Summary
Because activation of Erk1/2 MAP kinase (MAPK) is critical for hippocampus-dependent memory, there is considerable interest in mechanisms for regulation of MAPK during memory formation. Here we report that MAPK and CREB-mediated transcription are negatively regulated by SCOP (SCN Circadian Oscillatory Protein) and that SCOP is proteolyzed by calpain when hippocampal neurons are stimulated by BDNF, KCl depolarization, or NMDA. Moreover, training for novel object memory decreases SCOP in the hippocampus. To determine if hippocampus-dependent memory is influenced by SCOP in vivo, we generated a transgenic mouse strain for the inducible overexpression of SCOP in the forebrain. Overexpression of SCOP completely blocked memory for novel objects. We conclude that degradation of SCOP by calpain contributes to activation of MAPK during memory formation.
Introduction
MAPK plays a critical role in several forms of neuroplasticity including memory formation (for reviews see Impey et al., 1999; Mazzucchelli and Brambilla, 2000; Frankland et al., 2000; Sweatt, 2004) and neuronal survival (for reviews see Hetman and Xia, 2000; Heerssen and Segal, 2002). Activation of MAPK may be required for memory consolidation because it mediates activity-dependent, Ca2+ stimulation of cAMP Response Element (CRE)-mediated transcription (Xing et al., 1996; Impey 1998a; Athos et al., 2002; Arthur et al., 2004) and dendritic translation (Kelleher et al., 2004). For example, MAPK is activated during training for hippocampus-dependent memory (Athos et al., 2002; Atkins et al., 1998; Blum et al., 1999; Sindreu et al.) and by stimulus paradigms that evoke long-term potentiation (LTP) (English and Sweatt, 1996; Impey et al., 1998). Inhibitors of MAPK signaling block memory formation (Athos et al., 2002; Atkins et al., 1998; Blum et al., 1999) and increases in CRE-mediated transcription which are required for contextual memory (Athos et al., 2002; Atkins et al., 1998; Blum et al., 1999). Gene disruption studies also support the hypothesis that MAPK activity is critical for synaptic plasticity and memory. Disruption of the gene for RasGRF, an activator of Ras, attenuates long-term memory (LTM) for cued fear conditioning and LTP in the basolateral amygdala (Brambilla et al., 1997). In addition, disruption of the Erk1 gene has implicated MAPK-dependent signaling in behavioral plasticity (Mazzucchelli et al., 2002). Consequently, it is important to understand how MAPK is activated in CNS neurons. Activity-induced increases in intracellular free Ca2+ can stimulate MAPK by several mechanisms including calmodulin stimulation of RasGRF (Farnsworth et al., 1995), or indirectly through stimulation of adenylyl cyclase (Wang and Storm, 2003), subsequent activation of PKA, and stimulation of Rap1 (de Rooij et al., 1998; Vossler et al., 1997).
Negative regulation of MAPK signaling may be just as important for memory as stimulatory mechanisms. Mice deficient in neurofibromatosis type 1, a RAS GTPase-activating protein, have defects in hippocampus-dependent spatial memory, suggesting that a balance between Ras stimulation and inhibition is critical for memory (Silva et al., 1997). Recently, it was discovered that suprachiasmatic nucleus circadian oscillatory protein (SCOP) (Shimizu et al., 1999) is a negative regulator of K-Ras in PC12 cells (Shimizu et al., 2003). SCOP interacts directly with K-Ras through its leucine-rich repeat and inhibits K-Ras function by associating with the nucleotide-free form of K-Ras. This prevents binding of GTP to K-Ras, inhibits its activity, and prevents neurotrophin stimulation of MAPK. In PC12 cells, SCOP overexpression inhibits depolarization stimulation of MAPK by blocking K-Ras activity (Shimizu et al., 2003). SCOP inhibition of K-Ras activity is especially interesting because the K-Ras/MEK/MAPK pathway is critical for synaptic and behavioral plasticity (Ohno et al., 2001).
Although SCOP attenuation of MAPK signaling has the potential to play an important role in neuroplasticity, mechanisms for regulation of SCOP have not been reported. Furthermore, it has not been established that SCOP regulates MAPK/CRE-mediated transcription in CNS neurons, a transcriptional pathway important for long-term memory (Athos et al., 2002; Atkins et al., 1998; Blum et al., 1999; Bourtchuladze et al., 1994; Pittenger et al., 2002) and neuronal survival (Hetman et al., 1999; Mabuchi et al., 2001; Watt et al., 2004). Here, we report that SCOP negatively regulates CRE-mediated transcription in hippocampal neurons and is rapidly degraded by calpain when neurons are stimulated with BDNF, NMDA or KCl-induced depolarization. Interestingly, SCOP is also rapidly degraded during training for hippocampus-dependent memory, and SCOP-overexpressing transgenic mice have no long-term memory for novel objects. These data suggest that SCOP constrains MAPK activity and that its degradation contributes to the transient activation of MAPK required for some forms of hippocampus-dependent memory.
Results
SCOP Expression in the Hippocampus
SCOP was originally discovered as a protein that undergoes a circadian oscillation in the SCN but not in other regions of brain (Shimizu et al., 1999). To explore the role of SCOP in regulation of MAPK signaling in the hippocampus, we examined its distribution in brain by immunohistochemistry (Figure 1). SCOP immunoreactivity was present predominantly in the somata and processes extending from somata in area CA1 of the rat and mouse hippocampus. SCOP is also expressed in area CA3, but not the dentate gyrus. The SCOP immunostaining in the hippocampus was completely blocked when the SCOP antibody was pre-absorbed with SCOP protein. Because of the importance of the hippocampus for memory formation, this study focused on the regulatory role of SCOP in hippocampal neurons in vitro and in vivo.
Figure 1. SCOP is Expressed in the Hippocampus.
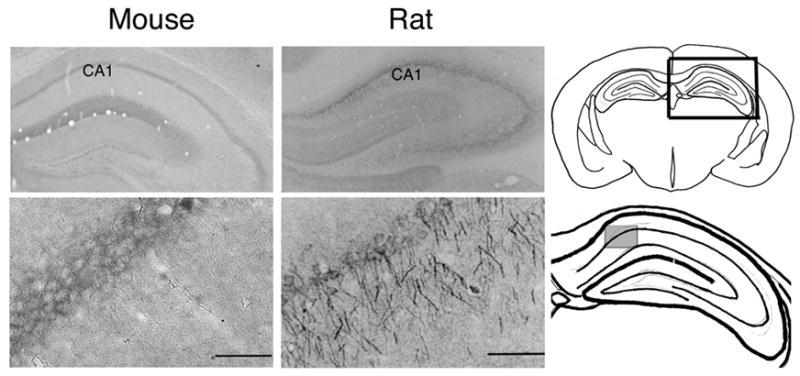
SCOP immunoreactivity was detectable in several areas of the rat and mouse hippocampus including area CA1. Coronal sections of the hippocampus were stained with SCOP antibody and visualized using 3-3’ diaminobenzidine as described in Experimental Procedures. The diagrams on the right side identify those areas of the hippocampus shown in the images. Scale bars represent 100 μm.
