Abstract
In human epidermis, functional symbiosis requires homeostatic balance between keratinocytes and melanocytes. Compelling evidence from co-culture studies demonstrated a sophisticated, multileveled regulation of normal melanocytic phenotype orchestrated by undifferentiated, basal-type keratinocytes. Keratinocytes control cell growth and dendricity, as well as expression of melanoma-associated cell surface molecules of normal melanocytes. In contrast, melanoma cells are refractory to the keratinocyte-mediated regulation. The loss of regulatory dominance by keratinocytes occurs in concert with down-regulation of E-cadherin expression in melanoma cells. To investigate the potential role of E-cadherin in melanoma-keratinocyte interaction, we transduced E-cadherin-negative melanoma cells with full-length E-cadherin cDNA using an adenoviral vector. Our results show that functional E-cadherin expression in melanoma cells leads to cell adhesion to keratinocytes rendering them susceptible for keratinocyte-mediated control. In a skin reconstruction model, ectopic E-cadherin expression inhibits invasion of melanoma cells into dermis by down-regulating invasion-related adhesion receptors, MelCAM/MUC18 and β3 integrin subunit, and by induction of apoptosis. Thus, disruption of the E-cadherin-mediated, normal regulatory control from keratinocytes may represent one of the mechanisms accounting for melanocyte transformation.
At the epidermal/dermal junction, melanocytes adhere to adjacent basal keratinocytes through expression of E-cadherin. 1,2 They synthesize and donate melanin through multiple dendrites to protect keratinocytes from deleterious effects of ultraviolet light. Despite the continuous differentiation and migration of cells during self-renewal, melanocytes exhibit controlled replication resulting in a life-long stable ratio of 1:5 to 6 with basal keratinocytes. After isolation and subsequent culture, melanocytes display different phenotypic characteristics: they grow continuously in vitro with doubling times of 48 to 96 hours, 3 assume bi- or tri-polar morphology, and acquire expression of melanoma-associated antigens, such as MelCAM/MUC18, β3 integrin subunit, gangliosidases, melanotransferrin, chondroitin sulfate proteoglycan, and growth factor receptors. 3,4 We and others have demonstrated that melanocytes regain their normal phenotype on co-culture with undifferentiated, basal-type keratinocytes, where the keratinocytes regulate melanocytic growth, dendrite formation, and cell surface receptor expression achieving a homeostatic balance resembling that in situ. 5,6 The keratinocyte-mediated phenotypic control over melanocytes is specific for the basal-type keratinocytes, which cannot be substituted by differentiated keratinocytes, dermal fibroblasts, or carcinoma cells. 6 Melanoma cells, unlike normal melanocytes, are refractory to phenotypic modulation by keratinocytes. 6 The loss of keratinocyte dominance over melanoma cells correlates with down-regulation of a cell adhesion molecule, E-cadherin, which was previously identified as the prime adhesion mediator between melanocytes and keratinocytes. 1 These observations led us to the hypothesis that in addition to intrinsic activation, melanocytic transformation may at least in part result from a disruption of E-cadherin-mediated, normal regulatory control of basal keratinocytes.
Cadherins comprise a growing family of surface membrane glycoproteins that mediate cell-cell adhesion in a calcium-dependent fashion. 7 The extracellular domains of cadherins serve as molecular zippers connecting neighboring cells, 8 whereas the cytoplasmic tails are linked noncovalently to the actin cytoskeleton via catenins. 9 Cadherins are not just biological glues. Signal mediators such as phosphokinases, 10 phosphatases, 11 and their substrates 12 are localized to the cadherin-catenin complexes. The organization of a hierarchy of multiple components allows cadherins to act as signaling receptors for intercellular communication with subsequent modulation of growth 13-16 and differentiation 17-19 in both normal and malignant cells. The physical association of the adenomatous polyposis coli tumor suppressor protein with catenins 20,21 and the ability of proto-oncogenes including c-erbB2, 22 Wnt-1, 23 and epidermal growth factor (EGF) receptor 24 to modulate E-cadherin expression also indicate an important link between tumor initiation and cell adhesion.
A tumor/invasion suppressor role of E-cadherin has been established in various human carcinomas. 25 Studies have demonstrated an association between E-cadherin down-regulation and dedifferentiation, invasiveness, and lymph node or distant metastasis in breast, 26 colorectal, 27 prostate, 28 bladder, 29 renal, 30 hepatocellular, 31 ovarian, 32 pancreatic, 33 endometrial, 34 and squamous cell carcinoma. 35 Furthermore, the functional modification of E-cadherin using blocking antibodies 27,31,36 demonstrated that disruption of E-cadherin-mediated homotypic cell adhesion facilitates tumor invasion, presumably by releasing the malignant cells from the primary site. Conversely, activation of E-cadherin resulted in growth retardation 13,14,16,37 and inhibition of the invasive and metastatic phenotype in carcinoma cells. 36,38-40
To obtain direct information on the role of E-cadherin-mediated adhesion and/or signal transduction in melanoma, we transduced E-cadherin-negative melanoma cell lines with full-length E-cadherin cDNA using an adenoviral vector. Our results show that functional restoration of E-cadherin in melanoma cells retards cell growth in anchorage-dependent and -independent cultures, reduces tumorigenicity in vivo, and confers keratinocyte-dependent normal melanocytic phenotype. The transduced cells grew in a controlled manner in the presence of keratinocytes and showed down-regulation of invasion-related antigens, such as MelCAM/MUC18 and β3 integrin subunit. In a three-dimensional reconstruction model, ectopic E-cadherin expression in melanoma cells inhibited invasion and induced apoptosis.
Materials and Methods
Cell Culture
The isolation and culture of normal human melanocytes and melanoma cells was performed as previously described. 41,42 Briefly, melanocytes were cultured in MCDB153/L15 medium (v/v: 4/1) supplemented with CaCl2 (2 mmol/L), insulin (5 μg/ml), EGF (5 ng/ml), 12-O-tetradecanoyl phorbol-13-acetate (10−7 mol/L), bovine pituitary extract (40 μg/ml), and 2% fetal bovine serum. Melanoma cells were cultured in melanocyte growth medium in the absence of EGF, phorbol ester, and bovine pituitary extract. WM115 is a vertical growth phase primary melanoma cell line, whereas, WM164, WM852, and 1205Lu are metastatic cells. All four cell lines are tumorigenic in severe combined immunodeficient mice and metastasis-competent. 42 Keratinocytes were grown in serum-free keratinocyte growth medium, containing modified MCDB153 supplemented with bovine pituitary extract (140 μg/ml), EGF (10 ng/ml), ethanolamine (0.1 mmol/L), hydrocortisone (5 × 10−7 mol/L), insulin (5 μg/ml), and O-phosphoryl ethanolamine (0.1 mmol/L). Primary human dermal fibroblasts were initiated as explant cultures from trypsin-treated and epidermis-stripped neonatal foreskin, and passaged in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Transcomplementing 293 cells, a cell line immortalized and transformed by adenovirus E1a and E1b, were obtained from the Vector Core of the Institute for Human Gene Therapy, University of Pennsylvania (Philadelphia, PA) and grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. All tissue culture reagents were purchased from Sigma Chemical Co. (St. Louis, MO) except for EGF (Collaborative Biochemical Products, Bedford, MA) and Dulbecco’s modified Eagle’s medium (Life Technologies, Inc., Gaithersburg, MD).
