Abstract
Originally identified as an essential component of the herpes simplex virus immediate early (IE) gene enhancer complex, the transcriptional coactivator host cell factor-1 (HCF-1) has been implicated in a broad range of cellular regulatory circuits. The protein mediates activation through multiple interactions with transcriptional activators, coactivators, and chromatin remodeling complexes. However, the mechanisms involved in HCF-1-dependent transcriptional stimulation were undefined. By using a minimal HCF-1-dependent promoter and a model activator, the varicella zoster IE62 protein, it was determined that HCF-1 was not required for the assembly of the RNAPII basal complex, which depended solely on IE62 in conjunction with the cellular factor Sp1. In contrast, HCF-1 was required for recruitment of the histone methyltransferases Set1 and MLL1 (mixed-lineage leukemia 1), leading to histone H3K4 trimethylation and transcriptional activation. Similarly, in a varicella zoster virus lytic infection, HCF-1, Set1, and MLL1 were recruited to the viral genomic IE promoter, suggesting an essential role for HCF-1 in chromatin modification and remodeling during initiation of lytic infection. The results indicate that one biological rationale for the incorporation of the viral IE activators in the viral particle is to recruit HCF-1/histone methyltransferase complexes and promote assembly of the viral IE gene promoters into transcriptionally active chromatin. These studies also contribute to the model whereby the induced nuclear transport of HCF-1 in sensory neurons may be critical to the reactivation of latent herpesviruses by promoting the activation of chromatin modifications.
Keywords: chromatin, histone methyltransferase, chromatin modifications, Sp1, transcription
The cellular transcriptional coactivator host cell factor-1 (HCF-1) was originally isolated as a component of the herpes simplex virus (HSV) immediate early (IE) gene enhanceosome complex containing the cellular POU domain protein Oct-1 and the viral transactivator VP16 (1–6). The protein has been most thoroughly studied in this context where it mediates the VP16 transcriptional activation of the viral IE genes (7, 8). In an analogous manner, HCF-1 also mediates the induction of the related varicella zoster virus (VZV) IE genes by the viral transactivators ORF10 and IE62 (8). The transcription of these α-herpesvirus genes is regulated by multiple mechanisms and factors via complex combinatorial enhancer-promoter domains. Strikingly, HCF-1 has been shown to be essential for IE gene expression, suggesting that it mediates a common rate-limiting step. Most intriguingly, both HSV and VZV establish latency in the neurons of sensory ganglia. In these cells, HCF-1 is uniquely sequestered in the cytoplasm of sensory neurons and is rapidly transported to the nucleus upon stimulation that results in viral reactivation (9). Therefore, whereas the protein is essential for viral lytic replication, it may also be a key component in the α-herpesvirus latency reactivation cycle.
Since its identification as a coactivator for the herpesvirus activators, several lines of evidence have indicated that HCF-1 also plays a broadly significant role in cellular transcription. The protein (i) is a binding partner and/or coactivator for numerous cellular transcription factors including those of the krupple (Sp1 and Krox20), Ets (GABP), ATF/CRE (CREB3), and E2F (E2F1 and E2F4) families (10–16), (ii) interacts with other coactivators (PGC and FHL2) (17, 18) where it has been hypothesized to mediate coactivator–activator interactions, (iii) is essential for multiple stages of cell cycle progression (19, 20), and (iv) has recently been identified as a component of complexes involved in chromatin modification and remodeling (21–23). Furthermore, expression array studies have identified a wide range of target genes whose expression is affected in cells in which the nuclear accumulation of HCF-1 has been inhibited (24). However, although activator partners and target genes have been identified, the biochemical mechanism by which HCF-1 stimulates transcription is undefined.
In this study, a model promoter (VZV IE62 promoter) and activator (VZV IE62) were studied to determine the role of HCF-1 in recruitment of the general transcription factor complex and in mediating activating chromatin modifications. The results demonstrate that IE62, in conjunction with the ubiquitous factor Sp1, mediates the assembly of the initiation complex. Promoter occupancy of HCF-1 is not required for this assembly but is essential for transcriptional activation via recruitment of the H3K4 methyltransferases Set1 and MLL1 (mixed-lineage leukemia 1). Strikingly, Sp1 is required to bridge or stabilize the interaction of the IE62 activator with the HCF-1 transcriptional coactivator, suggesting that this protein may play a general role in mediating HCF-1-activator interactions. Finally, the recruitment of HCF-1 and the Set1/MLL1 histone methyltransferases (HMTs) to the viral IE promoter during the initiation of infection indicates that chromatin modification and remodeling represent critical stages in the activation of α-herpesvirus transcription. The data support the model in which HCF-1-dependent chromatin modulation would play a critical role in the regulation of the viral lytic and latency reactivation cycle.
