Abstract
Epithelial cancer of the ovary spreads by implantation of tumor cells onto the mesothelial cells lining the peritoneal cavity. The aim of this study was to identify the adhesion molecules involved in the interaction of ovarian carcinoma cells with mesothelial cells. The human ovarian carcinoma cell lines SKOV3 and NIH:OVCAR5 as well as LP9 cells, a human peritoneal mesothelial cell line, were analyzed by flow cytometry for the expression of CD44 and the β1 integrin subunit. An in vitro adhesion assay was developed whereby LP9 cells were grown as confluent monolayers, and radiolabeled ovarian carcinoma cells were monitored for their ability to adhere to the mesothelial monolayer in the presence of potential inhibitors. Each cell line was evaluated for the presence of a pericellular matrix by a particle exclusion assay. A monoclonal antibody (MAb) against the β1 integrin subunit significantly reduced the adhesion of SKOV3 cells to LP9 cells, whereas NIH:OVCAR5 adhesion to LP9 cells was significantly inhibited by a CD44 MAb. The LP9 cells produced both hyaluronic acid (a ligand for CD44) as well as several extracellular matrix molecules (ligands for the β1 integrin heterodimers). These results suggest that both CD44 and the β1 integrin heterodimers may play a role in mediating the adhesion of ovarian carcinoma cells to mesothelial cells.
Epithelial cancer of the ovary is the fourth leading cause of cancer death in women in the United States, with little change in its incidence in recent decades. 1 A current working model for the metastatic process of ovarian carcinoma suggests that cancer cells are shed from the ovary and are present in the peritoneal fluid. The cancer cells then may attach to the layer of mesothelial cells that line the inner surface of the peritoneal cavity. Once ovarian carcinoma cells adhere to mesothelial cells, the cancer cells may migrate through the layer of mesothelial cells, invade the local organs, and spread to distant sites. This multistep process of cancer cell adhesion, migration, and invasion is believed to eventually result in the death of the patient. Curing advanced ovarian cancer is difficult because of both the inability to completely resect diffuse tumor involvement on the peritoneal surface and the eventual resistance of the cancer cells to chemotherapy. 2,3
Although an early step of metastasis likely involves the adhesion of ovarian carcinoma cells to mesothelial cells, few studies have focused on this interaction. In recent studies, in vitro adhesion assays were performed using mesothelial cells and ovarian carcinoma cells isolated from patients. 4,5 A CD44 monoclonal antibody (MAb) was able to partially inhibit this interaction, leading to the conclusion that CD44 on the surface of ovarian cancer cells may mediate binding to mesothelium-associated hyaluronic acid. 6,7 Furthermore, a CD44 MAb reduced the number of intra-abdominal tumor implants of a human ovarian carcinoma xenograft in nude mice. 8 However, as the CD44 MAb did not cause complete inhibition in either the in vitro or the in vivo studies, it is likely that some other cell surface molecules may also be involved in the adhesion of the ovarian carcinoma cells to the mesothelial cells.
Other studies have shown that CD44 on the surface of ovarian carcinoma cells is important in the binding to mesothelium-associated hyaluronic acid. For example, it has been demonstrated that hyaluronic acid resides in a cell-associated matrix, also termed a pericellular matrix, around the mesothelial cells that could be destroyed by aspirating the mesothelial cells’ medium or by treating the mesothelial cells with hyaluronidase. 5 Another study showed that six different ovarian carcinoma cell lines that expressed CD44 were able to adhere to plates coated with varying concentrations of hyaluronic acid, further demonstrating that CD44 on ovarian cancer cells may bind to hyaluronic acid present on mesothelial cells. 9 In support of a role for CD44 and hyaluronic acid in cancer, CD44 variant expression has been shown to be a common feature in epithelial ovarian cancer, 10 and tumor-cell-associated hyaluronic acid has been shown to be an unfavorable prognostic factor in colorectal cancer. 11
Other families of molecules that are involved in cell-cell interactions include integrins, selectins, cadherins, and the immunoglobulin superfamily. Integrins are a widely expressed family of cell surface adhesion receptors that are composed of an α subunit noncovalently associated to a β subunit. 12,13 Together, these two integrin subunits confer specificity to extracellular matrix (ECM) proteins. In many cases, one integrin heterodimer may interact with more than one ECM protein. 14 Recently, Cannistra et al focused on the role of integrins in the interaction of ovarian carcinoma cells with mesothelial cells. 15 They determined that mesothelial cells synthesize the ECM molecule fibronectin and that ovarian cancer cells express integrins. However, they did not provide evidence that integrins play a role in the interaction of ovarian carcinoma cells with mesothelial cells.
In this study, an in vitro adhesion assay was developed by growing a human peritoneal mesothelial cell line, LP9, as confluent monolayers in 96-well microtiter plates. After 2 days, [35S]methionine-labeled human ovarian carcinoma cell lines SKOV3 and NIH:OVCAR5 were added and allowed to adhere to the mesothelial monolayer and washed, and the radioactivity was quantitated. The assay was optimized for such variables as the number of cells added, incubation time, and the washing conditions. The SKOV3, NIH:OVCAR5, and LP9 cell lines were analyzed by flow cytometry to determine the surface expression of various cell adhesion molecules. MAbs against CD44 and the β1 integrin subunit were tested for their ability to alter the adhesion of the ovarian carcinoma cells to LP9 cells. In addition, the expression of ECM proteins by the mesothelial cells was evaluated by ELISA and radioimmunoprecipitation. Furthermore, particle exclusion assays were performed to determine whether the various cell lines synthesized a pericellular matrix. Our results suggest that both the β1 integrin subunit and CD44 may play a role in mediating the adhesion of ovarian carcinoma cells to mesothelial cells.
Materials and Methods
Cell Cultures
The human ovarian carcinoma cell line SKOV3, originally isolated from the ascites fluid of a patient with adenocarcinoma of the ovary, 16 was obtained at passage 42 from Dr. Robert C. Bast Jr., M.D. Anderson Cancer Center, University of Texas. They were used between passages 45 and 55 and grown as confluent monolayers in 75-cm 2 tissue culture flasks. The cells were maintained in McCoy’s 5A medium (Sigma Chemical Co., St. Louis, MO) supplemented with 15% fetal bovine serum (FBS), 2 mmol/L glutamine, and 50 U/ml penicillin G/streptomycin (Celox Labs, Minneapolis, MN). The human ovarian carcinoma cell line NIH:OVCAR5 was obtained from Dr. Thomas C. Hamilton, Fox Chase Cancer Center, Philadelphia, PA. 17 They were maintained in RPMI 1640 medium (Sigma), supplemented with 10% FBS, 2 mmol/L glutamine, 50 U/ml penicillin G/streptomycin (Celox Labs), and 0.2 U/ml insulin (Sigma). The human mesothelial cell line LP9 was obtained from the NIA Aging Cell repository (Camden, NJ) at passage 5. They were maintained in a medium containing a 1:1 ratio of M199 and MCDB10 basal medium (Sigma), supplemented with 15% FBS, 5 ng/ml epidermal growth factor (Sigma), 0.4 μg/ml hydrocortisone, 50 U/ml penicillin G/streptomycin (Celox Labs), and 2 mmol/L glutamine (Sigma). The LP9 cells were routinely used in the assay at passage 8. Cells were maintained in an incubator with 5% CO2 at 37°C. Photographs of cell morphologies were taken using Kodak PX125 film and an Olympus OM-4T camera mounted on an Olympus CK2 inverted microscope (Olympus Corp., Lake Success, NY).