SCOP Inhibits CRE-Mediated Transcription in Cultured Hippocampal Neurons
One of the major MAPK-activated transcriptional pathways implicated in hippocampus-dependent memory is CRE-mediated transcription (Athos et al., 2002; Bourtchuladze et al., 1994; Impey et al., 1998; Xing et al., 1996). To determine if SCOP inhibits stimulation of this transcriptional pathway in hippocampal neurons, neurons were co-transfected with a reporter for CRE-mediated transcription with varying amounts of a SCOP expression vector (Figure 2A). BDNF stimulation of CRE-mediated transcription in hippocampal neurons was progressively inhibited by increasing amounts of SCOP, with almost complete inhibition at the highest levels of SCOP. This indicates that SCOP can function as a negative regulator of CRE-mediated transcription in hippocampal neurons.
Figure 2. CRE-Mediated Transcription in Hippocampal Neurons is Inhibited by SCOP.
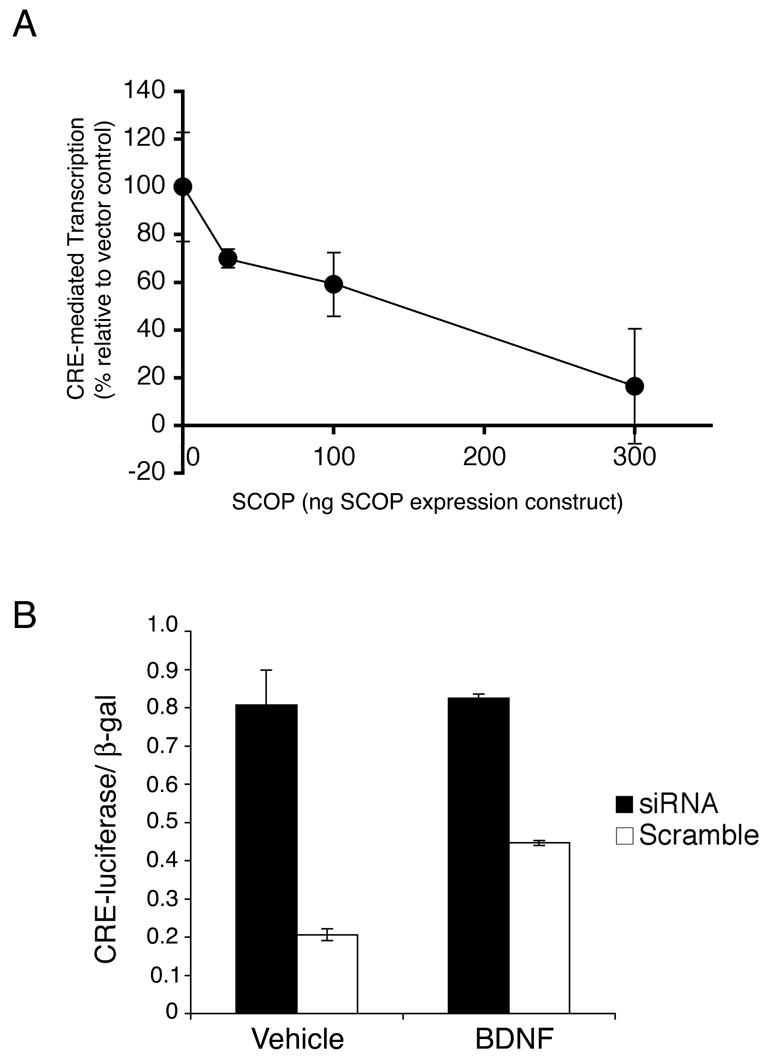
(A) SCOP overexpression inhibits BDNF stimulation of CRE-mediated transcription. Cultured hippocampal neurons were cotransfected with a CRE-luciferase reporter construct and varying amounts of a SCOP expression plasmid or empty vector (total 300 ng). An expression vector for β-galactosidase was cotransfected for normalization of transfection efficiencies. Luciferase activity was measured 6 hr after BDNF (5ng/ml) or vehicle treatment. Data are reported as the percent activity relative to the vector control. Error bars, SEM (n=3). (B) Inhibition of SCOP expression by siRNA stimulates CRE-mediated transcription. Cultured hippocampal neurons were cotransfected with a CRE-luciferase reporter construct with SCOP siRNA, or a scrambled RNA of the same composition. Luciferase activity was measured 24 hr after transfection and were normalized to β-galactosidase activity. Error bars, SEM (n=3).
If endogenous SCOP inhibits MAPK and CRE-mediated transcription, inhibition of SCOP expression should increase CRE-mediated transcription. To test this, hippocampal neurons were cotransfected with SCOP siRNA, or a scrambled RNA sequence of the same composition, and a reporter for CRE-mediated transcription. In unstimulated cells, SCOP siRNA increased CRE-mediated transcription four fold, a stimulation twice that obtained with BDNF alone (Figure 2B). These data indicate that endogenous SCOP constrains CRE-mediated transcription in hippocampal neurons. We confirmed that SCOP siRNA attenuates MAPK activation using a lenti-virus construct to express the SCOP siRNA. Lenti-virus expression of the SCOP siRNA depressed SCOP protein and activated pERK in unstimulated and BDNF-treated hippocampal neurons (Supplemental Fig. 1).
Treatment of Hippocampal Neurons with BDNF, NMDA or KCl Decreases SCOP Protein
Because SCOP inhibits MAPK signaling and blocks stimulation of CRE-mediated transcription, it was important to determine how SCOP itself is regulated in hippocampal neurons. Since we were unable to detect phosphorylation of SCOP (data not shown), we considered the possibility that SCOP may be degraded when neurons are stimulated with BDNF or other activators of MAPK. We monitored SCOP protein levels by Western analysis after hippocampal neurons were treated with BDNF. Within 5 min of BDNF treatment, SCOP protein was reduced by 50 % compared to controls and returned to unstimulated levels by 60 min (Figure 3A). We also found that depolarization of cultured hippocampal neurons with KCl or treatment with NMDA, reduced SCOP levels 52% and 44%, respectively (Figure 3B). Similar results were obtained when cultured cortical neurons were treated with BDNF; SCOP protein was rapidly degraded and returned to baseline within 3 hr (data not shown). The fact that SCOP protein was never totally degraded suggests that SCOP levels may be maintained by a balance between protein synthesis and proteolysis.
Figure 3. BDNF Stimulates Rapid Degradation of SCOP in Hippocampal Neurons.
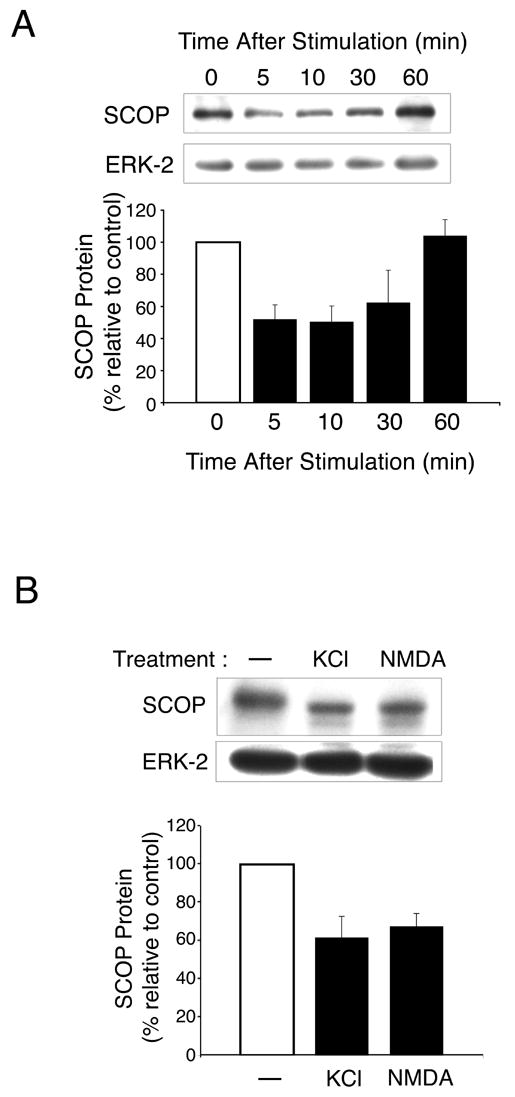
(A) SCOP protein in cultured hippocampal neurons (Day 18 in vitro: DIV 18) was monitored by Western analysis various times after treatment with BDNF (50ng/ml). The bar graph shows SCOP protein normalized to ERK-2. Error bars, SEM (n= 3) (B) SCOP protein in cultured hippocampal neurons (DIV18) was monitored by Western analysis 10 min after treatment with KCl (50mM) or NMDA (50μM). SCOP protein was normalized relative to ERK-2. Error bars, SEM (n=3).
