Abstract
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) with increased selectivity for the cyclooxygenase-2 (COX-2) isoform reduce gastrotoxicity but may increase adverse cardiovascular events.
Methods
We searched the literature for studies that reported the odds ratio (OR) for such events following exposure to NSAIDs.
Results
For studies comparing NSAID use with no use, increased COX-2 selectivity was significantly related to cardiovascular risk (log OR) amongst observational studies (R = −0.34, P < 0.001) and randomized controlled trials (RCTs) (R = −0.56, P < 0.001). For studies comparing NSAIDs, difference in selectivity was related to risk for observational studies (R = −0.28, P = 0.005) but not for RCTs (R = −0.23, P = 0.15).
Conclusions
Although increased COX-2 selectivity may reduce gastrotoxicity, this may be at the cost of increasing cardiovascular risk.
Keywords: NSAID, coxib, cardiovascular, myocardial infarction, COX-2 selectivity
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used to treat inflammatory disorders but their major drawback has been their tendency to cause serious gastrointestinal adverse effects [1]. The major target of NSAIDs, the enzyme cyclooxygenase (COX), exists as two closely related isoforms. COX-1 is a ‘house-keeping’ enzyme in most tissues, including the gastric mucosa, where it is intimately related to mucosal protection, whereas COX-2 is induced by inflammatory stimuli in many different tissues.
NSAIDs with enhanced selectivity for COX-2 (coxibs) were developed with the aim of maintaining the anti-inflammatory benefits but reducing gastrotoxicity. However, there have been growing concerns about the potential for coxibs to increase the frequency of adverse cardiovascular events. These culminated in the recent worldwide withdrawal of rofecoxib and further questions about the safety of other coxibs [2]. Although the cause for this unexpected adverse effect is unknown, studies examining the basic pharmacology of coxibs have pointed to a possible mechanism [3]. COX-2 is expressed in the wall of blood vessels, where it is the major source of prostacyclin, which inhibits platelet aggregation and causes vasodilation. In contrast, a major product of COX-1 activity in platelets is thromboxane A, which has the opposite effects [4]. Consequently, it might be anticipated that COX inhibitors with increasing selectivity for COX-2 over COX-1 might induce changes in prostanoid production that could lead to thrombotic events. Indeed, such changes have been demonstrated in healthy volunteers after short-term exposure to coxibs [4].
The aim of this study was to examine the hypothesis that there is a direct relationship between the extent of NSAID selectivity for the COX-2 isoform and adverse cardiovascular outcome using data currently available in the published literature.
Methods
We searched for all studies relevant to NSAIDs and cardiovascular disease outcomes published prior to 21 February 2005 using Medline and Embase. The NSAID and cardiovascular searches were combined and limited to human adult studies, of publication type randomized controlled trial (RCT), case–control or cohort study (CCS) or clinical trials (Medline) and article, journal or report (Embase). This yielded 1120 reports, and reduced to 95 full reports after assessing suitability from abstracts. Reports using pooled RCT data were excluded if data were available from original articles. Further reports were rejected if they did not include any cardiovascular data, had follow-up periods <14 days or included <100 subjects. Of the remaining reports, 24 CCSs, 24 RCTs and five studies containing pooled RCT data were accepted.
Data were extracted from these studies in the following way. Where the multivariate adjusted odds ratio (OR) for a combined cardiovascular endpoint was available, this value was extracted from that study along with its 95% confidence interval. If no combined endpoint was available, ORs for the individual cardiovascular outcomes of myocardial infarction, stroke or congestive heart failure were used. If more than one outcome was reported, reports for myocardial infarction were taken in preference to avoid duplication. Where data were available for all subjects including those on aspirin, and for subjects not on aspirin, the former was used. Where OR was not reported (e.g. in several RCTs) an unadjusted value was calculated directly and the standard error of the logarithm of the OR was derived via Woolf's formula. Where there were no events in one or other population, OR was calculated using the convention of adding 0.5 to the number with and without events.
For studies comparing NSAID use with placebo or no use, ORs for cardiovascular outcomes have been plotted against NSAID selectivity (S) based on recently reported studies of COX inhibition in human whole blood and expressed as S = log10 IC80 (COX-2/COX-1) [5]. For studies comparing two NSAIDs, the ORs have been plotted against the difference in antilog of the selectivity values for the two drugs. Where the comparison for an NSAID was against pooled data for all other NSAIDs, selectivity for pooled NSAIDs was taken as log(1).
Weighted linear regression analysis was performed for log OR and either selectivity (where comparator was no use or placebo) or difference in selectivity (where comparator was another NSAID). Weighting was based on the reciprocal of the standard error of log OR.