Antibodies
Purified goat polyclonal IgG against human collagen types I, III, and IV were purchased from Chemicon International (Temecula, CA). Polyclonal rabbit IgG against human vitronectin was purchased from Celsus Labs (Cincinnati, OH). Affinity-purified rabbit polyclonal IgG against human fibronectin was provided by Dr. James McCarthy (University of Minnesota, Minneapolis, MN). Affinity-purified rabbit polyclonal IgG against murine laminin was provided by Dr. Leo Furcht (University of Minnesota). Purified IgG of mouse MAb P5D2 against the human β1 integrin subunit was provided by Dr. Leo Furcht. Affinity-purified CD44 MAb IM7 IgG was purchased from Pharmingen (San Diego, CA), and affinity-purified CD44 MAb BU52 IgG was purchased from Binding Site Limited (San Diego, CA). Mouse MAb ascites against the human integrin α2 subunit (clone P1E6) and against the human integrin αv subunit (clone VNR147) were purchased from Gibco BRL (Gaithersburg, MD). Affinity-purified mouse MAb IgG against the human integrin α5 subunit was purchased from Chemicon International, and affinity-purified rat MAb IgG against the human integrin α6 subunit (clone GoH3) was purchased from AMAC (Westbrook, ME).
ECM Molecules
Mouse type IV collagen was purchased from Gibco BRL. Mouse EHS laminin, prepared as described, 18 was provided by Dr. Leo Furcht. Human plasma fibronectin, purified as described previously, 19 was provided by Dr. James McCarthy.
Flow Cytometry Analysis
Confluent SKOV3, NIH:OVCAR5, or LP9 cells were harvested from 75-cm 2 tissue culture flasks by gentle treatment with 1% trypsin in PBS containing 2 mmol/L EDTA as previously described. 20 Cells at 2 × 10 5 per sample were blocked at 4°C for 30 minutes in a solution of base medium (M199/MCDB110 media for LP9 cells, McCoy’s 5A media for SKOV3 cells, or RPMI 1640 for NIH:OVCAR5 cells) containing 1% goat serum and 0.02% sodium azide. Cells were then collected by centrifugation at 400 × g for 5 minutes, incubated with the indicated MAbs at 4°C for 1 hour, and washed twice with a wash buffer of base medium containing 1% goat serum, 20 mmol/L HEPES, and 0.2% NaN3. Cells were incubated at 4°C for 30 minutes with goat IgG against mouse IgG conjugated with fluorescein isothiocyanate (FITC; Tago, Burlingame, CA) that had been diluted 1:100 in base medium, 20 mmol/L HEPES, and 0.02% NaN3. Cells were then washed once with wash buffer, fixed in 2% formaldehyde in PBS, and analyzed on a Becton Dickinson FACSCalibur (San Jose, CA), in conjunction with CellQuest software for data acquisition and analysis. Approximately 10,000 fluorescent events were counted to determine the mean fluorescence intensity (MFI) for each sample.
Cell-Cell Adhesion Assay
LP9 mesothelial cells were trypsinized from 75-cm 2 flasks and added to clear-bottom 96-well microtiter ViewPlates (Packard Instruments, Meriden, CT) at 12,000 cells/well and grown to confluency over a 48-hour period. The mesothelial monolayer was then washed twice with 150 μl of assay buffer (phenol-red-free Dulbecco’s modified Eagle’s medium (Sigma) containing 1.8 mmol/L calcium chloride and 80 mmol/L magnesium sulfate, 2 mg/ml ovalbumin, and 20 mmol/L HEPES, pH 7.4. SKOV3 or NIH:OVCAR5 cells were labeled with l-[35S]methionine (1175 Ci/mmol, NEN, Boston, MA) for 24 hours and trypsinized as previously described. 20 They were then washed two times, added to monolayers of mesothelial cells, and incubated for various lengths of time, and non-adherent SKOV3 or NIH:OVCAR5 cells were removed by washing with the assay buffer. Radioactivity was measured by adding Microscint 40 (Packard Instruments) to wells and placing them in a TopCount microplate scintillation counter (Packard Instruments). The assay was performed in eight replicates for each condition.
Inhibition of Cell-Cell Adhesion
Inhibition assays were performed in the same way as the adhesion assay described above, except that the SKOV3 or NIH:OVCAR5 cells were pretreated with antibodies or ECM proteins for 30 minutes at 37°C before their addition at a density of 10,000 cells per well to the mesothelial cell monolayer. The ovarian carcinoma cells were incubated with the mesothelial cells in the continued presence of the potential inhibitors for 20 minutes at 37°C and washed two times with the assay buffer.
Inhibition of Cell-ECM Adhesion
SKOV3 or NIH:OVCAR5 cells were pretreated with MAbs against integrin subunits or normal mouse IgG for 30 minutes at 37°C before their addition at a density of 5000 cells per well to microtiter plates coated with either laminin (7 μg/ml), fibronectin (5 μg/ml), or type IV collagen (1.5 μg/ml). These concentrations of ECM molecules were selected because they caused 50% of the maximal adhesion of cells in a 20-minute adhesion assay. The ovarian carcinoma cells were incubated in the continued presence of the IgG for 20 minutes at 37°C and washed two times with the assay buffer.
ELISA
Mesothelial cells were seeded at 12,000 cells/well into clear-bottom 96-well microtiter plates and incubated for 48 hours until they were confluent. The cells were washed three times with PBS, incubated with the primary antibodies for 1 hour, and washed three times with 0.02% Tween 20 in PBS. The wells were then incubated with a horseradish-peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG for 1 hour at 37°C. The wells were washed again and allowed to react with the substrate reagent, 2 mmol/L ortho-phenylenediamine in 0.1 mol/L citric acid. After 1 minute, the reaction was stopped by the addition of 2.5 mol/L sulfuric acid, and the absorbance at 490 nm was measured using a UV max ELISA plate reader (Molecular Devices Corp., Sunnyvale, CA).
Radiolabeling and Isolation of Mesothelial Cellular Proteins
LP9 cells were labeled with [35S]methionine (1175 Ci/mmol; NEN) for 24 hours at 37°C. The medium was then collected, and 1 mmol/L phenylmethylsulfonyl fluoride (PMSF; Sigma) and 1 mmol/L N-ethyl maleimide (NEM; Sigma) were added to inhibit proteases. Cells were washed twice with PBS, and proteins were extracted by incubation in extraction buffer (PBS, pH 7.4, containing 1% Triton X-100, 10 mmol/L EDTA, 1 mmol/L PMSF, and 1 mmol/L NEM) on a rocker for 30 minutes at 4°C. Nuclei and cellular debris were removed by centrifugation at 8000 × g for 1 hour at 4°C.
