Abstract
Immunotherapy targeting the amyloid β (Aβ) peptide is a novel therapy under investigation for the treatment of Alzheimer's disease (AD). A clinical trial using Aβ1–42 (AN1792) as the immunogen was halted as a result of development of meningoencephalitis in a small number of patients. The cytokine TGF-β1 is a key modulator of immune responses that is increased in the brain in AD. We show here that local overexpression of TGF-β1 in the brain increases both meningeal and parenchymal T lymphocyte number. Furthermore, TGF-β1 overexpression in a mouse model for AD [amyloid precursor protein (APP) mice] leads to development of additional T cell infiltrates when mice were immunized at a young but not old age with AN1792. Notably, only mice overproducing both Aβ (APP mice) and TGF-β1 experienced a rise in T lymphocyte number after immunization. One-third of infiltrating T cells were CD4 positive. We did not observe significant differences in B lymphocyte numbers in any of the genotypes or treatment groups. These results demonstrate that TGF-β1 overproduction in the brain can promote T cell infiltration, in particular after Aβ1–42 immunization. Likewise, levels of TGF-β1 or other immune factors in brains of AD patients may influence the response to Aβ1–42 immunization.
Keywords: Aβ peptide, Alzheimer's disease, immunity, immunotherapy, inflammation, lymphocyte, neuropathology, T cell, vaccination
Introduction
Patients with Alzheimer's disease (AD) have prominent neurodegeneration hypothesized to be caused by increased levels of the amyloid β (Aβ) peptide in the brain. Aβ is a neurotoxic peptide generated from cleavage of the amyloid precursor protein (APP), and immunotherapy against Aβ is a promising treatment for AD (Bayer et al., 2005; Gilman et al., 2005). Administration of Aβ1–42 peptide in conjunction with Freund's adjuvant resulted in decreased formation of Aβ plaques in young APP transgenic mice and reduced AD-related neuropathology in older mice (Schenk et al., 1999). This was confirmed in several other APP transgenic mouse models and correlated with improved cognitive function (for review, see Schenk, 2002). A phase I trial demonstrated that immunotherapy with Aβ1–42 (AN1792) was well tolerated (Bayer et al., 2005). However, a phase II trial of AN1792 was discontinued because of symptoms of meningoencephalitis in 18 of 300 (6%) treated patients (Orgogozo et al., 2003). Many patients that developed symptoms had generated anti-Aβ titers, but symptom severity did not correlate with titer levels. CSF testing demonstrated a predominantly mononuclear cell meningitis, and brain pathology from two patients treated with AN1792 who had experienced encephalitis revealed prominent perivascular infiltrates that contained T lymphocytes (Nicoll et al., 2003; Ferrer et al., 2004). Neuropathology from a third patient, who was asymptomatic after immunization, demonstrated less prominent perivascular T lymphocyte infiltrates (Masliah et al., 2005), so the degree of T cell infiltration may correlate with meningoencephalitis symptoms.
TGF-β1 is a multifunctional cytokine that is a major regulator of the immune response. It can exert either anti-inflammatory or pro-inflammatory effects in a context-dependent manner. In T cell-mediated diseases, including experimental autoimmune encephalitis, systemic TGF-β1 inhibits disease, whereas increased TGF-β1 at the site of antigen presentation exacerbates disease (Wyss-Coray et al., 1997; Luethviksson and Gunnlaugsdottir, 2003). This is consistent with the chemotactic effects of TGF-β1 on T lymphocytes (Adams et al., 1991).
To determine whether TGF-β1 plays a role in the recruitment of T lymphocytes to the brain after treatment with AN1792, we immunized APP transgenic mice that overexpress TGF-β1 chronically from astrocytes and studied T lymphocyte recruitment into the brain. We found that chronic TGF-β1 overexpression increased the number of CD3-positive (CD3 +) T lymphocytes in the brains of both TGF-β1 and doubly transgenic APP/TGF-β1 transgenic mice. This number was elevated several-fold more in young APP/TGF-β1 mice treated with AN1792. In contrast to this, AN1792 treatment did not increase the number of T cells in the brains of mice carrying the APP or TGF-β1 transgenes alone. We conclude that local TGF-β1 overexpression in the brain results in additional T cell infiltration after Aβ1–42 immunization of APP mice. APP/TGF-β1 mice may thus be useful to better understand the infiltration of T lymphocytes into the brains of the subset of patients who exhibited symptoms after treatment with AN1792.
