Abstract
A ubiquitous herpesvirus that establishes life-long infection, the Epstein-Barr virus (EBV) has yielded little insight into how a single agent in general accord with its host can produce diverse pathologies ranging from oral hairy leukoplakia to nasopharyngeal carcinoma, from infectious mononucleosis to Hodgkin’s disease (HD) and Burkitt’s lymphoma. Its pathogenesis is further confounded by the less than total association of virus with histologically similar tumors. In other viral systems, defective (interfering) viral genomes are known to modulate outcome of infection, with either ameliorating or intensifying effects on disease processes initiated by prototype strains. To ascertain whether defective EBV genomes are present in HD, we examined paraffin-embedded tissue from 56 HD cases whose EBV status was first determined by cytohybridization for nonpolyadenylated EBV RNAs (EBERs). Using both standard polymerase chain reaction (PCR) and PCR in situ hybridization, we successfully amplified sequences that span abnormally juxtaposed BamHI W and Z fragments characteristic of defective heterogeneous (het) EBV DNA from 10 of 32 (31%) EBER-positive tumors. Of 24 EBER-negative HD, 8 yielded PCR products indicating presence of het EBV DNA. Two of these contained defective EBV in the apparent absence of the prototype virus. Of the 42 tumors analyzed for defective EBV by both PCR techniques, there was concordance of results in 38 (90%). Detection of defective EBV genomes with the potential to disrupt viral gene regulation suggests one mechanism for pathogenic diversity that may also account for loss of prototypic EBV from individual tumor cells.
The Epstein-Barr virus (EBV) has been linked to approximately half the cases of Hodgkin’s disease (HD), with virus localized by EBER in situ hybridization to the malignant Reed-Sternberg cell that characteristically makes up less than 1% of the tumor mass. Recent descriptions of relapsed HD, shown to be EBV-positive at initial diagnosis but EBV-negative on reoccurrence, raise the possibility of viral DNA loss during tumor progression in some individuals. 1,2 If similar loss of the EBV genome should occur in infected Reed-Sternberg cell precursors at subclinical stages of the initial disease, a viral contribution to tumor initiation would go unrecognized. Because of a proposed role for defective rearranged EBV DNA in the elimination of standard viral episomes from Burkitt’s lymphoma (BL) tumor cells, 3,4 we examined EBER-positive and -negative Hodgkin’s lymphoma biopsies for presence of the defective viral genome previously termed heterogeneous (het) EBV DNA. 5
Materials and Methods
Patient Tissues and Cell Lines
Formalin-fixed or B5-fixed paraffin-embedded diagnostic specimens from 56 children and adolescents with HD were studied, 26 from patients treated at Hospital de Clinicas in Curitiba, Brazil, and 30 treated at St. Jude Children’s Research Hospital, Memphis, TN. Histological subtypes included 17 cases of nodular sclerosing, 31 mixed cellularity, 4 lymphocyte depletion, and 4 lymphocyte predominance. Patients had a median age of 8 years (range, 4 to 21 years) and were human immunodeficiency virus-negative. Formalin-fixed paraffin-embedded Akata cells (EBV-infected BL cell line) and BL2 cells (EBV-negative BL cell line) were used as positive and negative controls, respectively, for EBER in situ hybridization. Paraffin-embedded BL-derived cell line P3HR-1 [subclone 5 (EBV strain P3HR1-positive and het EBV DNA-positive) and subclone 16 (EBV strain P3HR1-positive and het EBV DNA-negative)] 6 served as controls in the assays described below.
EBER in Situ Hybridization
EBV status of tumors was determined on paraffin sections by in situ cytohybridization using digoxigenin-labeled sense and anti-sense riboprobes specific for EBER1. 7 Bound probe was detected by an anti-digoxigenin antibody-alkaline phosphatase conjugate (Boehringer-Mannheim, Mannheim Germany) as per the manufacturer’s protocol. The EBER status of 44 of these samples had been reported in a previous publication. 8
Polymerase Chain Reaction (PCR) Techniques
Standard PCR and PCR in situ cytohybridization for defective EBV DNA were performed by separate individuals on serial sections taken from identical blocks using common primers and probes. Results by each technique were not compared until all samples had been processed.