SCOP is Proteolyzed by Calpain
The rapid reduction in SCOP protein when neurons are treated with BDNF, KCl, or NMDA suggests that it may be degraded by a protease. The fact that SCOP levels return to baseline relatively quickly suggest that transient activation of a protease stimulates SCOP degradation. We focused on Ca2+-stimulated proteases because activation of NMDA receptors (Dingledine, 1983), BDNF (Berninger et al., 1993; Li et al., 1998) and KCl depolarization (Di Virgilio et al., 1987) all cause transient increases in intracellular free Ca2+.
In cell-free, hippocampal extracts, SCOP was rapidly degraded in the presence of free Ca2+ but not in it absence, suggesting that SCOP is susceptible to degradation by a Ca2+-activated protease (Figure 4A). To identify the protease responsible for degradation of SCOP we examined inhibitors of several proteases including caspase-3 (Chan and Mattson, 1999; Dash et al., 2000) proteasome (Cline, 2003; Hegde, 2004; Lopez-Salon et al., 2001) and calpain (Chan and Mattson 1999). These proteases are present at synapses and have been implicated in neural plasticity and memory formation (For reviews see Cline, 2003; Chan and Mattson, 1999). Neither the caspase-3 inhibitor nor lactacystin, a proteasome inhibitor, blocked Ca2+-induced SCOP degradation (Figure 4B). Only calpeptin inhibited SCOP degradation, indicating that SCOP may be degraded by Ca2+-stimulated calpain. We confirmed that calpain is activated when hippocampal neurons are treated with BDNF (data not shown).
Figure 4. Calpain Catalyzes the Proteolysis of SCOP.
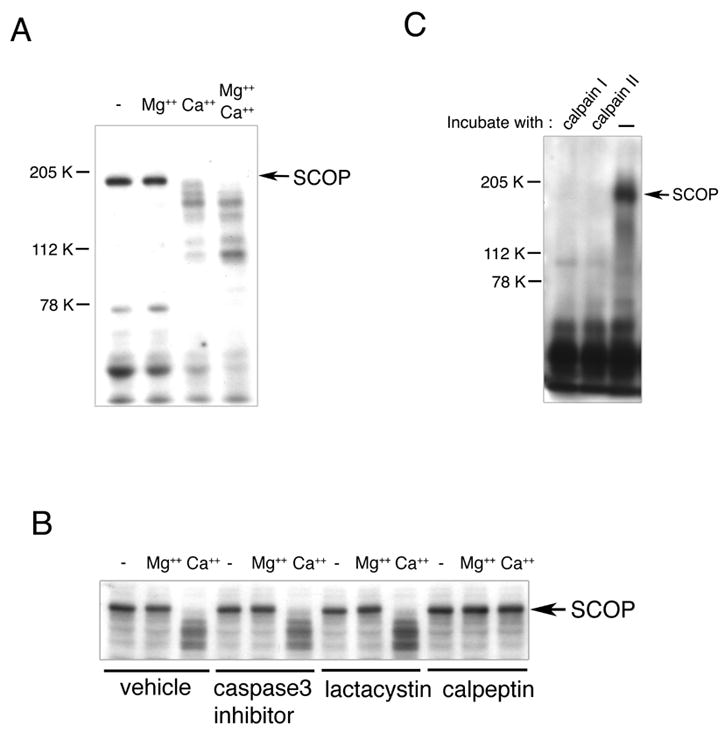
(A) SCOP protein was monitored by Western analysis after adding Mg2+ (10 mM) and/or Ca2+ (4 mM) to mouse brain lysates and incubating for three hours at 4°C. (B) Calpeptin inhibited Ca2+-stimulated proteolysis of SCOP. The mouse brain extract was pretreated with a caspase-3 inhibitor (10 μM), lactacystin (5 μM) or calpeptin (10 μM) 30 min before adding Ca2+. SCOP protein was monitored by Western analysis. (C) Calpain I and II both catalyzed the proteolysis of SCOP in vitro. Calpain I or calpain II was incubated with immunoprecipitated SCOP at 37 °C for one hr. SCOP protein was monitored by Western analysis.
There are two major calpain isoforms, calpain I (μ-form) and calpain II (m-form), which are activated at different Ca2+concentrations. Within the brain, calpain I is predominantly neuronal, existing at higher levels in the dendrites and cell bodies, whereas relatively higher levels of calpain II have been observed in axons and glia (Nixon, 1986; Onizuka et al., 1995). To determine if SCOP is selectively proteolyzed by calpain I or II, we incubated purified calpain I and II with immunoprecipitated SCOP. Both calpains degraded SCOP protein in vitro (Figure 4C).
To ascertain if calpain proteolyzes SCOP in hippocampal neurons, we treated neurons with calpain inhibitor III and monitored SCOP degradation when BDNF was applied (Figure 5A). The decrease in SCOP levels caused by BDNF was completely blocked by calpain inhibitor III and furthermore, there was a significant increase in SCOP levels after treatment with the protease inhibitor. This suggests that SCOP levels are controlled by a balance between synthesis of the protein and degradation by calpain. This was confirmed by examining SCOP levels when hippocampal neurons were treated with calpain inhibitor III, without BDNF (Figure 5B). Endogenous SCOP protein increases approximately two-fold by 90 min after treatment with the protease inhibitor. If SCOP negatively regulates MAPK signaling and calpain degrades SCOP, proteolytic degradation of SCOP may contribute to MAPK activation. Indeed, stimulation of MAPK activity in hippocampal neurons by BDNF was decreased at least two-fold when calpain activity was blocked (Figure 5C).
Figure 5. Inhibition of Calpain in Cultured Hippocampal Neurons Blocks SCOP Degradation and Inhibits Activation of MAPK.