Preclearing of Radiolabeled Cell Extracts
To eliminate nonspecific binding of primary antibody to mesothelial cellular protein, rabbit anti-mouse IgG (Sigma) or rabbit anti-goat IgG (Sigma) were coupled to protein-A agarose beads (Sigma) in PBS for 2 hours at 4°C (1 mg of IgG per 1 ml of packed volume of beads). The coupled beads were collected by centrifugation at 400 × g for 5 minutes, washed twice with immunoprecipitation buffer (50 mmol/L Tris, pH 7.5, 1% Triton X-100, 0.5% ovalbumin, and 0.02% NaN3), and resuspended as a 20% suspension. These beads were further coupled to primary antibodies for 2 hours at 4°C. The beads were then pelleted by centrifugation at 400 × g for 5 minutes and washed twice with the immunoprecipitation buffer. Each milliliter of the [35S]methionine-labeled cell extract or proteins secreted into the medium was precleared three times by incubation on a rocker for 1 hour at 4°C with 0.25 ml of 10% protein A immobilized on cross-linked 4% agarose beads (Sigma) coupled to rabbit anti-mouse IgG in the presence of normal mouse IgG or rabbit anti-goat IgG in the presence of normal goat IgG in PBS. The supernatant was collected by centrifugation at 400 × g for 5 minutes.
Immunoprecipitation
Immunoprecipitation of the precleared radiolabeled proteins was performed by adding 4 × 10 7 cpm of the cell extracts or 8 × 10 7 cpm of the spent cell medium to 0.1 ml of primary antibody-coupled beads. More cpm were used from the spent cell medium as compared with those deposited into the cell layer, as the majority of radioactivity in the spent cell medium is free [35S]methionine that is not incorporated into protein. In contrast, the majority of radioactivity in the cell extract is incorporated into protein. The immunoprecipitation process was allowed to proceed overnight at 4°C. The immunoprecipitates were collected by centrifugation at 14,000 × g for 3 minutes and then washed five times with a wash buffer (50 mmol/L Tris, pH 7.5, 400 mmol/L NaCl, 1% Triton X-100, 1 mmol/L EDTA, and 0.02% NaN3). The beads were then boiled for 5 minutes in a reducing sample buffer (62.5 mmol/L Tris/HCl, pH 6.8, 2% SDS, 20% glycerol, 5% β-mercaptoethanol, and 0.1% bromophenol blue) and centrifuged at 13,000 × g for 5 minutes. The supernatant was analyzed on a 7.5% SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Rainbow colored molecular weight standards were purchased from Amersham Life Science (Arlington Heights, IL).
Particle Exclusion Assay
Pericellular matrices were visualized using a particle exclusion assay. 21 Cells were seeded in their respective growth media at a density of 1 × 10 5 cells per well in six-well plates for 24 hours. The growth media were replaced with fresh growth media alone or contained one of the following three reagents: 1) 80 U/ml bovine testicular hyaluronidase (3000 U/mg; Sigma), 2) 4 U/ml Streptomyces hyaluronlyticus hyaluronidase (Calbiochem-Novabiochem Co., La Jolla, CA), or 3) 2 mg/ml rat chondrosarcoma proteoglycan (RCS-PG). The hyaluronidase was incubated in the wells for 1 hour at 37°C, whereas the RCS-PG was incubated in the wells for 2 hours at 37°C. After these treatments, the medium was removed from the monolayer of cell cultures, and 750 μl of a suspension of formalin-fixed goat erythrocytes (10 8 cells/ml in PBS, pH 7.4, containing 0.1% ovalbumin) was added to each well and allowed to settle for 15 minutes. Photographs of cell cultures were taken using Kodak PX125 film and an Olympus OM-4T camera mounted on an Olympus CK2 inverted microscope (Olympus Corp., Lake Success, NY).
Fixation and Hyaluronidase Treatment of Mesothelial Cells
Mesothelial cells were grown in 96-well microtiter ViewPlates (Packard Instruments) to confluency over a 48-hour period as described above. The mesothelial cell monolayer was washed twice with 150 μl of assay buffer and then treated with bovine testicular hyaluronidase or Streptomyces hyaluronlyticus hyaluronidase at various concentrations for 1 hour at 37°C. Next, they were fixed in 2% formaldehyde in PBS at room temperature for 15 minutes and then washed three times with the assay buffer.
Statistical Analysis
Student’s t-test was performed as a test of significance with the use of a Microsoft Excel program (release 5.0, Microsoft Co., Redmond, WA). All P values resulted from two-sided statistical tests. P values of <0.05 were considered to indicate statistically significant differences.
Results
Identification of Cell Adhesion Molecules Present on Ovarian Carcinoma and Mesothelial Cells
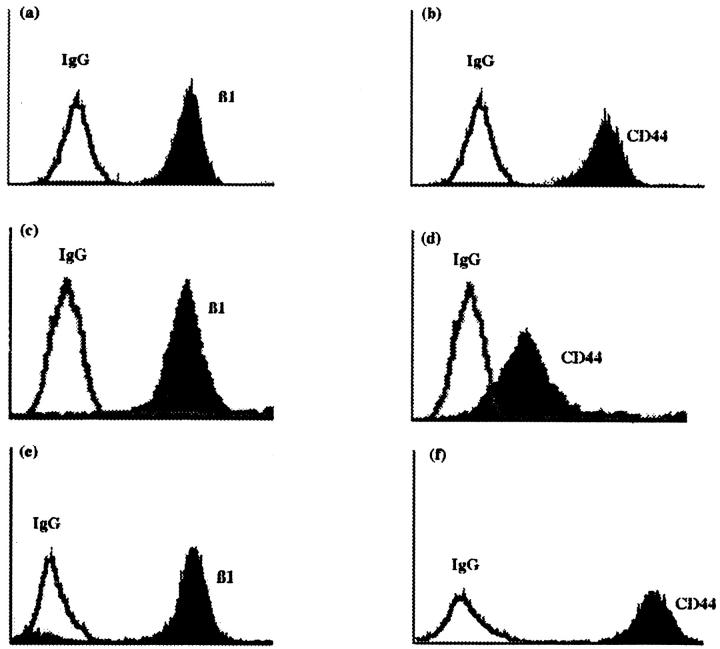
The expression of CD44 and the β1 integrin subunit on the ovarian carcinoma cell lines SKOV3 and NIH:OVCAR5, as well as the mesothelial cell line LP9, was determined by flow cytometry analysis. All three cell lines expressed very high levels of both CD44 and the β1 integrin subunit (Figure 1 ▶ , black peaks) when compared with the background values obtained with normal mouse IgG (Figure 1 ▶ , white peaks).
Figure 1.
Expression of the β1 integrin subunit and CD44 on ovarian carcinoma or mesothelial cell lines. The SKOV3 ovarian carcinoma cell line (a and b), NIH:OVCAR5 ovarian carcinoma cell line (c and d), and LP9 mesothelial cell line (e and f) were analyzed by flow cytometry using normal mouse IgG (white peaks) as a negative control or MAb P5D2 against the β1 integrin subunit (black peaks; a, c, and e) or CD44 MAb BU52 (black peaks; b, d, and f). The y axis is the number of positive cells and the x axis is the mean channel fluorescence.