For standard PCR, 5-μm-thick paraffin ribbons were treated with xylene and the DNA extracted as previously described. 9 PCR was performed on 100 ng and 500 ng of total cellular DNA with Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT) for 30 cycles of amplification on a DNA thermal cycler (Perkin-Elmer Cetus). Primers were selected that framed the junction of rearranged DNA (5′-GCACATTAGCAATGCCTGTG-3′ and 5′-GTCCAGCGCGTTTACGTAAG-3′; base coordinates 1381 and 1649, respectively). 10 Electrophoresed PCR products were hybridized after Southern transfer with 32P-labeled oligonucleotide probes specific for sequences internal to primers: first, to sequences in BamHI Z (5′-CATGCAGCAGACATTCATCATTTAGAAATG-3′ base coordinate 1498), 10 after which blots were striped and rehybridized for sequences in BamHI W (5′-AGTGGTCCCCCTCCCTAGAACTGACAATTG-3′, base coordinate 1588) 10 as described previously. 11 In select cases, PCR products were cloned into a pCRII vector (TA Cloning kit; Invitrogen, Carlsbad, CA) and sequenced (Sequenase Version 2.0 DNA Sequencing; Amersham Pharmacia Biotech, Inc., Piscataway, NJ) as per the manufacturers’ protocols.
To delineate what cell type within tumor sections bore defective EBV DNA, small lymphocytes or morphologically distinct Reed-Sternberg cells, we used PCR in situ cytohybridization to examine paraffin sections, as described in detail elsewhere. 12,13 Briefly, tissue sections affixed to glass slides were deparaffinized with xylene and digested with proteinase K. Twenty-five μl of reaction mix [250 nmol/L of each primer described above, 10 μmol/L (each) dNTP, PCR buffer] and 2.5 U of Taq polymerase were added beneath glass coverslips and 25 cycles of amplification (1 cycle = 95°C for 1 minute, 45°C for 2 minutes, 72°C for 2 minutes) performed on a Hybaid Omnislide thermocycler (National Labnet Co., Woodbridge, NJ). Slides were washed with 2× standard saline citrate, heated to 85°C for 5 minutes, then hybridized overnight at 49°C. The hybridization mixture contained 50% formamide, 0.1% single-stranded DNA, 10× Denhardt’s solution, 0.1% sodium dodecyl sulfate, and 20 pg/ml BamHI W-specific oligonucleotide probe labeled with digoxigenin (Boehringer Mannheim, Indianapolis, IN). Bound probe was detected by anti-digoxigenin antibody conjugated to alkaline phosphatase (Boehringer Mannheim).
For select HD biopsies, DNA derived above for standard PCR was also used in a real-time quantitative PCR assay to document presence of the standard EBV genome, 14,15 regardless of EBER expression status. Targeting the BamHI K fragment of EBV DNA, present in prototype virus but deleted from het EBV DNA, 16,17 allows such a determination. A 106-bp region of EBV EBNA1 gene in the BamHI K fragment was amplified (primers 5′-CCGGTGTGTTCGTATATGGAG-3′ and 5′-GGGAGACGACTCAATGGTGTA-3′, base coordinates 109463 and 109568, respectively, National Center for Biotechnology Information GenBank accession no. VO1555), together with a 101-bp DNA sequence of the human C-reactive protein (CRP) gene (primers 5′-CTTGACCAGCCTCTCTCATGC-3′ and 5′-TGCAGTCTTAGACCCCACCC-3′, base coordinates 132705 and 132605, respectively; accession no. AL445528). The TaqMan Fluorogenic System (PE Applied Biosystems, Foster City, CA), in which real-time amplification is measured by cleavage of fluorescent dye-labeled probes by the 5′ to 3′ exonuclease activity of Taq DNA polymerase, was used as described by others. 14,15 EBNA1 probe (5′TGCCCTTGCTATTCCACAATGTCGTCTT 3′, base coordinate 109521) and the CPR probe (5′TTTGGCCAGACAGGTAAGGGCCACC 3′, base coordinate 132682) were labeled with VIC and FAM (PE Applied Biosystems), respectively, two fluorescent dyes whose spectra emitted after laser excitation at 488 nm are easily distinguished by the ABI Prism 7700 Sequence Detection System (PE Applied Biosystems). Reactions were performed in a 50-μl volume using TaqMan Universal Master Mix (PE Applied Biosystems), 300 nm primers, 200 nmol/L probe, and 500 ng of HD DNA. Amplification consisted of 2 minutes at 50°C, 10 minutes at 95°C, and 40 two-step cycles of 15 seconds at 95°C and 60 seconds at 60°C. Each sample was run in duplicate, together with multiple template-negative controls. Serial dilutions of Namalwa DNA, a BL cell line (ATCC CRL-1432) containing two copies of EBV per cell and cellular DNA from the EBV-negative BL2 line served as standards. For precise EBV DNA quantification in samples, the amount of cellular CRP DNA present was analyzed and EBV copy number per sample was normalized to the amount of CRP DNA representing the actual amount of amplifiable cellular DNA in each sample. The lower detection limit of the method as determined by serial dilutions of Namalwa DNA in BL2 DNA was two viral genome copies.