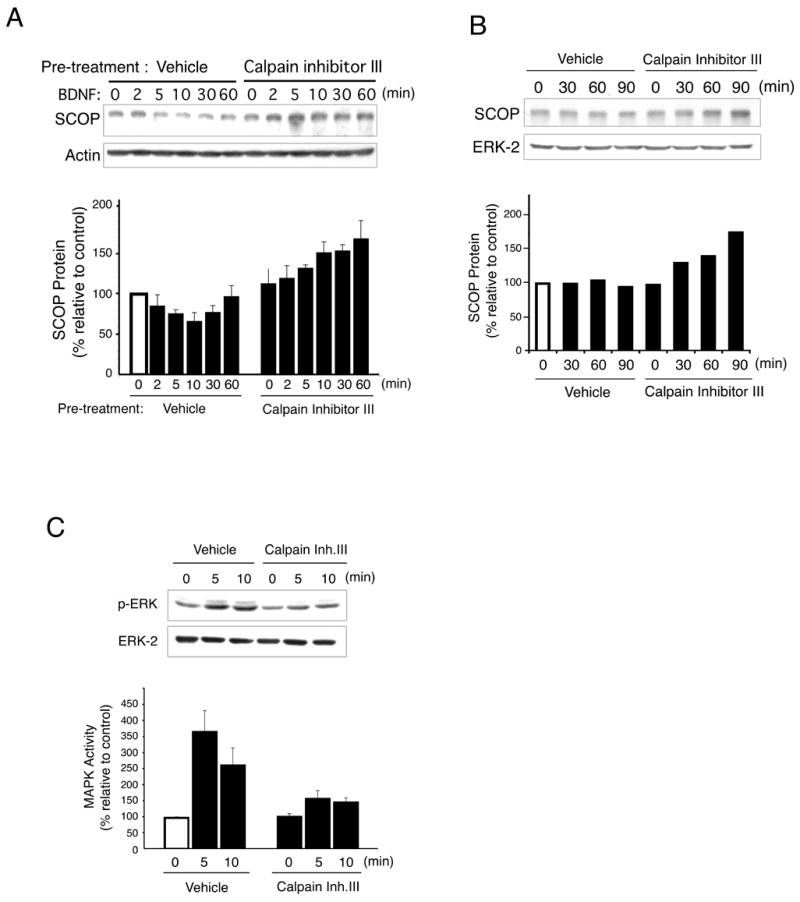
(A) Calpain inhibitor III inhibited BDNF-induced SCOP degradation in cultured hippocampal neurons. Neurons were pretreated with calpain inhibitor III (25μM) or vehicle 30 min before BDNF (5ng/ml) stimulation and harvested at various times. SCOP protein was monitored by Western analysis. SCOP protein was normalized to actin. Error bar, SEM (n=3). (B) Treatment of hippocampal neurons with calpain inhibitor III increases SCOP. Neurons were pretreated with calpain inhibitor III (25μM) or vehicle and harvested at various times after applying calpain inhibitor III (25μM) or vehicle. SCOP was quantitated by Western analysis. SCOP levels were normalized to ERK-2. (C) Calpain inhibitor III inhibits BDNF stimulation of MAPK activity measured as pERK in cultured hippocampal neurons. Total ERK-2 measured on the same Western blot was used as loading controls. The percentage change in pERK relative to the zero time point is reported. Error bars, SEM (n=3).
SCOP Degradation Following Training for Novel Object Memory
Since activation of MAPK is required for object recognition memory (Kelly et al., 2003) , it was of considerable interest to determine if the levels of SCOP protein decrease during training for object recognition memory. We measured SCOP 5 min after training for novel object recognition (Figure 6). In this paradigm, a mouse is allowed to briefly examine two novel objects, A and B. When the mouse is tested at a later time, it is presented with one of the original objects, A, and a new object C. If the mouse remembers object A, it will spend more time examining object C because of natural curiosity for new objects. Memory for novel objects is reflected by greater preference for the new object C during testing (Figure 6A). We examined SCOP levels following training for novel objects because this is a milder form of training that does not depend on shocking the animal. Untrained controls were handled and placed in the training cage without novel objects. Although the decrease in SCOP seen at 5 min after training for novel objects was only 16%, it was significant (p<.01), and was paralleled by an increase in MAPK activity (p<.05) (Figure 6B). SCOP protein also decreased when animals were trained for context (Supplemental Fig. 4). Presumably changes in SCOP and MAPK activity are much greater in neurons directly affected during training than that measured by Western analysis of extracts from the entire hippocampus.
Figure 6. Training for Novel Object Memory Decreases SCOP in the Hippocampus.
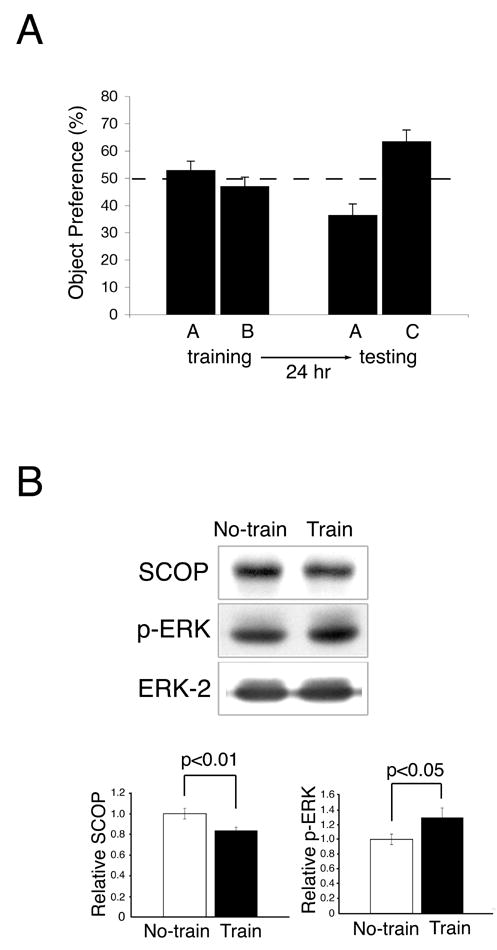
(A) The time spent exploring two novel objects (A and B) was measured for 5 min during training. Preference for one of the original objects (A) and a new object (C) was quantified 24 hr after training. The dotted line represents no object preference. Error bars, SEM (n=8 mice) (B) SCOP and pERK in the hippocampus from trained and untrained mice was analyzed by Western analysis 5 min after training. Untrained mice were handled and placed in the training chamber without novel objects. SCOP and p-ERK were normalized to ERK-2 from the Western blot. Error bars, SEM (n=9).
Overexpression of SCOP in the Hippocampus Blocks Memory for Novel Objects
To determine if hippocampus-dependent memory is sensitive to the amount of SCOP expressed in the hippocampus in vivo, we made a tetracycline-inducible, SCOP overexpressing mouse strain using the CamKIIα promoter to limit overexpression to the forebrain. The integration of transgene was verified by PCR based genotyping using specific primers for tetracycline response element- SCOP (TRE-SCOP) and CamKII-rtTA2 transgene, respectively (Figure 7A). Doxycycline treated TRE-SCOP x CamKll-rtTA2 mice showed 1.5 to 2.5-fold higher SCOP in the hippocampus than those not treated with doxycyline (Figure 7A, right panel of western blot and graph); doxycycline treated TRE-SCOP, or CamKll-rtTA2 mice showed no increase in SCOP compared to littermate controls. In control experiments, we verified that doxycycline treatment did not affect the memory of wild-type, TRE-SCOP, or CamKll-rtTA2 mice for novel objects (data not shown). Overexpression of SCOP did not alter open field activity or contextual memory indicating that general mobility and vision were unaffected (data not shown). Doxycyline treatment of TRE-SCOP x CamKll-rtTA2 mice, which increases SCOP in the hippocampus, had no effect on memory for novel objects measured 8 min after training (Figure 7B) but completely blocked memory for novel objects measured 24 hr after training (Figure 7C). These data are consistent with other experiments showing that MAPK activity is required for memory consolidation, but not acquisition or short-term memory (Blum et al., 1999; Kelleher et al., 2004). We confirmed that overexpression of SCOP in the transgenic mouse strain blocks decreases in SCOP protein and the increase in pERK associated with training for novel objects (Supplemental Fig. 2). When Doxycyline-treated mice were allowed to recover for 3 months, they exhibited normal 24 hr memory for novel objects (Figure 7D). Overexpression of SCOP did not completely block the increases in pERK observed during training for context (Supplemental Fig. 5) and did not block contextual memory. We conclude that memory for novel objects is sensitive to the concentration of SCOP in the hippocampus.