Results
HCF-1-Dependent Coactivation of IE62 Target Genes.
Previously it was demonstrated that HCF-1 was an essential component required for the combinatorial regulation of the expression of the HSV and VZV α-herpesvirus IE genes by the respective viral transactivators VP16, ORF10, and IE62 (8). As illustrated in Fig. 1A, the VZV IE62 promoter contains enhancer core elements that assemble the multiprotein Oct-1/HCF-1/ORF10 complex as well as sites for costimulatory factors such as GABP and Sp1 (25). In addition, this promoter contains an IE62 binding site that responds to the viral transactivator (26, 27). As previously demonstrated, IE62 stimulated the expression of an IE62 promoter-reporter gene and this induction was severely compromised in HCF-1-depleted cells (Fig. 1B).
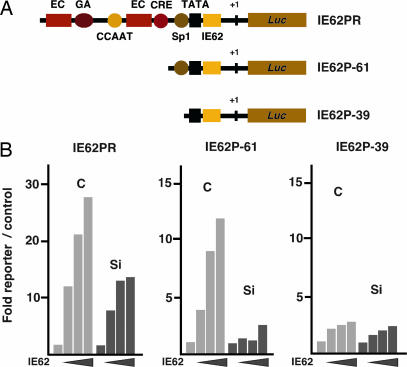
Fig. 1.
HCF-1-dependent coactivation of a minimal IE62 target gene. (A) The IE62 promoter–reporters are schematically illustrated, depicting the binding sites for various cellular and viral factors. EC represents the VZV IE enhancer core that assembles the Oct-1, ORF10, and HCF-1 multiprotein complex. GA, Sp1, CCAAT, CRE, and IE62 are the putative binding sites for GA-binding protein, Sp1, CCAAT-binding proteins, ATF/CRE factors, and VZV IE62, respectively. Luc, luciferase reporter gene coding region; +1, transcription initiation site. (B) HeLa cells were transfected with HCF-1 RNAi vector pU6-si-HCF-1 (Si) or the control RNAi pU6-si (C). The indicated reporter genes were transfected or cotransfected 48 h later with increasing amounts of pCMV-IE62 (0, 50, 100, and 200 ng). Reporter expression levels were normalized to the activity of the control vector (fold expression of reporter relative to control). The data are representative of three independent experiments. HCF-1 depletion was determined to be 70% by quantitative Western blot analysis, whereas no effect on the expression of IE62 in HCF-1-depleted cells was detected.
Similarly, an IE62 promoter-reporter construct containing only the IE62 binding site, a TATA element, and an adjacent Sp1 binding site was induced by IE62 in an HCF-1-dependent manner. In contrast, deletion of the TATA proximal Sp1 binding site abrogated IE62-mediated induction, thereby defining the minimal promoter requirements for IE62-mediated activation and HCF-1 coactivation. As shown in supporting information (SI) Fig. 8, the requirement for Sp1 for IE62 activation of the IEP-61 promoter may be explained by the lack of stable IE62 binding in the absence of the Sp1 binding site.
In addition to its autoregulatory function, IE62 also induces transcription of the early and late VZV gene classes (28). As shown in SI Fig. 9, promoter–reporter constructs representing early and late VZV gene classes were induced in the presence of IE62. In each case, depletion of HCF-1 resulted in a significant reduction in the IE62-dependent induction, indicating that the requirement for HCF-1 in IE62 transcriptional activation is a general one and is not restricted to the regulation of the IE genes.
IE62 Recruitment of the Coactivator HCF-1 to the IE62 Minimal Model Promoter.
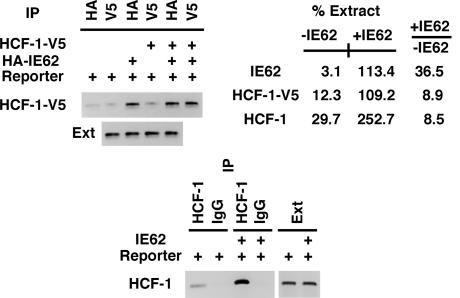
The HCF-1 dependence of IE62-mediated activation suggested that IE62 might directly recruit HCF-1 to the minimally responsive promoter target gene. Therefore, HCF-1 promoter occupancy was determined by ChIP assays in the presence and absence of IE62. As shown in Fig. 2, both cotransfected V5-tagged HCF-1 (Fig. 2 Upper) and endogenous HCF-1 (Fig. 2 Lower) were recruited to the IE62P-61 promoter in the presence of IE62. Although a low level of endogenous HCF-1 could be detected in the absence of IE62, this occupancy was strongly stimulated in the presence of IE62 (8.5-fold).