Results
Of the 56 patients studied, tumor biopsies from 32 (57%) were EBER-positive, indicating an association with EBV (Table 1) ▶ . In all cases, the hybridization signal localized to the morphologically distinct Reed-Sternberg cell (Figure 1) ▶ . Occasionally, sections contained infrequent smaller infiltrating lymphocytes that were also EBER-positive. Sections that stained with anti-sense EBER riboprobes were negative on hybridization with the control sense riboprobes (not shown).
Table 1.
Summary of Results
| Histologic subtype (n) | Het EBV*(+)/EBER†(+) | Het EBV(+)/EBER(−) |
|---|---|---|
| Nodular sclerosis (17) | 2 /6 | 4 /11 |
| Mixed cellularity (31) | 7 /23 | 3 /8 |
| Lymphocyte predominance (4) | 0 | 1 /4 |
| Lymphocyte depletion (4) | 1 /3 | 0 /1 |
| Total (56) | 10 /32 | 8 /24 |
n, Number of cases.
*Defective, heterogeneous EBV DNA genome as determined by PCR analysis.
†EBER in situ cytohybridization analysis as a marker for prototypic EBV DNA association with tumor.
Figure 1.
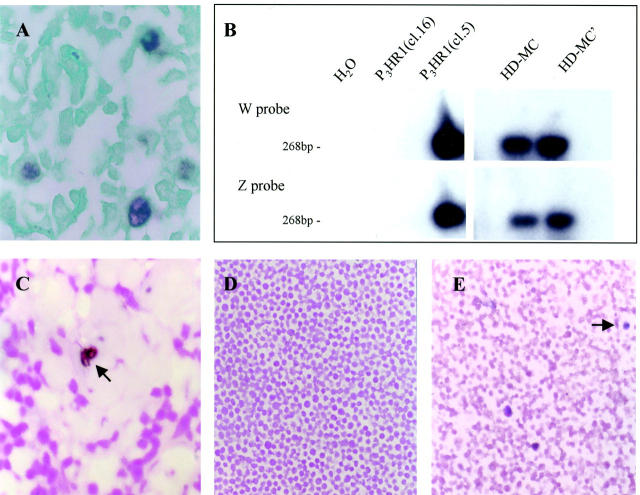
Detection of defective heterogeneous EBV DNA in HD, mixed cellularity subtype. A: EBER in situ cytohybridization showing Reed-Sternberg cells positive for EBV RNA (brown stain). B: Radiograph of Southern blot showing PCR products derived from tumor in A, using primers framing the junction of abnormally juxtaposed BamHI W and Z fragments of EBV DNA. Blotted product was first hybridized to a radiolabeled BamHI Z-specific probe, then to a BamHI W-specific probe after the blot had been striped. H2O is template-negative PCR control; Burkitt lymphoma-derived P3HR1 cell line (clone 16) is EBV-positive but het DNA-negative; P3HR1 clone 5 contains the parental EBV strain plus het DNA; HD-MC and HD-MC’ are samples (1× and 2.5× concentrations of template, respectively) from HD case in A. C: PCR in situ amplification for defective EBV genome in HD-MC paraffin section taken serially from block used in A and B. Brown hybridization signal localized to binucleate cell with nucleolar sparing (arrow) indicates PCR products derived from defective EBV DNA. D: P3HR1 cell line, clone 16, negative control for PCR in situ amplification. E: P3HR1 clone 5, positive control (het DNA is in a rare cell subset; three positive cells shown, one indicated by arrow). Sections counterstained with Light Green FS yellowish in (A) and with Nuclear Fast Red in (C–E). Original magnifications: ×600 (A); ×200 (C); ×100 (D and E).