Figure 7. Overexpression of SCOP in the Hippocampus Blocks Long-Term but not Short-Term Memory for Novel Objects.
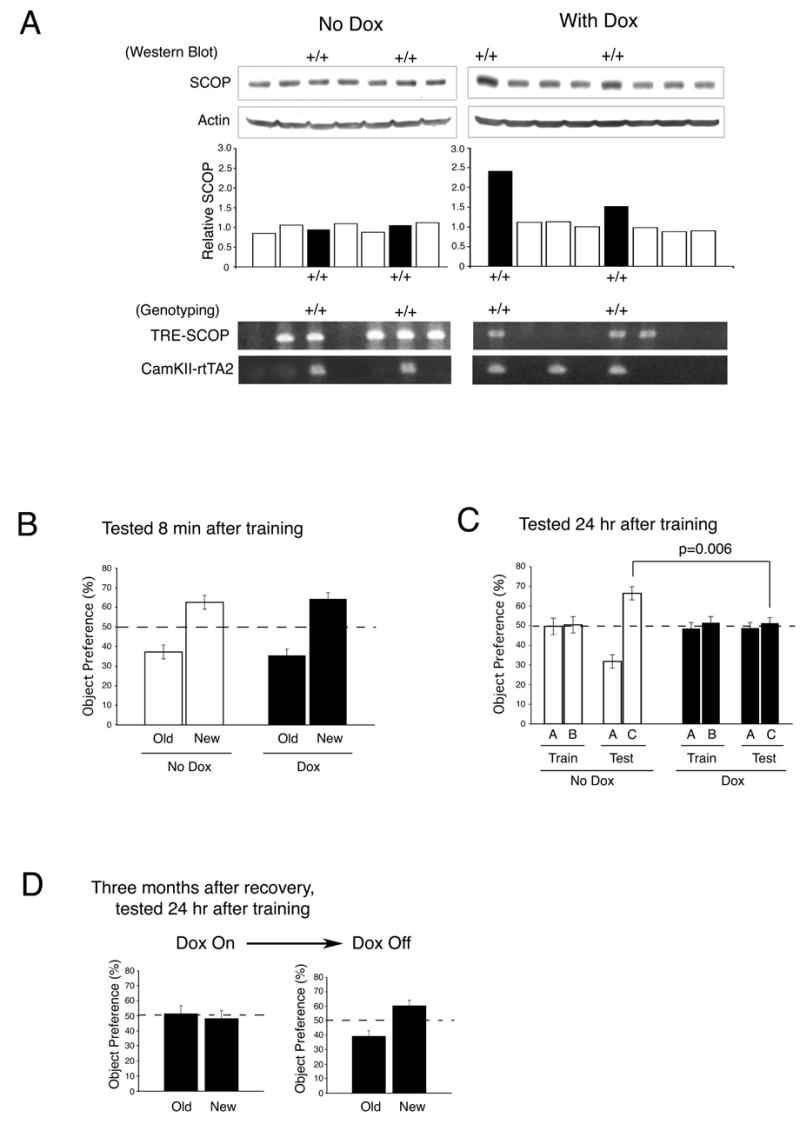
(A) SCOP in the hippocampus of TRE-SCOP x CamKII rtTA2 transgenic mice was increased by feeding doxycycline (Dox). Upper panels show the SCOP Western blot for each mouse and the bar graphs reports SCOP normalized to actin. Lower panels show the corresponding PCR genotyping of the transgenic mice. (B) Overexpression of SCOP (Dox) in the forebrain did not block short-term memory for novel objects measured 8 min after training. Error bars, SEM ( no DOX, n=8 mice; DOX , n=13). (C) Overexpression of SCOP in the forebrain blocked memory for novel objects measured 24 hr after training. Error bars, SEM (no Dox, n=12 mice; Dox, n=22 mice. (D) Doxycyline-treated mice were allowed to recover for three months after removal of doxycyline, trained for novel objects, and tested 24 hr after training. Error bars, SEM (n=8 mice).
The data described above indicates that memory for novel objects may depend on the levels of SCOP protein in the hippocampus. If calpain degradation of SCOP contributes to memory for novel objects, inhibition of calpain, in vivo, should block memory. Indeed, when a mixture of calpain inhibitors was bilaterally administered into area CA1 of the hippocampus, memory for novel objects measured 24 hr after training was completely blocked with no effect on short-term memory measured 8 min after training (Figure 8). This indicates that calpain activity is required for novel object long-term memory, possibly because calpain degrades SCOP in vivo. We confirmed that the decrease in SCOP and increase in pERK observed 5 min after training for novel objects is blocked by infusion of calpain inhibitors into are CA1 of the hippocampus (Supplemental Fig. 3).
Figure 8. Inhibition of Calpain in vivo Blocks Memory for Novel Objects.
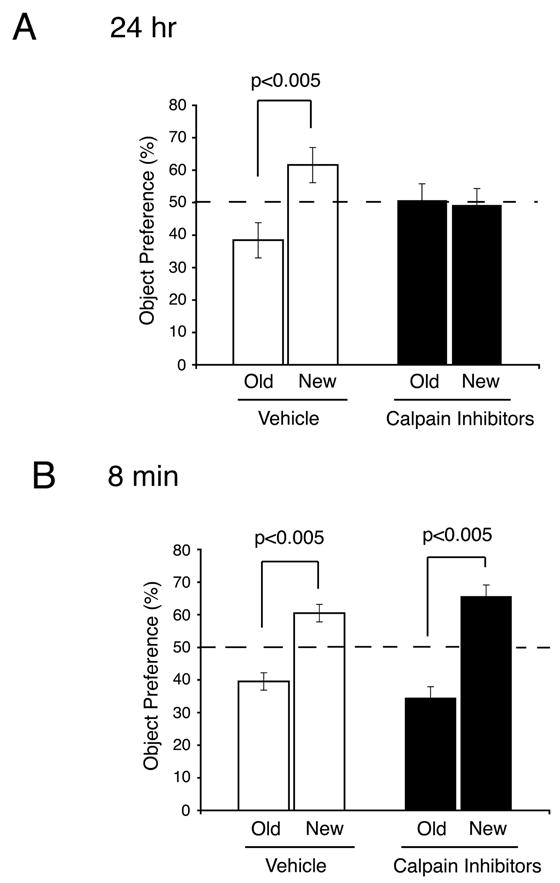
A calpain Inhibitor cocktail containing a mixture of calpeptin, calpain inhibitor I and calpain inhibitor III or vehicle was bilaterally infused in area CA1 in the hippocampus. Mice were trained 45 min after infusion. Object preference was measured 24 hr after training (A) or 8 min after training (B). Error bars, SEM (n=10 (24 hr), n=8 (8 min) mice in each group). The dotted line represents performance by chance 50%.