Fig. 2.
Recruitment of transfected and endogenous HCF-1 in the presence and absence of IE62. ChIP assays were done 48 h after transfection by using the indicated antibodies or control antibodies (HA, V5, and IgG). The signal intensities were quantitated as described in Materials and Methods and are expressed as a percentage of the extract signal. The data shown are derived from a single ChIP assay but are representative of at least two independent experiments. +IE62/−IE62 is the fold ratio of the intensities in the presence of IE62 to those in the absence of IE62. (Upper) HeLa cells were transfected with the IE62P-61 reporter plasmid (Reporter), the HA-tagged IE62 expression plasmid (HA-IE62), and/or the V5-tagged HCF-1 expression plasmid (HCF-1-V5) as indicated. (Lower) HeLa cells were transfected with the IE62P-61 reporter plasmid (Reporter) or cotransfected with the pCMV-IE62 expression plasmid. Ext, input extract before immunoprecipitation.
IE62-Mediated Assembly of the Basal Transcription Complex Is Not HCF-1-Dependent.
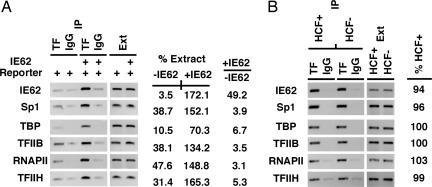
To investigate the biochemical mechanism(s) by which IE62/HCF-1 stimulates transcription, ChIP assays were done to assess the promoter occupancy of basal transcription factors in the presence and absence of IE62 or HCF-1. As shown in Fig. 3A, in the absence of IE62, low levels of Sp1 and the basal factors TBP, TFIIB, TFIIH, and RNAPII could be detected. However, a strong stimulation of promoter occupancy of all factors was found in the presence of IE62. In particular, stimulation of Sp1 occupancy in the presence of IE62 underscores the cooperative DNA binding suggested previously (SI Fig. 8) (29).
Fig. 3.
Assembly of the RNAPII complex by IE62-Sp1. Recruitment of Sp1 and factors of the RNAPII initiation complex in the presence and absence of IE62 or HCF-1. ChIP assays were done by using the indicated transcription factor (TF) antibodies or control IgG. The signal intensities are expressed as a percentage of the extract signal. Intensities and fold (+IE62/−IE62) were quantitated as described in Materials and Methods. The data shown are derived from a single ChIP assay but are representative of at least two independent experiments. (A) HeLa cells were transfected with the IE62P-61 reporter plasmid or cotransfected with pCMV-IE62. (B) HeLa cells were transfected with either the HCF-1 RNAi vector pU6-si-HCF-1 (HCF−) or the control RNAi pU6-si (HCF+). The IE62P-61 reporter was cotransfected with pCMV-IE62 48 h later. %HCF+ represents the ratio of signal intensity in the absence of HCF-1 to that in the presence of HCF-1. Ext, extract.
To determine whether HCF-1 was required for the assembly of the RNAPII transcription complex, occupancy was also assessed in HCF-1-positive and HCF-1-depleted cells (Fig. 3B). Strikingly, the depletion of HCF-1 had no effect on the recruitment of the RNAPII complex to the minimal promoter, indicating that the IE62-Sp1 activators were solely responsible for this assembled but transcriptionally inactive complex.
Transcriptional Induction by IE62 Results in HCF-1-Dependent Set1- and MLL1-Mediated Trimethylation of Histone H3K4.
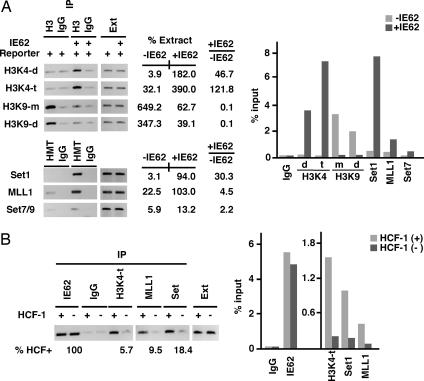
Recently, HCF-1 was identified as a component of the MLL family of HMTs (22, 23). As HCF-1 is critical for IE62-mediated transcriptional induction yet it is not required for recruitment/assembly of the RNAPII complex, the state of selected chromatin modifications was determined in HCF-1-positive and HCF-1-depleted cells (Fig. 4A). In the absence of IE62, the IE62 responsive promoter was marked by the repressive dimethyl-H3K9. However, in the presence of IE62, the activating trimethyl-H3K4 was significantly enhanced, whereas dimethyl-H3K9 was significantly reduced. In addition, IE62 specifically resulted in significant recruitment of the H3K4 HMTs Set1 and MLL1, relative to the HMT Set7/9. Interestingly, IE62-mediated occupancy by MLL1 increased only 4-fold. In contrast, IE62 resulted in a 30-fold increase in the promoter occupancy by Set1, suggesting that IE62 preferentially recruits this H3K4 HMT.