Antibodies and Streptavidin Conjugates
Mouse monoclonal anti-human E-cadherin antibodies, HECD-1 and SHE78–7 were purchased from Zymed Laboratories (San Francisco, CA). SHE78–7 was used in neutralizing experiments for inhibition of E-cadherin-dependent adhesion. HECD-1 was used for flow cytometry. A third monoclonal antibody (mAb) against E-cadherin which recognizes the C-terminus of the molecule was obtained from Transduction Laboratories, Inc. (C20820, Lexington, KY) and used for immunoprecipitation. Anti-β-catenin mAb (C19220) was also purchased from Transduction Laboratories, Inc. Mel-5 is a mouse mAb of the immunoglobulin-2a (IgG2a) subclass against tyrosinase-related protein-1 (a melanocytic marker) and was obtained from Signet (Dedham, MA). Fluorescein isothiocyanate-, Cy3-, and peroxidase-conjugated goat anti-mouse IgG as well as fluorescein isothiocyanate-conjugated streptavidin were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Mouse mAb SAP (IgG1) directed to the β3 integrin subunit was generated by immunizing mice with platelet membranes. 43 Mouse mAb A32 (IgG1) against MelCAM/MUC18 was previously characterized. 44 Antibody purification and biotinylation were performed following procedures described. 6,44
Construction of Replication-Deficient E-Cadherin Adenoviral Vector (E-cad/Ad5)
Full length human E-cadherin cDNA was a kind gift from Dr. David L. Rimm (Yale University, New Haven, CT). The adenoviral vector was constructed according to methods described by Graham and Prevec. 45 Briefly, full-length human E-cadherin cDNA was inserted into the multiple cloning site of pAd.cytomegalovirus (CMV)-Link.1 46 (obtained from the Vector Core, Institute for Human Gene Therapy, University of Pennsylvania, Philadelphia, PA) under the control of the CMV intermediate/early enhancer-promoter element and SV40 polyadenylation signal using a two-step subcloning strategy. Correct orientation of the insert was confirmed by restriction analyses. The resulting shuttle vector (E-cad/pAd.CMV-Link.1) was linearized and co-transfected with the ClaI-digested, E1–E3-deleted human adenoviral DNA dl7001 47 into 293 cells using calcium phosphate precipitation. When cytopathic effects were evident, individual plaques were picked and screened for incorporation of the E-cadherin sequence by Southern blotting. Positive plaques were repurified, propagated, and titrated in permissive 293 cells. Control adenoviral vector, lacZ/Ad5, encoding β-galactosidase was purchased from the Vector Core.
Infection of Melanoma Cells by Adenoviral Constructs
Optimal viral titer was defined as the minimum amount of virus required to yield the highest overall gene transfer efficiency without apparent alteration in cellular phenotype. In melanoma cells, 20 plaque forming units (pfu)/cell result in 100% gene transfer efficiency. Subconfluent melanoma cells were transduced with 20 pfu/cell of replication-deficient adenoviruses for 2 hours at 37°C in a minimum amount of serum-free Dulbecco’s modified Eagle’s medium sufficient to cover the culture vessels. Viral suspensions were then replaced by regular medium. Cells were allowed to recover for 24 hours before use.
Flow Cytometry
Cultured cells were detached with 10 mmol/L ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS), washed once with 0.1% bovine serum albumin in PBS, and stained for 40 minutes with 10 μg/ml of primary mAb at 4°C. After final incubation with fluorescein isothiocyanate-conjugated goat anti-mouse IgG, cells were analyzed by fluorescence-activated cell sorting using an Ortho Cytofluorograf 50H connected to a 2150 Data Handling System (Ortho Diagnostics, Inc., Westwood, MA). As a negative control, unrelated mouse IgG was used.
Immunoprecipitation and Western Blot Analyses
Subconfluent monolayers of cells were scraped off, washed with PBS, and followed by extraction in lysis buffer containing 1% Triton X-100, 1% deoxycholic acid, 2 mmol/L CaCl2, and protease inhibitors (10 μg/ml leupeptin, 10 μg/ml aprotinin, 1.8 mg/ml iodoacetamide, and 1 mmol/L phenylmethyl sulfonyl fluoride in PBS. After immunoprecipitation with anti-E-cadherin mAb- (2 μg/ml) or nonimmune mouse IgG-conjugated protein A Sepharose CL-4B (Pharmacia Biotech, Uppsala, Sweden), samples were washed three times with lysis buffer, boiled in Laemmli buffer containing β-mercaptoethanol, and then subjected to electrophoresis on a 6% sodium dodecyl sulfate-polyacrylamide gel. Immunoblotting was performed by sequential incubation with an anti-β-catenin mAb (0.5 μg/ml) and a peroxidase-labeled goat anti-mouse secondary antibody. For Western blot analyses, cell lysates were quantified by a BCA protein assay kit (Pierce, Rockford, IL). An equal amount (100 μg) of total protein from each sample was subjected to electrophoresis on a 6% sodium dodecyl sulfate-polyacrylamide gel, transblotted onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Richmond, CA), and probed with an anti-β3-integrin mAb (SAP) and a peroxidase-conjugated secondary antibody. Immunoreactive bands were detected using enhanced chemiluminescence (Amersham, Arlington Heights, IL).
Cell Adhesion Assay
Melanoma cells transduced with E-cad/Ad5 or lacZ/Ad5 were prelabeled with a fluorescent dye DiI (10 μg/ml; Molecular Probes, Eugene, OR) for 4 hours, and harvested by treatment with 0.01% trypsin in Hanks’ balanced salt solution containing 1 mmol/L calcium for 30 minutes at 37°C. Under these conditions, cadherins are specifically protected from proteolytic digestion. For blocking experiments, E-cad/Ad5-infected cells were incubated with E-cadherin blocking mAb SHE78–7 (5 μg/ml) at 4°C for 30 minutes, washed with Hanks’ balanced salt solution, and resuspended in assay medium containing 1% bovine serum albumin and 1 mmol/L calcium in Hanks’ balanced salt solution. A total of 2 × 10 5 cells in a volume of 400 μl was added to differentiated keratinocyte monolayers in 4-well chamber slides and allowed to adhere for 30 minutes. Keratinocyte differentiation was induced by preincubation in 2 mmol/L of calcium for 1 hour. After removal of nonadherent cells, slides were fixed. Numbers of adherent cells per high power field (×250) in triplicate wells were counted under a fluorescence microscope. Statistical analyses were done by Student’s t-test.
