, Omar Badjie, Fatou Bah, Saihou Bobb, Janko Camara, Sulayman Colley, Isatou Drammeh, Mam Maram Drammeh, Baboucarr Jobe, Momodou Jobe, Albert Magnusen, John S. Mendy, Ngui Ndow, Faalou Njie, Bala Musa Sambou, Sarjo Sanneh, and Mamadi Sidibeh. Akram Zaman, Paul Snell, and Sarah Crozier helped with data handling. We are extremely grateful for the support of Sally Savage of Sukuta Health Centre and Abi Khan of Western Division Health Team. Louis-Marie Yindom and Assan Jaye carried out HLA typing.
This work was supported by GSK Biologics and MRC.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. [DOI] [PubMed] [Google Scholar]
- 2.Bello, C., and H. Whittle. 1991. Cytomegalovirus infection in Gambian mothers and their babies. J. Clin. Pathol. 44:366-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budd, R. 2001. Activation-induced cell death. Curr. Opin. Immunol. 13:356-362. [DOI] [PubMed] [Google Scholar]
- 4.Bunce, M., C. O'Neill, M. Barnardo, P. Krausa, M. Browning, P. Morris, and K. Welsh. 1995. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 and DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46:355-367. [DOI] [PubMed] [Google Scholar]
- 5.Bunde, T., A. Kirchner, B. Hoffmeister, D. Habedank, R. Hetzer, G. Cherepnev, S. Proesch, P. Reinke, H.-D. Volk, H. Lehmkuhl, and F. Kern. 2005. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J. Exp. Med. 201:1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalandon, Y., S. Degermann, J. Villard, L. Arlettaz, L. Kaiser, S. Vischer, S. Walter, M. Heemskerk, R. van Lier, C. Helg, B. Chapuis, and E. Roosnek. 2006. Pretransplantation CMV-specific T cells protect recipients of T-cell-depleted grafts against CMV-related complications. Blood 107:389-396. [DOI] [PubMed] [Google Scholar]
- 7.Champagne, P., G. Ogg, A. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. Rizzardi, S. Fleury, M. Lipp, R. Förster, S. Rowland-Jones, R.-P. Sékaly, A. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 8.Chen, S., W.-W. Tu, M. Sharp, E. Tongson, X.-S. He, H. Greenberg, T. Holmes, Z. Wang, G. Kemble, A.-M. Manganello, S. Adler, C. Dekker, D. Lewis, and A. Arvin. 2004. Antiviral CD8 T cells in the control of primary human cytomegalovirus infection in early childhood. J. Infect. Dis. 189:1619-1627. [DOI] [PubMed] [Google Scholar]
- 9.De Wit, D., V. Olislagers, S. Goriely, F. Vermeulen, H. Wagner, M. Goldman, and F. Willems. 2004. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103:1030-1032. [DOI] [PubMed] [Google Scholar]
- 10.Gamadia, L., E. Remmerswaal, J. Weel, F. Bemelman, R. van Lier, and I. Ten Berge. 2003. Primary immune responses to human CMV: a critical role for IFN-γ-producing CD4+ T cells in protection against CMV disease. Blood 101:2686-2692. [DOI] [PubMed] [Google Scholar]
- 11.Gavin, M. A., M. J. Gilbert, S. R. Riddell, P. D. Greenberg, and M. J. Bevan. 1993. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J. Immunol. 151:3971-3980. [PubMed] [Google Scholar]
- 12.Gerdes, J., H. Lemke, H. Baisch, H. H. Wacker, U. Schwab, and H. Stein. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710-1715. [PubMed] [Google Scholar]
- 13.Gillespie, G., M. Wills, V. Appay, C. O'Callaghan, M. Murphy, N. Smith, P. Sissons, S. Rowland-Jones, J. Bell, and P. Moss. 2000. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J. Virol. 74:8140-8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goriely, S., B. Vincart, P. Stordeur, J. Vekemans, F. Willems, M. Goldman, and D. de Wit. 2001. Deficient IL-12(p35) gene expression by dendritic cells derived from neonatal monocytes. J. Immunol. 166:2141-2146. [DOI] [PubMed] [Google Scholar]
- 15.Gorozny, J., J. Fulbright, C. Crowson, G. Poland, W. O'Fallon, and C. Weyland. 2001. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J. Virol. 75:12182-12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamann, D., M. Roos, and R. van Lier. 1999. Faces and phases of human CD8+ T-cell development. Immunol. Today 20:177-180. [DOI] [PubMed] [Google Scholar]