Using both standard PCR as well as PCR in situ hybridization, we successfully amplified sequences that span abnormally juxtaposed BamHI W and Z fragments in defective het EBV DNA from 18 of 56 (32%) of samples. Of the EBER-positive tumor subset, 10 of 32 (31%) contained defective EBV. Eight of 24 EBER-negative HD yielded a PCR product indicative of defective genomes (Table 1) ▶ . In the standard PCR assay, the expected product of 268 bp was confirmed by successive hybridization of blots with probes specific to both BamHI W and Z restriction fragments of EBV DNA (Figure 1) ▶ , regions normally separated by ∼55 kb in the standard viral genome but now approximated in the rearranged genome. No product was obtained from negative controls containing reaction mixture alone or P3HR-1 clone 16 bearing the EBV genome without defective virus (Figure 1) ▶ . In two cases PCR products were sequenced and the rearrangement occurred at the locus described previously. 10
By PCR in situ hybridization, signal denoting defective het EBV DNA localized to scattered cells with morphology consistent with Reed-Sternberg cells (Figure 1) ▶ . Positive cells were rare, with never more than two per microscopic (×40) field. Of the 42 tumors analyzed by both standard PCR and PCR in situ hybridization techniques, there was concordance of results in 38 (90%). The similarity of outcome by divergent methodologies performed by separate individuals makes it unlikely that these findings can be attributed to PCR contamination. Instances in which the results are at variance for a single tumor may reflect chance-sampling differences incurred during serial sectioning. Of the eight EBER-negative tumors in which defective EBV DNA was detected, all were positive for the defective genome by standard PCR with six of the eight also positive by PCR in situ hybridization.
To ascertain whether lack of EBER expression in the eight tumors containing defective EBV was synonymous with absence of the standard viral genome, we used real-time quantitative PCR to amplify regions of BamHI K fragment of EBV DNA encoding the EBNA1 gene that is contained in prototypic but not in the defective EBV genome. In the six EBER-negative/het-DNA-positive samples still available for study, we detected the standard EBV genome (BamHI K DNA) in four (Table 2) ▶ . Two samples, however, did not yield a PCR product despite successful amplification of cellular CRP DNA and het DNA, indicating the absence of PCR inhibitors. As expected, all four EBER(+)/het(+) HD samples included as controls contained EBNA1 DNA sequences, with a mean of 0.33 EBV genome equivalents per cell (Table 2) ▶ . That copy number is consistent with previously published estimates of 50 to 100 EBV genomes per Reed-Sternberg cell, presuming the latter comprises less than 1% of the tumor mass. 18,19
Table 2.
PCR Quantification of Standard EBV Genome in Hodgkin’s Disease Samples Containing EBV het DNA
| EBER status | Histologic subtype | EBV genome equivalents/cell* |
|---|---|---|
| Negative | Mixed cellularity | 0.1 |
| Negative | Nodular sclerosis | 0.04 |
| Negative | Nodular sclerosis | 0 |
| Negative | Mixed cellularity | 0.61 |
| Negative | Nodular sclerosis | 0 |
| Negative | Mixed cellularity | 1.98 |
| Positive | Mixed cellularity | 0.65 |
| Positive | Mixed cellularity | 0.01 |
| Positive | Nodular sclerosis | 0.37 |
| Positive | Mixed cellularity | 0.29 |
*Calculated from total cellular DNA by normalizing EBV copy number (EBNA1 sequences) to the amount of human CRP DNA representing the actual amount of amplifiable cellular DNA in each HD sample. Zero values indicate EBV DNA was not amplified despite an ample PCR product from CRP DNA. Because Reed-Sternberg cells comprise <1% of all cells in HD tumors, EBV DNA content in each Reed-Sternberg cell is at least 100 fold higher than the value above.