Anchorage-Dependent Growth Assay
Subconfluent cultures were trypsinized and seeded in triplicate 35-mm wells at 4 × 10 5 cells/well. Cells were refed twice weekly. At days 1, 4, and 7, cells were harvested and counted in a Coulter counter (Coulter Electronics; Luton, Beds, England). Statistical analyses were performed using the Student’s t-test.
Soft Agar Assay
Melanoma cells were suspended in MCDB153/L15 medium (v/v: 4/1) supplemented with 25 μg/ml bovine pituitary extract, 2 ng/ml EGF, 2 μg/ml insulin, 4% fetal bovine serum, and 0.25% agar and plated in triplicate at 6 × 10 4 cells/well in 6-well plates. After 3 weeks, colonies were counted using an inverted microscope. Student’s t-test was used for statistical analyses.
In Vivo Tumorigenicity
The tumorigenicity was examined in severe combined immunodeficient mice. Melanoma cells (5 × 106/mouse) were suspended in 0.1 ml of growth medium and injected subcutaneously in the dorsal skin. Tumor size was monitored on days 3, 5, and 7. The size of tumors was determined as follows: (maximum dimensions × minimum dimensions)2/2.
Monolayer Co-Culture and Double Immunofluorescence
Melanocytic cells were detached by trypsinization, mixed with keratinocytes at a 1:10 ratio and seeded in 8-well chamber slides (Lab-Tek; Nunc, Inc., Naperville, IL). After 4 days in co-culture, double immunofluorescence was performed as described. 6 Briefly, cells were fixed, permeabilized, and incubated sequentially with antibodies Mel-5, Cy3-conjugated goat anti-mouse IgG, biotinylated SAP or A32, and fluorescein isothiocyanate-conjugated streptavidin. As a negative control, normal mouse serum was used instead of a primary antibody. All incubations were performed at room temperature for 1 hour. For cell growth experiments, slides were counterstained with Höechst reagent (Bisbenzimide; Sigma Chemical Co.). Cell growth was monitored by counting cells in five random high-power fields (×250). The ratio of keratinocytes and melanocytic cells was determined by the following equation: keratinocytes/melanocytic cells = (total number of Höechst positive nuclei − red cell number)/red cell number.
Cell Invasion in Three-Dimensional Skin Reconstructs
Skin reconstructs were prepared as previously described. 48,49 Invasion of melanoma cells was tested in artificial skin reconstructs, in which human foreskin dermal fibroblasts in rat tail collagen were placed on a precast collagen gel. After 6 days, the constricted collagen gels formed a concave surface, serving as a cradle for seeding of epidermal cells. Melanoma cells were then mixed with keratinocytes at a 1:5 ratio and seeded onto the dermal constructs. After 5 days, cultures were lifted to the air-liquid level for an additional 10 days to allow stratification of epidermal keratinocytes. The reconstructs were then harvested, fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Apoptosis was evaluated using the ApopTag in situ apoptosis detection kit (Oncor, Gaithersburg, MD).
Results
Characterization of E-cad/Ad5-Transduced Melanoma Cells
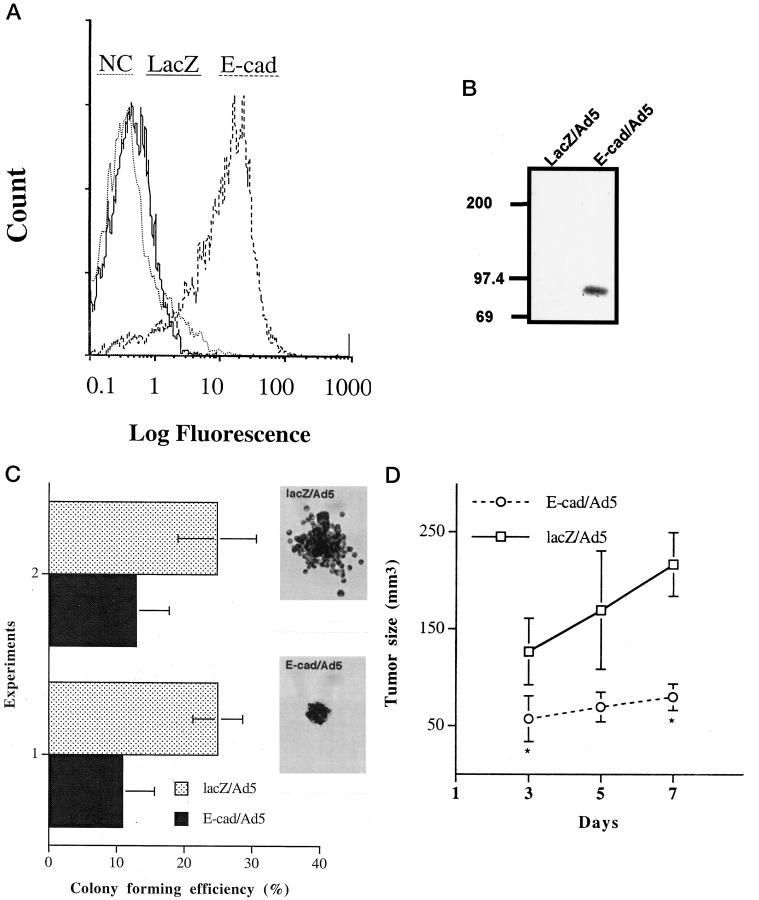
Forty-eight hours after transduction with E-cad/Ad5 at 20 pfu/cell, more than 90% of the WM115 cells expressed E-cadherin, whereas control cells transduced with the lacZ reporter gene (lacZ/Ad5) or nontransduced cells remained negative by fluorescence analysis (Figure 1A) ▶ . Similar results were obtained with the additional three melanoma cell lines (WM164, WM852, and 1205Lu). The expression of exogenous E-cadherin in these melanoma cells was further confirmed by Western blotting (data not shown). Because cadherin function depends on the cytoplasmic anchorage, we tested the physical association of transduced E-cadherin with endogenous signal transduction proteins by immunoprecipitation. Forty-eight hours after transduction, extracts of melanoma cells (WM115 and 1205Lu) were immunoprecipitated with an E-cadherin mAb. After electrophoresis, samples were subjected to immunoblotting with an anti-β-catenin mAb. A band of 92 kd corresponding to β-catenin was detected in the E-cadherin-transduced cells, whereas no specific band was present in the cells transduced with lacZ/Ad5. Figure 1B ▶ shows representative data of WM115. In addition, the functional activity of the E-cadherin transgene products in melanoma cells was demonstrated by a fourfold increase in adhesion of E-cadherin-transduced WM115 melanoma cells to keratinocytes when compared to cells transduced with the control vector. Furthermore, the adhesion of E-cadherin-transduced cells to keratinocytes was significantly reduced in the presence of an E-cadherin blocking mAb (data not shown). Transduction of E-cadherin into four melanoma cell lines (WM115, WM164, WM852, and 1205Lu) reduced cell growth in monolayer by 25 to 50% (data not shown) and colony formation in soft agar by 15 to 30% (Figure 1C) ▶ . The E-cadherin-transduced cells grew as compact colonies of 40 to 60% smaller size when compared to lacZ-transduced or nontransduced cells (Figure 1C ▶ , inserts). The reduction in colony size was apparently due to the tight cell-cell adhesive interactions. In tumorigenicity assays, E-cadherin-overexpressing 1205Lu melanoma cells remained tumorigenic when injected subcutaneously into severe combined immunodeficient mice but the tumor size was significantly reduced by 60 to 70% in comparison to those arising from control vector-transduced counterparts (Figure 1D) ▶ .