Cell Adhesion Assay Optimization
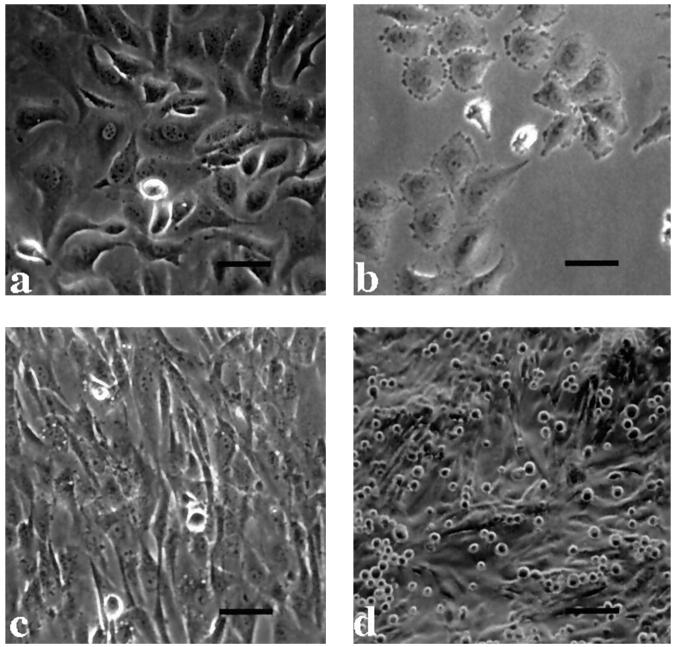
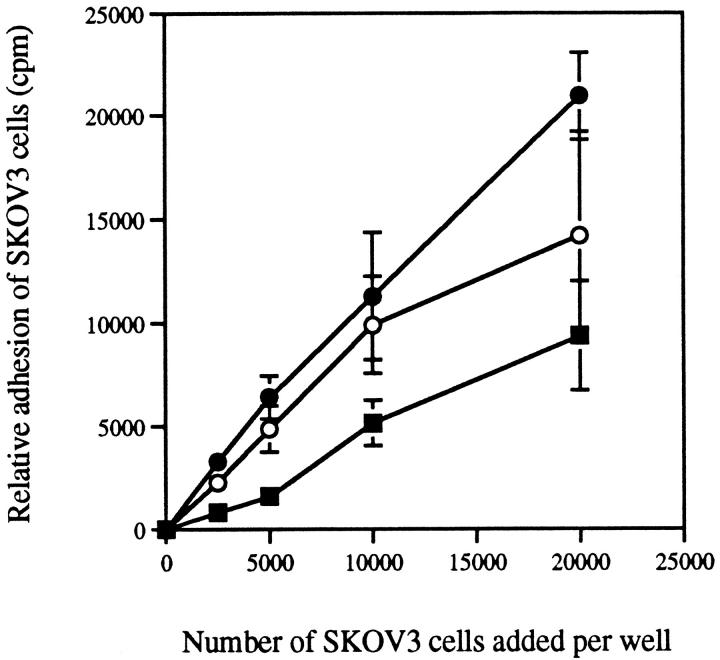
To design an assay to quantitate the adhesion of ovarian carcinoma cells to mesothelial cells, we first determined the optimal number of ovarian carcinoma cells to add to each well containing mesothelial cells, as well as the optimal length of time for the assay. Ovarian carcinoma cell lines SKOV3 (Figure 2a) ▶ and NIH:OVCAR5 (Figure 2b) ▶ were radiolabeled, and 2500, 5000, 10,000, or 20,000 cells were added to each well containing a confluent monolayer of mesothelial cells (Figure 2c) ▶ . Ovarian carcinoma cells were allowed to bind to the mesothelial cells for 5, 15, and 30 minutes. The ovarian carcinoma cells remained rounded up during these adhesion assays and did not spread out on the mesothelial cell monolayer (Figure 2d) ▶ . The relative adhesion of ovarian carcinoma cells to mesothelial cells increased with increasing numbers of ovarian carcinoma cells added, as shown for SKOV3 cells in Figure 3 ▶ . When fewer than 5000 SKOV3 cells were added, the cpm were too low to be useful for inhibition studies. Likewise, when SKOV3 cells were allowed to adhere to the monolayer of mesothelial cells for only 5 minutes, significant levels of adhesion (∼30%) did occur; however, the cpm values were relatively low. The percentage of SKOV3 cells that adhered to mesothelial cells at 15 and 30 minutes were comparable when 0 to 10,000 SKOV3 cells were added (Figure 3) ▶ . However, when 20,000 SKOV3 cells were added, ∼40% of the cells adhered in 15 minutes, whereas >60% of the cells adhered in 30 minutes (Figure 3) ▶ . The data from these experiments were then used to design an inhibition assay. Based on previous studies, 20 we have observed that optimal inhibition occurs in these types of assays if the level of adhesion is <50%. This can be achieved when the two parameters, the number of cells used and the length of the assay, have been selected to give high enough cpm to be significantly over background but not so high as to make it difficult to observe a decrease in adhesion when screening potential inhibitors of adhesion. Therefore, based on these results, 10,000 SKOV3 cells were added to each microtiter well containing a confluent monolayer of mesothelial cells and allowed to adhere for 20 minutes for all subsequent experiments. Similar results were obtained with NIH:OVCAR5 cells (not shown).
Figure 2.
Morphology of the ovarian carcinoma cell lines and LP9 mesothelial cells. Morphology of SKOV3 ovarian carcinoma cells (a) and NIH:OVCAR5 ovarian carcinoma cells (b) when grown on tissue culture plastic; bar, 17.5 μm. c: A confluent monolayer of LP9 mesothelial cells; bar, 17.5 μm. d: When SKOV3 cells were trypsinized and added to a confluent monolayer of mesothelial cells, they remained rounded up during the 20-minute assay; bar, 35 μm. The NIH:OVCAR5 cells appeared similar to the SKOV3 cells during the assay (not shown).
Figure 3.
Cell adhesion assay optimization. SKOV3 cells were [35S]methionine labeled, and increasing numbers of cells were added to each well in quadruplicate, which contained confluent monolayers of mesothelial cells. Binding was allowed to occur for 5 minutes (▪), 15 minutes (○), or 30 minutes (•), and then unbound cells were washed two times. Relative cell adhesion was assessed by measuring the amount of radioactivity as described in Materials and Methods. In this experiment, 20,000 cells corresponded to 33,000 cpm. Data are expressed as mean ± SD.
