Abstract
Synaptotagmins form a family of calcium-sensor proteins implicated in exocytosis, and these vesicular transmembrane proteins are endowed with two cytosolic calcium-binding C2 domains, C2A and C2B. Whereas the isoforms syt1 and syt2 have been studied in detail, less is known about syt9, the calcium sensor involved in endocrine secretion such as insulin release from large dense core vesicles in pancreatic β-cells. Using cell-based assays to closely mimic physiological conditions, we observed SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor)-independent translocation of syt9C2AB to the plasma membrane at calcium levels corresponding to endocrine exocytosis, followed by internalization to endosomes. The use of point mutants and truncations revealed that initial translocation required only the C2A domain, whereas the C2B domain ensured partial pre-binding of syt9C2AB to the membrane and post-stimulatory localization to endosomes. In contrast with the known properties of neuronal and neuroendocrine syt1 or syt2, the C2B domain of syt9 did not undergo calcium-dependent membrane binding despite a high degree of structural homology as observed through molecular modelling. The present study demonstrates distinct intracellular properties of syt9 with different roles for each C2 domain in endocrine cells.
Keywords: Ca2+, exocytosis, membrane, secretory granule, translocation
Abbreviations: CCD, charged-coupled-device; eGFP, enhanced green fluorescent protein; KRB, Krebs–Ringer buffer; LDCV, large dense core vesicle; MAb, monoclonal antibody; PKC, protein kinase C; SNAP-25, 25 kDa synaptosome-associated protein; SNARE, soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor; TfR, transferrin receptor; VAMP, vesicle-associated membrane protein
INTRODUCTION
Insulin secretion in pancreatic β-cells proceeds by exocytosis of LDCVs (large dense core vesicles) and Ca2+ constitutes the final trigger for fusion of vesicle and plasma membrane [1,2]. Synaptotagmins have been identified as a major Ca2+ sensor in neuronal exocytosis based on genetic, electrophysiological and biochemical evidence [3,4]. They form a family of transmembrane proteins containing two cytosolic C2 domains, C2A and C2B [3,4]. The most studied synaptotagmins, syt1 and syt2, bind phospholipids and SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) proteins in a Ca2+-dependent fashion via the C2 domains and thereby attach to lipid membranes and protein complexes involved in exocytosis [3]. In reconstituted systems this mechanism seems to be instrumental for membrane fusion [5]. In addition to their interaction with exocytotic proteins, synaptotagmins may also intervene in endocytosis as suggested previously [6]. They are indeed known to interact with several components required for endocytosis [7–9]. Certain observations suggest that this may be mediated by the second C2 domain, C2B, although it is probably not its sole function [10–12].
The 16 synaptotagmins currently known can be classified according to their biochemical properties and by sequence alignment [13]. Ca2+-dependent phospholipid binding has been reported for syt1, syt2, syt3, syt5, syt7 and syt9 [14] and these isoforms have been implicated in the regulation of membrane fusion in different models. In insulin-secreting cells the use of dominant-negative mutants, recombinant proteins and knockdowns has demonstrated a role in exocytosis for syt1, syt2 and syt9, the latter being the only isoform expressed in primary β-cells [15–17]. Syt9 represents a close homologue to syt1 on sequence alignments with a consensus of 69% (C2A) and 72% (C2B), although its structure has not yet been reported. Whereas the kinetics of membrane translocation in the case of syt9 are compatible with endocrine exocytosis, the Ca2+-sensitivity of the cytosolic C2AB domains is not easily to reconcile [18–20]. In addition, divergent results have been published concerning the ability of the syt9 domains and especially the C2B domain, to interact with phospholipids and SNARE proteins [21,22].
In view of the importance of syt9 as an endocrine Ca2+ sensor for the exocytosis of LDCVs, in the present study we have addressed its translocation and Ca2+-dependency as well as the role of the two C2 domains. We have used cell-based assays employing transient expression and fluorescence or biochemical characterization. This also allows us to determine the site of translocation and to circumvent problems inherent to synaptotagmins expressed in bacteria [23,24]. In the present study we report Ca2+-dependent translocation of C2AB with an EC50 compatible with endocrine secretion and solely supported by the C2A domain. In contrast, the C2B domain impeded translocation but was required for subsequent localization of C2AB to endocytotic structures.
MATERIALS AND METHODS
Antibodies
The following antibodies were employed: MAb (monoclonal antibody) anti-TfR (transferrin receptor; H684, Sigma), MAb anti-syt2, MAb anti-syt9 (BD Biosciences), anti-syntaxin (clone HPC1, Sigma), anti-β-actin (Abcam), polyclonal anti-eGFP (enhanced green fluorescent protein; SantaCruz Biotechnologies) and anti-SNAP-25 (25 kDa synaptosome-associated protein; Sternberger Monoclonals, Covance Research Products). Antibodies against chromogranin A, insulin, VAMP (vesicle-associated membrane protein) 2 and synaptophysin were as previously described [25–27]. HRP (horseradish peroxidase)-linked anti-mouse or anti-rabbit secondary antibodies were purchased from Amersham Biosciences. A Cy3-labelled anti-mouse secondary antibody was obtained from Jackson ImmunoResearch.
Plasmids
The plasmid peGFP-Rab7 was provided by B. van Deurs (Department of Medical Anatomy, Panum Institute, University of Copenhagen, Copenhagen, Denmark), peGFP-CD63 by G. Griffiths (Sir William Dunn School of Pathology, University of Oxford, Oxford, U.K.), mRFP-Phogrin by W. Almers (Vollum Institute, Oregon Health Science University, Portland, OR, U.S.A.), BONT/C and BOTN/E by H. Niemann, H. Binz and T. Binz, (Institut für Biochemie, Medizinische Hochschule Hannover, Hannover, Germany) and peGFP-PKC by C. Larsson (Department of Laboratory Medicine, University of Lund, Sweden). The vector pRSET-BmStrawberry was donated by R. Tsien [28]. The plasmid pKS-Syt2 was as described previously [15].
Plasmid construction and mutagenesis
cDNA encoding mouse syt9 was generated by RT (reverse transcriptase)-PCR from total brain RNA and inserted in a pGEMT-T cloning vector. Truncated syt2 or syt9 was generated by PCR from pGEMT-syt9 or pKS-syt2 plasmids respectively, using the corresponding sense and antisense primers with an EcoRI site (underlined) inserted in the forward primer and a BamHI in the reverse primer. Primers were as follows: syt9C2AB (amino acids 77–386), sense primer 5′-TTGGAATTCATGCTGGGCCGGAGTTACATAG-3′, antisense primer 5′-TCTGGATCCTCCGGGTGCAGGTATTGGCC-3′; syt9C2A (77–233): sense primer 5′-TTGGAATTCATGCTGGGCCGGAGTTACATAG-3′, antisense primer 5′-TCTGGATCCAGCCACCTGCAGCTCTCTC-3′; syt9C2B (215–386): sense primer 5′-TTGGAATTCATGAGTTCAGTGAACCTGG-3′, antisense primer 5′-TCTGGATCCTCCGGGTGCAGGTATTGGCC-3′; syt2C2AB (101–422): sense primer 5′-TTGGAATTCATGAAGGGCAAAGGCATGAAG-3′, antisense primer 5′-AGTGGATCCTTGTTCTTGCCCAGAAGAG-3′. The amplified coding sequences were inserted in the EcoRI/BamHI cloning sites either of peGFPN3 (Clontech Laboratories) for syt9 or in the EcoRI/BamHI cloning sites of peGFPN1 for syt2. Syt9C2AB-mStrawberry was constructed by PCR amplification of syt9C2AB using the primers 5′-CCCAAGCTTATGCTGGGCCGGAGTTACATAG-3′ and 5′-TCTGGATCCGGTCCGGGTGCAGGTATTGGCC-3′, HindIII and BamHI sites are underlined. After digestion with HindIII/BamHI, the amplicon was inserted in-frame into pCDNA3-mStrawberry. The pcDNA3-mStrawberry vector was constructed by insertion of the mStrawberry coding sequence into the restriction sites for BamHI and EcoRI of pcDNA3. All constructs were verified by sequencing of both strands. Point mutations were introduced into the syt9 coding sequence leading to the substitution of an aspartic acid residue to an asparagine residue using the Quikchange® site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The forward primers corresponding to each mutation were as follows (exchanged nucleotides given in bold) D145N: 5′-CCTAGGAGGTTCCTCAAATCCCTATGTTAGTGTCT-3′; D197,199N: 5′-GGTCATGGCGGTGTATAACTTTAATCGGTTCTCCCGCAACG-3′; D330,332N: 5′-CTGACTGTTCTGAATTATAACAAACTGGGGAAGAATGAG-3′.