Figure 1.
Characterization of E-cad/Ad5-transduced melanoma cells. A: Expression of exogenous E-cadherin in E-cad/Ad5-transduced WM115 melanoma cells (E-cad, broken line) as determined by fluorescence-activated cell sorting analysis with an anti-E-cadherin mAb 48 hours after transduction. The solid line represents lacZ/Ad5-transduced cells and the dotted line represents the negative control (NC) cells stained with an isotype-matched negative control antibody. Similar results were obtained with three other melanoma cell lines. B: Detection of E-cadherin/catenin complex in E-cad/Ad5-transduced WM115 melanoma cells by immunoprecipitation. Forty-eight hours after transduction, cell lysates were immunoprecipitated with an anti-E-cadherin mAb. Samples were subjected to electrophoresis and probed with an anti-β-catenin mAb. A 92-kd band representing β-catenin co-immunoprecipitated with E-cadherin in E-cad/Ad5-transduced cells but not in lacZ-transduced cells. C: Soft agar growth of 1205Lu melanoma cells overexpressing E-cadherin. Cells were transduced with lacZ/Ad5 or E-cad/Ad5, and 24 hours later, resuspended in 0.25% agar and seeded in triplicate 35-mm wells at 6 × 10 4 cells/well. After 3 weeks, colony-forming efficiency was determined as the percentage of cells forming colonies containing four or more cells. Ten random fields were examined for each condition. Data represent mean ± SD from two independent experiments. Asterisks indicate significant difference by Student’s t-test (experiment 1: P < 0.001; experiment 2: P = 0.001). Inserts show the micrographs of representative colonies. D: Tumorigenicity of melanoma cells after induction of E-cadherin. One day after transduction, 1205Lu melanoma cells in subconfluent cultures were trypsinized and resuspended in growth medium at 5 × 10 7 cells/ml. Suspended cells (100 μl) were injected subcutaneously onto the back of SCID mice (3 mice/condition). Tumor size was monitored on days 3, 5, and 7. Asterisks indicate significant differences between groups (day 3, P = 0.045; day 5, P = 0.052; and day 7, P = 0.003).
Re-Expression of E-Cadherin Restores Keratinocyte-Dependent Growth Control and Melanoma-Associated Antigen Down-Regulation in Melanoma Cells
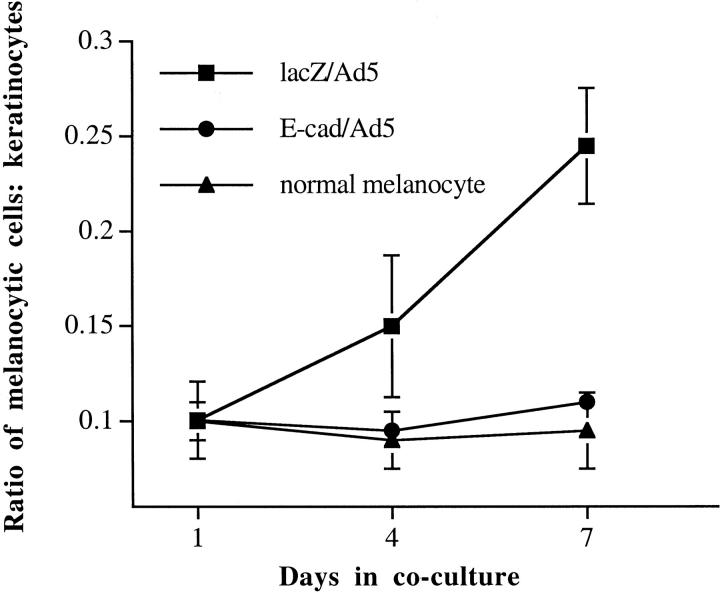
Valyi-Nagy et al 5 had shown that normal melanocytes, co-cultured with human keratinocytes at a physiological 1:5 or 1:10 ratio, maintained the initial seeding ratio throughout a 14-day observation period, despite continuing proliferation of both cell types. E-cadherin-transduced WM115 melanoma cells when seeded with keratinocytes exhibited similar controlled growth as normal melanocytes (Figure 2) ▶ . In contrast, lacZ/Ad5-transduced melanoma cells rapidly propagated resulting in a significant increase of the ratio beginning at approximately 4 days after seeding (Figure 2) ▶ . Similar results were obtained using 1205Lu melanoma cells. In control experiments, when E-cadherin-transduced WM115 melanoma cells were cultured separately from keratinocytes, the ratios between the two cell types increased (although at a slower rate in comparison to those of lacZ-transduced cells; data not shown) indicating that keratinocytes regulate growth of E-cadherin-transduced melanoma cells through direct cell-cell contact.
Figure 2.
Growth control and regulation of cell surface molecule expression. Keratinocytes control growth of human melanoma cells expressing E-cadherin. Melanoma cells WM115, transduced with either E-cad/Ad5 (•) for overexpression of E-cadherin or lacZ/Ad5 control vector (▪), and normal human melanocytes as positive control (▴) were co-cultured with keratinocytes at an initial seeding ratio of 1:10. On days 1, 4, and 7, co-cultures were stained with mAb Mel5, an anti-TRP-1 antibody, to identify melanocytic cells and then counterstained with Höechst dye to visualize all cells. Cell ratios were determined by counting under a fluorescence microscope. E-cadherin- and lacZ-transduced melanoma cells showed significant differences in cell ratios (P < 0.05 on days 4 and 7 by Satterthwaite’s method for unequal variances).