Discussion
SCOP was originally identified as a protein whose expression is regulated in a circadian manner within the SCN (Shimizu et al., 1999). The discovery that SCOP inhibits MAPK activity (Shimizu et al., 2003) provides an explanation for the circadian oscillation of MAPK activity and CRE-mediated transcription in the SCN (Obrietan et al., 1998). The presence of SCOP in the hippocampus raised the interesting possibility that it may inhibit MAPK in the hippocampus and play an important role in MAPK signaling during memory formation and other MAPK-regulated events. The objectives of this study were to determine if SCOP regulates CRE-mediated transcription in hippocampal neurons, and to identify mechanisms for signaling through SCOP. Furthermore, it was important to determine if SCOP is regulated during training for hippocampus-dependent memory in vivo and if varying the levels of SCOP in vivo affects memory formation.
We discovered that SCOP inhibits CRE-mediated transcription in hippocampal neurons and that treatment with BDNF, KCl, or NMDA causes rapid degradation of SCOP protein. The decrease in SCOP is dependent upon increased intracellular Ca2+ and blocked by calpain inhibitors, suggesting that activity-dependent increases in Ca2+ stimulate calpain which degrades SCOP. When cultured neurons are treated with calpain inhibitors there is a constant rise in SCOP protein suggesting that the levels of SCOP reflect a balance between newly translated protein and proteolysis. Furthermore, BDNF stimulation of MAPK activity in hippocampus neurons is greatly reduced by calpain inhibitors supporting the hypothesis that calpain-stimulated degradation of SCOP is necessary for full activation of MAPK in neurons.
We believe that SCOP may regulate MAPK signaling in vivo because SCOP decreased and MAPK activity increased during training for novel objects or contextual memory. The decrease in SCOP protein, stimulation of MAPK, and memory for novel objects were completely blocked by overexpressing SCOP in the hippocampus. When SCOP was over expressed, training for contextual fear still gave a measurable decrease in SCOP protein and an increase in MAPK activity. Since the MAPK activation is only partially blocked by SCOP overexpression during contextual training, the mice still exhibit memory for context. This suggests that the training stimulus during contextual training is more robust and leads to greater reduction in SCOP levels. We hypothesize that proteolytic degradation of SCOP releases a constraint on K-Ras activity and contributes to MAPK activation (Figure 9). This hypothesis is supported by data showing that memory for novel objects depends on calpain activity (Figure 8). Our data does not exclude other mechanisms by which calpain may contribute to activation of MAPK. Although calpain-1 knockout mice show no apparent defects in LTP or fear conditioning (Grammer et al., 2005), SCOP is degraded by both calpain-1 and calpain-2. Consequently, definition of the role of calpains in memory formation through gene disruption may require conditional double-knockouts of calpain-1 and calpain2.
Figure 9. Model for Role of SCOP in MAPK Activation.
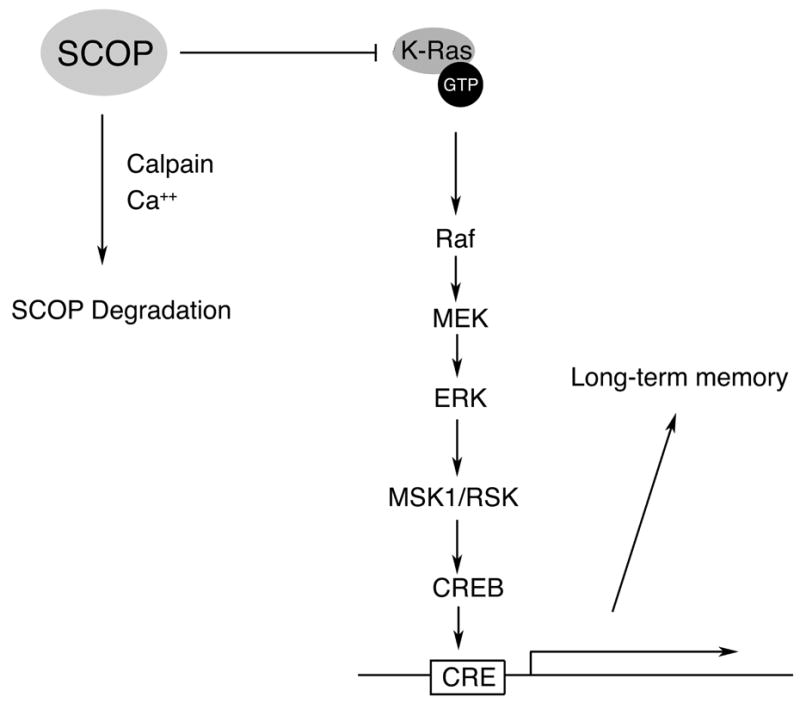
It is hypothesized that SCOP attenuates MAPK activity in hippocampal neurons by binding to the nucleotide-free form of K-Ras, thereby inhibiting stimulation of the MAPK pathway. Calapin may contribute to ERK activation by degrading SCOP.
Considering the general importance of MAPK for neuroplasticity, one would expect that its activity in CNS neurons would be tightly regulated (for a review see Agell et al., 2002). There are several mechanisms for activation of MAPK by Ca2+ including direct activation through calcium-stimulated Ras GEFs (Farnsworth et al., 1995) and indirect mechanisms mediated through calcium-stimulated adenylyl cyclases and PKA. We propose that in unstimulated neurons, SCOP restrains MAPK activity and that transient decreases in SCOP caused by Ca2+ stimulation of calpain is required for activation of MAPK caused by neurotrophins and Ca2+ signals. Thus, activation of MAPK activation in neurons may depend upon a dynamic balance between SCOP degradation and MAPK-activating mechanisms.
Although this study focused on regulation of SCOP in hippocampal neurons and its relationship to memory formation, the data have broad implications because of the central role played by MAPK and CRE-mediated transcription in neuroplasticity (Frankland et al., 2003; Impey et al., 1999; Mazzucchelli and Brambilla, 2000; Sweatt, 2004). It seems likely that other MAPK regulated events in CNS neurons including synaptic plasticity (Coogan et al., 1999; English and Sweatt, 1996), protein synthesis (Kelleher et al., 2004), circadian rhythm (Obrietan et al., 1999; Obrietan et al., 1998), modulation of K+ion channels (Yuan et al., 2002), and neuronal survival (Hetman et al., 1999), may depend upon SCOP protein and its regulated degradation. For example, the circadian oscillation of SCOP protein in the SCN correlates with the changes in MAPK activity during the circadian cycle (Shimizu et al., 1999). The extent to which regulation of SCOP levels contribute to these processes remains to be established.
In summary, maximal activation of MAPK activity in hippocampal neurons depends on degradation of SCOP by calpain. Training for novel object memory lowers SCOP in brain, and memory for novel objects was blocked when SCOP was increased two-fold in the hippocampus. We hypothesize that calpain-catalyzed degradation of SCOP in response to activity-dependent Ca2+ increases is required for activation of MAPK during formation of hippocampus-dependent memory.