Materials and Methods
Mice.
TGF-β1 mice contain a constitutively active mutant of porcine TGF-β1 under control of a GFAP promoter (lines 64 and 115; low and medium expressor mice, respectively) (Wyss-Coray et al., 2000). Higher levels of TGF-β1 result in the development of communicating hydrocephalus; the lines used here do not develop this complication. Human APP mice (J20 line) contain a PDGF–APP transgene and develop robust AD-like neuropathology (Mucke et al., 2000). All transgenic lines are maintained on an inbred, C57BL/6J genetic background. Twelve-month-old C57BL/6J–APP mice exhibit extensive amyloid plaques, whereas C57BL/6J–APP/TGF-β1 mice have less overall amyloid and amyloid deposits tend to be vascular in location (Wyss-Coray et al., 2001). To construct the B6:SJL F2 cross for immunization studies, APP and TGF-β1 (line 115) transgenic mice were crossed with SJL/J mice to produce C57BL/6J:SJL/J F1 transgenic mice. F1 mice were used to produce the four experimental genotypes, APP, APP/TGF-β1, TGF-β1, and wild type. SJL/J and C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal care and use was in accordance with institutional guidelines and approved by the Palo Alto Veterans Administration Committee on Animal Research. Mice were perfused with heparinized saline, and one hemibrain was postfixed in 4% paraformaldehyde (PFA) and then cryoprotected in 30% sucrose. The other hemibrain was snap frozen on dry ice.
Immunization.
In the first experiment, B6SJL–APP, APP/TGF-β1, TGF-β1, or wild-type mice were immunized with 75 μg of AN1792 plus 25 μg of QS21 adjuvant (AN1792) or with QS21 alone from 2–3 months of age monthly for 12 months. The first injection was intraperitoneal, and all subsequent ones were subcutaneous. Blood was sampled from tail veins, and anti-Aβ antibody titers were determined by ELISA (Schenk et al., 1999). After nine injections, mice that did not generate a titer were removed regardless of genotype. Final n in the analysis was n = 4–7 for APP and APP/TGF-β1 genotypes and n = 1–7 for wild-type and TGF-β1 mice (only one group, the adjuvant-treated wild-type mice, had only one mouse at the end of the experiment). There were no significant differences in the number of deaths or acquisition of Aβ antibody titers in any group or between genotypes. In the second experiment, 15- to 18-month-old B6SJL–APP, APP/TGF-β1, TGF-β1, or wild-type mice (n = 3–16 per group) were immunized subcutaneously every other week for three doses and then monthly for a total of 6 months.
Immunohistochemistry.
Most immunohistochemistry was performed using standard techniques on PFA-fixed 40 μm sagittal brain sections. Primary antibodies were against β-dystroglycan (1:100; Novocastra, Newcastle, UK), CD3 (1:1000; BD Biosciences, San Jose, CA), CD68 (1:50; DakoCytomation, High Wycombe, UK), B220 (1:500; BD Biosciences), CD4 (1:100; BD Biosciences), and ionized calcium binding associated protein-1 (Iba1) (1:2500; Wako Bioproducts, Richmond, VA). 3D6 antibody against Aβ was used at 1:1000. Sodium citrate antigen retrieval was used for B220 staining. Tyramide amplification (Invitrogen, Carlsbad, CA) was required to visualize CD3 for florescent double stains. CD8 (1:20; BD Biosciences) immunostaining was performed on 10 μm frozen sections postfixed in methanol.