Discussion
Previous studies have addressed a possible viral association for cases of HD that by standard screening assays tested negative for EBV, the premise being that detection is prevented by absent viral gene expression or an integrated EBV genome harboring deletions. 20,21 Although vestiges of previous EBV infection were not found in those examples, we report here the detection of defective het EBV DNA in 2 of 24 (8%) EBER-negative HD tumors in which the standard viral genome could not be demonstrated. Our inquiry was directed by the presumption that the absence of standard viral DNA may be consequent to a defective EBV genome, termed het DNA, previously implicated in viral episome loss from BL cells. 3,4 Rather than look for randomly integrated viral DNA fragments, we sought a helper virus-dependent, defective genome with a putative capability of eradicating standard EBV episomes from infected cells. Unlike the study by Staratschek-Jox and colleagues 20 that examined principally young adult HD cases in which EBV positivity in tumor tissue is lowest, we chose cases predominantly of children in which EBV presence in tumors is highest. 8,22,23 Here, any effector of EBV DNA loss would intuitively be most in evidence, potentially even in EBER-negative tumors. Accordingly, results demonstrated a defective EBV genome not only in 31% of EBER-positive tumors, but also in an EBER-negative subset of tumors as well. A second methodological difference setting this study apart from earlier work is that, instead of a standard in situ hybridization approach, we used highly sensitive PCR methodologies capable of distinguishing the defective genome from standard EBV DNA. Finally, the number of patients surveyed in our study was considerably larger than previously examined, increasing the likelihood for a conclusive result.
The defective EBV DNA genome as originally described in BL cell cultures is made up of four nonadjacent regions of viral DNA comprising approximately one-third of the standard genome: two larger segments from terminal regions and two smaller fragments originating from the central portion of the genome but in reverse orientation. 10,16,17,26-28 These regions contain an origin of replication, terminal sequences needed for packaging of the DNA into virions, as well as open reading frames (BZLF1, BSMLF1, and BI’LF1) known to encode proteins that function as transactivators of lytic gene expression. 10,16,17,26,28 The resultant new linkages in het DNA formed during genomic rearrangement have been shown to be remarkably consistent from BL cell lines to clinical samples. 3,4,10,11 As a marker for the defective EBV genome, we targeted abnormally juxtaposed BamHI W and Z fragments in het DNA. 10 This rearrangement causes constitutive expression of immediate early gene BZLF1 (BamHI Z Leftward ORF 1), the transient transfection of which has been shown to produce partial elimination of EBV episomes from infected cells. 29,30 The BZLF1-encoded protein (Zta) is thought to down-regulate the EBV latency promoter (Qp). 31 Qp is used to express latency protein EBNA-1, which is essential for the maintenance of the EBV episome in dividing cells. Thus, transient Zta induction provides a mechanistic basis for EBV DNA loss from cells within a tumor that is otherwise routinely associated with EBV. Impaired in its ability to replicate, the defective genome would presumably be lost once standard EBV DNA was eliminated. 4 Previous reports of BZLF1 expression in a small subset of Reed-Sternberg cells are consistent with our findings of het DNA in the occasional Reed-Sternberg cell. 32,33
The infrequency of Reed-Sternberg cells positive for defective EBV by PCR in situ hybridization implies that only a subclone within the total population of infected Reed-Sternberg precursors may lose virus by this mechanism. This observation gives rise to two predictions. First, expansion of the affected subclone to produce an EBV-negative tumor would require that a selective advantage be conferred by loss of virus. In this regard, the EBV-encoded latent membrane proteins LMP1 and LMP2 expressed in EBV-positive HD not only play a role in tumorigenesis, 34,35 but also provide immunological targets for the anti-tumor cytotoxic T-cell response. 36,37 Second, if viral DNA loss is indeed a by-product of the defective genome, it is likely that Reed-Sternberg cells within so-called EBV-positive HD may be heterogeneous with respect to presence of EBV DNA.
Even though abundant EBER expression is characteristic of latent EBV infection and routinely used as a diagnostic tool to detect EBV within tumor tissue, 38 we demonstrated presence of prototype EBV in four EBER-negative HD tumors. Absence of EBERs despite infection with the standard EBV genome may relate to variances in fixation within the tissue block and RNA degradation. However, altered patterns of EBER expression have been reported in both normal and malignant tissues. 39-41 Notably, two EBER-negative samples contained only defective genomes in absence of standard EBV, a finding that has been previously documented in fresh BL biopsies. 3
Identification of defective viral genomes either in the presence or absence of prototype EBV must be viewed in terms of potential biological relevance. Dysregulated, rearranged viral genes may have an impact beyond mere disruption of EBV latency and loss of viral episomes. We cannot exclude the possibility that defective het EBV DNA, which is packaged and spreads cell-to-cell, 5 is in itself pathogenic. For example, the defective genome also retains gene segments producing some of the highly spliced complementary strand transcripts (BamHI A rightward transcripts, or BARTS) overexpressed in EBV-associated cancers. 42-45 Questions regarding the dynamics of defective EBV with respect to viral gene regulation, cellular gene expression, and virus stability within cells form the basis for future investigation.