- 17.Hamprecht, K., J. Maschmann, M. Vochem, K. Dietz, C. Speer, and G. Jahn. 2001. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 357:513-518. [DOI] [PubMed] [Google Scholar]
- 18.Hill, P., R. Brookes, A. Fox, K. Fielding, D. Jeffries, D. Jackson-Sillah, M. Lugos, P. Owiafe, S. Donkor, A. Hammond, J. Otu, T. Corrah, R. Adegbola, and K. McAdam. 2004. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin. Infect. Dis. 38:966-973. [DOI] [PubMed] [Google Scholar]
- 19.Jaimes, M., O. Rojas, A. González, I. Cajiao, A. Charpilienne, P. Pothier, E. Kohli, H. Greenberg, M. Franco, and J. Angel. 2002. Frequencies of virus-specific CD4+ and CD8+ T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J. Virol. 76:4741-4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaech, S., E. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 21.Kanai, T., E. Thomas, Y. Yasutomi, and N. Letvin. 1996. IL-15 stimulates the expansion of AIDS virus-specific CTL. J. Immunol. 157:3681-3687. [PubMed] [Google Scholar]
- 22.Kern, F., I. P. Surel, N. Faulhaber, C. Frömmel, J. Schneider-Mergener, C. Schönemann, P. Reinke, and H.-D. Volk. 1999. Target structures of the CD8+-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan, N., N. Shariff, M. Cobbold, R. Bruton, J. Ainsworth, A. Sinclair, L. Nayak, and P. Moss. 2002. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol 169:1984-1992. [DOI] [PubMed] [Google Scholar]
- 24.Komatsu, H., A. Inui, T. Sogo, T. Fujisawa, H. Nagasaka, S. Nonoyama, S. Sierro, J. Northfield, M. Lucas, A. Vargas, and P. Klenerman. 2006. Large scale analysis of pediatric antiviral CD8+ T cell populations reveals sustained, functional and mature responses. Immun. Ageing 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohman, B., J. Slyker, B. Richardson, C. Farquhar, J. Mabuka, C. Crudder, T. Dong, E. Obimbo, D. Mbori-Ngacha, J. Overbaugh, S. Rowland-Jones, and G. John-Stewart. 2005. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J. Virol. 79:8121-8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Looney, R., A. Falsey, D. Campbell, A. Torres, J. Kolassa, C. Brower, R. McCann, M. Menegus, K. McCormick, M. Frampton, W. Hall, and G. Abraham. 1999. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin. Immunol. 90:213-219. [DOI] [PubMed] [Google Scholar]
- 27.Marchant, A., V. Appay, M. van der Sande, N. Dulphy, C. Liesnard, M. Kidd, S. Kaye, O. Ojuola, G. Gillespie, A. Vargas Cuero, V. Cerundolo, M. Callan, K. McAdam, S. Rowland-Jones, C. Donner, A. McMichael, and H. Whittle. 2003. Mature CD8+ T lymphocyte response to viral infection during fetal life. J. Clin. Investig. 111:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson, J., A. Wikby, B. Johansson, S. Löfgren, B.-O. Nilsson, and F. Ferguson. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121:187-201. [DOI] [PubMed] [Google Scholar]
- 29.Pass, R. F. 1985. Epidemiology and transmission of cytomegalovirus. J. Infect. Dis. 152:243-248. [DOI] [PubMed] [Google Scholar]
- 30.Patki, A., M. Quiñones-Mateu, D. Dorazio, B. Yen-Lieberman, W. Boom, E. Thomas, and M. Lederman. 1996. Activation of antigen-induced lymphocyte proliferation by interleukin-15 without the mitogenic effect of interleukin-2 that may induce human immunodeficiency virus-1 expression. J. Clin. Investig. 98:616-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawelec, G., A. Akbar, C. Caruso, B. Grubeck-Loebenstein, R. Solana, and A. Wikby. 2005. Human immunosenescence: is it infectious? Immunol. Rev. 25:257-268. [DOI] [PubMed] [Google Scholar]
- 32.Pawelec, G., and C. Gouttefangeas. 2006. T-cell dysregulation caused by chronic antigenic stress: the role of CMV in immunosenescence? Aging Clin. Exp. Res. 18:171-173. [DOI] [PubMed] [Google Scholar]