Inhibition of Ovarian Carcinoma Cell Binding to Mesothelial Cells by MAbs against CD44 or the β1 Integrin Subunit
To determine the role of CD44 and the β1 integrin heterodimers in the adhesion of ovarian carcinoma cells to mesothelial cells, we preincubated SKOV3 and NIH:OVCAR5 cells with MAbs against either the β1 integrin subunit or CD44 at varying concentrations for 30 minutes at 37°C. Normal mouse IgG was used as a negative control. Ten thousand ovarian carcinoma cells were preincubated with either the MAb against the β1 integrin subunit, the CD44 MAb, or normal mouse IgG, and the cells were added to confluent mesothelial cells and incubated for 20 minutes. Pretreatment of SKOV3 cells with the MAb against the β1 integrin subunit significantly (P < 0.001) decreased SKOV3 cell adhesion to mesothelial cells in a concentration-dependent manner (Figure 4A) ▶ , with maximal inhibition of >50% at 10 μg/ml. However, the CD44 MAb did not cause significant inhibition, nor did normal mouse IgG (Figure 4A) ▶ . In contrast, pretreatment of NIH:OVCAR5 cells with the CD44 MAb significantly (P < 0.001) inhibited NIH:OVCAR5 cell adhesion to mesothelial cells by ∼40% (Figure 4B) ▶ , whereas neither the MAb against the β1 integrin subunit nor normal mouse IgG caused significant inhibition (Figure 4B) ▶ . When SKOV3 or NIH:OVCAR5 cells were pretreated simultaneously with MAbs against the β1 integrin subunit and the CD44 MAb, no additive inhibitory effect was observed (data not shown).
Figure 4.
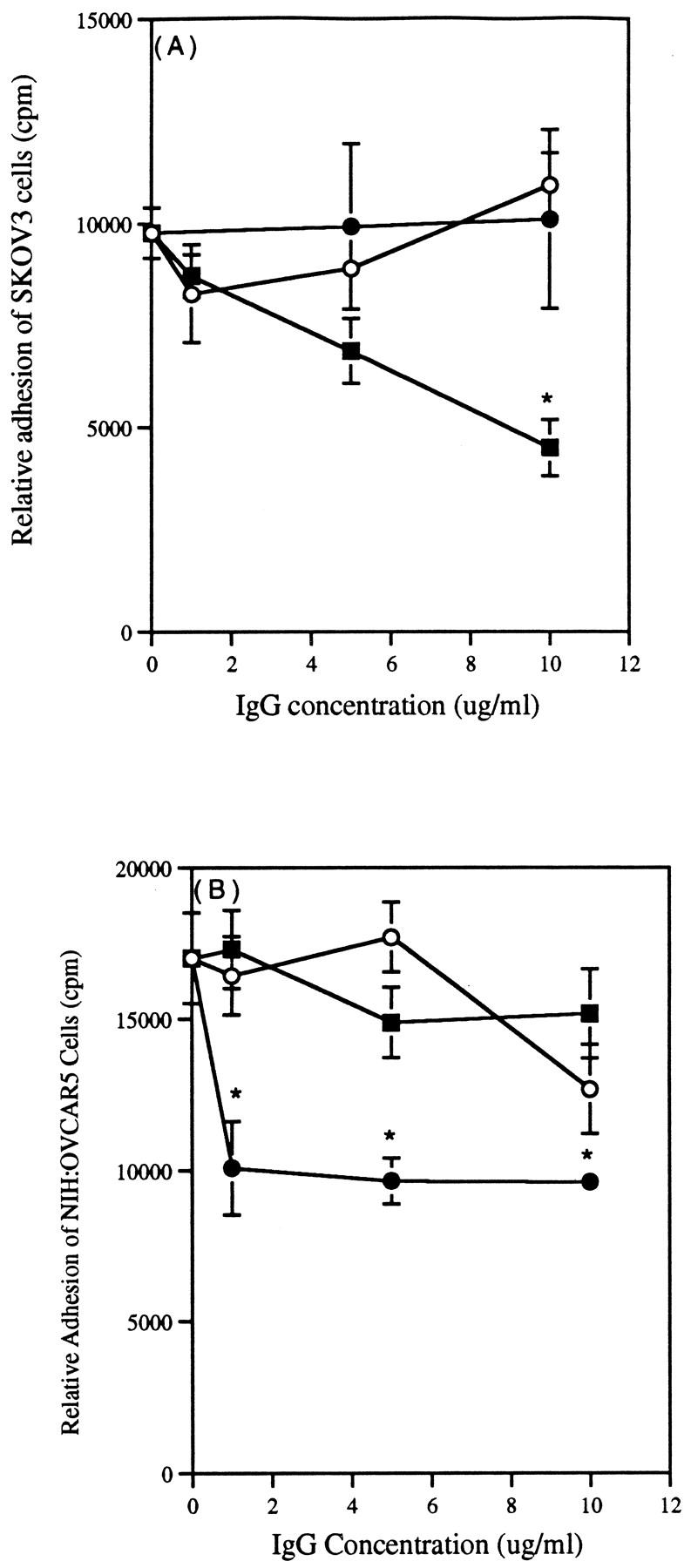
Inhibition of ovarian carcinoma cell adhesion to mesothelial cells by MAbs against the β1 integrin subunit or CD44. Ovarian carcinoma cell lines SKOV3 (A) or NIH:OVCAR5 (B) were labeled with [35S]methionine and preincubated with increasing concentrations of P5D2, a MAb against the β1 integrin subunit (▪); IM7, a CD44 MAb (•); or normal mouse IgG (negative control, ○). Cells were then added to confluent monolayers of mesothelial cells in the continued presence of the IgG and allowed to adhere for 20 minutes. Data are expressed as mean ± SD. *P < 0.001 compared with the normal mouse IgG control.
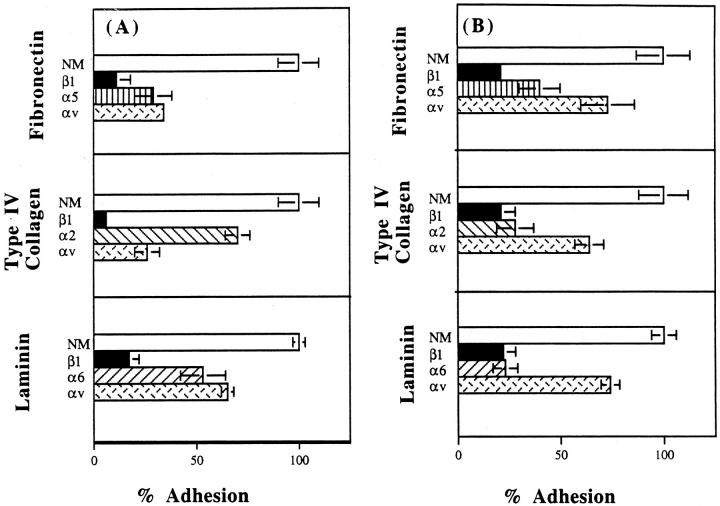
Identification of Integrin Subunits Involved in Ovarian Cancer Cell Adhesion to ECM Proteins
Next, we determined which integrin subunits could potentially be serving as receptors on SKOV3 and NIH:OVCAR5 cells for three major ECM molecules: laminin, fibronectin, and type IV collagen. SKOV3 cells (Figure 5A) ▶ and NIH:OVCAR5 cells (Figure 5B) ▶ were preincubated with MAbs against the β1 integrin subunit, various α integrin subunits, or normal mouse IgG. In the continued presence of the IgG, the cells were added to wells coated with either laminin (7 μg/ml), type IV collagen (1.5 μg/ml), or fibronectin (5 μg/ml) and allowed to adhere for 20 minutes. SKOV3 and NIH:OVCAR5 cell adhesion to fibronectin, laminin, and type IV collagen was almost completely blocked in the presence of the MAb against the β1 integrin subunit (Figure 5) ▶ . Furthermore, the adhesion of SKOV3 and NIH:OVCAR5 cells to fibronectin appears to be mediated by α5 and αv integrin subunits, adhesion to laminin appears to be mediated by α6 and αv integrin subunits, and adhesion to type IV collagen appears to be mediated by α2 and αv integrin subunits (Figure 5) ▶ .