Cell culture, transient transfection and biochemical membrane translocation assay
MIN6 cells (passage 21–30) and HIT-T15 cells (passage 75–85) were cultured as described previously [28,29]; after plating (72 h), MIN6 and HIT-T15 cells were transfected with plasmids using Lipofectamine™2000 (Invitrogen) or JetPEI™ (PolyPlus-Transfection) respectively according to the manufacturer's instructions. HIT-T15 cells seeded at 5×104 cells/ml 72 h prior transfection were grown in 24-well dishes and transfected with plasmid encoding for different cytosolic syt9 fusion proteins. After transfection (72 h), cells were washed with PBS (pH 7.4), detached by PBS containing 10 mM EDTA and centrifuged at 3000 g for 5 min at 4 °C. Pelleted cells were resuspended and incubated in intracellular buffer [140 mM L-glutamic acid/monopotassium salt, 5 mM NaCl, 7 mM MgSO4, 20 mM Hepes (pH 7), 1 μg/ml aprotinin, 1 μg/ml pepstatin, 1 μg/ml leupeptin and 0.5 mM PMSF] containing either 2 mM EGTA (non-stimulating conditions/in the absence of Ca2+) or 2 mM CaCl2 with 10 μM ionomycin (stimulating conditions/in the presence of Ca2+) for 5 min at 37 °C. Cells were then disrupted by sonication using pulses of 10 s for 1 min at 4 °C [27,29]. The cell debris and the nuclei were eliminated by a centrifugation at 500 g for 10 min at 4 °C. Supernatants were centrifuged at 55000 rev./min for 1 h at 4 °C (using a TLA120.1 rotor) to separate the membrane and the cytosolic fractions. The corresponding samples were suspended in Laemmli buffer, heated at 95 °C for 5 min and resolved on SDS/PAGE (10% gels). Proteins were electroblotted on to PVDF, stained, blocked and incubated with the appropriate antibodies [27,29]. The signal obtained with the ECL (enhanced chemiluminescence) kit Lumi-LightPlus Western blotting substrate (Roche Diagnostics) was detected using a CCD (charged-coupled-device)-camera (Roperts Scientific) and quantified with FluorChem v2.00 (Alpha Innotech) [26].
In vivo membrane translocation assay
MIN6 cells (1×104 cell/cm2) grown on 25 mm round glass coverslips were washed twice with 1 ml of KRB (Krebs–Ringer buffer), pH 7.4, supplemented with 0.05% BSA and 3 mM glucose at 37 °C [26,27]. In experiments using digitonin, cells were incubated in intracellular buffer supplemented with EGTA (0.4 mM) and then stimulated in the same buffer with a defined Ca2+ concentration in the presence of 30 μM digitonin. Buffered Ca2+ solutions were obtained as described previously [26,27] or, in the case of concentrations of free Ca2+ above 10 μM, calculated using Winmaxc (http://www.stanford.edu/∼cpatton/maxc.html) employing the chelators NTA (nitrilotriacetic acid) and HEDTA (N-hydroxyethylethylenediaminetriacetic acid) [29]. When ionomycin was used, cells were first incubated in KRB (pH 7.4) supplemented with 0.05% BSA and 3 mM glucose and stimulation was performed with 1 mM Ca2+ and 10 μM ionomycin in KRB. Cells were kept on a heated microscope stage during acquisition as described previously [26,27]. The stimulating buffer was pressure ejected (≈20.7 kPa for 10 s) from a micropipette held at approximately 20 μm from the cell. Imaging of living cells was performed at 1 frame/0.5 s using an inverted microscope (Nikon TD300 equipped with a Z-drive) coupled to a monochromator (Till Photonics) and appropriate emission filters. Images were recorded by a CCD-camera (Micromax 1300Y HS, Roperts Scientific) using Metamorph software (Universal Imaging) [30]. The percentage of cells in which translocation was observed was determined. Due to leakage of unbound syt9-constructs through the pores generated by the detergent, translocation is an all-or-nothing event in digitonin-permeabilized cells.
Fluorescence microscopy of fixed cells
MIN6 cells seeded at 2.6×104 cells/cm2 were grown on 12-mm round glass coverslips and transfected with the corresponding plasmid. After transfection (72 h), cells were washed twice with KRB and exposed to either KRB (non-stimulating conditions) or KRB containing 5 mM Ca2+ and 10 μM ionomycin (stimulating conditions) for 10 min at room temperature (22 °C). Cells were then fixed for 20 min at room temperature in 4% paraformaldehyde/PBS in the absence (non-stimulating conditions) or presence of 5 mM CaCl2 (stimulating conditions). Coverslips were either directly mounted or first stained with antibodies. In the latter case, coverslips were washed once with KRB and three times with PBS containing 2% BSA. Permeabilization was performed with 0.1% saponin in PBS containing 2% BSA for 30 min at room temperature and cells were stained with corresponding antibodies. Coverslips were mounted using an antifade kit (Vectashield® mounting medium, Vector Laboratories). Imaging was performed on a LMS 510 Meta confocal laser microscope (Zeiss). Co-localization of antibody staining was quantified using Metamorph software (Universal Imaging) by analysing localization and intensities of pixels (n=3) after thresholding. Values are given as means±S.E.M. [28,30]. Co-localization masks were generated with ImageJ using the plugin ‘RG2B co-localization’ with a minimum ratio between channels of 0.2 and thresholds of 26 (over 255) for each channel [30].
Molecular modelling
Simulations were carried out for following systems: (i) domain C2B of syt1 (NM001033680, amino acids 272–421 for modelling) [32] with 12323 water molecules plus 5 chloride ions, giving a total of 38.544 atoms in an initial box size of 7.37 nm3; (ii) C2B domain of syt9 (NM016908, amino acids 239–386) with 10967 water molecules plus 12 chloride ions giving a total of 34.388 atoms in an initial box size of 7.06 nm3. These systems were solvated with SPC (simple point charge) water and energy minimized. Molecular dynamic simulations were run using Gromacs [33]. A twin-range cut-off was used for longer range interactions: 1 nm for Van der Waals interactions and 1.8 nm for electrostatic interactions. The time step was 2 fs using Shake and NPT conditions were used to constrain bond lengths (i.e. constant number of particles, pressure and temperature in the simulation). A constant pressure of 100 kPa was employed in all three directions with a coupling constant of θr=1 ps [34]. Water and protein were coupled separately to a temperature bath at 300 K, with a coupling constant θr=0.1 ps. The system was equilibrated for 2 ns and the simulation run for another ns. Results were depicted as ribbon diagrams using VMD 1.8.4 (http://www.ks.uiuc.edu/Research/vmd/).