To quantify lymphocytes, we counted immunopositive cells in hippocampus-containing sagittal brain sections. We counted every cell except for cells in the cerebellum and olfactory bulb, which were excluded because they were not present on every section. In TGF-β1 mice, we quantified T lymphocytes (CD3 + cells) in two randomly selected sections per mouse. For both immunization experiments, sections were collected sequentially, and all quantifications were performed on three sections per mouse spaced 640 μm apart. All mice in the first immunization experiment were analyzed for CD3 + and B220 + cells (T and B lymphocytes). CD4 immunostaining (helper T cells) was performed on APP and APP/TGF-β1 mice from all three treatment groups. For the final immunization experiment, CD3 immunostaining was performed on all mice.
Quantification of the percentage area covered by Iba1 immunostaining was performed on sections from adjuvant and AN1792-treated APP and APP/TGF-β1 mice in the first immunization experiment, three per mouse. MetaMorph software (Molecular Devices, Sunnyvale, CA) was used to analyze images, and the results of all three were averaged.
Statistical analysis.
All results were analyzed by an investigator blinded to genotype and treatment group. Statistical analysis was performed using Statview 5.0 software (SAS Institute, Cary, NC) for Macintosh (Apple Computers, Cupertino, CA). A p value of ≤0.05 was considered significant. Unpaired Student's t tests were used to determine whether the results were significantly different between mouse groups. ANOVA, followed by Fisher's PLSD, was used to compare means between groups.
Results
Chronic TGF-β1 overexpression increases the number of meningeal and parenchymal T lymphocytes
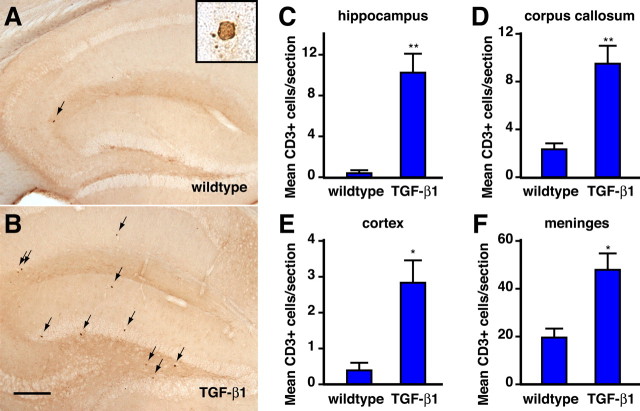
TGF-β1 is a potent modulator of immune responses, can increase immune cell infiltration in experimental autoimmune encephalitis (Wyss-Coray et al., 1997), and is increased in the brain in AD. To determine whether chronic TGF-β1 overproduction affects the baseline number of T lymphocytes in the brain, we performed immunohistochemistry for CD3, a pan T cell marker, on sagittal brain sections from 6-month-old GFAP–TGF-β1 transgenic mice (line T64). These mice secrete constitutively active TGF-β1 from astrocytes (Wyss-Coray et al., 1995) and have levels of TGF-β1 mRNA that are approximately twofold higher than wild type. On immediate inspection, there appeared to be more T cells in the TGF-β1 mice than in their nontransgenic littermates (Fig. 1 A, B). We quantified the positive cells and found that wild type mouse brains contained very few T lymphocytes, with the largest number seen in the meninges (Fig. 1). In contrast, TGF-β1 mice had higher numbers of T lymphocytes, ranging from twice normal in the meninges to a 25-fold increase in the hippocampus. We did not observe perivascular T lymphocyte infiltrates. Thus, chronic TGF-β1 overexpression increases the number of T lymphocytes in the brain, and it affects the parenchymal pool more dramatically than the meningeal pool of cells.
Figure 1.
T lymphocytes are increased in brains of TGF-β1 mice. Six-month-old TGF-β1 transgenic (n = 7) and nontransgenic littermate control (n = 5) mice were analyzed for the number of T lymphocytes using immunohistochemistry on 40 μm sagittal vibratome sections. A, B, Immunohistochemistry for CD3 + lymphocytes in the hippocampus of wild-type and TGF-β1 mice. Scale bar, 100 μm. C–F, Average number of T cells in two sections per mouse in hippocampus, corpus callosum, cortex, and meninges. Error bars indicate mean ± SEM. *p ≤ 0.05, **p ≤ 0.005, unpaired Student's t test.