Figure 5.
Identification of integrin subunits involved in ovarian cancer cell adhesion to ECM proteins. SKOV3 (A) or NIH:OVCAR5 (B) cells labeled with [35S]methionine were preincubated with MAbs against the β1 integrin subunit (dark bars), or various α-integrin subunits, or normal mouse IgG (open bars). Cells were then added to wells coated with either laminin, type IV collagen, or fibronectin and allowed to adhere for 20 minutes as described in Materials and Methods. Relative cell adhesion was assessed by measuring the amount of radioactivity. Data are expressed as mean ± SD.
Expression of ECM Proteins by LP9 Human Mesothelial Cells
The β1 integrin heterodimers serve as cell surface receptors for a number of ECM molecules, including fibronectin, laminin, vitronectin, and collagens. 14,22 In this study, the β1 integrin heterodimers appears to play a major role in the adhesion of the SKOV3 cells to the ECM components fibronectin, laminin, and type IV collagen (Figure 5) ▶ . Therefore, we tried to identify the ECM molecules associated with mesothelial cells that may be involved in the adhesion of the SKOV3 cells. We first tested for the presence of ECM molecules on the mesothelial cell monolayer by designing an ELISA in which mesothelial cells were incubated with polyclonal antibodies against the ECM molecules fibronectin, laminin, vitronectin, and collagens types I, III, and IV as described in Materials and Methods. Fibronectin appeared to be expressed at a much higher level than any of the other ECM molecules (data not shown); however, each of the ECM molecules tested was present to some extent on the mesothelial cells.
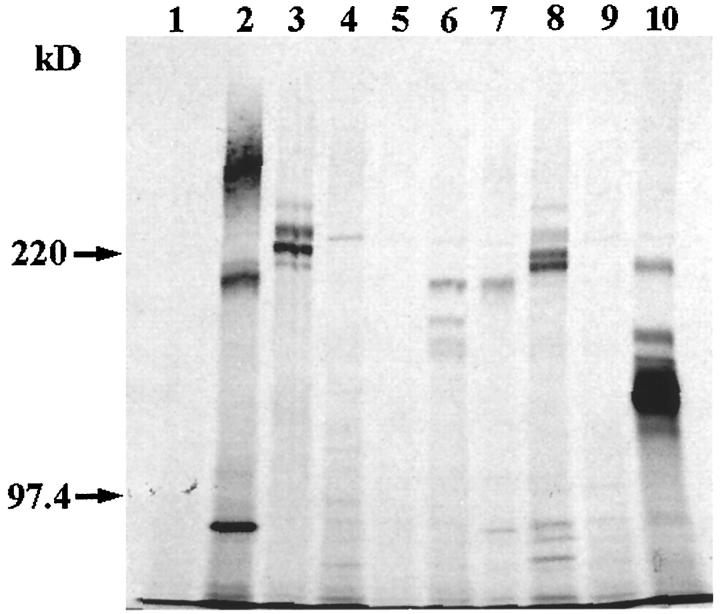
As the ELISA was qualitative, a second type of experiment was conducted to confirm the ELISA results. In this experiment, proteins that were either extracted from the mesothelial cell layer or secreted into the medium were immunoprecipitated using polyclonal antibodies to ECM molecules as described in Materials and Methods. The mesothelial LP9 cells were found to express the following ECM molecules on their surface: fibronectin (Figure 6 ▶ , lane 2), laminin (Figure 6 ▶ , lane 3), type I collagen (Figure 6 ▶ , lane 6), type III collagen (Figure 6 ▶ , lane 7), and type IV collagen (Figure 6 ▶ , lane 8). No vitronectin was detected in the mesothelial cell surface extract (Figure 6 ▶ , lane 4). As metabolic radiolabeling was performed, several radioactive bands were observed for some of these ECM proteins. These multiple bands may be due to the ECM molecules being present in various stages of processing. For example, some of these ECM molecules, in particular collagens, undergo extensive processing as procollagens. In addition, the multiple bands observed may be due to the fact that several of the ECM molecules are composed of multiple chains; for example, laminin is composed of three chains (α, β, and γ) whereas fibronectin is composed of A and B chains. Although protease inhibitors were present during all stages of the immunoprecipitation procedure, it is possible that some degradation might have occurred, giving rise to minor bands. Based on flow cytometry analysis (Figure 1e) ▶ , the MAb against the β1 integrin subunit was used as a positive control for immunoprecipitation. As expected, the β1 integrin subunit was immunoprecipitated from the cell extract (Figure 6 ▶ , lane 10). The negative controls, normal rabbit IgG (Figure 6 ▶ , lane 1), normal goat IgG (Figure 6 ▶ , lane 5), and normal mouse IgG (Figure 6 ▶ , lane 9), did not precipitate any detectable protein. Autoradiographs of the immunoprecipitated radiolabeled material secreted into the media by LP9 cells showed that fibronectin, laminin, and collagen types I, III, and IV were synthesized and secreted by the LP9 cells (data not shown). The relative amounts of these ECM components were similar to that seen in the cell extract.
Figure 6.
Immunoprecipitation of extracellular matrix proteins expressed by mesothelial cells. Mesothelial cells were labeled with [35S]methionine and lysed, and the cell lysate was immunoprecipitated with polyclonal antibodies to fibronectin (lane 2), laminin (lane 3), vitronectin (lane 4), type I collagen (lane 6), type III collagen (lane 7), and type IV collagen (lane 8) or a MAb against the β1 integrin subunit, P5D2 (lane 10). Negative controls were normal rabbit IgG (lane 1), normal goat IgG (lane 5), and normal mouse IgG (lane 9). The immunoprecipitates were separated on a 7.5% SDS-PAGE under reducing conditions, and the gels were dried and then exposed to Kodak X-Omat autoradiograph film. Molecular weight standards were myosin (220 kd) and phosphorylase b (97.4 kd).
Inhibition of SKOV3 Cell Adhesion to Mesothelial Cells by Pretreating SKOV3 Cells with ECM Molecules
We next examined the ability of exogenous soluble ECM molecules to inhibit SKOV3 cell adhesion to mesothelial cells. SKOV3 cells were preincubated with fibronectin, laminin, or type IV collagen at various concentrations. The pretreated SKOV3 cells were then added to wells of confluent mesothelial cells to allow for adhesion. All three ECM proteins inhibited the adhesion of SKOV3 cells to mesothelial cells, with maximal inhibition of ∼60% at 50 μg/ml (data not shown).