Statistical analyses
For the comparison of two groups, the Student's t test was used.
RESULTS
Biochemical characterization of syt9C2 domains in situ
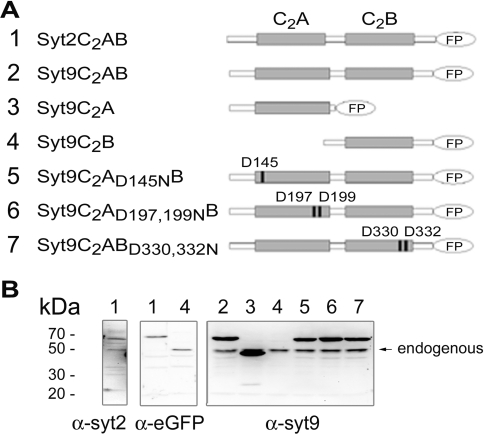
In our attempt to investigate the behaviour and translocation of the cytosolic domain of syt9 we have generated constructs and mutants encompassing the C2A and the C2B domain (see Figure 1) and compared it with syt2C2AB. All constructs harboured a fluorescent protein at their C-terminus, either eGFP or Strawberry [28]. Moreover, point mutations were introduced into the Ca2+-binding sites. Indeed, replacement of cognate aspartic acid residues by asparagine residues in the C2A domain of syt1 has been shown to partially (D145, numbering for syt9) or completely (D197/199 in syt9) abolish Ca2+-mediated effects of this domain [15,35]. Similarly, a double mutation in the Ca2+-binding site of the C2B domain of syt1 (corresponding to D330/332 of syt9) completely abolished its function in vivo [11]. The encoded proteins were expressed in insulin-secreting cells (Figure 1B) as demonstrated by the use of antibodies against syt9, which also recognized the endogenous forms (right-hand panel and middle panel), except for syt9C2B. The protein expressed by this construct no longer contained the antigenic epitope for the anti-syt9 antibody, but still reacted with anti-eGFP (Figure 1B, middle panel). Note that degradation products of fluorescent proteins were not detected.
Figure 1. Expression of syt2 and syt9 constructs.
(A) Schematic diagram representing the different syt9 and syt2 constructs used: syt2C2AB (101–422), syt9C2AB (77–386), syt9C2A (77–233) and syt9C2B (215–386). Mutation of the aspartic acid residue to an asparagine residue in the C2A and/or the C2B domains gave rise to D145N, D197N, D199N, D330N and D332N. All constructs are C-terminally tagged with a fluorescent protein (FP). (B) Expression of the constructs in HIT-T15 cells. Cells were transfected with the different variants. After transfection (72 h), cells were harvested and 20 μg of total proteins were separated by SDS/PAGE and immunoblotted with anti-syt2, anti-syt9 or anti-eGFP antibodies. The lane number corresponds to the construct number.
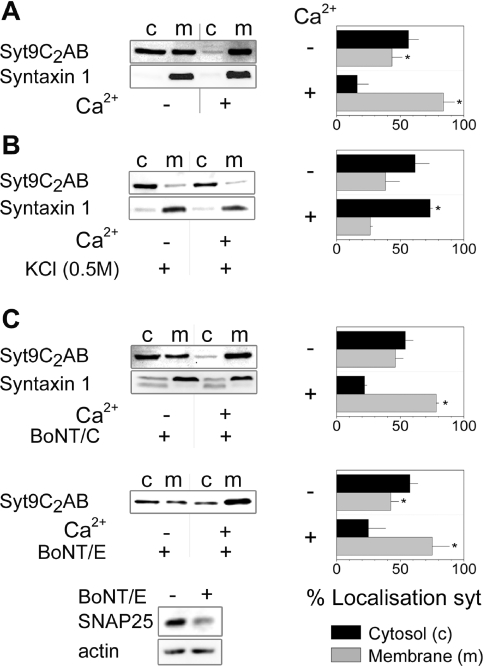
We subsequently used these constructs to examine the translocation of syt9 using a biochemical approach to characterize the behaviour of syt9C2AB. To this end cells were incubated in the absence or presence of Ca2+ (2 mM) and ionomycin (10 μM) prior to their fractionation into supernatant (cytosol) and membrane pellets. As shown in Figure 2(A), a considerable amount of syt9C2AB was already present at membranes in the absence of Ca2+ and the cation induced a complete shift of syt9C2AB to the membrane fraction. Membrane binding of syt9C2AB was sensitive to a high concentration of salt (Figure 2B), indicating the electrostatic nature of the interaction. Ca2+-sensitive synaptotagmins bind to membrane SNARE proteins such as syntaxin or SNAP-25 in a Ca2+-sensitive manner and this interaction is sensitive to the action of clostridial neurotoxins [36–38]. To test whether these SNARE proteins were involved in the translocation observed in the present study, we co-expressed syt9C2AB and botulinum neurotoxin E or C prior to analysis. Neither of the two toxins altered the Ca2+-sensitive distribution of syt9C2AB despite cleavage of syntaxin 1 and SNAP-25 (Figure 2C). Note that cleavage was not complete, as transient transfection led to expression only in a fraction of cells. Taken together these data demonstrate that syt9C2AB translocates to membranes in response to Ca2+ in these insulin-secreting cells. This event occured independently of SNARE proteins and most likely implies ionic interactions with membrane phospholipids.
Figure 2. Membrane binding of syt9C2AB–eGFP in HIT-T15 cells.
(A) After transfection (72 h) with syt9C2AB–eGFP, HIT-T15 cells were incubated for 5 min at 37 °C in the presence of Ca2+ (2 mM CaCl2 supplemented with 10 μM ionomycin) or in absence of Ca2+ (2 mM EGTA) and fractioned by ultracentrifugation at 55000 rev./min. Distribution of syt9C2AB–eGFP in supernatants (c) and membrane pellets (m) were analysed by Western blot using antibodies against syt9 and against the transmembrane protein syntaxin 1 as a control for fractionation. The distribution was quantified by densitometry as given by the histogram (on the right-hand side), Means±S.D.s are indicated (n=8). (B) Transfected cells were incubated in the absence or in the presence of Ca2+ supplemented with 0.5 M KCl. Fractionation and analysis were performed as described above (n=3). (C) HIT-T15 cells were co-transfected with syt9C2AB–eGFP and plasmids coding for botulinum neurotoxins BoNT/C and BoNT/E. Translocation and the effect of each toxin were analysed by immunoblotting using anti-syt9, anti-syntaxin1 or anti-SNAP-25 antibodies. Errors bars represent the S.D. (n=3); *2P<0.05 as compared with membranes.