Local TGF-β1 overexpression causes brain T lymphocyte number to increase after treatment with Aβ1–42 in APP mice
We next examined the effect of chronic TGF-β1 overexpression on the response of the brain to immunization with Aβ1–42. Two- to 3-month-old APP, APP/TGF-β1, TGF-β1, and wild type mice on a B6SJL F2 genetic background were immunized for 1 year. Mice received AN1792, QS21 adjuvant, or no treatment. Mice were weighed every 2 weeks and weights did not differ significantly in any group of mice, nor were there obvious clinical signs of encephalitis in the AN1792-treated mice.
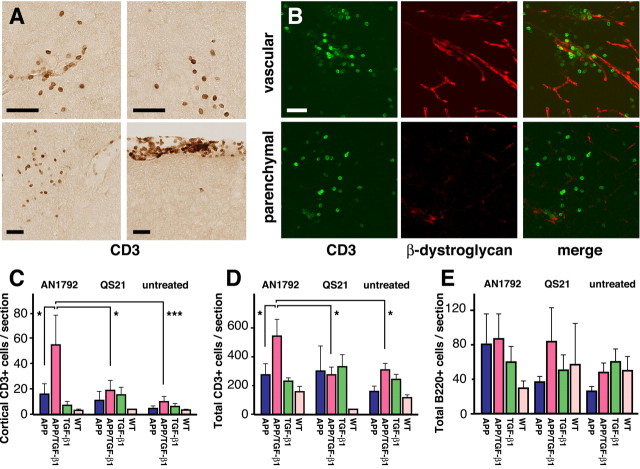
After the animals were killed, immunohistochemistry for the pan T cell marker CD3 demonstrated significant T lymphocyte infiltrates in some of the AN1792-treated doubly transgenic APP/TGF-β1 mice (Fig. 2 A, B). Infiltrates were observed in all brain regions and were both parenchymal and perivascular. The most dramatic effects were observed in the cortex (Fig. 2C), but AN1792-treated APP/TGF-β1 mice also had significantly more total brain T cell numbers than adjuvant-treated or untreated mice (Fig. 2 D). AN1792 treatment did not affect the number of T cells in the brains of APP mice, and T cell number did not correlate with serum anti-Aβ antibody titers. There were no significant differences in the number of B lymphocytes between treatment groups or genotypes (Fig. 2 E). Thus, chronic TGF-β1 overexpression in APP mice leads to increased brain T lymphocyte number that is further increased after AN1792 treatment.
Figure 2.
Active immunotherapy of APP/TGF-β1 mice with Aβ1–42 increased the number of T lymphocytes in the brain. A, Immunohistochemistry for the T cell marker CD3 on brain sections from an APP/TGF-β1 mouse treated with AN1792/QS21. Top row, Cortical infiltrates; bottom row, hippocampal and meningeal infiltrates. B, Examples of vascular (top) and parenchymal (bottom) infiltrates in an APP/TGF-β1 mouse treated with AN1792/QS21. β-Dystroglycan labels blood vessels. C, D, Mean number of CD3 + cells in cortex and total per sagittal section from APP, APP/TGF-β1, TGF-β1, and wild-type mice treated with AN1792, adjuvant alone (QS21), or untreated. E, B cells per sagittal section in the same mice as C and D. Graphs show means ± SEM. *p ≤ 0.05, ***p ≤ 0.001, Fishers PSLD. Scale bars, 25 μm.