Visualization of the Pericellular Matrix
To determine whether a pericellular matrix may be surrounding the mesothelial, SKOV3, or NIH:OVCAR5 cells, cells were grown in six-well plates for 24 hours, and the pericellular matrix was visualized by the particle exclusion assay using fixed goat red blood cells (RBCs; Figure 7 ▶ ). The LP9 mesothelial cells exhibited a large pericellular matrix (Figure 7a) ▶ as did NIH:OVCAR5 cells (Figure 7b) ▶ . In both cases, the pericellular matrix was removed by enzymatic treatment with 80 U/ml bovine testicular hyaluronidase for 1 hour at 37°C (Figure 7, d and e) ▶ , although the pericellular matrix was not completely removed from NIH:OVCAR5 cells after hyaluronidase treatment. The pericellular matrix surrounding the mesothelial and NIH:OVCAR5 cells was enlarged by incubation with exogenous RCS-PG (Figure 7, g and h ▶ , respectively). This is consistent with the presence of hyaluronic acid bound to the cell surface, which then binds the exogenous proteoglycan and excludes the RBCs more effectively. In contrast, SKOV3 cells did not exhibit a pericellular matrix (Figure 7c) ▶ until exogenous RCS-PG was added (Figure 7i) ▶ as visualized by the particle exclusion assay. These results are summarized in Table 1 ▶ . When each of the three cell lines were treated with 80 U/ml bovine testicular hyaluronidase for 1 hour, washed, and then incubated with 2 mg/ml exogenous RCS-PG for 2 hours at 37°C, the pericellular matrix was absent (Table 1) ▶ , indicating the importance of hyaluronic acid in the maintenance of a pericellular matrix. Similar results were obtained using either 80 U/ml bovine testicular hyaluronidase or 4 U/ml Streptomyces hyaluronlyticus hyaluronidase.
Figure 7.
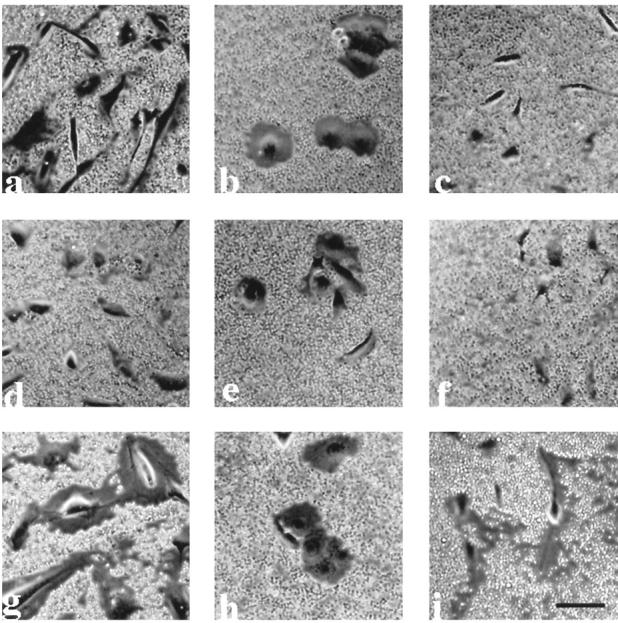
Visualization of the pericellular matrix on LP9 mesothelial cells, NIH:OVCAR5 cells, and SKOV3 cells. LP9 cells (a, d, and g), NIH:OVCAR5 cells (b, e, and h), and SKOV3 cells (c, f, and i) were seeded at a low density and then cultured for 24 hours. The pericellular matrix was visualized by the particle exclusion assay using fixed goat RBCs as described in Materials and Methods. Cells were incubated in the assay buffer for 1 hour before adding the RBCs (a to c) or treated with either 80 U/ml bovine testicular hyaluronidase (d to f) or 2 mg/ml RCS-PG (g to i). Bar, 21 μm.
Table 1.
Summary of the Particle Exclusion Assay Data Using LP9, NIH:OVCAR5, and SKOV3 Cells
| Cell line | No treatment | Hyaluronidase treatment | RCS-PG treatment | Hyaluronidase treatment followed by RCS-PG treatment |
|---|---|---|---|---|
| LP9 | +++ | − | ++++ | − |
| NIH:OVCAR5 | ++ | + | +++ | − |
| SKOV3 | − | − | + | − |
LP9, NIH:OVCAR5, and SKOV3 cells were seeded into six-well plates at a low density and then cultured for 24 hours. The pericellular matrix was visualized by the particle exclusion assay using fixed goat RBCs (see Figure 7 ▶ ). Cells were incubated in the assay buffer for 1 hour before adding the RBCs or treated with either hyaluronidase, RCS-PG, or hyaluronidase for 1 hour followed by incubation with 2 mg/ml RCS-PG. The size of the pericellular matrices visualized under these conditions were scored as follows: −, absent; +, small; ++, medium; +++, large; ++++, very large.
Hyaluronidase Treatment of Mesothelial Cells
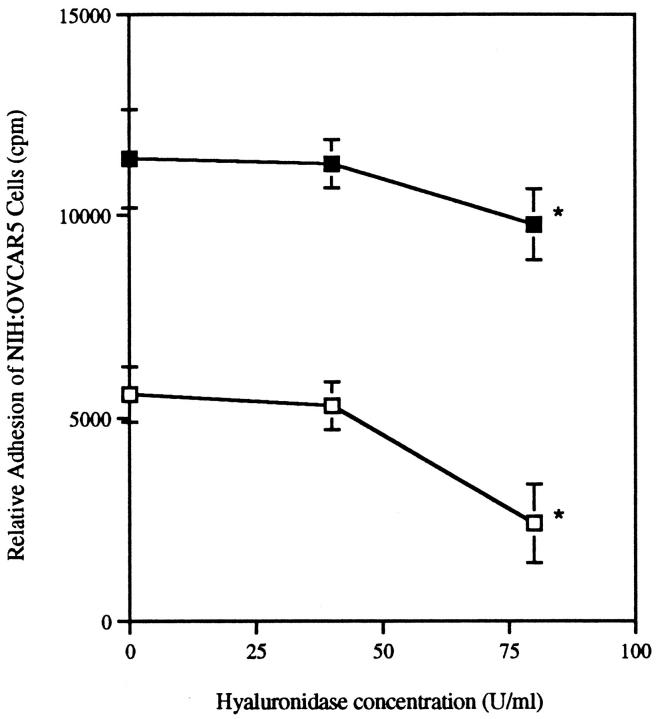
A second assay was designed to further demonstrate that the adhesion of NIH:OVCAR5 cells to LP9 cells is dependent on the availability of hyaluronic acid, a ligand for CD44. In this assay, LP9 mesothelial cells were grown to confluency in 96-well microtiter plates and then treated with various concentrations of hyaluronidase to enzymatically remove the hyaluronic acid on the LP9 cells. The hyaluronidase was washed off of the mesothelial cells, and the mesothelial cells were incubated for 15 minutes at room temperature. Mesothelial cells were washed with the adhesion buffer, and NIH:OVCAR5 cells were incubated in the wells for 30 minutes at 37°C. The adhesion of NIH:OVCAR5 cells to mesothelial cells was partially dependent on the presence of hyaluronic acid, as removal of hyaluronic acid by hyaluronidase resulted in a small, albeit significant decrease in NIH:OVCAR5 cell adhesion (Figure 8 ▶ , solid squares). These data suggest that hyaluronic acid has a rapid turnover on mesothelial cells, as it appears to be resynthesized and secreted in the short time period of the assay after its enzymatic digestion. In parallel experiments, the hyaluronidase was washed off of the mesothelial cells, and the mesothelial cells were fixed with formaldehyde for 15 minutes at room temperature to prevent the re-expression of hyaluronic acid onto their surface (Figure 8 ▶ , open squares). In these experiments, the adhesion of NIH:OVCAR5 cells to fixed mesothelial cells was ∼50% less than the adhesion to the unfixed mesothelial cells. This may be due to the destruction of the functionally active sites of many cell surface molecules, not just hyaluronic acid, as a result of fixation with 2% formaldehyde. In both the fixed and unfixed conditions, the adhesion of NIH:OVCAR5 cells to mesothelial cells significantly decreased in the presence of 80 U/ml hyaluronidase (P < 0.001), suggesting that hyaluronic acid plays an important role in the adhesion of NIH:OVCAR5 cells to mesothelial cells. Furthermore, as ∼20% of the NIH:OVCAR5 cells still adhere to the mesothelial cells after hyaluronidase treatment and fixation, it is likely that cell surface molecules in addition to hyaluronic acid and CD44 are involved in this cell-cell interaction.