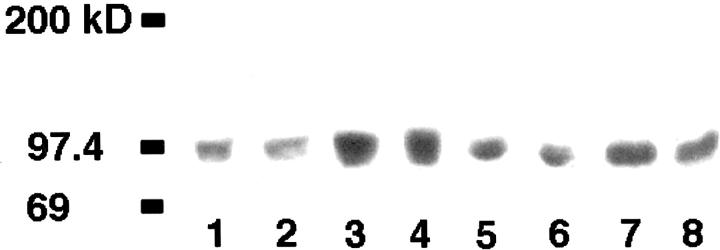
To investigate whether E-cadherin contributes to the phenotypic plasticity of melanocytic cells in response to keratinocyte contact-mediated control, we tested cell surface antigen expression in E-cadherin-transduced WM115 and 1205Lu melanoma cells co-cultured with keratinocytes. Figure 3 ▶ shows representative data from WM115. Nontransduced or lacZ/Ad5-transduced melanoma cells retained the expression of melanoma-associated antigens such as the cell-cell adhesion molecules, MelCAM/MUC18 and the β3 subunit of the αvβ3 vitronectin receptor (Figure 3) ▶ . By contrast, E-cadherin-transduced melanoma cells expressed neither antigen at detectable levels after 7 days in co-culture. In the absence of keratinocytes, E-cadherin transduction of melanoma cells had no effect on the expression of these invasion-related adhesion molecules (Figure 4) ▶ .
Figure 3.
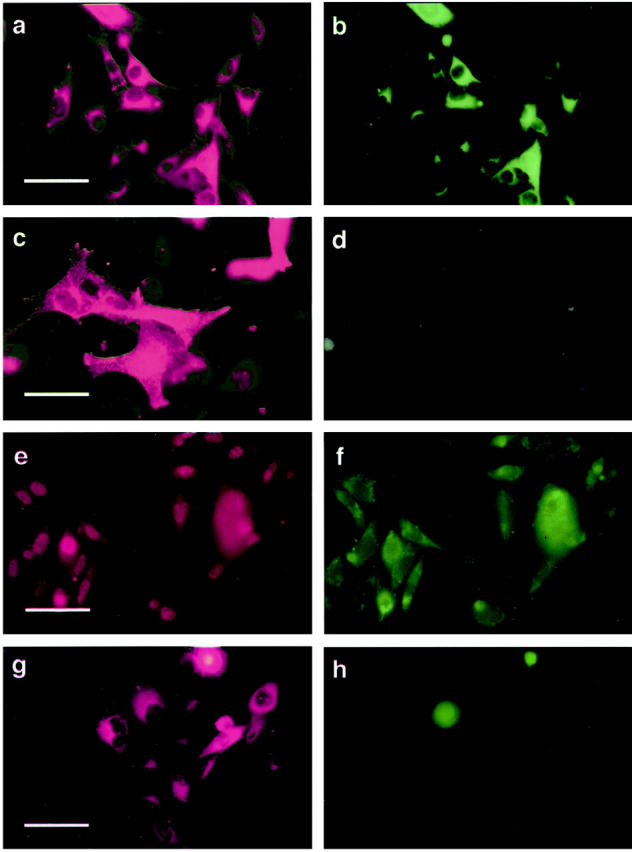
Keratinocytes down-regulate cell surface expression of Mel-CAM (a–d) and β3 integrin subunit (e–h) by E-cadherin-expressing melanoma cells. Melanoma cells were transduced with either E-cad/Ad5 or lacZ/Ad5. After 24 hours, cells were mixed with normal human keratinocytes at a 1:5 ratio and the co-cultures were stained 7 days later. a: Identification of lacZ-transduced WM115 melanoma cells in co-cultures using mAb Mel-5, a melanosomal marker, followed by Cy3-conjugated secondary antibody. b: lacZ-transduced melanoma cells double-stained with mAb A32 defining Mel-CAM. c: E-cadherin-transduced melanoma cells identified in the co-cultures using mAb Mel-5. d: E-cadherin-transduced melanoma cells double-stained with anti-Mel-CAM mAb A32. Similar down-regulation was observed using SAP mAb against β3 subunit of the vitronectin receptor (e–h): lacZ-transduced 1205Lu melanoma cells in co-culture double-stained with Mel5 (e) and SAP (f); E-cadherin-transduced 1205Lu melanoma cells in co-culture double-stained with Mel5 (g) and SAP (h). Scale bars: a, e, and g, 40 μm; c, 20 μm.
Figure 4.
Western blot analyses of β3 integrin expression in melanoma cells. Melanoma cells were transduced with either E-cad/Ad5 or lacZ/Ad5. After 24 hours, cell lysates were prepared, quantified, resolved by electrophoresis, and immunoblotted with mAb SAP. Lane 1: lacZ/Ad5-transduced normal melanocytes; lane 2: E-cad/Ad5-transduced normal melanocytes; lane 3: lacZ/Ad5-transduced 1205Lu melanoma cells; lane 4: E-cad/Ad5-transduced 1205Lu melanoma cells; lane 5: lacZ/Ad5-transduced WM115 melanoma cells; lane 6: E-cad/Ad5-transduced WM115 melanoma cells; lane 7: lacZ/Ad5-transduced WM164 melanoma cells; lane 8: E-cad/Ad5-transduced WM164. Expression of β3 integrin subunit of melanoma cells after transduction of E-cadherin is not altered in the absence of keratinocytes. Total protein loaded per lane: 100 μg.
E-Cadherin Expression in Melanoma Cells Inhibits Invasion and Triggers Apoptosis in Three-Dimensional Skin Reconstructs
To determine whether the E-cadherin-induced down-regulation of tumor-associated antigens on the melanoma cell surface has biological consequences, a three-dimensional reconstruct was used to test the invasive capacity of the melanoma cells. This model consists of a dermal compartment containing fibroblasts in a collagen gel, which is separated from an epidermal compartment composed of melanocytic cells and keratinocytes by a naturally formed basement membrane. 49 Control vector-transduced 1205Lu metastatic melanoma cells grew deep into the dermis, forming strands of cell nests (Figure 5 ▶ , B and D), whereas, E-cadherin-transduced melanoma cells remained in the epidermis and upper dermis (Figure 5 ▶ , A and C). Those melanoma cells located in the upper dermis showed typical signs of apoptotic death (Figure 5C) ▶ , including nuclear condensation and apoptotic bodies. Free 3′-OH ends resulting from DNA fragmentation were detected in the apoptotic cells using the ApopTag in situ apoptosis detection kit (Oncor) (Figure 5E) ▶ , whereas, invading cells in control reconstructs showed no evidence of apoptosis (Figure 5F) ▶ . Similar results were obtained using WM115 vertical growth phase melanoma cells (data not shown).
Figure 5.