Fig. 4.
HCF-1-dependent Set1- and MLL1-mediated H3K4 trimethylation. Recruitment of HMTs and H3K4 trimethylation in the presence and absence of IE62 or HCF-1. ChIP assays were done by using the indicated antibodies or control IgG. The signal intensities are expressed as a percentage of the extract signal. Intensities and fold (+IE62/−IE62) were quantitated as described in Materials and Methods. The data shown are derived from a single ChIP assay but are representative of at least two independent ChIP assays. The results of the real-time PCR are graphed as a percentage of the input sample. m, d, and t represent mono-, di-, and trimethylation, respectively. (A) HeLa cells were transfected with the IE62P-61 reporter plasmid or cotransfected with pCMV-IE62. ChIP assays were done 48 h later by using the indicated modified histone, HMT, or control IgG. (B) HeLa cells were transfected with either the HCF-1 RNAi vector pU6-si-HCF-1 (HCF−) or the control RNAi pU6-si (HCF+). The reporter plasmid pGL3-IE62P-61 was cotransfected with pCMV-IE62 48 h later. %HCF+ represents the ratio of signal intensity in the absence of HCF-1 to that in the presence of HCF-1. Ext, extract.
Most significantly, in the absence of HCF-1 (Fig. 4B), the recruitment of Set1 and MLL1 and the resulting H3K4 trimethylation were all severely reduced (9.5%, 18.4%, and 5.7% of HCF-1+, respectively). This reduction was not due to reduced levels of the critical protein components in HCF-1-depleted cells as demonstrated by quantitative Western blot analysis (SI Fig. 10). Thus, recruitment of the coactivator HCF-1 results in Set1- and MLL1-dependent H3K4 trimethylation.
Sp1 Is Required to Bridge/Stabilize the IE62-HCF-1 Interaction.
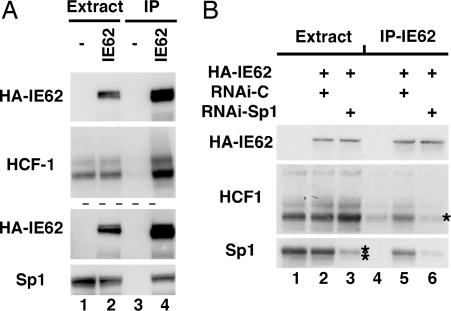
Sp1 is clearly an important component of the IE62-HCF-1-mediated transcriptional induction as shown by the removal of the TATA proximal Sp1 site, which abrogates stable IE62 binding (SI Fig. 8) and by the increased promoter occupancy by Sp1 in the presence of IE62 (Fig. 3). Interestingly, both IE62 and HCF-1 interact directly with Sp1 (12, 29), leading to the possibility that, in addition to cooperative DNA binding interactions with IE62, Sp1 may also function to mediate or stabilize the interaction of IE62 with HCF-1. As shown in Fig. 5A, both endogenous HCF-1 and Sp1 were coimmunoprecipitated with IE62 (lane 4). However, RNAi-mediated depletion of Sp1 reduced the coimmunoprecipitation of HCF-1 to background levels (Fig. 5B, compare lanes 4 and 5 with lane 6). Thus, Sp1 is required to bridge or stabilize the interaction of the activator IE62 with its coactivator HCF-1.
Fig. 5.
Sp1 is required to mediate IE62–HCF-1 interaction. HA-IE62 immunoprecipitates (IP-IE62) of HeLa cell extracts were subjected to Western blot analysis with HA (HA-IE62), HCF-1, and Sp1 antisera. (A) HeLa cells were transfected with the HA-IE62 expression plasmid (HA-IE62) or control vector (−). (B) HeLa cells were transfected with Sp1 RNAi (RNAi-Sp1) or control RNAi (RNAi-C) and subsequently retransfected with the HA-IE62 expression plasmid. *, HCF-1 background level (compare lanes 4 and 6); **, 78% depletion of Sp1 as determined by quantitative Western blot analysis.
Recruitment of HCF-1 and Chromatin Modifications at the VZV IE62 Promoter During VZV Lytic Infection.