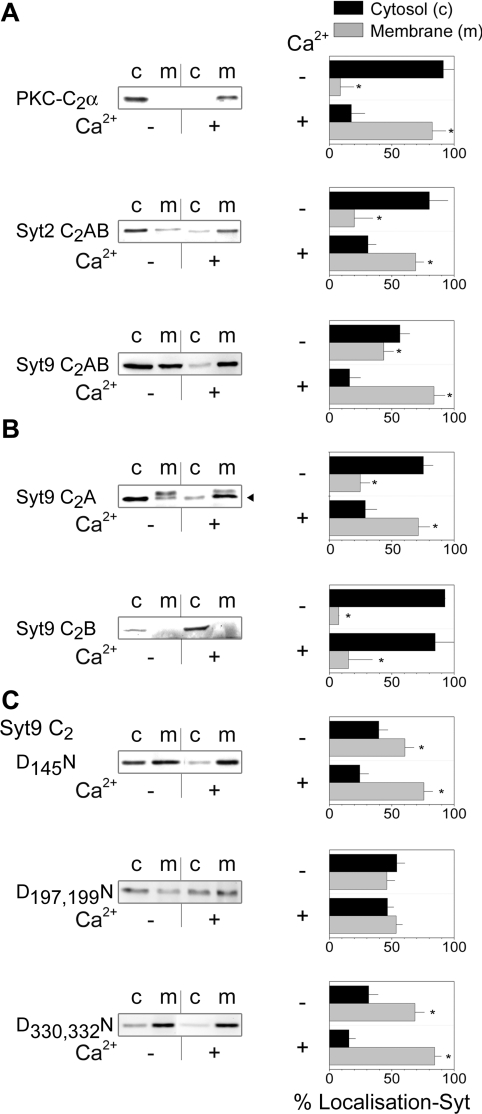
Next we addressed the role of the different domains of syt9C2AB in translocation by biochemical means and compared first the C2 domain of PKC (protein kinase C) with the C2AB domain of syt2 and syt9 (Figure 3A). Interestingly both PKC-C2α and syt2C2AB did not bind to membranes in the absence of Ca2+ in contrast with syt9C2AB. As expected, a rise in Ca2+ resulted in a complete translocation for all three constructs. The first C2 domain of syt9 alone, syt9C2A, was largely cytosolic in the absence of Ca2+ and fully translocated. It thus behaved as syt2C2AB and PKC-C2α. Note that a double band was observed (indicated with an arrowhead) as syt9C2A migrated just below endogenous syt9 and both were stained by the antibody used. In contrast with the C2A domain, the C2B domain remained mainly cytosolic despite the increase in Ca2+. Mutation of Asp145 to an asparagine residue in syt9C2AB did not alter translocation, whereas mutation of Asp197 and Asp199 rendered syt9C2AB insensitive to Ca2+ (Figure 3C). We also tested the role of the aspartic acid residues in the second C2 domain known to be required for Ca2+ co-ordination in syt1 and its function in neuroendocytosis [12]. Contrary to the mutations in syt9C2A, the mutations in the second C2 domain did not influence the Ca2+-induced distribution of syt9C2AB. However, syt9C2ABD330N, D332N exhibited a considerable increase in membrane binding in the absence of Ca2+ similar to the reported observations on the cognate mutation in syt1C2AB [35].
Figure 3. The C2A but not the C2B domain is responsible for Ca2+-dependent binding of syt9 to membranes.
(A) HIT-T15 cells expressing PKC-C2α–eGFP, syt2C2AB–eGFP and syt9C2AB–eGFP were incubated for 5 min at 37 °C in the presence (2 mM CaCl2 and 10 μM ionomycin) or the absence (2 mM EGTA) of Ca2+. The subsequent fractionation was analysed by Western blot using anti-GFP, anti-syt2 or anti-syt9 antibodies for PKC-C2α–eGFP, syt2C2AB–eGFP and syt9C2AB–eGFP respectively. Histograms on the right-hand side represent the distribution of the protein quantified by densitometry. Errors bars represent the S.D (n=3); *2P<0.05 as compared with membranes. (B) The same experiments in (A) were performed on HIT-T15 cells transfected with syt9C2A–eGFP and syt9C2B–eGFP. Syt9C2A–eGFP was revealed with the anti-syt9 antibody that detected the fluorescent protein (indicated by the arrowhead) and the endogenous protein. Syt9C2B–eGFP was revealed with the anti-GFP antibody as it does not contain the epitope for the anti-syt9 antibody. (C) Syt9C2AB–eGFP and its mutants were detected using anti-syt9.
Modelling of the syt9C2B domain
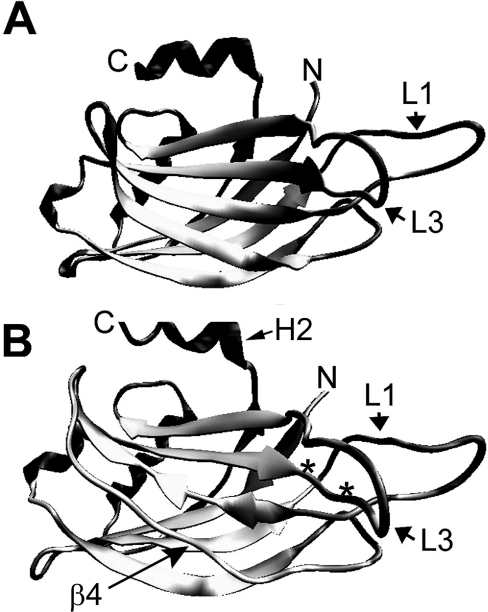
To get more insight into the differences between syt1 and syt9 we modelled the structure of syt9C2B using the published crystal structure of syt1C2B as a template, and performed molecular dynamic simulations following equilibration of the system (Figure 4). The syt9 sequence showed a striking overall resemblance to syt1, though some differences were apparent. The main distinction concerned the β-sheets 3 and 4 (Figure 4) which were partially or completely absent in syt9C2B. Sheet 4 contains seven lysine residues, which are generally unfavourable for β-sheet formation and modelling has probably failed to detect the sheets documented previously by NMR and crystallography for this reason (http://rsb.info.nih.gov/ij/ and [37]. As a control, we modelled syt1C2B alongside syt9C2B and again, both β-sheets were partially unfolded or were absent (results not shown). The sole additional, albeit minor, difference concerned the C-terminal acidic α-helix H2, which was shorter in syt9C2B compared with syt1C2B. In contrast, the main Ca2+ co-ordination sites were well-conserved and no difference was seen in the spatial arrangement around β-sheets 6 and 7, from which they emerge. Actually exchange of this portion in syt9C2B by the corresponding sequence of syt1C2B conferred ‘syt1-like’ biochemical properties to the mutated syt9C2B. Thus differences in spatial arrangement do not seem to be responsible for the particular behaviour of syt9C2B.
Figure 4. Molecular simulation of the syt9C2B domain.
Ribbon diagrams are shown. (A) Syt1C2B according to the crystal structure 1UOW. (B) C2B domain of syt9 after 3 ns simulation. The loops 1 and 3 (L1 and L3), the mutation D330N/D332N (*), the unstructured part corresponding to the β-sheet 4 in syt1 and the shortened α-helix H2 are indicated. Views are given to highlight the differences between the structures.
Translocation of syt9C2 domains in living cells
These biochemical assays suggest that the C2B domain does not positively influence translocation of syt9C2AB but rather impedes it. However, this approach may be influenced by the lengthy fractionation protocol and does not allow determination of the nature of the membrane(s) syt9 is binding to. We therefore resorted to the observation of membrane translocation in living cells by the use of fluorescent videomicroscopy. Moreover, we combined this approach with the use of a detergent, digitonin, to permeabilize the membrane and the use of defined Ca2+ buffers to equilibrate intracellular concentrations of the cation [26,37]. A typical experiment is shown in Figure 5(A) depicting the translocation observed for syt9C2A and syt9C2AB. Subsequent to the stimulus and membrane attachment, we observed the disappearance of fluorescent proteins over time. This is most likely due to leakage of fluorescent proteins through the pores formed by digitonin as it was also observed for eGFP itself and only minor bleaching occurred (results not shown). Indeed, chelation of the small volume of Ca2+ ejected from the pipette by EGTA present in the bath solution will rapidly decrease the concentration of free Ca2+. Consequently, even translocated syt9C2 constructs will detach and dilute in the bath. In the case of syt9C2B we only observed disappearance of the fluorescence without any attachment to membranes. We also observed some nuclear localization of the fluorescent proteins. This probably represents transiently expressed full-length syt9C2 constructs, as no degradation products containing eGFP were apparent on immunoblots (see Figure 1) and the proteins dissolved through the pores arguing against aggregation.