Acknowledgments
We thank Ann Watson (St. Jude Children’s Research Hospital, Memphis, TN) for sequence analysis, George Miller (Yale University, New Haven CT) for P3HR-1 clones 5 and 16, Carmen Mendonça (Hospital de Clinicas, Curitiba, Brazil) for Brazilian samples, J. J. Jenkins (St. Jude Children’s Research Hospital, Memphis, TN) for sections and histological subtyping, and the Louisiana State University Health Sciences Center-Shreveport Research Core Facility for access to real-time quantitative PCR technology.
Footnotes
Address reprint requests to John W. Sixbey, M.D., Department of Microbiology and Immunology, Louisiana State University Health Sciences Center-Shreveport, 1501 Kings Highway, Shreveport, LA 71130. E-mail: jsixbe@lsuhsc.edu.
Supported by grants RO1 CA67372 and RO1 DE12187 from the National Institutes of Health.
References
- 1.Delecluse HJ, Marafioti T, Hummel M, Dallenbach F, Anagnostopoulos I, Stein H: Disappearance of the Epstein-Barr virus in a relapse of Hodgkin’s disease. J Pathol 1997, 182:475-479 [DOI] [PubMed] [Google Scholar]
- 2.Nerurkar AY, Vijayan P, Srinivas V, Soman CS, Dinshaw KA, Advani SH, Magrath I, Bhatia K, Naresh KN: Discrepancies in Epstein-Barr virus association at presentation and relapse of classical Hodgkin’s disease: impact on pathogenesis. Ann Oncol 2000, 11:475-478 [DOI] [PubMed] [Google Scholar]
- 3.Razzouk BI, Srinivas S, Sample CE, Singh V, Sixbey JW: Epstein-Barr virus DNA recombination and loss in sporadic Burkitt’s lymphoma. J Infect Dis 1996, 173:529-535 [DOI] [PubMed] [Google Scholar]
- 4.Srinivas S, Sample JT, Sixbey JW: Spontaneous loss of viral episomes accompanying Epstein-Barr virus reactivation in a Burkitt’s lymphoma cell line. J Infect Dis 1998, 177:1705-1709 [DOI] [PubMed] [Google Scholar]
- 5.Miller G, Heston L, Countryman J: P3HR-1 Epstein-Barr virus with heterogeneous DNA is an independent replicon maintained by cell-to-cell spread. J Virol 1985, 54:45-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabson M, Heston L, Miller G: Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci USA 1983, 80:2762-2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu TC, Mann RB, Charache P, Hayward SD, Staal S, Lambe BC, Ambinder RF: Detection of EBV gene expression in Reed-Sternberg cells of Hodgkin’s Disease. Int J Cancer 1990, 46:801-804 [DOI] [PubMed] [Google Scholar]
- 8.Razzouk BI, Gan YJ, Mendonça C, Jenkins JJ, Liu Q, Hudson M, Sixbey JW, Ribeiro RC: Epstein-Barr virus in pediatric Hodgkin disease: age and histiotype are more predictive than geographic region. Med Pediatr Oncol 1997, 28:248-254 [DOI] [PubMed] [Google Scholar]
- 9.Wright DK, Manos MM: Sample preparation from paraffin-embedded tissues. Innis MA Gelfand DH Simsky J White TJ eds. PCR Protocols: A Guide to Methods and Applications. 1990, :pp153-158 Academic Press, San Diego [Google Scholar]
- 10.Jenson HB, Miller G: Polymorphisms of the region of the Epstein-Barr virus genome which disrupts latency. Virology 1988, 165:549-564 [DOI] [PubMed] [Google Scholar]
- 11.Patton DF, Shirley P, Raab-Traub N, Resnick L, Sixbey JW: Defective viral DNA in Epstein-Barr virus-associated oral hairy leukoplakia. J Virol 1990, 64:397-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bagasra O, Seshamma T, Pomerantz RJ: Polymerase chain reaction in situ: intracellular amplification and detection of HIV-1 proviral DNA and other specific genes. J Immunol Methods 1993, 158:131-145 [DOI] [PubMed] [Google Scholar]
- 13.Rooney CM, Smith CA, Ng CYC, Loftin SK, Sixbey JW, Gan YJ, Srivastava D-K, Bowman LC, Krance RA, Brenner MK, Heslop HE: Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood 1998, 92:1549-1555 [PubMed] [Google Scholar]