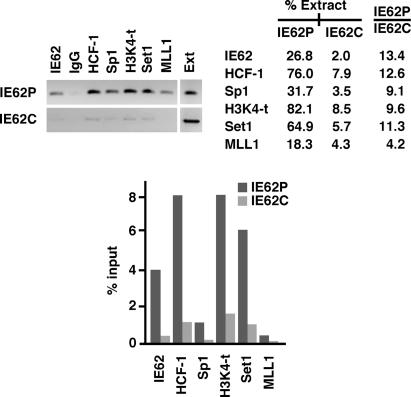
The data presented here using a model IE62-responsive promoter argue that HCF-1-mediated modifications would be represented at the viral IE promoter during the early stages of infection. Therefore, cells were infected with VZV, and 4 h after infection, the occupancy of the viral IE62 promoter by IE62, Sp1, HCF-1, Set1/MLL1 HMTs, and trimethyl-H3K4 was assessed. As shown in Fig. 6, results comparable with those obtained by using the transfected model promoter were found at the viral genomic IE promoter, including clear occupancy of IE62, Sp1, HCF-1, Set1, and MLL1. As anticipated, based on the HCF-1 and Set/MLL occupancy, the promoter exhibited a strong trimethyl-H3K4 signal. In contrast, the IE62 coding region did not reveal any significant HCF-1 occupancy or Set1/MLL1-mediated H3K4 trimethylation.
Fig. 6.
HCF-1–HMT occupancy and chromatin modifications at the viral IE62 promoter during VZV infection. BS-C-1 cells were infected with VZV as described. Four hours after infection, ChIP assays were done by using the indicated antisera or control IgG. The signal intensities of the PCR products are represented as percent extract for the promoter (IE62P) and control coding domains (IE62C). Intensities and fold (+IE62/−IE62) were quantitated as described in Materials and Methods. The data shown are derived from a single ChIP assay but are representative of at least two independent experiments. The results of the real-time PCR are graphed as a percentage of the input sample. H3K4-t, trimethyl-H3K4; Ext, extract.
The requirement of HCF-1 in mediating these modifications during a viral infection was similarly addressed by infection of a cell culture that had been partially depleted of HCF-1. In these cells, a 52% HCF-1 depletion resulted in similar decreases in HCF-1, Set1, MLL1, and trimethyl-H3K4 signals at the viral IE promoter, whereas no significant change in the promoter occupancy by the viral IE62 protein was found (data not shown). The results indicate that HCF-1-mediated chromatin modulation of the IE gene promoter domain is likely to play a critical role in the initiation of the viral infectious cycle.
Discussion
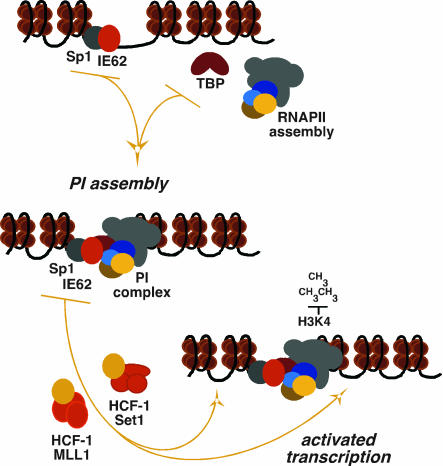
Numerous lines of evidence have indicated that HCF-1 is a cellular coactivator of broad significance. In this study, the VZV IE62 activator and an IE62-responsive model promoter were used to define the functional role of HCF-1 in transcriptional regulation. As shown in the model depicted in Fig. 7, HCF-1 was not required for the recruitment of the RNAPII basal factor complex, which depended on the presence of the viral IE62 activator in concert with the ubiquitous cellular activator Sp1. The ability of IE62 to mediate the formation of this complex is consistent with in vitro studies indicating that IE62 interacts directly with TBP and TFIIB (26) and enhances the binding of TBP/TFIID (29). Additionally, the requirement for Sp1 for stable IE62 promoter occupancy underscores the importance of factors such as Sp1, which has been observed in studies of IE62-mediated activation (30). However, despite the formation of this initiation complex, IE62-Sp1 was unable to stimulate transcription in the absence of HCF-1.
Fig. 7.
Model of HCF-1-dependent IE62-mediated transcriptional activation. The role of HCF-1 in IE62-mediated transcriptional activation is schematically represented. TBP, TATA binding protein; PI, preinitiation assembly.