Figure 5. Stimulation of living cells by low micromolar Ca2+ translocates the C2A, but not the C2B domain, of syt9.
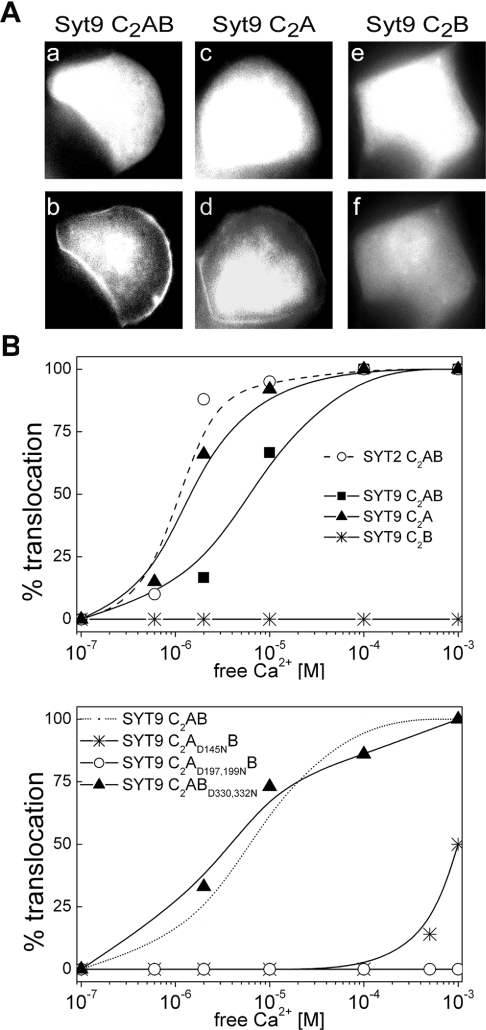
(A) MIN6 cells expressing syt9C2AB–eGFP, syt9C2A–eGFP or syt9C2B–eGFP were grown on coverslips and stimulated by pressure ejection of buffer containing 10 μM free Ca2+ supplemented with 30 μM digitonin. Cells were imaged at 37 °C by time-lapse microscopy. Images a, c and e were taken at 0 s and, b, d and f 3 s after stimulation. (B) Membrane binding affinity of syt9 variants. Experiments were performed in MIN6 cells as described in (A). Defined concentrations of free Ca2+ supplemented with digitonin were used to stimulate the cells.
Syt2C2AB and syt9C2AB exhibited distinct Ca2+ sensitivities EC50s at around 1 μM and 10 μM free Ca2+ respectively (Figure 5B). In contrast, syt9C2A behaved as syt2C2AB in terms of Ca2+ sensitivity in line with our observations in the biochemical assays (see above). Again syt9C2B remained purely cytosolic and no evidence, even on close direct inspection during the experiment or by confocal microscopy (results not shown), could be found for translocation (Figure 5B). This approach also revealed that syt9C2AB carrying the mutation D145N exhibited some degree of translocation but only at millimolar concentrations of free Ca2+, whereas translocation was completely abolished by the double mutation D197N/D199N. Again, the mutation D330N/D332N in the C2B domain did not alter the Ca2+ sensitivity of syt9C2AB. Thus in both the biochemical and the in vivo approaches the C2B domain of syt9 actually reduced the Ca2+-sensitivity and the extent of translocation.
Syt9C2B is required for late translocation to endosomes
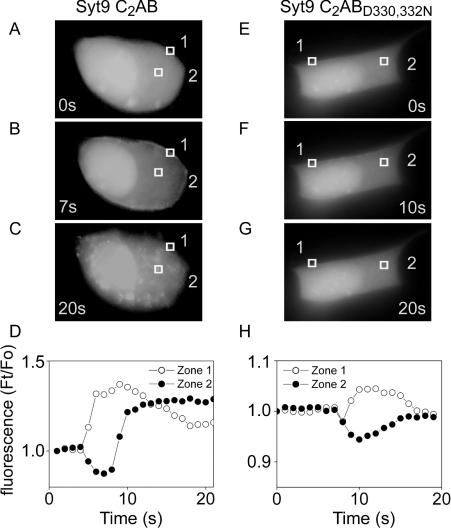
On close inspection of films it became apparent that syt9C2AB rapidly detached from the membrane once the perfusion with buffers containing elevated Ca2+ was stopped, and translocated to granular intracellular structures. However, as a large amount of syt9C2AB leaked out of the cell when using digitonin, imaging of those structures was difficult. We therefore used ionomycin and Ca2+ as a stimulus to improve visualization of these structures (Figures 6A–6D). At about 5 s after the beginning of membrane translocation, staining of these structures became apparent. Most interestingly, the D330N/D332N mutant of syt9C2AB translocated to the membrane, but never subsequently moved to intracellular structures, thus indicating a role for the C2B domain in the second step. Syt9C2B alone never translocated and both mutants of the C2A Ca2+-binding site (syt9C2AB D145N and D197N, D199N) failed to translocate to the plasma membrane under the conditions used here (intact cells, ionomycin) and were not found on intracellular structures (results not shown). It seems therefore that prior passage to the plasma membrane is required for localization on intracellular structures.
Figure 6. Syt9C2AB–eGFP but not syt9C2ABD330N/D332N–eGFP distributes to intracellular structures after stimulation with Ca2+.
MIN6 cells expressing syt9C2AB–eGFP (A, B and C) or syt9C2ABD330N/D332N–eGFP (E, F and G) were stimulated by 5 mM CaCl2 and 10 μM ionomycin. The distribution of the two fusion proteins was imaged and the three panels represent the distribution of the proteins at 0 s (A and E), 10 s (B and F) and 20 s after stimulation (C and G). GFP fluorescence was quantified in two different areas corresponding to plasma membrane (zone 1) and to the intracellular space (zone 2) (D and H). Fo, fluorescence at t=0 s; Ft, fluorescence at t. Images are representative of at least ten independent experiments.