Results
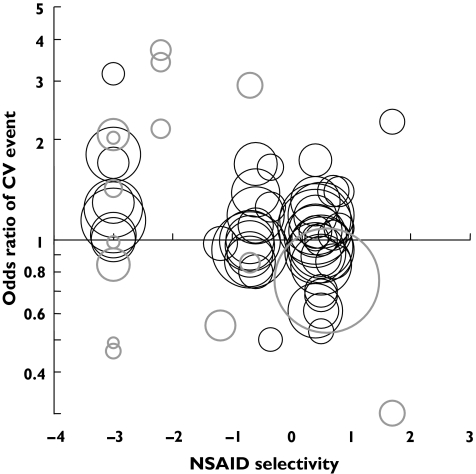
Figure 1 shows the relationship between COX-2 selectivity and the log OR for cardiovascular events in studies (50 CCS, 16 RCT) comparing NSAID use with no use. For both CCSs (R = −0.34, P < 0.001) and RCTs (R =−0.56, P < 0.001) there was a highly significant association between the extent of COX-2 selectivity and increased cardiovascular risk. This relationship was still apparent amongst CCSs (R = −0.38, P < 0.001) and RCTs (R = −0.64, P < 0.001) if a single weighted mean log OR was calculated for each NSAID.
Figure 1.
The relationship of COX-2 selectivity to cardiovascular outcome (NSAID v. no use). Randomised controlled trials (RCTs) and pooled RCT data are indicated by bold grey circles and cohort/case control studies are indicated by thin black circles. The circle area is based on weightings used for regression analysis (see text for details). OR is plotted on a log scale
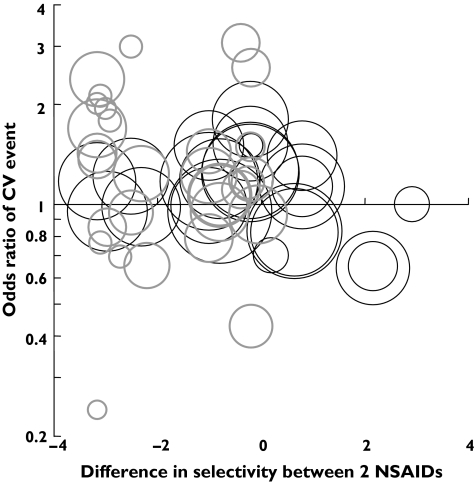
Figure 2 shows the relationship between the difference in COX selectivity and the log OR for cardiovascular events in studies (23 CCS, 30 RCT) comparing two different NSAIDs. For CCSs (R = −0.28, P = 0.005) there was a significant association between the difference in selectivity and cardiovascular risk, although this was not apparent amongst RCTs (R = −0.23, P = 0.15).
Figure 2.
The relationship of COX-2 selectivity to cardiovascular outcome (NSAID v. NSAID). Randomised controlled trials (RCTs) and pooled RCT data are indicated by bold grey circles and cohort/case control studies are indicated by thin black circles. The circle area is based on weightings used for regression analysis (see text for details). OR is plotted on a log scale
Discussion
We present preliminary evidence to support the hypothesis that the degree of COX-2 selectivity is directly associated with the increased risk of cardiovascular events amongst a group of NSAIDs with widely varying properties. This result has several important implications. First, while attention has been focused on the adverse effects of rofecoxib, concerns that similar problems might be found with other coxibs such as celecoxib, valdecoxib and lumiracoxib have been increasing [2]. Our data suggest that increased cardiovascular risk may be an inevitable problem for newer NSAIDs with increased COX-2 selectivity, i.e. a class effect. The development of more highly selective compounds may be a trade-off between increased gastrointestinal safety and cardiovascular risk. Second, our data support the concept that the mechanism linking coxibs to cardiovascular events is directly related to the relative activity of COX-1 and COX-2 isoforms in the vascular wall [3, 4]. Finally, this approach might provide a rational basis for modelling the extent of the risk posed by individual coxibs based on the extent of their COX-2/COX-1 selectivity.
These data have important limitations. They include a heterogeneous group of observations with respect to study design, patient population, cardiovascular outcome variable and comparator group. It might also be argued that they are potentially biased by the number of observations relating to individual NSAIDs. There is also the potential for confounding in observational studies. Amongst other factors, the emerging relationship between coxibs and cardiovascular risk may have meant that patients at higher risk for events may have been more likely to receive nonselective drugs [6]. The overall picture is likely to be clarified by further data concerning the impact of newer highly selective coxibs. Nevertheless, following the withdrawal of rofecoxib there is a pressing need to assess the safety of the remaining coxibs. If a direct relationship with selectivity is confirmed then the continued availability and prescription of NSAIDs with enhanced selectivity will have to be based on a careful judgement of the extent of the benefits and risks for individual patients.
Acknowledgments
Conflict of interest
None declared.
S.R.J.M., G.D.M. and D.J.W. are supported by the Scottish Higher Education Funding Council. R.A.P. is supported by the Edinburgh Technology Fund.
References
- 1.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–99. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell S, Webb DJ. COX-2 selective inhibitors—important lessons learned. Lancet. 2005;365:449–51. doi: 10.1016/S0140-6736(05)17876-3. [DOI] [PubMed] [Google Scholar]
- 3.Cheng Y, Austin SC, Rocca B, Koller BH, Coffman TM, Grosser T, Lawson JA, FitzGerald GA. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–41. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 4.McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA. 1999;96:272–7. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 6.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case–control analysis. BMJ. 2005;330:1366–72. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]