Recruitment of the coactivator HCF-1 to the model promoter by the IE62-Sp1 activators results in Set1 and MLL1 HMT promoter occupancy, H3K4 trimethylation, and transcriptional activation. H3K4 trimethylation is a hallmark of transcriptional activation and probably serves as a signal for the recruitment of remodeling and activation components (31–35). Therefore, although HCF-1 may play additional roles, one primary function of this coactivator is to promote chromatin modifications characteristic of activated transcription. Interestingly, in the absence of the IE62 activator, the model promoter is marked with dimethyl-H3K9 modifications, a repressive mark that precludes H3K4 trimethylation. It remains unclear whether the recruitment of HCF-1/Set1/MLL1 prevents the accumulation of these marks or whether there are additional components, such as an H3K9 demethylase, that may also be required for HCF-1-mediated transcriptional activation. Recent studies have demonstrated the cooperative role of these enzymes in transcriptional activation by removal of repressive H3K9 methylation (36, 37). It would therefore be of interest to determine whether HCF-1 might coordinately recruit an H3K9 demethylase in conjunction with the Set1/MLL1 H3K4 HMTs.
In this study, the significance of the results obtained by using the model promoter and IE62 activator is shown by the recruitment of HCF-1 and the Set1/MLL1 HMTs to the genomic viral IE promoter during early infection. The data highlight the biological relevance of chromatin modulation for the initiation of a herpesvirus infection and complement the study of Huang et al. (38), which implicated Set1 as an important regulatory component of the HSV viral lytic cycle. For both HSV and VZV, the IE transactivators are packaged in the virus and released upon initial infection. The requirement for these activators and their ability to recruit the HCF-1/HMT complexes to the IE promoters may be necessary to prevent or circumvent the assembly of these regions into repressive chromatin.
Most significantly, α-herpesviruses establish latency in sensory neurons. In these cells, the coactivator HCF-1 is uniquely sequestered in the cytoplasm of the cell but is rapidly relocalized to the nucleus upon stimuli that result in viral reactivation. In the model, the viral transactivators are not present during latency, and the relocalized HCF-1 is recruited to the viral IE promoters by alternative transcription factors that recognize the complex promoter–enhancer domains (9). The data presented here enrich this model by suggesting that recruitment of HCF-1/HMT complexes to the latent viral IE promoters would promote chromatin remodeling and viral IE gene expression. In this respect, the ability of Sp1 to bridge or stabilize the interaction of HCF-1 with the IE62 activator places new emphasis on this factor whose cooperative interactions with numerous transcription factors may be important to a more general targeting/recruitment of HCF-1.
Materials and Methods
Cell Culture and Virus.
HeLa and BS-C-1 cells were maintained according to standard procedures. VZV (Ellen) was obtained from American Type Culture Collection (Manassas, VA). VZV viral infections were done by overlaying cell-associated virus on naive BS-C-1 cells. Cells were harvested for ChIP assays 4 h after infection.
Reporter Assays.
Luciferase reporter genes contained ORF29, ORF28, and gI VZV promoter sequences cloned into pGL3-basic (SI Fig. 9). IE62P-61 and IE62P-39 were derived from IE62PR that contained IE62 promoter sequences from −269 to +73. pCMV-IE62 expresses the IE62 activator under control of the CMV IE promoter and has been previously described (8). For all reporter assays, 4 × 104 HeLa cells were transfected with 300 ng of pU6-Si-HCF-1 or pU6-Si control RNAi using FuGENE (Roche Diagnostics Corporation) according to the manufacturer's recommendations. At 48 h after transfection, the cells were cotransfected with the reporter constructs and increasing amounts of pCMV-IE62, or control vector. The reporter gene activity was measured 24 h later by using a Dual-Luciferase assay kit (Promega, Madison, WI) in a luminometer (Berthold, Bad Wildbad, Germany). All activity units were normalized by protein concentration and by the activity of the control vector. The data presented are representative of three independent experiments.
ChIP Assays.
