Abstract
The carotid artery shows a common response to many forms of injury, including a rapid activation of smooth muscle cell (SMC) proliferation in the media and migration of SMCs into the intima to form a neointima. Platelet-derived growth factor (PDGF) is believed to play a role in this response to injury, but it has proven difficult to distinguish whether it is stimulating cell migration or cell proliferation, and whether the action is direct or indirect. To determine this, we created chimeric mice composed of both wild-type (WT) and marked PDGF receptor β (PDGFRβ)-deficient cells, and determined the consequences of PDGFRβ expression for SMC participation in response to ligation of the left common carotid artery. The proportion of PDGFRβ−/− SMCs increased 4.5-fold in the media and decreased 1.8-fold during formation of the neointima, consistent with migration of WT SMCs out of the media and into the intima, leaving the PDGFRβ−/− cells behind. The fibrotic reaction in the adventitia, which does not involve cell migration, did not result in any change in relative abundance of WT and PDGFRβ-deficient fibroblasts. We conclude that the most significant direct role of PDGFRβ is to mediate responses that involve cell migration rather than proliferation.
In an uninjured mouse or rat carotid artery, the intima, the portion of the vessel inside the internal elastic lamina, consists of a single layer of endothelial cells. In response to many forms of experimental injury, the carotid shows a common response, including rapid activation of smooth muscle cell (SMC) proliferation in the media, migration of SMCs into the intima to form a neointima, and continued proliferation of SMCs in the neointima. 1 Carotid injury also results in activation and proliferation of fibroblasts in the adventitial connective tissue layer surrounding the media, though this has been the focus of less attention. An extensive body of data supports the involvement of platelet-derived growth factor (PDGF) (from platelets and macrophages) and fibroblast growth factor (FGF) (released from injured SMCs) in driving these changes, but the relative roles of these and other growth factors in stimulating cell migration and/or proliferation have still not been fully elucidated.
PDGF receptor β (PDGFRβ) is expressed by vascular SMCs and other stromal and connective tissue cells. 2 It can mediate both chemotaxis and proliferation of cultured cells in response to binding of its ligands, PDGF B-chain 2-4 or the recently discovered protease-activated PDGF-D chain. 5,6 This suggested the hypothesis that injury results in the release of PDGF from activated platelets and macrophages, and that this PDGF contributes to driving the cell migration and proliferation involved in the response to injury. 2 This general hypothesis has been supported by many studies in animal models in which the effects of augmenting or inhibiting the PDGF/PDGF receptor system have been evaluated. 7-12 These studies have generally concluded that PDGF is acting primarily as a chemotactic agent rather than as a mitogen. For example, when rats were depleted of platelets, a rich source of PDGF B-chain, 13 the neointima that formed after balloon injury was smaller, but there was no detectable difference in SMC replication. 14 Studies using exogenous PDGF-BB 9 and neutralizing antibodies against PDGF-BB, 7 or specific inhibitor of PDGF receptor tyrosine kinase, 15 further suggested that the primary role of PDGF-BB was to induce migration of SMCs from the media into the intima, with little or delayed effects on replication.
Embryos that are homozygous for disruption of PDGF B-chain 16 or PDGFRβ 17 die at or before birth, with comparable abnormalities in vascular development that reflect reduced presence of vascular SMCs, or SMC-like cells, in small vessels. 18,19 The phenotype of PDGFRβ−/− mice supports a role for PDGFRβ in vascular development, but the early lethality prevents their use in evaluating the role of PDGFRβ system in adult tissue responses to injury. However, chimeric embryos, prepared by fusing wild-type (WT) and PDGFRβ−/− pre-implantation mouse embryos, are viable and develop into normal adults. 20 During developmental processes that use PDGFRβ ligands to drive cell proliferation or recruitment, PDGFRβ−/− cells in a chimeric embryo become progressively less abundant as they are “out-competed” by the WT control cells which do express PDGFRβ. 20 This competitive disadvantage becomes the basis for calculating the magnitude of the role that PDGFRβ plays in the development of different cell lineages. Chimera analysis revealed that PDGFRβ plays an important role in the development of all muscle lineages, including pericytes, 20 mesangial cells, 21 and arterial SMCs, 20 and it revealed that, counter to expectation, PDGFRβ does not play a significant role in the development of fibroblast or endothelial cell populations.