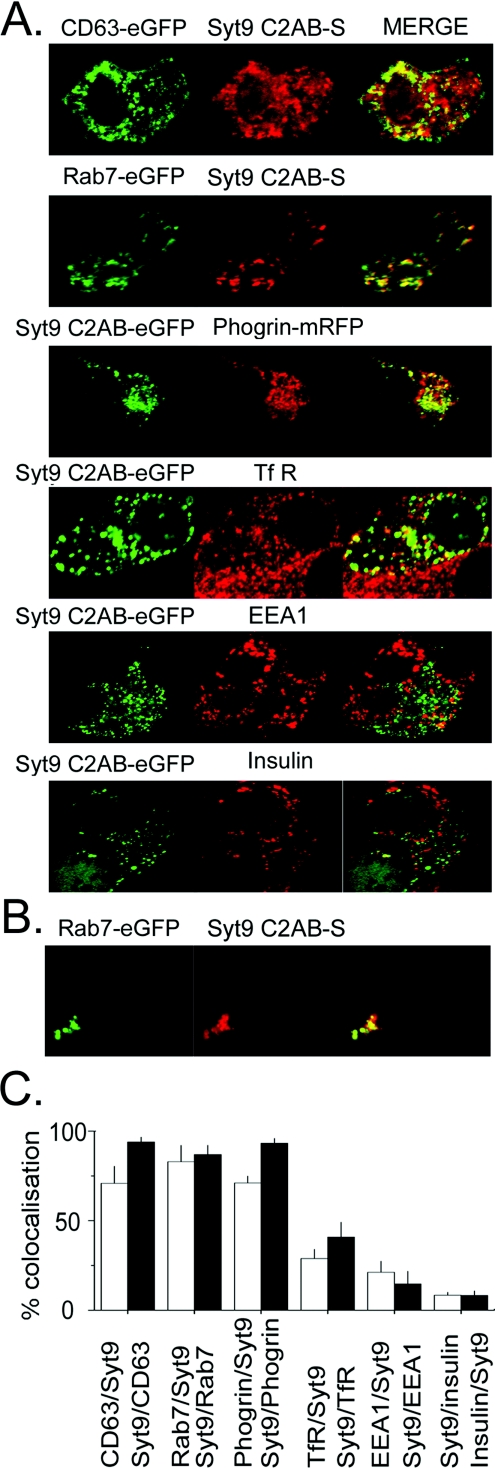
In view of the requirement of the C2B domain for this event we attempted to characterize the compartment(s) involved by co-expression of fluorescent fusion proteins and the use of antibodies. As the fusion proteins used as markers were tagged with eGFP, we used syt9C2AB linked to the fluorescent protein Strawberry (syt9C2AB–S) known to be monomeric [28]. Syt9C2AB–S behaved as syt9C2AB–eGFP and co-localized with eGFP-tagged syt9 upon co-transfection (results not shown). To exclude a potential interaction between fluorescent proteins, we tested the combination of syt9C2AB–S and LPH–eGFP, a plasma-membrane marker [29,30] and SVP38–eGFP [39]. Neither of these constructs co-localized with syt9C2AB–S suggesting that interactions between fluorescent proteins did not play any significant role (results not shown). A considerable number of compartments tested using antibodies also did not reveal any substantial degree of co-localization such as SNAP-25, syntaxin 1, VAMP2/synaptobrevin 2, insulin and chromogranin A. This excluded compartments harbouring these SNARE proteins or secretory granules as localization for syt9C2AB–eGFP. In contrast, we observed a high degree of co-localization for the endosomal markers CD63–eGFP [40] and rab7–eGFP [41] in fixed cells (see Figures 7A and 7C). In addition, co-localization was evident for phogrin–eGFP, known to be recycled through endosomal pathways [42], and to a lesser degree for the TfR. We also determined whether co-localization can be observed in living cells by videomicroscopy (see Figure 7B). Indeed, a considerable degree of co-localization was apparent for Rab7–eGFP and syt9C2AB–S after stimulation of cells with ionomycin. These observations are in line with an attachment of syt9C2AB to endosomal membranes.
Figure 7. Syt9C2AB redistributes to endosomes.
MIN6 cells transfected with indicated constructs were incubated for 10 min at room temperature in the presence of Ca2+ (5 mM CaCl2 and 10 μM ionomycin) to allow the translocation of syt9C2AB-FP [eGFP or S (Strawberry)] to the plasma membrane and to intracellular structures. Images corresponding to the different channels are given in the left-hand panels and middle panels; co-localization is indicated by ‘MERGE’ in the right-hand panels. EEA1, early endosomal antigen. (A) Cells were subsequently fixed and co-localization of the FP constructs analysed by confocal microscopy. (B) Living cells observed by videomicroscopy 10 min after stimulation. (C) Percentage of co-localization given as obtained from at least five experiments.
DISCUSSION
Synaptotagmins play a major role as Ca2+ sensors in exocytotic membrane fusion and in endocytosis [43,44]. Despite a considerable conservation in the Ca2+ and phospholipid-binding C2 domains among several isoforms, such as syt1, syt2 and syt9, differences are apparent which may be of functional importance. Our data indicate that the C2AB domain of syt9 is operational in living cells at Ca2+ levels present during exocytosis in pancreatic β-cells [20,45]. In contrast with syt1, however, the C2B domain lowers the Ca2+-affinity for membrane translocation, but is required for ensuing localization on endosomal structures.
In contrast with previous studies, we used transient expression of synaptotagmins in mammalian cells for our biochemical and fluorescent assays. Although liposomal systems permit a good control of the variables, they do not reproduce the lipid composition or membrane protein/lipid ratio present in native preparations as glycerophospholipids are largely over-represented. It is thus important to obtain affinities at intracellular conditions. Moreover, the use of recombinant synaptotagmins expressed in bacteria has been difficult due to precipitation [46,47] or to the presence of contaminants [23,24]. Our initial biochemical characterization revealed that the interactions are sensitive to ionic forces and can occur independently from SNARE proteins. Indeed, their cleavage by clostridial neurotoxins did not alter the distribution or extent of translocation. Note that divergent results on Ca2+-dependent binding of syt9C2AB to SNARE proteins had been reported previously [18,21]. Our observation does not exclude a role for the interaction with SNARE proteins in the membrane attachment of syt9, as they may direct synaptotagmins to specific sites, or in the function of syt9 during membrane fusion, which was not tested in the present study.
Two major differences were apparent when comparing the C2AB of syt9 and syt2. First, in both assays syt9C2AB was already in part attached to the membrane in non-stimulated living cells or in the absence of free Ca2+ in biochemical assays, whereas syt2C2AB was completely cytosolic under those conditions. The differential membrane attachment of the two isoforms in the absence of Ca2+ has been observed in some but not all liposome-based assays using phosphatidylcholine/phosphatidylserine vesicles and was more pronounced in the presence of PtdIns(4,5)P2 [21,22]. Cleavage of SNARE proteins by clostridial neurotoxins did not diminish the attachment in the absence of Ca2+ in our assays. Interestingly, neither syt9C2A alone nor syt9C2B alone demonstrated any membrane binding in the absence of Ca2+. Pre-binding of syt9C2AB to membranes is therefore independent of SNARE proteins and requires co-operation between the two C2 domains. Secondly, the EC50 for Ca2+ required in membrane translocation differed by one order of magnitude between syt2C2AB and syt9C2AB. Interestingly, syt9C2A alone was indistinguishable from syt2C2AB in this respect. The most likely explanation is provided by the increased pre-binding of syt9 in the presence of the C2B domain which abolishes a high-affinity component. Importantly, the EC50 observed here for syt9C2AB in living cells corresponds well with the values reported for Ca2+-stimulated exocytosis of insulin [20,45].
Whereas the C2A domains of syt9 behaved as expected, the C2B domain clearly differed from syt2C2B in respect to reported Ca2+-dependent membrane binding [22,48]. As to the behaviour of the C2B domain, divergent results have been reported using either the C2B domain alone [21] or a C2B domain containing in addition 23 amino acids of the sequence linking C2A and C2B [22]. Our modelling data support the notion that amino acids 238–386 are sufficient to form a stable C2B domain and additional N-terminal residues are not required. The observations reported in the present study clearly indicate that syt9C2B is not capable of translocating in the cellular environment. This difference is surprising in view of the high degree of sequence conservation as well as structural arrangement between the isoforms. As membrane attachment of syt1C2B is less resistant to salt washes than syt1C2A, minor changes in the C2B domain may be important [49]. Indeed, domain swapping between syt1 and syt9 around loop 3 restored Ca2+ sensitivity [21]. As the structure of this part of the sequence is conserved between the two isoforms in modelling, very subtle changes may be responsible for their distinct behaviour.