- 14.Jabs WJ, Hennig H, Kittel M, Pethig K, Smets F, Bucsky P, Kirchner H, Wagner HJ: Normalized quantification of Epstein-Barr virus load by means of real-time PCR in patients at risk for posttransplant lymphoproliferative disorder. J Clin Microbiol 2001, 39:564-569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wandinger K-P, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H-J, Wessel K, Kirchner H, Hennig H: Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 2000, 55:178-184 [DOI] [PubMed] [Google Scholar]
- 16.Cho MS, Bornkamm GW, zur Hausen H: Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J Virol 1984, 51:199-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho MS, Gissman L, Hayward SD: Epstein-Barr virus (P3HR-1) defective DNA codes for components of both the early antigen and viral capsid antigen complexes. Virology 1984, 137:9-19 [DOI] [PubMed] [Google Scholar]
- 18.Gulley ML, Eagan PA, Quintanilla-Martinez L, Picado AL, Smir BN, Childs C, Dunn CD, Craig FE, Williams Jr JW, Banks PM: Epstein-Barr virus DNA is abundant and monoclonal in the Reed-Sternberg cells of Hodgkin’s disease: association with mixed cellularity subtype and Hispanic American ethnicity. Blood 1994, 83:1595–1602 [PubMed]
- 19.Weiss LM, Movahed LA, Warnke RA, Sklar J: Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin’s disease. N Engl J Med 1989, 320:502-506 [DOI] [PubMed] [Google Scholar]
- 20.Staratschek-Jox A, Kotkowski S, Belge G, Rüdiger T, Bullerdiek J, Diehl V, Wolf J: Detection of Epstein-Barr virus in Hodgkin-Reed-Sternberg cells: no evidence for the persistence of integrated viral fragments in latent membrane protein-1 (LMP-1)-negative classical Hodgkin’s disease. Am J Pathol 2000, 156:209-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambinder R: Gammaherpesviruses and ‘hit-and-run’ oncogenesis. Am J Pathol 2000, 156:1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarrett RF, Gallagher A, Jones DB, Alexander FE, Krajewski AS, Kelsey A, Adams J, Angus B, Gledhill S, Wright DH, Cartwright RA, Onions DE: Detection of Epstein-Barr virus genomes in Hodgkin’s disease: relation to age. J Clin Pathol 1991, 44:844-848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady JO, Hummel M, Preciado MV, Knecht H, Chan JKC, Claviez A: Epstein-Barr virus-associated Hodgkin’s Disease: epidemiologic characteristics in international data. Int J Cancer 1997, 70:375-382 [DOI] [PubMed] [Google Scholar]
- 24.Heller MT, Dambaugh T, Kieff E: Epstein-Barr virus DNA IX: variation among viral DNAs from producer and nonproducer infected cells. J Virol 1981, 38:632-648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delius H, Bornkamm GW: Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol 1978, 137:9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenson HB, Rabson MS, Miller G: Palindromic structure and polypeptide expression of 36 kilobase-pairs of heterogeneous Epstein-Barr virus (P3HR-1) DNA. J Virol 1986, 58:475-486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenson HB, Farrell PJ, Miller G: Sequences of the Epstein-Barr virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J Virol 1987, 61:1495-1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff E: Epstein-Barr virus and its replication. Fields BN Knipe DM Howley PM Chanock RM Melnick JL Monath TP Roizman B Straus SE eds. Fields Virology. 1996, :pp 2342-2396 Lippincott-Raven, Philadelphia [Google Scholar]