Because the requirement for PDGFRβ expression during development is not absolute, every cell lineage and tissue in an adult PDGFRβ−/− ⇔ WT chimera includes at least some PDGFRβ−/− cells. This makes it possible to evaluate the further roles of PDGFRβ in adult disease processes by comparing the responses of adjacent WT versus PDGFRβ−/− cells within the tissue. Because the positive and negative cells share the same local environment in the same chimeric individual, animal-to-animal variation and indirect and systemic effects of the gene deletion are minimized. Chimera analysis of adult responses to injury have revealed some surprises. In granulation tissue formed in response to subcutaneous implantation of a surgical sponge, we found that fibroblasts and endothelial cells, which did not depend on PDGFRβ expression during development, 20 were virtually excluded from accumulation in developing granulation tissue unless they expressed normal levels of PDGFRβ. 22 In this report, we describe the use of chimeric mice to support the hypothesis that, after arterial injury, PDGFRβ plays an important direct (ie, cell-autonomous) role in driving SMC migration from the media to the intima, but not in the response of fibroblasts in the adventitia.
Materials and Methods
Preparation of Chimeric Mice
Two lines of mice were used to generate morulae for the creation of aggregation chimeras, as previously described. 20 In brief, the wild-type SWR (PDGFRβ+/+) line provided control (WT) component morulae. PDGFRβ+/− C57Bl/6 mice 17 homozygous for a pBR322/globin insert 23 provided marked PDGFRβ+/+, +/−, and −/− morulae. The pBR322/globin insert is not expressed and serves as a target for non-isotopic in situ hybridization which allowed us to identify cells from the experimental line. 20 Morulae from these two lines were fused ex vivo to produce PDGFRβ+/+ ⇔ WT, PDGFRβ+/− ⇔ WT, or PDGFRβ−/− ⇔ WT chimeric embryos, which were implanted into pseudopregnant CD-1 females.
Mouse Model of Vascular Injury
All mice were conventionally housed in the University of Washington vivarium and all procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Adult chimeric mice of both sexes were anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 8 mg/kg xylazine. A ventral midline cervical incision was made, the left common carotid artery was isolated by blunt dissection and ligated with 6−0 silk just proximal to the bifurcation into the internal and external branches, as described by Kumar and Lindner. 24 The skin was closed using Michel wound clips. Mice were euthanized 4 weeks post-surgery, and the left and right carotid arteries were harvested, fixed with methyl Carnoy’s (60% methanol, 30% acetic acid, 10% chloroform), embedded in paraffin, and 5-micron sections cut.
Identification of Cells of the Experimental Genotype
The marker for the experimental component of the chimera (which was PDGFRβ+/+, PDGFRβ+/−, or PDGFRβ−/−) is a non-expressed 1000-copy tandem repeat of a mouse β-globin/pBR322 construct 23 which can be detected by non-isotopic in situ hybridization using digoxygenin-labeled probes as previously described. 20 Nuclei were counter-stained with methyl green. Our primary interest was to evaluate vascular SMCs and adventitial fibroblasts. To identify and exclude leukocytes from our evaluation, all sections were immunostained for the pan-leukocyte marker CD45, using anti-mouse CD45 (clone 30-F11, PharMingen, San Diego, CA) followed by biotinylated rabbit anti-rat IgG then Vectastain elite ABC peroxidase (Vector Laboratories, Burlingame, CA) and diaminobenzidine. Peroxidase activity was then quenched by 30 minutes in 3% hydrogen peroxide after which the in situ hybridization was begun. We also identified SMCs by direct immunostaining for smooth muscle α-actin combined with cell shape. This approach identified the same numbers of cells identified by cell shape and non-CD45-positivity, but was more difficult to score for the nuclear genotype marker. We obtained comparable results using the two methods for identifying SMCs.
Data Analysis and Normalization
500 to 1000 cells of each type of interest from sections of left carotid (ligated) and right carotid (uninjured control) were counted as either marked (experimental component) or unmarked (WT component) by the pBR322-targeted in situ hybridization. To control for interindividual differences in percent initial carotid artery chimerism, the values for the media and neointima in the ligated left carotid were compared to the value for the media of the unmanipulated right carotid in the same individual, and results are expressed as the “relative abundance,” ie, the ratio of the marked percentage in the ligated left carotid to the marked percentage of the same cell type in the unmanipulated right carotid. Comparisons between groups were evaluated for statistical significance using Student’s t-test.