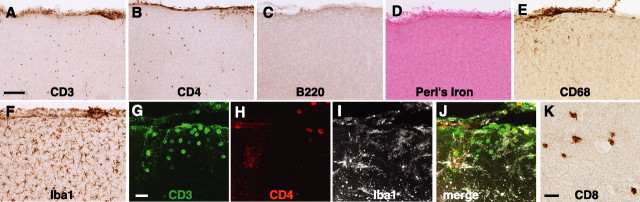
There were wide variations in the amount of amyloid deposition in untreated APP mice, and this study was not powered to determine the percentage of β-amyloid clearance after treatment with Aβ1–42. Because vascular amyloid deposition has been hypothesized to be linked to microhemorrhages (Pfeifer et al., 2002), we determined whether microhemorrhages played a role in increasing brain T cell numbers in immunized mice. We examined the three actively immunized APP/TGF-β1 mice that had the highest number of cortical T lymphocytes. T cell infiltrates were not associated with Aβ deposits, B lymphocytes, or with microhemorrhages (Fig. 3A–D). T cell infiltrates were associated with activated microglia (Fig. 3 A, E–J). Indeed, increased immunostaining for the microglial marker Iba1 correlated tightly with hippocampal T cell number in immunized APP and APP/TGF-β1 mice (p = 0.04, r2 = 0.918 for APP; p = 0.0370, r2 = 0.703 for APP/TGF-β1). There was no correlation between CD3+ cell number and Iba1 immunostaining in untreated or adjuvant-treated controls.
Figure 3.
T cell infiltrates in Aβ1–42-immunized APP/TGF-β1 mice contained helper and cytotoxic T cells and were associated with activated microglia but not with B lymphocytes or hemorrhages. Sagittal brain sections from AN1792-treated APP/TGF-β1 mice are shown. A–C, Immunohistochemistry for the pan-T cell marker CD3, the helper T cell marker CD4, and the B cell marker B220. D, Perl's iron stain for hemorrhages, counterstained with nuclear fast red. E, F, Staining for CD68, which labels activated microglia, and Iba1, which labels all microglia. G–J, Triple immunolabeling of a cortical infiltrate containing CD3 + T lymphocytes (green), some of which colabel with the helper T cell marker CD4 (red). The microglial marker Iba1 (white) reveals activated microglia. K, Immunostaining for the cytotoxic T cell marker CD8. Scale bars: A, 50 μm; G, K, 25 μm.
We quantified the number of helper T lymphocytes in APP and APP/TGF-β1 mice using CD4 immunostaining and found that approximately one-third of CD3 + T cells were CD4 + in both APP and APP/TGF-β1 mice, regardless of treatment (quantification data not shown; immunohistochemistry, Fig. 3 A, B, G–J). Immunostaining also revealed many T lymphocytes that stained for CD8, a cytotoxic T cell marker (Fig. 3H).
Although aged APP/TGF-β1 mice continued to have the highest numbers of T cells, AN1792 treatment did not increase brain T cell number
An additional experiment was performed in old mice. We began immunization at 15–17 months of age, after the development of parenchymal and vascular amyloid deposits, and treated mice for 6 months. We did not observe any differences in weight or neurological abnormalities in any genotype or treatment group. Mice who received AN1792 generated similar anti-Aβ antibody titers to those mice in the first experiment. When analyzed for T lymphocytes, we again observed the highest number of T lymphocytes in the brains of doubly transgenic APP/TGF-β1 mice (data not shown). However, there was no difference in T cell number between treatment groups, and, in fact, the total number of brain T lymphocytes in treated mice was similar to that seen in untreated mice. Thus, although old mice could generate an antibody response, we did not see evidence of additional T cell infiltration in the adjuvant or AN1792 immunized groups.
Discussion
Our studies show that local overexpression of TGF-β1 increases both meningeal and parenchymal T lymphocyte number. In addition, TGF-β1 overexpression in APP mice caused them to develop additional T cell infiltrates when they were immunized at young but not old age with AN1792. Notably, only mice that had local Aβ overproduction in addition to TGF-β1 overexpression experienced a statistically significant rise in T lymphocyte number after immunization. Excess brain T cells do not appear to make the TGF-β1 mice grossly ill, and even the immunized mice with the highest number of T lymphocytes in their brains did not experience weight loss or appear ill during our experiments.