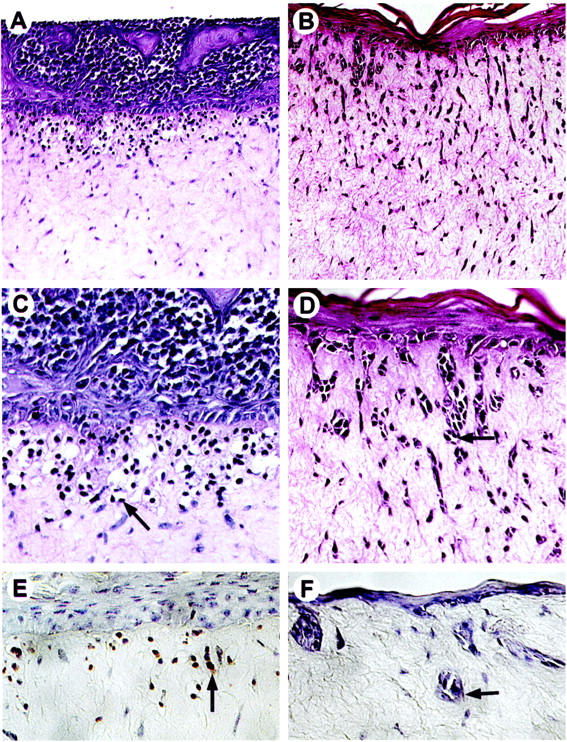
Keratinocytes inhibit invasion of E-cadherin-expressing melanoma cells into the dermis in human skin reconstructs. 1205Lu metastatic melanoma cells were transduced with either E-cad/Ad5 or lacZ/Ad5 and incorporated into the epidermal compartments of skin reconstructs. At maturation, reconstructs were harvested, fixed, and embedded in paraffin for hematoxylin and eosin staining. E-cadherin-expressing melanoma cells grew exclusively in the epidermis, at the epidermal/dermal junction, and in the upper dermis (A and C), displaying typical signs of apoptosis (C). These cells stained positive by the ApopTag in situ apoptosis detection kit (Oncor) (E). Arrows in C and E indicate a melanoma cell with apoptotic bodies. In contrast, lacZ-transduced cells (B, D, and F) formed strands of cell nests (arrows) that invaded deep into the dermis and were not apoptotic (F). Magnification: A and B, ×100; C–F, ×250.
Discussion
Melanoma progression involves several discrete stages which have been well characterized in terms of their clinical appearance and histopathology. 50 The relative importance of some of these lesions is controversial however, there is little doubt that progression involves alterations in spatial and functional relationships between normal melanocytes and keratinocytes. Most evidently, there is a loss of association with epidermis, and growth and invasion of melanocytic cells in the dermis. Although it has long been postulated that interactions between tumor cells and their microenvironment contribute to tumorigenesis, the role of disrupted epidermal homeostasis in melanoma development and progression has received surprisingly little attention. A large body of data now exists indicating that the control of gene expression and maintenance of tissue function is dependent on multiple extracellular signals originating not only from growth factors, hormones, and extracellular matrix molecules, but also from direct interactions with other cells. 51 Here, we present data suggesting that the melanocytic phenotype is environmentally regulated, and that the malignant phenotype (associated with neoplastic transformation) reflects a disturbance in normal control exerted by E-cadherin-dependent signals from the surrounding keratinocytes.
Although our data demonstrated that E-cadherin induced inhibition of growth in human melanoma cells in vitro (Figure 1C) ▶ and in vivo (Figure 1D) ▶ , there is no evidence that down-regulation of E-cadherin initiates melanoma. Earlier cytogenetic analyses of melanoma cell lines failed to identify frequent abnormalities at 16q where the E-cadherin gene is mapped. 52 Clearly, with new markers currently available, additional genetic evidence is required to establish a true tumor suppressor role of E-cadherin in human melanoma. Our data demonstrated that functional expression of E-cadherin in melanoma cells resulted in growth retardation as well as inhibition of cell motility and local invasion. However, malignant properties such as soft agar growth and tumor formation remained relatively intact. Recently, Rubinfeld et al 53 demonstrated accumulation of β-catenin as a result of gene mutations in human melanoma cells, which in turn activated aberrant Lef/Tcf-dependent gene transcription, including transcription factors c-jun and fra-1, and urokinase-type plasminogen activator receptor. 54 E-cadherin may exert its growth-suppressive effect directly through tight cell-cell adhesion or indirectly by sequestrating β-catenin and thus preventing aberrant Lef/Tcf-related transcriptional activation. One caveat is that E-cadherin-induced growth retardation in melanoma cell lines renders our study on keratinocyte-mediated growth control more difficult to interpret. Nevertheless, a specific growth regulation by keratinocytes was evident because E-cadherin-transduced melanoma cells when physically separated from keratinocytes exhibited increased relative cell ratios over time.
In our co-culture model, keratinocytes down-regulated melanoma-associated cell adhesion molecules, MelCAM/MUC18 and β3 integrin subunit, in E-cadherin-transduced melanoma cells (Figure 3) ▶ . This suggests that E-cadherin-mediated signal transduction may contribute to the regulation of cell adhesion molecules expression. Indeed, cross-talk between adhesion receptors has been described recently. Hodivala and Watt 55 provided evidence that cadherins play a role in integrin down-regulation during keratinocyte terminal differentiation. Monier-Gavelle and Duband 56 reported that β1 and β3 integrins control cell surface distribution and activity of N-cadherin in migrating neural crest cells. Forced expression of α5 integrin subunit in primary quail myoblasts also up-regulates N-cadherin expression. 57 Therefore, cross-talk between adhesion molecules may represent one of the mechanisms through which specific cellular functions are coordinated.
Tumor invasion is the hallmark of malignancy. However, the invasive phenotype does not originate solely from the cancer cells themselves but also depends on microenvironmental factors including local growth factors, cytokines, and interactions between the cancer cells and surrounding host cells. 58 Our skin reconstruct model, which allows evaluation of the invasive potential of melanoma cells in a physiological milieu, demonstrated an anti-invasive effect of E-cadherin (Figure 5) ▶ . This effect may rely in a decreased mobility of melanoma cells due to E-cadherin-enhanced adhesion to adjacent keratinocytes, or alternatively, to E-cadherin-induced modulation of invasion-related cell adhesion molecules (such as MelCAM/MUC18 and β3 integrin subunit) and proteolytic enzymes important for matrix degradation. 59 The importance of adhesion receptors for the tumorigenic phenotype was demonstrated in our previous study where overexpression of the β3 integrin subunit in nontumorigenic and noninvasive radial growth phase primary melanoma cells induced conversion and progression to the tumorigenic and invasion-competent vertical growth phase primary melanoma. 48 The observed down-regulation of MelCAM/MUC18 and β3 integrin subunit in co-cultures further pointed to the plasticity of the antigenic phenotype of malignant cells suggesting plausible means to modulate expression of tumor-associated molecules and reverse natural progression.
It remains, however, unclear whether signals from keratinocytes for the control of growth and expression of melanoma-associated adhesion receptors of melanocytes/melanoma cells are transduced directly through E-cadherin/catenin pathway or indirectly through other molecules whose function requires E-cadherin-mediated cell-cell adhesion. It is hoped that once the molecular details of these mechanisms are discovered, it may be possible to activate them in the absence of keratinocytes. Therapeutic applications of this strategy could lead to a static tumor without further proliferation, invasion, and metastasis.