Unless otherwise indicated, 1.3 × 106 HeLa cells were transfected with 1 μg of the appropriate reporter plasmids (IE62P-61 or IE62P-39) or cotransfected with 2 μg of the IE62 expression plasmid (pCMV-IE62). For HCF-1 recruitment, 2 μg of HA-tagged IE62 (pCMV-HA-IE62) and 4 μg of V5-tagged HCF-1 (pHCF-1-V5) (18) were cotransfected with the IE62P-61 reporter. For depletion of HCF-1, cells were transfected with 3 μg of pU6-HCF-RNAi or pU6-control RNAi and were retransfected with reporter and IE62 expression plasmids 48 h later. ChIP assays were done essentially as previously described (39–41). Cell lysates were sonicated to obtain DNA fragments ranging from 300 to 700 bp. Chromatin from 7 × 106 cells was used for each immunoprecipitation with the following antibodies: IE62 (29); HCF-1 (2); IgG, RNAPII, dimethyl-H3K4, trimethyl-H3K4, monomethyl-H3K9, and dimethyl-H3K9 (catalog nos. 12-370, 05-952, 07-030, 05-745, 07-450, and 07-441, respectively; Upstate Biotechnology, Lake Placid, NY); Sp1, TBP, TFIIB, TFIIH, and HA (catalog nos. SC-59, SC-273, SC-274, SC-6857/6859, and SC-805, respectively; Santa Cruz Biotechnology, Santa Cruz, CA); MLL1 and Set1 (catalog nos. 1408/1289 and 1193, respectively; Bethyl Laboratories, Montgomery, TX); and Set7/9 (catalog no. 13731; Abcam, Cambridge, MA). Immunoprecipitates were washed and eluted, and the cross-linking was reversed. Recovered DNA was subjected to standard PCR with dilutions of input DNA to ensure linearity, resolved in ethidium bromide agarose gels, and the signal intensities were quantitated by using a 4000MM Image Station (Kodak, Rochester, NY). The signal intensities of individual bands were calculated as a percentage of the intensity of the input extract after subtraction of the appropriate background antibody controls. Where indicated, samples were also subjected, in triplicate, to real-time PCR using SYBR Green (Qiagen, Valencia, CA) on a Prism 7900 system (Applied Biosystems, Foster City, CA) and analyzed with SDS 2.2.2 software. The data presented are the quantity means. All ChIP data shown are derived from a single ChIP assay but are representative of at least two independent experiments. The location and sequence of primer sets used are shown in SI Fig. 11.
Coimmunoprecipitations.
HeLa cells (1.3 × 106) were transfected with 5 μg of pHA-IE62 expression plasmid or control vector DNA. For depletion of Sp1, 5 μg of pU6-Sp1 vector (Panomics, Redwood City, CA) or control RNAi was transfected on day 1 and retransfected on day 2 with 2.5 μg of pU6-Sp1 or control and 48 h later pHA-IE62 expression vector. Extracts were made 48 h later as described in ref. 18, sonicated briefly, and clarified by centrifugation. Protein extract was incubated for 2 h at 4°C with HA-Sepharose beads, washed five times with binding buffer, eluted in SDS sample buffer, and resolved in 4–20% Tris-glycine gels. Western blot analyses of resolved extracts and immunoprecipitates were done with the indicated antibodies (HA, HCF-1 AB2131, and Sp1), developed for chemiluminescence by using Super Signal Dura (Pierce, Rockford, IL), and quantitated by using a Kodak 4000MM Image Station.
Supplementary Material
Acknowledgments
We thank J. Vogel, Yu Liang G. Kolb, H. Peng, and A. McBride for discussions and advice; J. Vogel, T. Pierson, P. Sharp, and B. Moss for critical reading of the manuscript; and members of the Laboratory of Viral Diseases for helpful discussions. This study was supported by the Laboratory of Viral Diseases and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (T.M.K.) and by National Institute of Allergy and Infectious Diseases Grant AI18449 (to W.T.R.).
Abbreviations
- HCF-1
host cell factor-1
- HSV
herpes simplex virus
- IE
immediate early
- VZV
varicella zoster virus
- HMT
histone methyltransferase.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704351104/DC1.
References
- 1.Kristie TM. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A, Campadielli-Fiume G, Roizman B, Whitley R, Yamanishi K, editors. Cambridge, UK: Cambridge Univ Press; 2006. pp. 395–452. [PubMed] [Google Scholar]
- 2.Kristie TM, Pomerantz JL, Twomey TC, Parent SA, Sharp PA. J Biol Chem. 1995;270:4387–4394. doi: 10.1074/jbc.270.9.4387. [DOI] [PubMed] [Google Scholar]
- 3.Kristie TM, Sharp PA. J Biol Chem. 1993;268:6525–6534. [PubMed] [Google Scholar]
- 4.Wilson AC, Cleary MA, Lai JS, LaMarco K, Peterson MG, Herr W. Cold Spring Harb Symp Quant Biol. 1993;58:167–178. doi: 10.1101/sqb.1993.058.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AC, LaMarco K, Peterson MG, Herr W. Cell. 1993;74:115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]