- 29.Rooney C, Taylor N, Countryman J, Jenson H, Kolman J, Miller G: Genomic rearrangements activate the Epstein-Barr virus gene whose product disrupts latency. Proc Natl Acad Sci USA 1988, 85:9801-9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada K, Ji Z, Fujiwara S, Shimizu N, Tanabe-Tochikura A: Partial elimination of Epstein-Barr virus plasmids from Burkitt’s lymphoma cells by transfection of the BZLF1 gene. J Virol 1992, 66:5590-5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Lee JM, Wang Y, Huang DP, Ambinder RF, Hayward SD: The Epstein-Barr virus latency BamHI-Q promoter is positively regulated by STATs and Zta interference with JAK/STAT activation leads to loss of BamHI-Q promoter activity. Proc Natl Acad Sci USA 1999, 96:9339-9344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pallesen G, Sandvej K, Hamilton-Dutoit SJ, Rowe M, Young LS: Activation of Epstein-Barr virus replication in Hodgkin and Reed-Sternberg cells. Blood 1991, 78:1162-1165 [PubMed] [Google Scholar]
- 33.Bibeau F, Brousset P, Knecht H, Meggetto F, Drouet E, Rubin B, Delsol G: Epstein-Barr virus replication in Hodgkin’s disease. Bull Cancer 1994, 81:114-118 [PubMed] [Google Scholar]
- 34.Kulwichit W, Edwards RH, Davenport EM, Baskar JF, Godfrey V, Raab-Traub N: Expression of the Epstein-Barr virus latent membrane protein 1 induces B cell lymphoma in transgenic mice. Proc Natl Acad Sci USA 1998, 95:11963-11968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholle F, Bendt KM, Raab-Traub N: Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol 2000, 74:10681-10689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sing AP, Ambinder RF, Hong DJ, Jensen M, Batten W, Petersdorf E, Greenberg PD: Isolation of Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes that lyse Reed-Sternberg cells: implications for immune-mediated therapy of EBV+ Hodgkin’s disease. Blood 1997, 89:1978-1986 [PubMed] [Google Scholar]
- 37.Roskrow MA, Suzuki N, Gan YJ, Sixbey JW, Ng CYC, Kimbrough S, Hudson M, Brenner MK, Heslop H, Rooney CM: Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin’s disease. Blood 1998, 91:2925-2934 [PubMed] [Google Scholar]
- 38.Chang KL, Chen YY, Shibata D, Weiss LM: Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol 1992, 1:246-255 [PubMed] [Google Scholar]
- 39.Gilligan K, Rajadurai P, Resnick L, Raab-Traub N: Epstein-Barr virus small nuclear RNAs are not expressed in permissively infected cells in AIDS-associated leukoplakia. Proc Natl Acad Sci USA 1990, 87:8790-8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greifenegger N, Jäger M, Kunz-Schughart LA, Wolf H: Epstein-Barr virus small RNA (EBER) genes: differential regulation during lytic viral replication. 1998, 72:9323–9328 [DOI] [PMC free article] [PubMed]
- 41.Sugawara Y, Mizugaki Y, Uchida T, Torii T, Imai S, Makuuchi M, Takada K: Detection of Epstein-Barr virus (EBV) in hepatocellular carcinoma tissue: a novel EBV latency characterized by the absence of EBV-encoded small RNA expression. Virology 1999, 256:196-202 [DOI] [PubMed] [Google Scholar]
- 42.Gilligan KJ, Rajadurai P, Lin JC, Busson P, Abdel-Hamid M, Prasad U, Tursz T, Raab-Traub N: Expression of the Epstein-Barr virus BamHI A fragment in nasopharyngeal carcinoma: evidence for a viral protein expressed in vivo. J Virol 1991, 65:6252-6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen HK, Lung MM, Sham JS, Choy DT, Griffin BE, Ng MH: Transcription of BamHI A region of the EBV genome in NPC tissues and B cells. Virology 1992, 191:193-201 [DOI] [PubMed] [Google Scholar]
- 44.Sadler RH, Raab-Traub N: Structural analysis of the Epstein-Barr virus BamHI A transcripts. J Virol 1995, 69:1132-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith PR, de Jesus O, Turner D, Hollyoake M, Karstegl CE, Griffin BE, Karran L, Wang Y, Hayward SD, Farrell PJ: Structure and coding content of CST (BART) family of RNAs of Epstein-Barr virus. J Virol 2000, 74:3082-3092 [DOI] [PMC free article] [PubMed] [Google Scholar]