Results
Using Aggregation Chimeras to Evaluate Cell Kinetics in Arterial Response to Injury
The most thoroughly studied model of arterial injury in the mouse, whose small size makes mechanical injury relatively difficult to control, is ligation of the left common carotid artery just proximal to the carotid bifurcation. 24 This results in formation of a neointima containing SMCs and leukocytes. We identified the leukocytes by immunostaining for CD45 and excluded them from our analysis. The adventitia of the ligated carotid also thickens and there is an increase in cellularity, constituted of increased numbers of fibroblasts and CD45-positive leukocytes. At 4 weeks after ligation, most of the adventitial cells are fibroblasts, with smaller numbers of leukocytes.
We created chimeric mice by in vitro aggregation of early embryos (morula stage) from two component mouse lines. The “experimental component” was marked by the homozygous presence of an expression-independent DNA marker 23 and was either PDGFRβ+/+, PDGFRβ+/−, or PDGFRβ−/−. The control component was unmarked and PDGFRβ+/+, ie, WT. The resulting chimeric mice are designated: PDGFRβ+/+ ⇔ WT, PDGFRβ+/− ⇔ WT, or PDGFRβ−/− ⇔ WT. We used these chimeras to compare, in the same individual, the behavior of WT and PDGFRβ-deficient cells in response to carotid injury. The PDGFRβ+/+ ⇔ WT chimeras served as a control for possible differences in cell behavior that did not result from difference in PDGFRβ expression, ie, that resulted from some other difference between the two parental lines. The media of an injured carotid in a chimeric mouse contained comparable numbers of cells in both PDGFRβ−/− ⇔ WT and WT ⇔ WT chimeras (data not shown).
We used the value of marked/unmarked cells in the right (uninjured) carotid as a reference for the value that would have been found in the left (injured) carotid vessel before the experimental injury. This is justified by previous data, in which we determined that marked/unmarked cells make the same relative contribution to different arteries within an individual mouse. 20 It is supported in this report by comparisons between vessels in PDGFRβ+/+ ⇔ WT chimeras: the percentage of medial SMCs (and adventitial fibroblasts) from the experimental line in the ligated carotid is equal to percentage in the contralateral carotid (ratio of 1, Figure 1 ▶ ), and the percentage of marked to unmarked SMCs in the neointima is equal to the percentage in the contralateral media (ratio of 1, Figure 1 ▶ ).
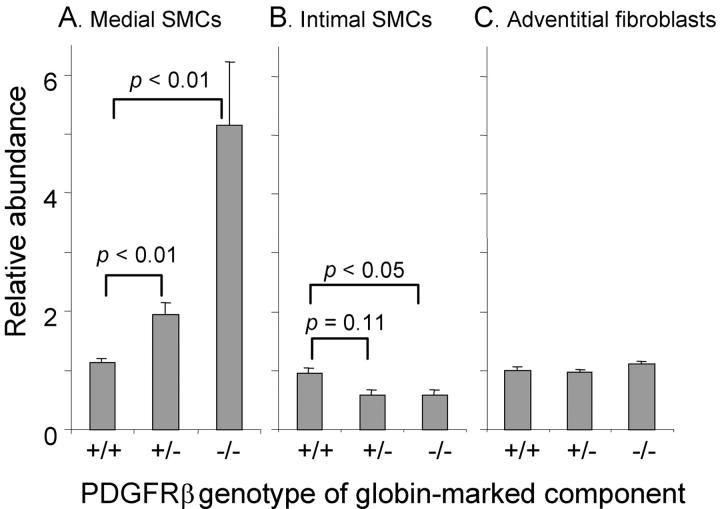
Figure 1.
Relative abundance of cells after carotid ligation as a function of PDGFRβ expression. Chimeric mice were prepared in which the genotype of the marked experimental component was PDGFRβ+/+, PDGFRβ+/−, or PDGFRβ−/− as indicated on the abscissa. The left carotid was injured by ligation to establish a neointima. After 4 weeks, the relative abundance of experimental versus WT cells was determined by in situ hybridization and plotted as mean ± SEM for 7 to 9 mice per group. A: Values for SMCs in the injured media compared to values in the uninjured media. B: Values for SMCs in the neointima compared to values in the uninjured media. C: Values for adventitial fibroblasts in the injured carotid compared to values for fibroblasts in the uninjured carotid.