TGF-β1 has pleiotropic effects on T lymphocytes, which together probably explain its site-specific effects during T cell-mediated diseases (Luethviksson and Gunnlaugsdottir, 2003). In the periphery, TGF-β1 inhibits proliferation of immature T cells. At the inflammatory site, TGF-β1 is chemotactic for T lymphocytes (Adams et al., 1991) and can polarize the initial response toward pro-inflammatory, Th1 helper T cells and away from less inflammatory Th2 cells (Smeltz et al., 2005). In addition, activated T cells are not as responsive to inhibitory signals from TGF-β1 (Cottrez and Groux, 2001). Accordingly, animal models for the T cell-mediated autoimmune diseases rheumatoid arthritis and multiple sclerosis have been shown to be exacerbated by local TGF-β1 expression and ameliorated by systemic increases in TGF-β1 (Wyss-Coray et al., 1997; Luethviksson and Gunnlaugsdottir, 2003). In our model, we did not see any overt harmful effects from TGF-β1-induced T lymphocyte infiltration.
In AN1792-immunized mice, we observed that the presence of both APP and TGF-β1 transgenes increased brain T lymphocyte number. T lymphocytes can migrate into the CNS, and CNS-specific T cells can be retained and undergo tolerance induction in the CNS (Engelhardt and Ransohoff, 2005). In APP/TGF-β1 mice, the presence of antigen in the brain (Aβ in this case) may recruit antigen-specific T cells to that site. Adjuvant choice and genetic background have also been shown to influence T lymphocyte infiltration after Aβ1–42 immunization (Seabrook et al., 2004). Increased levels of brain interferon-γ can produce transient T lymphocyte infiltration after Aβ1–42 immunization (Monsonego et al., 2006). Our data demonstrate that overall T lymphocyte number is increased chronically in the brain by local TGF-β1 overexpression; similarly, local TGF-β1 overexpression in the brains of AD patients may predispose them to T lymphocyte accumulation. In both cases, T cells were present at sites in which we observe TGF-β1 immunostaining (data not shown). T cells are localized to both perivascular and parenchymal sites in our mouse model, in which TGF-β1 is produced from both perivascular and parenchymal astrocytes. In immunized patients, T cell infiltrates were predominantly perivascular in patients with and without symptoms of meningoencephalitis (Nicoll et al., 2003; Ferrer et al., 2004; Masliah et al., 2005). We see a correspondingly prominent vascular pattern to TGF-β1 immunostaining in untreated AD patients (Wyss-Coray et al., 1997).
We did not see increased T lymphocyte infiltration after Aβ1–42 immunization in elderly mice, although we did observe higher brain T cell numbers in elderly APP/TGF-β1 and TGF-β1 mice compared with wild-type and APP littermates. The absence of a response to AN1792 could be because older mice have decreased production of naive T lymphocytes and an impaired ability to mount CD4 and CD8-dependent T cell responses (Linton and Dorshkind, 2004). These mice might have also developed T cell tolerance with age (Monsonego et al., 2001). Also, active TGF-β1 may increase in the serum with age (Forsey et al., 2003), and this could quench T cell-mediated responses.
Immunotherapy for AD remains a promising treatment, with multiple clinical trials for both active and passive immunotherapy ongoing. In the AN1792 trial, patients did not show a treatment benefit on most cognitive tests, such as the Alzheimer's Disease Assessment Scale–cognitive subscale, but did demonstrate reduced deterioration on a neuropsycholometric battery and had reduced CSF levels of tau (Orgogozo et al., 2003; Gilman et al., 2005). A small subgroup of patients with serum antibodies that bound to brain sections from APP mice also did better cognitively than patients without such antibodies (Hock et al., 2003). Passive immunization, in which a recombinant anti-Aβ antibody is directly injected and the host T cell response is bypassed, has shown efficacy in several mouse models (Bard et al., 2000; Schenk, 2002; Levites et al., 2006), and this approach is currently in phase 2 clinical trials (Bapineuzumab). New techniques that modulate the immune response to Aβ are being developed (Kim et al., 2005; Maier et al., 2006). Peripheral blood from patients in the AN1792 immunization trial demonstrated increases in multiple pro-inflammatory genes in those who would go on to develop symptoms after immunization, implying that peripheral immune status influenced the possibility of development of meningoencephalitis after treatment with AN1792 (O'Toole et al., 2005). Our study demonstrates that increases in brain TGF-β1 can influence the number of T cells in the brain and provides a starting point for understanding how to design therapies that produce a targeted, safe, specific immune response to Aβ in AD patients.