Acknowledgments
We are grateful to Dr. David L. Rimm for the generous gift of cDNA. Dr. David J. Easty (University College Dublin, Dublin, United Kingdom) is acknowledged for tireless proofreading and stimulating discussions.
Footnotes
Address reprint requests to Dr. Meenhard Herlyn, The Wistar Institute, 3601 Spruce Street, Philadelphia, PA 19104. E-mail: herlynm@wistar.upenn.edu.
Supported by National Institutes of Health grants CA-76674, CA-25874, and CA-15479, by the Wistar Cancer Center Core Grant (CA-10815), and NASA grant NAG-9–832.
M.-Y. Hsu’s current address: Department of Pathology, Thomas Jefferson University, Philadelphia, PA 19107.
F. E. Meier’s current address: Department of Dermatology, University of Tübingen, Tübingen, Germany.
J.-Y. Hsu’s current address: Sidney Kimmel Cancer Center, San Diego, CA 92121.
References
- 1.Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA: E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci 1994, 107:983-992 [DOI] [PubMed] [Google Scholar]
- 2.Hsu M-Y, Wheelock MJ, Johnson KR, Herlyn M: Shifts in cadherin profiles between human normal melanocytes and melanomas. J Invest Dermatol Symp Proc 1996, 1:188-194 [PubMed] [Google Scholar]
- 3.Herlyn M, Rodeck U, Mancinati ML, Cardillo FM, Lang A, Ross AH, Jambrosi J, Koprowski H: Expression of melanoma-associated antigens in rapidly dividing human melanocytes in culture. Cancer Res 1987, 47:3057-3061 [PubMed] [Google Scholar]
- 4.Elder DE, Rodeck U, Thurin J, Cardillo F, Clark WH, Stewart R: Antigenic profile of tumor progression in human melanocytic nevi and melanomas. Cancer Res 1989, 49:5091-5096 [PubMed] [Google Scholar]
- 5.Valyi-Nagy IT, Hirka G, Jensen PJ, Shih I-M, Juhasz I, Herlyn M: Undifferentiated keratinocytes control growth, morphology, and antigen expression of normal melanocytes through cell-cell contact. Lab Invest 1993, 69:152-159 [PubMed] [Google Scholar]
- 6.Shih I-M, Elder DE, Hsu M-Y, Herlyn M: Regulation of Mel-CAM/MUC18 expression on melanocytic cells of different stages of tumor progression by normal keratinocytes. Am J Pathol 1994, 145:837-845 [PMC free article] [PubMed] [Google Scholar]
- 7.Takeichi M: Cadherin cell adhesion receptors as a morphogenetic regulator. Science 1991, 251:1451-1455 [DOI] [PubMed] [Google Scholar]
- 8.Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehman MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA: Structural basis of cell-cell adhesion by cadherins. Nature 1995, 374:327-337 [DOI] [PubMed] [Google Scholar]
- 9.Cowin P: Unraveling the cytoplasmic interactions of the cadherin superfamily. Proc Natl Acad Sci USA 1994, 91:10759-10761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsukita S, Oishi K, Akiyama T, Yamanashi Y, Yamamoto T, Tsukita S: Specific proto-oncogenic tyrosine kinases of src family are enriched in cell-to-cell adherens junctions where the level of tyrosine phosphorylation is elevated. J Cell Biol 1991, 113:867-879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rim DL, Del Vecchio RL, Tonks NK: Dynamic interaction of PTPmu with multiple cadherins in vivo. J Cell Biol 1998, 141:287-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reynolds AB, Daniel JM, Mo YY, Wu J, Zhang Z: The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res 1996, 225:328-337 [DOI] [PubMed] [Google Scholar]
- 13.Watabe M, Nagafuchi A, Tsukita S, Takeichi M: Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol 1994, 127:247-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyaki M, Tanaka K, Kikuchi-Yanoshita R, Muraoka M, Konishi M, Takeichi M: Increased cell-substratum adhesion, and decreased gelatinase secretion and cell growth, induced by E-cadherin transfection of human colon carcinoma cells. Oncogene 1995, 11:2547-2552 [PubMed] [Google Scholar]
- 15.Kandikonda S, Oda D, Niederman R, Sorkin BC: Cadherin-mediated adhesion is required for normal growth regulation of human gingival epithelial cells. Cell Adhesion Commun 1996, 4:13-24 [DOI] [PubMed] [Google Scholar]
- 16.Soler C, Grangeasse C, Baggetto LG, Damour O: Dermal fibroblast proliferation is improved by β-catenin overexpression and inhibited by E-cadherin expression. FEBS Lett 1999, 442:178-182 [DOI] [PubMed] [Google Scholar]
- 17.Lewis JE, Jensen PJ, Wheelock MJ: Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J Invest Dermatol 1994, 102:870-877 [DOI] [PubMed] [Google Scholar]
- 18.Jawhari A, Farthing M, Pignatelli M: The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homeostasis: more than intercellular glue? Gut 1997, 41:581-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redfield A, Nieman MT, Knudsen KA: Cadherins promote skeletal muscle differentiation in three-dimensional cultures. J Cell Biol 1997, 138:1323-1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P: The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem 1995, 270:5549-5555 [DOI] [PubMed] [Google Scholar]
- 21.Su LK, Vogelstein B, Kinzler KW: Association of the APC tumor suppressor protein with catenins. Science 1993, 262:1734-1737 [DOI] [PubMed] [Google Scholar]
- 22.Kanai Y, Ochiai A, Shibata T, Oyama T, Ushijima S, Akimot S, Hirohashi S: c-erbB-2 gene product directly associates with β-catenin and plakoglobin. Biochem Biophys Res Commun 1995, 208:1067-1072 [DOI] [PubMed] [Google Scholar]
- 23.Shimamura K, Hirano S, McMahon AP, Takeichi M: Wnt-1-dependent regulation of local E-cadherin and alpha N-catenin expression in the embryonic mouse brain. Development 1994, 120:2225-2234 [DOI] [PubMed] [Google Scholar]
- 24.Hazan RB, Norton L: The epidermal growth factor receptor modulates the interaction of E-cadherin with the actin cytoskeleton. J Biol Chem 1998, 273:9078-9084 [DOI] [PubMed] [Google Scholar]
- 25.Tamura S: The E-cadherin-mediated cell-cell adhesion system in human cancers. Br J Surg 1997, 84:899-900 [DOI] [PubMed] [Google Scholar]
- 26.Hunt NC, Douglas-Jones AG, Jasani B, Morgan JM, Pignatelli M: Loss of E-cadherin expression associated with lymph node metastases in small breast carcinomas. Virchows Arch 1997, 430:285-289 [DOI] [PubMed] [Google Scholar]
- 27.Kinsella AR, Lepts GC, Hill CL, Jones M: Reduced E-cadherin expression correlates with increased invasiveness in colorectal carcinoma cell lines. Clin Exp Metastasis 1994, 12:335-342 [DOI] [PubMed] [Google Scholar]
- 28.Umbas R, Isaacs WB, Bringuier PP, Schaafsma HE, Karthaus HF, Oosterhof GO, Debruyne FM, Schalken JA: Decreased E-cadherin expression is associated with poor prognosis in patients with prostate cancer. Cancer Res 1994, 54:3929-3933 [PubMed] [Google Scholar]