PDGFRβ Mediates SMC Migration from the Media into the Intima
In rodent models of arterial response to injury, many publications demonstrate that SMCs proliferate in the injured media and migrate into the intima, where they continue to proliferate to form the neointima. 1,24,25 What role does PDGFRβ play in these processes? If medial SMCs respond to PDGF (via PDGFRβ) largely as a mitogen, then the ratio of PDGFRβ−/− to WT SMCs in the media should decrease after injury, as the WT SMC proliferate and the PDGFRβ−/− SMCs do not. If PDGFRβ mediates largely a chemotactic response, then the ratio should increase, as the WT SMCs migrate out of the media into the intima and leave the PDGFRβ−/− cells behind. The data in Figure 1 ▶ , and illustrated in Figure 2 ▶ , (many “globin-marked” SMC nuclei in the media of injured carotid compared to uninjured control carotid and very few globin-marked SMC in the neointima) support the hypothesis that PDGFRβ is predominantly mediating a chemotactic response. In PDGFRβ−/− ⇔ WT chimeras there is an approximately 4.5-fold increase (P < 0.01) in the percentage of PDGFRβ−/− SMCs remaining in the ligated media as compared to the control media (Figure 1) ▶ .
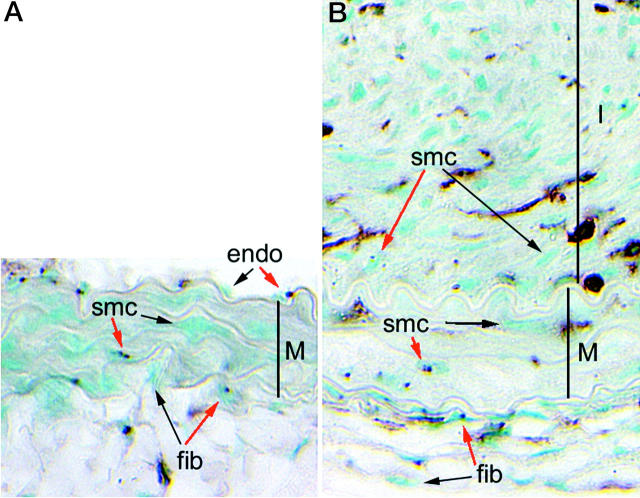
Figure 2.
Distribution of WT and PDGFRβ−/− cells in the carotid artery from a PDGFRβ−/− ⇔ WT chimera before (A) and after (B) ligation-induced neointima formation. The tunica media (M) is defined by the elastic laminae. The neointima (I) in the ligated vessel occupies the top half of B. All nuclei are counterstained with methyl green. PDGFRβ−/− cells are identified by non-isotopic in situ hybridization for the nuclear marker, which produces dark nuclear dots. Representative marked (red arrows) and unmarked (black arrows) SMCs, fibroblasts (fib), and endothelial cells (endo) are noted. Leukocytes are identified by immunostaining for CD45 (brown).
If PDGFRβ−/− SMCs cells remain in the media while the PDGFRβ+/+ SMCs migrate into the intima, then PDGFRβ−/− cells should be under-represented in the neointima compared to the media from which they migrated. Figure 1B ▶ documents that this is the case. In both PDGFRβ+/− ⇔ WT and PDGFRβ−/− ⇔ WT chimeras, the intima is composed of approximately 40% fewer PDGFRβ-deficient SMCs then in the uninjured contralateral media. In control PDGFRβ+/+ ⇔ WT chimeras there is no difference between the relative abundance of marked and unmarked cells (representing the two components of the chimera) in the injured media, the contralateral media, or the neointima. This demonstrates that the difference in the PDGFRβ−/− ⇔ WT chimeras was due to absence of PDGFRβ expression rather than to some other difference between the two lines used to generate the chimeras. In PDGFRβ+/− ⇔ WT chimeras, in which the experimental component is heterozygous for PDGFRβ and expresses half the normal level of PDGFRβ, there is an intermediate magnitude of change (1.75-fold increase in the media, P < 0.01). The dose dependence of behavior on PDGFRβ expression level was also observed during SMC development 20 and fibroblast participation in granulation tissue formation, 22 and demonstrates how sensitive SMCs are to relatively small differences in PDGFRβ expression level. This supports the possibility that the magnitude of changes in level of ligand and receptor expression that have been observed in various pathophysiologic responses could be of consequence in fine tuning cell responses. 26
The Participation of Adventitial Fibroblasts in the Fibrotic Response Is Not Affected by PDGFRβ Expression
In addition to the formation of a neointima, carotid injury stimulates changes in the adventitial connective tissue layer around the vessel, which becomes thicker and more cellular. Leukocytes are abundant in the adventitia soon (1 week) after injury, but by 4 weeks, the adventitia is composed largely of an expanded population of fibroblasts and a more abundant extracellular matrix. To determine whether PDGFRβ mediates this fibrotic response in the adventitia, we compared the behavior of PDGFRβ-deficient fibroblasts to WT fibroblasts. As shown in Figure 1 ▶ , the relative numbers of PDGFRβ-deficient fibroblasts did not change in response to ligation in either the PDGFRβ+/+ ⇔ WT, PDGFRβ+/− ⇔ WT or PDGFRβ−/− ⇔ WT chimeras. This demonstrates that the increased number and density of adventitial fibroblasts is not dependent on PDGFRβ expression.