- 6.Wysocka J, Herr W. Trends Biochem Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 7.Luciano RL, Wilson AC. Proc Natl Acad Sci USA. 2002;99:13403–13408. doi: 10.1073/pnas.202200399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan A, Nogueira ML, Ruyechan WT, Kristie TM. J Biol Chem. 2005;280:1369–1375. doi: 10.1074/jbc.M410178200. [DOI] [PubMed] [Google Scholar]
- 9.Kristie TM, Vogel JL, Sears AE. Proc Natl Acad Sci USA. 1999;96:1229–1233. doi: 10.1073/pnas.96.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delehouzee S, Yoshikawa T, Sawa C, Sawada J, Ito T, Omori M, Wada T, Yamaguchi Y, Kabe Y, Handa H. Genes Cells. 2005;10:717–731. doi: 10.1111/j.1365-2443.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- 11.Freiman RN, Herr W. Genes Dev. 1997;11:3122–3127. doi: 10.1101/gad.11.23.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunther M, Laithier M, Brison O. Mol Cell Biochem. 2000;210:131–142. doi: 10.1023/a:1007177623283. [DOI] [PubMed] [Google Scholar]
- 13.Knez J, Piluso D, Bilan P, Capone JP. Mol Cell Biochem. 2006;288:79–90. doi: 10.1007/s11010-006-9122-x. [DOI] [PubMed] [Google Scholar]
- 14.Lu R, Yang P, Padmakumar S, Misra V. J Virol. 1998;72:6291–6297. doi: 10.1128/jvi.72.8.6291-6297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luciano RL, Wilson AC. J Biol Chem. 2003;278:51116–51124. doi: 10.1074/jbc.M303470200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel JL, Kristie TM. EMBO J. 2000;19:683–690. doi: 10.1093/emboj/19.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 18.Vogel JL, Kristie TM. Proc Natl Acad Sci USA. 2006;103:6817–6822. doi: 10.1073/pnas.0602109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goto H, Motomura S, Wilson AC, Freiman RN, Nakabeppu Y, Fukushima K, Fujishima M, Herr W, Nishimoto T. Genes Dev. 1997;11:726–737. doi: 10.1101/gad.11.6.726. [DOI] [PubMed] [Google Scholar]
- 20.Julien E, Herr W. EMBO J. 2003;22:2360–2369. doi: 10.1093/emboj/cdg242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guelman S, Suganuma T, Florens L, Swanson SK, Kiesecker CL, Kusch T, Anderson S, Yates JR, III, Washburn MP, Abmayr SM, et al. Mol Cell Biol. 2006;26:871–882. doi: 10.1128/MCB.26.3.871-882.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wysocka J, Myers MP, Laherty CD, Eisenman RN, Herr W. Genes Dev. 2003;17:896–911. doi: 10.1101/gad.252103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khurana B, Kristie TM. J Biol Chem. 2004;279:33673–33683. doi: 10.1074/jbc.M401255200. [DOI] [PubMed] [Google Scholar]
- 25.Moriuchi H, Moriuchi M, Cohen JI. J Virol. 1995;69:4693–4701. doi: 10.1128/jvi.69.8.4693-4701.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera LP. J Biol Chem. 2000;275:487–496. doi: 10.1074/jbc.275.1.487. [DOI] [PubMed] [Google Scholar]
- 27.Perera LP, Mosca JD, Sadeghi-Zadeh M, Ruyechan WT, Hay J. Virology. 1992;191:346–354. doi: 10.1016/0042-6822(92)90197-w. [DOI] [PubMed] [Google Scholar]
- 28.Ruyechan WT, Peng H, Yang M, Hay J. J Med Virol. 2003;70(Suppl 1):S90–S94. doi: 10.1002/jmv.10328. [DOI] [PubMed] [Google Scholar]
- 29.Peng H, He H, Hay J, Ruyechan WT. J Biol Chem. 2003;278:38068–38075. doi: 10.1074/jbc.M302259200. [DOI] [PubMed] [Google Scholar]
- 30.Ruyechan WT. In: Alpha Herpesviruses. Sandri-Goldin RM, editor. Norfolk, VA: Caister Academic; 2006. pp. 1–20. [Google Scholar]
- 31.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, et al. Nat Genet. 2007;39:311–318. doi: 10.1038/ng1966. [DOI] [PubMed] [Google Scholar]
- 32.Kouzarides T. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Ruthenburg AJ, Allis CD, Wysocka J. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Sims RJ, III, Chen CF, Santos-Rosa H, Kouzarides T, Patel SS, Reinberg D. J Biol Chem. 2005;280:41789–41792. doi: 10.1074/jbc.C500395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, et al. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- 36.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 37.Wissmann M, Yin N, Muller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Gunther T, Buettner R, et al. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Kent JR, Placek B, Whelan KA, Hollow CM, Zeng PY, Fraser NW, Berger SL. J Virol. 2006;80:5740–5746. doi: 10.1128/JVI.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee TI, Johnstone SE, Young RA. Nat Protocols. 2006;1:729–748. doi: 10.1038/nprot.2006.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oberley MJ, Tsao J, Yau P, Farnham PJ. Methods Enzymol. 2004;376:315–334. doi: 10.1016/S0076-6879(03)76021-2. [DOI] [PubMed] [Google Scholar]
- 41.Wells J, Farnham PJ. Methods. 2002;26:48–56. doi: 10.1016/S1046-2023(02)00007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.