- 29.Otto T, Birchmeier W, Schmidt U, Hinke A, Schipper J, Rubben H, Raz A: Inverse relation of E-cadherin and autocrine motility factor receptor expression as a prognostic factor in patients with bladder carcinomas. Cancer Res 1994, 54:3120-3123 [PubMed] [Google Scholar]
- 30.Katagiri A, Watanabe R, Tomita Y: E-cadherin expression in renal cell cancer and its significance in metastasis and survival. Br J Cancer 1995, 71:376-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slagle BL, Zhou YZ, Birchmeier W, Scorsone KA: Deletion of the E-cadherin gene in hepatitis B virus-positive Chinese hepatocellular carcinomas. Hepatology 1993, 18:757-762 [DOI] [PubMed] [Google Scholar]
- 32.Veatch AL, Carson LF, Ramakrishnan S: Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer 1994, 58:393-399 [DOI] [PubMed] [Google Scholar]
- 33.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, Kloppel G, Lemoine NR: Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol 1994, 174:243-248 [DOI] [PubMed] [Google Scholar]
- 34.Otto T, Rembrink K, Goepel M, Meyer-Schwickerath M, Rubben H: E-cadherin: a marker for differentiation and invasiveness in prostatic carcinoma. Urol Res 1993, 21:359-362 [DOI] [PubMed] [Google Scholar]
- 35.Andrews NA, Jones AS, Helliwell TR, Kinsella AR: Expression of the E-cadherin-catenin cell adhesion complex in primary squamous cell carcinomas of the head and neck and their nodal metastases. Br J Cancer 1997, 75:1474-1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vleminckx K, Vakaet L, Jr, Mareel M, Fiers W, van Roy F: Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991, 66:107-119 [DOI] [PubMed] [Google Scholar]
- 37.Hermiston ML, Wong MH, Gordon JI: Forced expression of E-cadherin in the mouse intestinal epithelium shows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev 1996, 10:985-996 [DOI] [PubMed] [Google Scholar]
- 38.Frixen UH, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W: E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 1991, 113:173-185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilding J, Vousden KH, Soutter WP, McCrea PD, Del Buono R, Pignatelli M: E-cadherin transfection down-regulates the epidermal growth factor receptor and reverses the invasive phenotype of human papilloma virus-transfected keratinocytes. Cancer Res 1996, 56:5285-5292 [PubMed] [Google Scholar]
- 40.Mbalaviele G, Dunstan CR, Sasaki A, Williams PJ, Mundy GR, Yoneda T: E-cadherin expression in human breast cancer cells suppresses the development of osteolytic bone metastases in an experimental metastasis model. Cancer Res 1996, 56:4063-4070 [PubMed] [Google Scholar]
- 41.Hsu M-Y, Herlyn M: Cultivation of normal human epidermal melanocytes. Jones GE eds. Human Cell Culture Protocols. 1996, :pp 9-20 The Humana Press Inc., Totowa, New Jersey, [DOI] [PubMed] [Google Scholar]
- 42.Hsu M-Y, Elder DE, Herlyn M: The Wistar melanoma (WM) cell lines. Masters JRW Palsson B eds. Human Cell Culture. 1999, :pp 259-274 Kluwer Academic Publishers, Norwell, Massachusetts, [Google Scholar]
- 43.Van Belle PA, Elentitsas R, Satyamoorthy K, Wolfe J, Guerry D, IV, Schuchter L, Van Belle TJ, Albelda S, Tahin P, Herlyn M, Elder DE: Progression-related expression of β3 integrin in melanoma and nevi. Hum Pathol 1999, 30:562-567 [DOI] [PubMed] [Google Scholar]
- 44.Shih I-M, Elder DE, Speicher D, Johnson JP, Herlyn M: Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Res 1994, 54:2514-2520 [PubMed] [Google Scholar]
- 45.Graham FL, Prevec L: Methods for construction of adenovirus vectors. Mol Biotechnol 1995, 3:207-220 [DOI] [PubMed] [Google Scholar]
- 46.Kozarsky K, Grossman M, Wilson JM: Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat Cell Mol Genet 1993, 19:449-458 [DOI] [PubMed] [Google Scholar]
- 47.Ranheim TS, Shisler J, Horton TM, Wold L, Gooding LR, Wold WS: Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J Virol 1993, 67:2159-2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu M-Y, Shih D-T, Meier FE, Van Belle P, Hsu J-Y, Elder DE, Buck CA, Herlyn M: Adenoviral gene transfer of β3 integrin subunit induces conversion from radial to vertical growth phase in primary human melanoma. Am J Pathol 1998, 153:1435-1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meier F, Nesbit M, Hsu M-Y, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Crombleholme T, Herlyn M: Human melanoma progression in skin reconstructs: biological significance of bFGF. Am J Pathol 2000, 156:193-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark WHJ: Tumor progression and the nature of cancer. Br J Cancer 1991, 64:631-644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones PL, Schmidhauser C, Bissell MJ: Regulation of gene expression and cell function by extracellular matrix. Crit Rev Eukaryot Gene Expr 1993, 3:137-154 [PubMed] [Google Scholar]
- 52.Huntsman DG, Caldas C: Assignment of the E-cadherin gene (CDH1) to chromosome 16q22.1 by radiation hybrid mapping. Cytogenet Cell Genet 1998, 83:82-83 [DOI] [PubMed] [Google Scholar]
- 53.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P: Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 1997, 275:1790-1792 [DOI] [PubMed] [Google Scholar]
- 54.Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, Kanski C: Target genes of β-catenin-T cell factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci USA 1999, 96:1603-1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodivala KJ, Watt FM: Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol 1994, 124:589-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monier-Gravelle F, Duband JL: Cross-talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by β1 and β3 integrins in migrating neural crest cells. J Cell Biol 1997, 137:1663-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huttenlocher A, Lakonishok M, Kinder MWS, Truong TM, Knudsen KA, Horwitz AF: Integrin and cadherin synergy regulates contact inhibition of migration and motile activity. J Cell Biol 1998, 141:515-526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mareel M, Berx G, Van Roy F, Bracke M: Cadherin/catenin complex: a target for anti-invasive therapy? J Cell Biochem 1996, 61:524-530 [DOI] [PubMed] [Google Scholar]
- 59.Frixen UH, Nagamine Y: Stimulation of urokinase-type plasminogen activator expression by blockage of E-cadherin dependent cell-cell adhesion. Cancer Res 1993, 53:3618-3623 [PubMed] [Google Scholar]