Discussion
PDGFRβ Expression Is Important for SMC Chemotaxis in Response to Arterial Injury
The formation of a neointima, and the remodeling of the media in response to injury, are known to involve migration, proliferation, and death. 7-12,25,27 Multiple growth factors have been implicated driving these processes, but it has been difficult to determine which growth factor is responsible for driving which process. The chimera analysis results presented above show that, during the formation of the neointima, the proportion of PDGFRβ-deficient SMCs increases in the media and decreases in the neointima. The simultaneous disappearance of medial and appearance of intimal PDGFRβ-expressing SMCs suggests that the PDGF B-chain/PDGFRβ system is acting largely as a chemotactic agent, to drive migration of SMC from the media to the neointima.
If PDGFRβ is mediating SMC migration from the media to the neointima, why isn’t the neointimal population enriched in PDGFRβ+/+ cells to the same extent that the media is depleted (compare Figure 1, A and B ▶ )? We suggest two non-exclusive explanations for this: 1) Technical limitations resulting from failure to distinguish between leukocytes and SMCs. Because PDGFRβ−/− SMCs are already under-represented in the media before injury (due to selection for WT SMCs during development), it is more difficult to precisely measure further decreases in the neointima than to measure an increase in the media. In addition, since the neointima contains many leukocytes, and since PDGFRβ−/− leukocytes are not selected against during development, 20 the occasional mis-identification of a leukocyte with a SMC would have a proportionately large effect on the calculated ratio of PDGFRβ−/− to WT SMCs in the neointima compared to the media, where there are few leukocytes. 2) A more interesting explanation is that the vessel wall is not a closed system for SMCs, and that circulating progenitors may contribute to the SMC population in the neointima. We have used the nuclear marking system in hematopoietic chimeras to demonstrate that up to 11% of the endothelial cells in newly formed capillaries are derived from the hematopoietic compartment. 28 In that study, we did not directly evaluate SMC origin from circulating hematopoietic cells, but cell culture evidence supports the existence of progenitors with capacity to become either endothelial cells or SMCs, 29 and other investigators, using Y chromosome markers, have suggested that SMCs can derive from circulating progenitors under certain pathological situations. 30,31 We plan to directly investigate this possibility in future studies.
Does PDGFRβ Expression Also Affect SMC Apoptosis and Proliferation?
We attempted to determine this by directly measuring the relative proliferation and apoptotic rates of PDGFRβ+/+ versus PDGFRβ−/− cells in injured chimeric vessels. In both cases, we were stymied by a technical limitation created by the nature of our nuclear genotype marker. We found that we could not, in practice, reliably distinguish the nuclear genotype marker while simultaneously immunostaining for nuclear BrdU incorporation to evaluate proliferation, or nuclear TUNEL staining to evaluate apoptosis. Nevertheless, the following arguments suggest that PDGF effects on proliferation and apoptosis are unlikely to play major roles in the changes that we observed.
If PDGFRβ signaling was stimulating medial SMC proliferation, we would expect the proportion of PDGFRβ deficient medial SMCs to decrease rather than increase. This suggests that any action of PDGF to stimulate proliferation of medial PDGFRβ+/+ SMCs is overwhelmed by its action as chemoattractant, drawing PDGFRβ+/+ SMCs out of the media. This conclusion is consistent with earlier observations that inhibition of PDGF using a neutralizing antibody did not affect SMC proliferation in the injured rat carotid artery 7 and that exogenous PDGF only minimally increased intimal and medial SMC proliferation. 9 Thus, although many publications over the last 20 years document the ability of PDGF to stimulate proliferation of cultured SMCs and fibroblasts, this does not appear to be the predominant activity of PDGF in vascular response to injury in vivo.