Experimental Procedures
Cell Culture
Neurons were cultured as previously described with some modifications (Chan et al., 1998). Primary hippocampal neurons were cultured from newborn C57BL6 pups and maintained in Neurobasal-A (GIBCO) with minimal supplements. Pups were sacrificed by decapitation and the hippocampi were digested in 5 ml of 10 units/ml papain at 37°C for 30 min. After two rinses, the tissue was triturated three times in dissociation medium (27 mM K2SO4, 15 mM MgCl2, 74 mM Na2SO4, 18 mM glucose, 225 μM CaCl2, 0.0012% phenol red and 2 mM HEPES, pH 7.4) using a 5 ml disposable plastic pipette. Cells were plated into 24-well plates or 3 cm dishes coated with poly-D-lysine (50 μg/ml) at 5X104 cells/cm2 in Neurobasal-A medium containing B-27 supplement with 10 units/ml penicillin, 10 μg/ml streptomycin, 0.5 μg/ml glutamine and 1% Fetal Bovine Serum. The medium was changed to Neurobasal-A with B-27 containing 0.5 μg/ml glutamine and 2 μM AraC 24 hr after plating. Neurons were maintained in Neurobasal-A with B-27 and 0.5 μg/ml glutamine and half of the media was changed every two days. Plates were incubated in a CO2 incubator at 37°C.
Preparation of siRNA
The siRNA targeted against SCOP mRNA was designed with a two-base 3’-overhang. The target site was nuceotides 292–312 from the start codon (AACTCGCTGCTGCTGAGGAGA). The single strand siRNA nucleotides were chemically synthesized with dTdT 3’-overhangs (CUCGCUGCUGCUGAGGAGAtt, UCUCCUCAGCAGCAGCGAGtt )(Ambion). A scrambled sequence of the same nucleotide composition as the SCOP siRNA with no significant sequence homology to the genome was used as a negative control (UAUGCCGGCUGGAGUACCGtt, CGGUACUCCAGCCGGCAUAtt). Complementary pairs of siRNA oligonucleotides were annealed in annealing buffer (6 mM HEPES, pH 7.4, 20 mM potassium acetate, 0.4 mM magnesium acetate). The annealed double stranded RNAs were confirmed by acrylamide electrophoresis. For transfection of neurons, 16 nM of siRNA was used.
Expression Plasmids
The full-length SCOP overexpression plasmid and CRE (α168)-luciferase have been described previously (Matthews et al., 1994; Shimizu et al., 2003).
Reporter Assays for CRE-Mediated Transcription
Cultured hippocampal neurons in 24-well plates at day 7 in vitro were transfected with a complex of 0.15 μg of CRE-luciferase, 0.15 μg of the internal control plasmid EF1α-lacZ, 0.3 μg of the described plasmid (in each well of a 24-well plate) and Lipofectamine 2000 (Invitrogen) according to the manufacture’s protocol. Twenty-four hours after transfection, 5 ng/ml BDNF in 0.2% BSA or vehicle (0.2% BSA) was applied. Reporter assays were conducted 6 hr post-BDNF stimulation. Luciferase activity was measured as previously described (Matthews et al., 1994) and normalized to β-galactosidase activity (Impey et al., 1996).
Immunohistochemistry
Animals were deeply anesthetized with Ketamine/Xylazine cocktail and intracardially perfused with saline containing 0.1% NaNO2 and 2.0 mM EDTA. They were then continuously perfused with ice-cold 2% paraformaldehyde in PBS. Brains were removed, post-fixed for 3 hrs and dehydrated in 30% sucrose in PBS overnight. The dehydrated brains were frozen in dry ice and sliced into 25 μm thick sections using a microtome. Sections were washed three times with 0.5% tritonX-100 in PBS and incubated in 0.5% tritonX-100, 0.5% BSA and 1% normal goat serum for 30 min at 37°C. They were then incubated with anti-SCOP antibody (αEC) (1:50) overnight at 4°C (Shimizu et al., 1999). Immunoreactivity was detected by the conventional avidin-biotin complex method (Vectastain ABC Kit; Vector Lab) using 3-3’ diaminobenzidine as chromogen. Brain sections were mounted using DPX mountant (Fluka).
Immunoprecipitation of SCOP
Whole brains from adult male mice were homogenized in 10 fold excess (v/v) of buffer A (50 mM tris-HCl, pH.7.4, 50 mM NaCl, 1 mM EDTA). The resulting homogenate was centrifuged at 10,000 X g for 15 min. The supernatant was used for immunoprecipitation and pre-cleared on protein A agarose beads (Oncogene) at 4 °C for 2 hr incubation on a rotating wheel. The pre-cleared cell lysate was incubated overnight at 4°C with a C-terminal SCOP antibody (Shimizu et al., 1999). New protein agarose beads were added and incubated for 2 h at 4 °C. The beads were washed four times with buffer A.
Western Analysis
Mice were sacrificed by cervical dislocation and the hippocampi were rapidly dissected and immediately frozen in dry ice. Two hippocampi were sonicated for 10 sec in the sonication buffer (20 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 2 mM EDTA, 5 mM EGTA, 5 mM β-mercaptoethanol, 50 mM NaF, 1 mM Na3VO4, 1 mM phenylmethanesulfonyl fluoride, pH7.4) with a protease inhibitor cocktail (complete mini, EDTA-free, Roche) and phosphatase inhibitor cocktail I and II (Sigma). Neurons from three cm culture dishes were treated with 150 μl of 2X SDS-sample buffer and cells were scraped from the dish. The resulting homogenates were treated with SDS-sample buffer, boiled for 3 min and subjected to SDS-PAGE and Western analysis.
Housing and Handling of mice
Male C57BL6 mice at 8–11weeks of age were used. Food and water were provided ad libitum, and animals were kept on a 12 hr light/dark cycle. To reduce handling-induced stress, mice were housed individually and handled daily for one week prior to experiments.
Behavioral Assays
Context-trained mice were placed into a conditioning chamber (Solid State Shocker/ Distributor; Coulbourn Instruments, Allentown, Pennsylvania) and allowed to explore. After 2 min, they were shocked 1 times (0.7 mA, two sec). Mice were then allowed to recover for one min and returned to home cages. Unpaired controls were shocked once for 2 sec immediately after being placed in context. Novel object-trained mice were first habituated in a training rat cage for 3 to 4 hr before training. After which two different objects A and B were presented for 5 min after which they were returned to their home cage. They were tested in the training cage with a new object C and one of the original objects A or B. During training and testing the time spent examining each object was recorded for 5 min. Memory for familiar objects is manifested as a preference for the new object C during testing. Control mice were placed into the training cage with no novel objects.
Cannulation and Calpain inhibitor infusion
Mice (8 weeks old) were cannulated as described previously (Athos et al., 2002). Briefly, mice were anesthetized with an intraperitonial injection (20 μl/g body weight) of a mixture of ketamine (7.0 mg/ml) and xylazine (0.44 mg/ml) dissolved in bacteriostatic saline. Anesthetized mice were mounted on a stereotaxic frame (10 micron model, Cartesian Research), and 30-gauge dummy cannulae were implanted just above the pyramidal cell layer of CA1 region of dorsal hippocampus. Cannulae resided 1.5 mm lateral, 1.25 mm ventral and 1.5 mm posterior from bregma. The cannulae were affixed with dental acrylic and fitted with 24-gauge guide cannulae to maintain cannula position. Mice were housed individually and allowed at least one week of postoperative recovery before handling. Calpain inhibitor I, calpain inhibitor III and calpeptin were dissolved in DMSO and further diluted in 10% Cremophor EL (SIGMA) in saline so that the final concentrations of inhibitors were 2 mM, each. Drug and vehicle solutions had a final concentration of 18% DMSO and 8.2% Cremophor EL. One-half microliter of drug or vehicle was infused bilaterally into the cannulae through a 30-gauge injection needle 45 min before object recognition training. The final concentrations of the inhibitors in the hippocampus were 20 μM. The infusions were carried out at a rate of 0.25 μl/min using a motorized syringe pump (kdScientific).