Although syt9C2B was fully dispensable for Ca2+-dependent membrane translocation, it was clearly implicated in the subsequent attachment to intracellular structures as syt9C2ABD330N, D332N still translocated to the plasma membrane, but was not internalized. Although syt9C2B does not bind Ca2+ in accordance to indirect assays as performed in the present study and by others [21], the mutation C2ABD330N, D332N is located at the base of a loop directed towards the membrane and may thus destabilize interactions [50]. Intracellular targeting of syt1C2A has been reported but was mainly directed to the trans-Golgi network in MDCK (Madin–Darby canine kidney) cells in contrast with our observation on both C2 domains of syt9 [51]. The reported localization may reflect distinct affinities for certain phosphoinositols or SNARE proteins. The co-distribution of marker proteins observed in the present study for syt9C2AB is compatible with late endosomes as a major location and a minor location on early endosomes. Syt9 has been previously reported on endocytotic compartments in mast cells and syt9C2B interacts with the clathrin adaptor complex AP-2, as had been shown for other synaptotagmins [7,52]. This provides a potential mechanism for internalization. As targeting to the intracellular structures required the presence of the C2A domain, we suggest that prior contact with the plasma membrane is required. The fluorescent protein probably accompanies membrane internalization, but is not directly transferred from the cytosol to endosomes. The functional implication is yet unclear in the case of syt9. The C2B domain of syt1 is implicated in the regulation of endocytosis and interestingly requires the Ca2+-co-ordinating aspartic acid residues in C2B cognate to those which abolished internalization of syt9C2AB [53].
What might be the functional outcome of the differences between syt1 and syt9 in terms of C2 domains? Whereas in clonal β-cells syt1, syt2 and syt9 are expressed on LDCVs and function in exocytosis, primary cells contain seemingly only syt9 and this isoform should therefore not be a redundant Ca2+-sensor [15–17,54]. The Ca2+ dependency of syt9C2AB fits well with the physiological Ca2+ requirements for insulin exocytosis and the observed right shift may be compensated by pre-binding to the membrane via the C2B domain. Such a mechanism may be an advantage in a cell that secretes mainly at limited time periods, i.e. in response to nutritional stimuli.
References
- 1.Henquin J. C. Pathways in β-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes. 2004;53:S48–S58. doi: 10.2337/diabetes.53.suppl_3.s48. [DOI] [PubMed] [Google Scholar]
- 2.Lang J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 1999;259:3–17. doi: 10.1046/j.1432-1327.1999.00043.x. [DOI] [PubMed] [Google Scholar]
- 3.Jahn R., Lang T., Sudhof T. C. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 4.Sudhof T. C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 5.Bhalla A., Chicka M. C., Tucker W. C., Chapman E. R. Ca2+-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion. Nat. Struct. Mol. Biol. 2006;13:323–330. doi: 10.1038/nsmb1076. [DOI] [PubMed] [Google Scholar]
- 6.DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J. Z., Davletov B. A., Sudhof T. C., Anderson R. G. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 8.von Poser C., Zhang J. Z., Mineo C., Ding W., Ying Y., Sudhof T. C., Anderson R. G. Synaptotagmin regulation of coated pit assembly. J. Biol. Chem. 2000;275:30916–30924. doi: 10.1074/jbc.M005559200. [DOI] [PubMed] [Google Scholar]
- 9.Fergestad T., Broadie K. Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis. J. Neurosci. 2001;21:1218–1227. doi: 10.1523/JNEUROSCI.21-04-01218.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llinas R. R., Sugimori M., Moran K. A., Moreira J. E., Fukuda M. Vesicular reuptake inhibition by a synaptotagmin I C2B domain antibody at the squid giant synapse. Proc. Natl. Acad. Sci. U.S.A. 2004;101:17855–17860. doi: 10.1073/pnas.0408200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackler J. M., Drummond J. A., Loewen C. A., Robinson I. M., Reist N. E. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- 12.Earles C. A., Bai J., Wang P., Chapman E. R. The tandem C2 domains of synaptotagmin contain redundant Ca2+ binding sites that cooperate to engage t-SNAREs and trigger exocytosis. J. Cell Biol. 2001;154:1117–1124. doi: 10.1083/jcb.200105020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craxton M. Synaptotagmin gene content of the sequenced genomes. BMC Genomics. 2004;5:43. doi: 10.1186/1471-2164-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickman C., Craxton M., Osborne S., Davletov B. Comparative analysis of tandem C2 domains from the mammalian synaptotagmin family. Biochem. J. 2004;378:681–686. doi: 10.1042/BJ20031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang J., Fukuda M., Zhang H., Mikoshiba K., Wollheim C. B. The first C2 domain of synaptotagmin is required for exocytosis of insulin from pancreatic β-cells: action of synaptotagmin at low micromolar calcium. EMBO J. 1997;16:5837–5846. doi: 10.1093/emboj/16.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gut A., Kiraly C. E., Fukuda M., Mikoshiba K., Wollheim C. B., Lang J. Expression and localization of synaptotagmin isoforms in endocrine β-cells: their function in insulin exocytosis. J. Cell Sci. 2001;114:1709–1716. doi: 10.1242/jcs.114.9.1709. [DOI] [PubMed] [Google Scholar]
- 17.Iezzi M., Eliasson L., Fukuda M., Wollheim C. B. Adenovirus-mediated silencing of synaptotagmin 9 inhibits Ca2+-dependent insulin secretion in islets. FEBS Lett. 2005;579:5241–5246. doi: 10.1016/j.febslet.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 18.Bhalla A., Tucker W. C., Chapman E. R. Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion. Mol. Biol. Cell. 2005;16:4755–4764. doi: 10.1091/mbc.E05-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rorsman P., Eliasson L., Renstrom E., Gromada J., Barg S., Gopel S. The cell physiology of biphasic insulin secretion. News Physiol. Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- 20.Vallar L., Biden T. J., Wollheim C. B. Guanine nucleotides induce Ca2+-independent insulin secretion from permeabilized RINm5F cells. J. Biol. Chem. 1987;262:5049–5056. [PubMed] [Google Scholar]
- 21.Shin O. H., Maximov A., Lim B. K., Rizo J., Sudhof T. C. Unexpected Ca2+-binding properties of synaptotagmin 9. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2554–2559. doi: 10.1073/pnas.0308477100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tucker W. C., Edwardson J. M., Bai J., Kim H. J., Martin T. F., Chapman E. R. Identification of synaptotagmin effectors via acute inhibition of secretion from cracked PC12 cells. J. Cell Biol. 2003;162:199–209. doi: 10.1083/jcb.200302060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin O. H., Rhee J. S., Tang J., Sugita S., Rosenmund C., Sudhof T. C. Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron. 2003;37:99–108. doi: 10.1016/s0896-6273(02)01145-5. [DOI] [PubMed] [Google Scholar]
- 24.Ubach J., Lao Y., Fernandez I., Arac D., Sudhof T. C., Rizo J. The C2B domain of synaptotagmin I is a Ca2+-binding module. Biochemistry. 2001;40:5854–5860. doi: 10.1021/bi010340c. [DOI] [PubMed] [Google Scholar]
- 25.Boal F., Le Pevelen S., Cziepluch C., Scotti P., Lang J. Cysteine-string protein isoform β (Cspβ) is targeted to the trans-Golgi network as a non-palmitoylated CSP in clonal β-cells. Biochim. Biophys. Acta. 2007;1773:109–119. doi: 10.1016/j.bbamcr.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 26.Boal F., Zhang H., Tessier C., Scotti P., Lang J. The variable C-terminus of cysteine string proteins modulates exocytosis and protein–protein interactions. Biochemistry. 2004;43:16212–16223. doi: 10.1021/bi048612+. [DOI] [PubMed] [Google Scholar]
- 27.Monterrat C., Boal F., Grise F., Hemar A., Lang J. Synaptotagmin 8 is expressed both as a calcium-insensitive soluble and membrane protein in neurons, neuroendocrine and endocrine cells. Biochim. Biophys. Acta. 2006;1763:73–81. doi: 10.1016/j.bbamcr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Shaner N. C., Steinbach P. A., Tsien R. Y. A guide to choosing fluorescent proteins. Nat. Methods. 2005;2:905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 29.Lajus S., Vacher P., Huber D., Dubois M., Benassy M. N., Ushkaryov Y., Lang J. α-latrotoxin induces exocytosis by inhibition of voltage-dependent K+ channels and by stimulation of L-type Ca2+ channels via latrophilin in β-cells. J. Biol. Chem. 2006;281:5522–5531. doi: 10.1074/jbc.M510528200. [DOI] [PubMed] [Google Scholar]
- 30.Lajus S., Lang J. Splice variant 3, but not 2 of receptor protein-tyrosine phosphatase σ can mediate stimulation of insulin-secretion by α-latrotoxin. J. Cell. Biochem. 2006;98:1552–1559. doi: 10.1002/jcb.20871. [DOI] [PubMed] [Google Scholar]
- 31. Reference deleted.