- 33.Retière, C., V. Prod'homme, B.-M. Imbert-Marcille, M. Bonneville, H. Vié, and M.-M. Hallet. 2000. Generation of cytomegalovirus-specific human T-lymphocyte clones by using autologous B-lymphoblastoid cells with stable expression of pp65 or IE1 proteins: a tool to study the fine specificity of the antiviral response. J. Virol. 74:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto, F., D. Lenig, R. Förster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 35.Sandberg, J., N. Fast, K. Jordan, S. Furlan, J. Barbour, G. Fennelly, J. Dobroszycki, H. Spiegel, A. Wiznia, M. Rosenberg, and D. Nixon. 2003. HIV-specific CD8+ T cell function in children with vertically acquired HIV-1 infection is critically influenced by age and the state of the CD4+ T cell compartment. J. Immunol. 170:4403-4410. [DOI] [PubMed] [Google Scholar]
- 36.Saurwein-Teissl, M., T. Lung, F. Marx, C. Gschösser, E. Asch, I. Blasko, W. Parson, G. Böck, D. Schönitzer, E. Trannoy, and B. Grubeck-Loebenstein. 2002. Lack of antibody production following immunization in old age: association with CD8+ CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168:5893-5899. [DOI] [PubMed] [Google Scholar]
- 37.Seder, R., and R. Ahmed. 2003. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat. Immunol. 4:835-842. [DOI] [PubMed] [Google Scholar]
- 38.Solache, A., C. L. Morgan, A. I. Dodi, C. Morte, I. Scott, C. Baboonian, B. Zal, J. Goldman, J. E. Grundy, and J. A. Madrigal. 1999. Identification of three HLA-A*0201-restricted cytotoxic T cell epitopes in the cytomegalovirus protein pp65 that are conserved between eight strains of the virus. J. Immunol. 163:5512-5518. [PubMed] [Google Scholar]
- 39.Sylwester, A., B. Mitchell, J. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. Sleath, K. Grabstein, Hosken, F. Kern, J. Nelson, and L. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202:673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 41.Tsegaye, A., D. Wolday, S. Otto, B. Petros, T. Assefa, T. Alebachew, E. Hailu, F. Adugna, W. Measho, W. Dorigo, A. L. Fontanet, D. van Baarle, and F. Miedema. 2003. Immunophenotyping of blood lymphocytes at birth, during childhood, and during adulthood in HIV-1-uninfected Ethiopians. Clin. Immunol. 109:338-346. [DOI] [PubMed] [Google Scholar]
- 42.Utz, U., S. Koenig, J. E. Coligan, and W. E. Biddison. 1992. Presentation of three different viral peptides, HTLV-1 Tax, HCMV gB, and influenza virus M1, is determined by common structural features of the HLA-A2.1 molecule. J. Immunol. 149:214-221. [PubMed] [Google Scholar]
- 43.van Lier, R., I. ten Berge, and L. Gamadia. 2003. Human CD8+ T-cell differentiation in response to viruses. Nat. Rev. Immunol. 3:1-8. [DOI] [PubMed] [Google Scholar]
- 44.Walter, E., P. Greenberg, M. Gilbert, R. Finch, K. Watanabe, E. Thomas, and S. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogenic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 45.Weekes, M., A. Carmichael, M. Wills, K. Mynard, and J. Sissons. 1999. Human CD28− CD8+ T cells contain greatly expanded functional virus-specific memory CTL clones. J. Immunol. 162:7569-7577. [PubMed] [Google Scholar]
- 46.Weekes, M., M. Wills, K. Mynard, R. Hicks, J. Sissons, and A. Carmichael. 1999. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28−CD8+ T-cell population. Immunology 98:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weekes, M. P., M. R. Wills, K. Mynard, A. J. Carmichael, and P. J. G. Sissons. 1999. The memory cytotoxic T-lymphocyte (CTL) response to human cytomegalovirus infection contains individual peptide-specific CTL clones that have undergone extensive expansion in vivo. J. Virol. 73:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wikby, A., B. Johansson, J. Olsson, S. Lofgren, B. O. Nilsson, and F. Ferguson. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37:445-453. [DOI] [PubMed] [Google Scholar]
- 49.Wills, M. R., A. J. Carmichael, K. Mynard, X. Jin, M. P. Weekes, B. Plachter, and P. J. G. Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wills, M. R., G. Okecha, M. P. Weekes, M. K. Gandhi, P. J. Sissons, and A. J. Carmichael. 2002. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J. Immunol. 168:5455-5464. [DOI] [PubMed] [Google Scholar]