Generation of tet-on SCOP over-expression mouse
Complementary DNA encoding rat SCOP was inserted into the multicloning site of pTRE2 (BD Biosciences) so that the expression of SCOP was placed under the control of Tet-responsive promoter (TRE). A TRE-SCOP-poly A cassette from the subcloned plasmid was injected (pronuclear) into C57BL6/C3H X C57BL6 embryos. The genotype was determined by PCR using transgene-specific primers. Transgenic mice were bred into the C57BL6 background for at least 6 generations. Manipulations were approved and in accordance with the animal care committee’s guidelines at University of Washington.
SCOP overexpression in the forebrain
CaMKIIα promoter-rtTA2 (CaM-rtTA2) mouse was generously provided by Dr. Isabelle Mansuy’s laboratory at the Swiss Federal Institute of Technology. CaM-rtTA2 mice were mated with TRE-SCOP mice to obtain double transgenic mice, which allowed SCOP overexpression in the forebrain to be mice controlled by tetracycline. Genotyping was determined by PCR. Control mice were littermates carrying no transgene or either one of the transgenes. Before testing, mice were fed doxycycline (SIGMA) at 6 mg per g food, as indicated. For all experiments, we used male mice 2–5 months old for behavioral studies.
Supplementary Material
Acknowledgments
We thank, Dr. Katsuya Nagai at Y.M.P. International Corporation, Dr. Masato Okada at the Research Institute for Microbial Diseases of Osaka University and Dr. Yoshitaka Fukada at University of Tokyo for laying the groundwork for SCOP protein. And we thank members of the Storm lab for critical reading of the manuscript. This research was supported by NIH grant NS 20498.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Berninger B, Garcia DE, Inagaki N, Hahnel C, Lindholm D. BDNF and NT-3 induce intracellular Ca2+ elevation in hippocampal neurones. Neuroreport. 1993;4:1303–1306. doi: 10.1097/00001756-199309150-00004. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Gnesutta N, Minichiello L, White G, Roylance AJ, Herron CE, Ramsey M, Wolfer DP, Cestari V, RossiArnaud C, et al. A role for the Ras signalling pathway in synaptic transmission and long-term memory. Nature. 1997;390:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci. 1998;18:5322–5332. doi: 10.1523/JNEUROSCI.18-14-05322.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Cline H. Synaptic plasticity: importance of proteasome-mediated protein turnover. Curr Biol. 2003;13:R514–516. doi: 10.1016/s0960-9822(03)00443-3. [DOI] [PubMed] [Google Scholar]
- Coogan AN, O'Leary DM, O'Connor JJ. P42/44 MAP kinase inhibitor PD98059 attenuates multiple forms of synaptic plasticity in rat dentate gyrus in vitro. J Neurophysiol. 1999;81:103–110. doi: 10.1152/jn.1999.81.1.103. [DOI] [PubMed] [Google Scholar]
- Dash PK, Blum S, Moore AN. Caspase activity plays an essential role in long-term memory. Neuroreport. 2000;11:2811–2816. doi: 10.1097/00001756-200008210-00040. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJT, Verheijen MHG, Cool RH, Hijman SMB, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide exchange factor directly activated by cAMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Milani D, Leon A, Meldolesi J, Pozzan T. Voltage-dependent activation and inactivation of calcium channels in PC12 cells. Correlation with neurotransmitter release. J Biol Chem. 1987;262:9189–9195. [PubMed] [Google Scholar]
- Dingledine R. Excitatory amino acids: modes of action on hippocampal pyramidal cells. Fed Proc. 1983;42:2881–2885. [PubMed] [Google Scholar]
- English JD, Sweatt DJ. Activation of p42 MAP kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ohno M, Takahashi E, Chen AR, Costa RM, Kushner SA, Silva AJ. Pharmacologically regulated induction of silent mutations (PRISM): combined pharmacological and genetic approaches for learning and memory. Neuroscientist. 2003;9:104–109. doi: 10.1177/1073858403252225. [DOI] [PubMed] [Google Scholar]
- Grammer M, Kuchay S, Baudry M. Lack of phenotype for LTP and fear conditioning learning in calpain 1 knock-out mice. Neurobiol Learning and Memory. 2005;84:222–227. doi: 10.1016/j.nlm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hegde AN. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog Neurobiol. 2004;73:311–357. doi: 10.1016/j.pneurobio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J Biol Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Impey S, Mark M, Villacres EC, Poser S, Chavkin C, Storm DR. Induction of CRE-mediated gene expression by stimuli that generate long-lasting LTP in area CA1 of the hippocampus. Neuron. 1996;16:973–982. doi: 10.1016/s0896-6273(00)80120-8. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–10240. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Kitagawa K, Kuwabara K, Takasawa K, Ohtsuki T, Xia Z, Storm D, Yanagihara T, Hori M, Matsumoto M. Phosphorylation of cAMP response element-binding protein in hippocampal neurons as a protective response after exposure to glutamate in vitro and ischemia in vivo. J Neurosci. 2001;21:9204–9213. doi: 10.1523/JNEUROSCI.21-23-09204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Fodrin degradation by calcium-activated neutral proteinase (CANP) in retinal ganglion cell neurons and optic glia: preferential localization of CANP activities in neurons. J Neurosci. 1986;6:1264–1271. doi: 10.1523/JNEUROSCI.06-05-01264.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR. Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem. 1999;274:17748–17756. doi: 10.1074/jbc.274.25.17748. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Ohno M, Frankland PW, Chen AP, Costa RM, Silva AJ. Inducible, pharmacogenetic approaches to the study of learning and memory. Nat Neurosci. 2001;4:1238–1243. doi: 10.1038/nn771. [DOI] [PubMed] [Google Scholar]
- Onizuka K, Kunimatsu M, Ozaki Y, Muramatsu K, Sasaki M, Nishino H. Distribution of mu-calpain proenzyme in the brain and other neural tissues in the rat. Brain Res. 1995;697:179–186. doi: 10.1016/0006-8993(95)00838-h. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Huang YY, Paletzki RF, Bourtchouladze R, Scanlin H, Vronskaya S, Kandel ER. Reversible inhibition of CREB/ATF transcription factors in region CA1 of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34:447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Okada M, Nagai K, Fukada Y. Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J Biol Chem. 2003;278:14920–14925. doi: 10.1074/jbc.M213214200. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Okada M, Takano A, Nagai K. SCOP a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Frankland PW, Marowitz Z, Friedman E, Lazlo G, Cioffi D, Jacks T, Bourtchuladze R. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat Genet. 1997;15:281–284. doi: 10.1038/ng0397-281. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Schiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. doi: 10.1016/j.neuron.2006.11.024. (in Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Storm DR. A Genetic increase in type I adenylyl cyclase in mouse brain enhances LTP and recognition memory. Nature Neuroscience Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Watt W, Sakano H, Lee Z, Reusch J, Trinh K, Storm D. Odorant stimulation enhances survival of olfactory sensory neurons via MAPK and CREB. Neuron. 2004;41:955–967. doi: 10.1016/s0896-6273(04)00075-3. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