A substantial frequency of SMC apoptosis has been reported in the ligated carotid model 24,25 and in other arterial injury models. 1,27,32,33 PDGF is generally reported to be anti-apoptotic for cultured SMCs, 34-36 and blocking PDGF-mediated signaling in the rat carotid injury model has been reported to increase SMC apoptosis. 32 If PDGF played a predominant role in inhibiting SMC apoptosis in the injured media, we would expect to find an increase, rather than the decrease we observed, in medial PDGFRβ-deficient SMCs in the injured chimeric vessels. This argues that the anti-apoptotic effect must be small relative to the effect on chemotaxis. However, under conditions in which proliferation is prevented or limited, PDGF has been reported to be pro-apoptotic. 36,37 Our results would be consistent with a pro-apoptotic effect of PDGF in this injury model.
PDGFRβ Does Not Mediate Responses of Fibroblasts in the Adventitia
In contrast to the importance of PDGFRβ expression for SMC migration, we found that PDGFRβ expression did not affect the kinetics of adventitial fibroblasts. The proportion of PDGFRβ-deficient fibroblasts did not decrease, as we would have expected if either PDGFRβ+/+ fibroblasts were drawn into the adventitia and/or proliferated more readily; nor did the proportion of PDGFRβ deficient cells increase as we would have expected if PDGFRβ+/+ cells were drawn out of the adventitia. This result may seem surprising, since, for cultured fibroblasts, PDGF is active as a direct mitogen and chemotactic agent, 2-4 and since chimera analysis of granulation tissue formation revealed that fibroblast participation depended on PDGFRβ expression. 22 In that study, in which we induced granulation tissue by implanting a subcutaneous sponge, we found that fibroblasts that accumulated within the sponge showed a greater dependence on PDGFRβ expression than did fibroblasts that formed the capsule around the sponge. 22 This is consistent with PDGF action as a chemoattractant, since fibroblasts that penetrate the sponge matrix need to travel farther than fibroblasts that remain at the periphery. Since fibroblasts are already present within the normal adventitia, there is no need for migration and no opportunity for selection based on the role of PDGFRβ expression in driving migration. The adventitial fibroblast proliferation and fibrosis that occurs in this model is thus largely independent of PDGFRβ expression and activation.
Advantages and Limitations of Chimera Analysis of Vascular Response to Injury
PDGF can stimulate increased expression of other growth factors and cytokines 38 and it affects extracellular matrix synthesis and degradation. 8,12,39,40 These secondary effectors could then act locally or systemically, to contribute to the overall consequences of PDGF action in the vessel wall. Because it detects only cell-autonomous effects, chimera analysis makes it possible to distinguish pathophysiologic changes that are direct effects of PDGF on PDGF-responsive cells, from changes that are downstream consequences of the action of secondary effectors. The chimera analysis reported here indicates that PDGFRβ mediates a direct, cell-autonomous, effect in driving SMC migration in response to arterial injury. However, chimera analysis does not reveal a role for PDGFRβ and PDGF B-chain in regulating the final cellularity/architecture of the artery. The media of PDGFRβ−/− ⇔ WT and PDGFRβ+/+ ⇔ WT chimeric carotids contain comparable numbers of SMCs, both before and after injury, despite the fact that the PDGFRβ−/− SMC are unable to respond via PDGFRβ. Similarly, hematopoietic chimeras, in which PDGF B-chain is eliminated in platelets and macrophages, still develop relatively normal granulation tissue 41 and fibrous neointimal lesions. 10 Finally, the size and structure of larger arteries and veins in late term non-chimeric PDGFRβ−/− embryos is relatively normal, 17 despite the complete absence of PDGFRβ expression. Chimera analysis thus confirms the conclusions of studies using inhibitors of the PDGF/PDGF receptor system: PDGF plays a direct role in driving SMC migration in response to injury, and in determining the rate at which the vessel establishes a new architecture, but the final size/cellularity of the vessel must be determined by other factors.
Footnotes
Address reprint requests to Daniel F. Bowen-Pope, Ph.D., University of Washington, Dept. of Pathology, Box 357470, Seattle, WA 98195-7470. E-mail: bp@u.washington.edu.