Footnotes
This work was supported by Elan Pharmaceuticals, the Veterans Administration Geriatric Research, Education, and Clinical Center (T.W.-C.), and the National Institute on Aging (T.W.-C.).
References
- Adams DH, Hathaway M, Shaw J, Burnett D, Elias E, Strain AJ. Transforming growth factor-beta induces human T lymphocyte migration in vitro. J Immunol. 1991;147:609–612. [PubMed] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke R, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, Millais SB, Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J Immunol. 2001;167:773–778. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsey RJ, Thompson JM, Ernerudh J, Hurst TL, Strindhall J, Johansson B, Nilsson BO, Wikby A. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, Eisner L, Kirby L, Rovira MB, Forette F, Orgogozo JM. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Kim HD, Maxwell JA, Kong FK, Tang DC, Fukuchi K. Induction of anti-inflammatory immune response by an adenovirus vector encoding 11 tandem repeats of Abeta1–6: toward safer and effective vaccines against Alzheimer's disease. Biochem Biophys Res Commun. 2005;336:84–92. doi: 10.1016/j.bbrc.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, Murphy MP, Golde TE. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Luethviksson BR, Gunnlaugsdottir B. Transforming growth factor-beta as a regulator of site-specific T-cell inflammatory response. Scand J Immunol. 2003;58:129–138. doi: 10.1046/j.1365-3083.2003.01297.x. [DOI] [PubMed] [Google Scholar]
- Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-β (Aβ) immunogens reduce cerebral Aβ load and learning deficits in an Alzheimer's disease mouse model in the absence of an Aβ-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Immune hyporesponsiveness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proc Natl Acad Sci USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsonego A, Imitola J, Petrovic S, Zota V, Nemirovsky A, Baron R, Fisher Y, Owens T, Weiner HL. Abeta-induced meningoencephalitis is IFN-{gamma}-dependent and is associated with T cell-dependent clearance of Abeta in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5048–5053. doi: 10.1073/pnas.0506209103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Masliah E, Yu G-Q, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- O'Toole M, Janszen DB, Slonim DK, Reddy PS, Ellis DK, Legault HM, Hill AA, Whitley MZ, Mounts WM, Zuberek K, Immermann FW, Black RS, Dorner AJ. Risk factors associated with beta-amyloid(1–42) immunotherapy in preimmunization gene expression patterns of blood cells. Arch Neurol. 2005;62:1531–1536. doi: 10.1001/archneur.62.10.1531. [DOI] [PubMed] [Google Scholar]
- Pfeifer M, Boncristiano S, Bondolfi L, Stalder A, Deller T, Staufenbiel M, Mathews PM, Jucker M. Cerebral hemorrhage after passive anti-Abeta immunotherapy. Science. 2002;298:1379. doi: 10.1126/science.1078259. [DOI] [PubMed] [Google Scholar]
- Schenk D. Amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat Rev Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Iglesias M, Bloom JK, Spooner ET, Lemere CA. Differences in the immune response to long term Abeta vaccination in C57BL/6 and B6D2F1 mice. Vaccine. 2004;22:4075–4083. doi: 10.1016/j.vaccine.2004.03.061. [DOI] [PubMed] [Google Scholar]
- Smeltz RB, Chen J, Shevach EM. Transforming growth factor-beta1 enhances the interferon-gamma-dependent, interleukin-12-independent pathway of T helper 1 cell differentiation. Immunology. 2005;114:484–492. doi: 10.1111/j.1365-2567.2005.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-β1. Am J Pathol. 1995;147:53–67. [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-β1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997;77:45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Sanan D, Mucke L, Masliah E. Chronic overproduction of TGF-β1 in astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156:139–150. doi: 10.1016/s0002-9440(10)64713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu G, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:614–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]