- 32.Cheng Y., Sequeira S. M., Malinina L., Tereshko V., Sollner T. H., Patel D. J. Crystallographic identification of Ca2+ and Sr2+ coordination sites in synaptotagmin I C2B domain. Protein Sci. 2004;13:2665–2672. doi: 10.1110/ps.04832604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Aalten D. M., Amadei A., Linssen A. B., Eijsink V. G., Vriend G., Berendsen H. J. The essential dynamics of thermolysin: confirmation of the hinge-bending motion and comparison of simulations in vacuum and water. Proteins. 1995;22:45–54. doi: 10.1002/prot.340220107. [DOI] [PubMed] [Google Scholar]
- 34.Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F., DiNola A., Haak J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 35.Robinson I. M., Ranjan R., Schwarz T. L. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C2A domain. Nature. 2002;418:336-340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- 36.Jahn R., Niemann H. Molecular mechanisms of clostridial neurotoxins. Ann. N.Y. Acad. Sci. 1994;733:245-255. doi: 10.1111/j.1749-6632.1994.tb17274.x. [DOI] [PubMed] [Google Scholar]
- 37.Lang J., Regazzi R., Wollheim C. B. Bacterial toxins: Tools in Cell Biology. In: Aktories K., editor. Weinheim: Chapman & Hall; 1997. pp. 217–240. [Google Scholar]
- 38.Lang J., Zhang H., Vaidyanathan V. V., Sadoul K., Niemann H., Wollheim C. B. Transient expression of botulinum neurotoxin C1 light chain differentially inhibits calcium and glucose induced insulin secretion in clonal β-cells. FEBS Lett. 1997;419:13–17. doi: 10.1016/s0014-5793(97)01411-7. [DOI] [PubMed] [Google Scholar]
- 39.Kaether C., Skehel P., Dotti C. G. Axonal membrane proteins are transported in distinct carriers: a two-color video microscopy study in cultured hippocampal neurons. Mol. Biol. Cell. 2000;11:1213–1224. doi: 10.1091/mbc.11.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi T., Vischer U. M., Rosnoblet C., Lebrand C., Lindsay M., Parton R. G., Kruithof E. K., Gruenberg J. The tetraspanin CD63/lamp3 cycles between endocytic and secretory compartments in human endothelial cells. Mol. Biol. Cell. 2000;11:1829-1843. doi: 10.1091/mbc.11.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vonderheit A., Helenius A. Rab7 associates with early endosomes to mediate sorting and transport of semliki forest virus to late endosomes. PLoS Biology. 2005;3:e233. doi: 10.1371/journal.pbio.0030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vo Y. P., Hutton J. C., Angleson J. K. Recycling of the dense-core vesicle membrane protein phogrin in Min6 β-cells. Biochem. Biophys. Res. Commun. 2004;324:1004–1010. doi: 10.1016/j.bbrc.2004.09.147. [DOI] [PubMed] [Google Scholar]
- 43.Geppert M., Goda Y., Hammer R. E., Li C., Rosahl T. W., Stevens C. F., Sudhof T. C. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 44.Nicholson-Tomishima K., Ryan T. A. Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16648–16652. doi: 10.1073/pnas.0406968101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bokvist K., Eliasson L., Ammala C., Renstrom E., Rorsman P. Co-localization of L-type Ca2+ channels and insulin-containing secretory granules and its significance for the initiation of exocytosis in mouse pancreatic B-cells. EMBO J. 1995;14:50–57. doi: 10.1002/j.1460-2075.1995.tb06974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damer C. K., Creutz C. E. Calcium-dependent self-association of synaptotagmin I. J. Neurochem. 1996;67:1661–1668. doi: 10.1046/j.1471-4159.1996.67041661.x. [DOI] [PubMed] [Google Scholar]
- 47.Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 48.Li C., Ullrich B., Zhang J. Z., Anderson R. G., Brose N., Sudhof T. C. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 49.Hui E., Bai J., Chapman E. R. Ca2+-triggered simultaneous membrane penetration of the tandem C2-domains of synaptotagmin I. Biophys. J. 2006;91:1767–1777. doi: 10.1529/biophysj.105.080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rufener E., Frazier A. A., Wieser C. M., Hinderliter A., Cafiso D. S. Membrane-bound orientation and position of the synaptotagmin C2B domain determined by site-directed spin labeling. Biochemistry. 2005;44:18–28. doi: 10.1021/bi048370d. [DOI] [PubMed] [Google Scholar]
- 51.Evans J. H., Gerber S. H., Murray D., Leslie C. C. The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells. Mol. Biol. Cell. 2004;15:371–383. doi: 10.1091/mbc.E03-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haberman Y., Ziv I., Gorzalczany Y., Fukuda M., Sagi-Eisenberg R. Classical protein kinase C(s) regulates targeting of synaptotagmin IX to the endocytic recycling compartment. J. Cell Sci. 2005;118:1641–1649. doi: 10.1242/jcs.02276. [DOI] [PubMed] [Google Scholar]
- 53.Poskanzer K. E., Fetter R. D., Davis G. W. Discrete residues in the C2B domain of synaptotagmin I independently specify endocytic rate and synaptic vesicle size. Neuron. 2006;50:49–62. doi: 10.1016/j.neuron.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 54.Xiong X., Zhou K. M., Wu Z. X., Xu T. Silence of synaptotagmin I in INS-1 cells inhibits fast exocytosis and fast endocytosis. Biochem. Biophys. Res. Commun. 2006;347:76–82. doi: 10.1016/j.bbrc.2006.06.045. [DOI] [PubMed] [Google Scholar]