Supported by the National Institutes of Health (grant HL03174 to D.F.B.-P.).
References
- 1.Clowes AW, Reidy MA, Clowes MM: Kinetics of cellular proliferation after arterial injury: I. smooth muscle growth in the absence of endothelium. Lab Invest 1983, 49:327-333 [PubMed] [Google Scholar]
- 2.Ross R, Raines EW, Bowen-Pope DF: The biology of platelet-derived growth factor. Cell 1986, 46:155-169 [DOI] [PubMed] [Google Scholar]
- 3.Seppa H, Grotendorst G, Seppa S, Schiffmann E, Martin GR: Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol 1982, 92:584-588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senior RM, Griffin GL, Huang JS, Walz DA, Deuel TF: Chemotactic activity of platelet α granule proteins for fibroblasts. J Cell Biol 1983, 96:382-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U: PDGF-D is a specific, protease-activated ligand for the PDGF β-receptor. Nat Cell Biol 2001, 3:512-516 [DOI] [PubMed] [Google Scholar]
- 6.LaRochelle WJ, Jeffers M, McDonald WF, Chillakuru RA, Giese NA, Lokker NA, Sullivan C, Boldog FL, Yang M, Vernet C, Burgess CE, Fernandes E, Deegler LL, Rittman B, Shimkets J, Shimkets RA, Rothberg JM, Lichenstein HS: PDGF-D, a new protease-activated growth factor. Nat Cell Biol 2001, 3:517-521 [DOI] [PubMed] [Google Scholar]
- 7.Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R: Inhibition of neointimal smooth-muscle accumulation after angioplasty by an antibody to PDGF. Science 1991, 253:1129-1132 [DOI] [PubMed] [Google Scholar]
- 8.Jackson CL, Raines EW, Ross R, Reidy MA: Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb 1993, 13:1218-1226 [DOI] [PubMed] [Google Scholar]
- 9.Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW: Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest 1992, 89:507-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozaki K, Kaminski WE, Tang J, Hollenbach S, Lindahl P, Sullivan C, Yu JC, Abe K, Martin PJ, Ross R, Betsholtz C, Giese NA, Raines EW: Blockade of platelet-derived growth factor or its receptors transiently delays but does not prevent fibrous cap formation in ApoE null mice. Am J Pathol 2002, 161:1395-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirois MG, Simons M, Edelman ER: Antisense oligonucleotide inhibition of PDGFR-β receptor subunit expression directs suppression of intimal thickening. Circulation 1997, 95:669-676 [DOI] [PubMed] [Google Scholar]
- 12.Zempo N, Koyama N, Kenagy R, Lea H, Clowes A: Regulation of vascular smooth muscle cell migration and proliferation in vitro and in injured rat arteries by a synthetic matrix metalloprteinase inhibitor. Arterioscler Thromb 1996, 16:28-33 [DOI] [PubMed] [Google Scholar]
- 13.Bowen-Pope DF, Hart CE, Seifert RA: Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem 1989, 264:2502-2508 [PubMed] [Google Scholar]
- 14.Fingerle J, Johnson R, Clowes AW, Majesky MW, Reidy MA: Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci USA 1989, 86:8412-8416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myllarniemi M, Calderon L, Lemstrom K, Buchdunger E, Hayry P: Inhibition of platelet-derived growth factor receptor tyrosine kinase inhibits vascular smooth muscle cell migration and proliferation. EMBO J 1997, 11:1119-1126 [DOI] [PubMed] [Google Scholar]
- 16.Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C: Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev 1994, 8:1875-1887 [DOI] [PubMed] [Google Scholar]
- 17.Soriano P: Abnormal kidney development and hematological disorders in PDGF β-receptor mutant mice. Genes Dev 1994, 8:1888-1896 [DOI] [PubMed] [Google Scholar]
- 18.Lindahl P, Johansson BR, Leveen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 1997, 277:242-245 [DOI] [PubMed] [Google Scholar]
- 19.Hellstrom M, Kal n M, Lindahl P, Abramsson A, Betsholtz C: Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 1999, 126:3047-3055 [DOI] [PubMed] [Google Scholar]
- 20.Crosby JR, Seifert RA, Soriano P, Bowen-Pope DF: Chimaeric analysis reveals role of Pdgf receptors in all muscle lineages. Nat Genet 1998, 18:385-388 [DOI] [PubMed] [Google Scholar]
- 21.Lindahl P, Hellstrom M, Kalen M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C: Paracrine PDGF-B/PDGF-Rβ signaling controls mesangial cell development in kidney glomeruli. Development 1998, 125:3313-3322 [DOI] [PubMed] [Google Scholar]
- 22.Crosby JR, Tappan KA, Seifert RA, Bowen-Pope DF: Chimera analysis reveals that fibroblasts and endothelial cells require platelet-derived growth factor receptorβ expression for participation in reactive connective tissue formation in adults but not during development. Am J Pathol 1999, 154:1315-1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lo CW, Coulling M, Kirby C: Tracking of mouse cell lineage using microinjected DNA sequences: analyses using genomic Southern blotting and tissue-section in situ hybridizations. Differentiation 1987, 35:37-44 [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Lindner V: Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol 1997, 17:2238-2244 [DOI] [PubMed] [Google Scholar]
- 25.Harmon KJ, Couper LL, Lindner V: Strain-dependent vascular remodeling phenotypes in inbred mice. Am J Pathol 2000, 156:1741-1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett TB, Seifert RA, Bowen-Pope DF: Regulation of platelet-derived growth factor receptor expression by cell context overrides regulation by cytokines. J Cell Physiol 1996, 169:126-138 [DOI] [PubMed] [Google Scholar]
- 27.Perlman H, Maillard L, Krasinski K, Walsh K: Evidence for the rapid onset of apoptosis in medial smooth muscle cells after balloon injury. Circulation 1997, 95:981-987 [DOI] [PubMed] [Google Scholar]
- 28.Crosby JR, Kaminski WE, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF: Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res 2000, 87:728-730 [DOI] [PubMed] [Google Scholar]
- 29.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K: Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 2000, 408:92-96 [DOI] [PubMed] [Google Scholar]
- 30.Glaser R, Lu MM, Narula N, Epstein JA: Smooth muscle cells, but not myocytes, of host origin in transplanted human hearts. Circulation 2002, 106:17-19 [DOI] [PubMed] [Google Scholar]
- 31.Han CI, Campbell GR, Campbell JH: Circulating bone marrow cells can contribute to neointimal formation. J Vasc Res 2001, 38:113-119 [DOI] [PubMed] [Google Scholar]
- 32.Leppanen O, Janjic N, Carlsson MA, Pietras K, Levin M, Vargeese C, Green LS, Bergqvist D, Ostman A, Heldin CH: Intimal hyperplasia recurs after removal of PDGF-AB and -BB inhibition in the rat carotid artery injury model. Arterioscler Thromb Vasc Biol 2000, 20:E89-E95 [DOI] [PubMed] [Google Scholar]
- 33.Pollman MJ, Hall JL, Gibbons GH: Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury: influence of redox state and cell phenotype. Circ Res 1999, 84:113-121 [DOI] [PubMed] [Google Scholar]
- 34.Bennett MR, Evan GI, Schwartz SM: Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest 1995, 95:2266-2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC: Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med 2002, 8:987-994 [DOI] [PubMed] [Google Scholar]
- 36.Romashkova JA, Makarov SS: NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 1999, 401:86-90 [DOI] [PubMed] [Google Scholar]
- 37.Kim HR, Upadhyay S, Li G, Palmer KC, Deuel TF: Platelet-derived growth factor induces apoptosis in growth-arrested murine fibroblasts. Proc Natl Acad Sci USA 1995, 92:9500-9504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poon M, Hsu WC, Bogadanov VY, Taubman MB: Secretion of monocyte chemotactic activity by cultured rat aortic smooth muscle cells in response to PDGF is due predominantly to the induction of JE/MCP-1. Am J Pathol 1996, 149:307-317 [PMC free article] [PubMed] [Google Scholar]
- 39.Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhia J, Wight TN: Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys 2001, 394:29-38 [DOI] [PubMed] [Google Scholar]
- 40.Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW: Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation 1997, 96:3555-3560 [DOI] [PubMed] [Google Scholar]
- 41.Buetow BS, Crosby JR, Kaminski WE, Ramachandran RK, Lindahl P, Martin P, Betsholtz C, Seifert RA, Raines EW, Bowen-Pope DF: Platelet-derived growth factor B-chain of hematopoietic origin is not necessary for granulation tissue formation and its absence enhances vascularization. Am J Pathol 2001, 159:1869-1876 [DOI] [PMC free article] [PubMed] [Google Scholar]