Figure 8.
Hyaluronidase treatment of mesothelial cells. Mesothelial cells were grown to confluency in 96-well microtiter plates and treated with various concentrations of bovine testicular hyaluronidase. Cells were washed and either not fixed (▪) or fixed (□) with 2% formaldehyde for 15 minutes at room temperature and then washed again as described in Materials and Methods. Radiolabeled NIH:OVCAR5 cells were added to each well and incubated at 37°C for 20 minutes. The unbound NIH:OVCAR5 cells were washed two times, and relative cell adhesion was assessed by measuring the amount of radioactivity. Data are expressed as mean ± SD. * P < 0.001 compared with 0 U/ml hyaluronidase.
Discussion
Epithelial cancer of the ovary spreads primarily by the implantation of the tumor cells onto the mesothelium that lines the peritoneal cavity. Our hypothesis is that specific adhesion molecules on the surface of ovarian cancer cells and mesothelial cells mediate this interaction. Previous studies have suggested that CD44 on the surface of ovarian cancer cells interacts with its ligand, hyaluronic acid, which is associated with mesothelial cells. 6,7 9
In this study, we focused on the integrin family of cell surface adhesion molecules in addition to CD44. By flow cytometry, we determined that the β1 integrin subunit and CD44 are both expressed at very high levels on the ovarian cancer cell lines SKOV3 and NIH:OVCAR5 and the mesothelial cell line LP9. We performed cell adhesion assays designed to inhibit the interaction of the ovarian carcinoma cells to monolayers of mesothelial cells. A MAb against the β1 integrin subunit was able to partially reduce the adhesion of SKOV3 cells to LP9 cells. In contrast, the CD44 MAb partially reduced the adhesion of NIH:OVCAR5 cells to LP9 cells. This suggests that SKOV3 and NIH:OVCAR5 cells may use different mechanisms for their adhesion to mesothelial cells.
We next identified the ECM molecules associated with the mesothelial cells that may be responsible for their interaction with the β1 integrin heterodimers on ovarian carcinoma cells. By ELISA and immunoprecipitation techniques, we found that collagen types I, III, and IV, fibronectin, and laminin were expressed by the LP9 mesothelial cell line. Other mesothelial cell lines have been shown to express fibronectin, laminin, vitronectin, and collagens types I, III, and IV; however, the ECM molecules that are expressed from one cell line to another vary. 23-26
Pretreatment of SKOV3 cells with fibronectin, laminin, or type IV collagen markedly reduced their adhesion to mesothelial cells in a concentration-dependent manner. Furthermore, pretreatment of SKOV3 cells with a MAb against the β1 integrin subunit caused significant inhibition of SKOV3 cell binding to wells that had been coated with fibronectin, type IV collagen, or laminin. In addition, the α2, α5, α6, and αv integrin subunits appear to play a role in ovarian carcinoma cell adhesion to these ECM molecules. Therefore, it is likely that the epitopes of the β1 integrin heterodimers that recognize fibronectin, laminin, or collagen IV are involved in the binding of SKOV3 cells to mesothelial cells.
Interestingly, pretreatment of the mesothelial cells with polyclonal antibodies against the individual ECM molecules did not reduce the adhesion of the SKOV3 cells to mesothelial cells (data not shown). This may be due to the ability of the β1 integrin heterodimers on SKOV3 cells to recognize multiple ECM molecules. Perhaps by blocking just one type of ECM molecule on the surface of the mesothelial cells by use of polyclonal antibodies against a specific ECM molecule, no significant effect on the overall adhesion of SKOV3 cells to mesothelial cells was observed, due to the presence of other ECM molecules on the surface of mesothelial cells. However, even by adding polyclonal antibodies against all of the ECM molecules simultaneously, we were unable to reduce the adhesion of the SKOV3 cells to the mesothelial cells (data not shown). As mesothelial cells have a pericellular matrix surrounding them, as shown in this and previous studies, 5 it is possible that the ECM epitopes may be sufficiently masked by the hyaluronic acid and proteoglycans, such that functionally active polyclonal antibodies against ECM components cannot compete.
The inhibition assay was designed such that SKOV3 cells were preincubated with the MAb against the β1 integrin subunit and allowed to adhere to the mesothelial cells in the continued presence of the MAb. One could imagine that, as mesothelial cells have high levels of the β1 integrin subunit on their surface, it would be possible that these β1 integrin heterodimers could bind to ECM molecules present on the surface of the SKOV3 cells. However, by flow cytometry, we did not detect laminin, fibronectin, or type IV collagen on the surface of SKOV3 cells before their addition to the inhibition assay (data not shown). Therefore, it is much more likely that the interaction of SKOV3 cells with mesothelial cells occurs via the β1 integrin heterodimers on the SKOV3 cells and the ECM molecules on the mesothelial cells.
By use of a particle exclusion assay, we have demonstrated the presence of a pericellular matrix that is rich in hyaluronic acid on LP9 mesothelial cells as well as NIH:OVCAR5 cells. The enlargement of the pericellular matrix by the addition of exogenous proteoglycan was more pronounced in the case of LP9 cells as compared with NIH:OVCAR5 cells, suggesting that the ratio of proteoglycan to hyaluronic acid on NIH:OVCAR5 cells is higher than that on LP9 cells. In contrast, SKOV3 cells did not exhibit a pericellular matrix that could be digested by hyaluronidase. However, when SKOV3 cells were treated with exogenous proteoglycan, a pericellular matrix was detected, suggesting that cell-associated hyaluronic acid is present on SKOV3 cells. The hyaluronic acid levels on the SKOV3 cells thus appear to be low when compared with mesothelial cells. When any of the three cell lines were treated with hyaluronidase and then incubated with RCS-PG, no pericellular matrix was observed, further suggesting that cell-associated hyaluronic acid is required for the assembly of proteoglycan into the pericellular matrix. The assembly of the pericellular matrix in the presence of proteoglycans is a specific event that requires the availability of both the proteoglycan and hyaluronic acid as shown by Knudson et al using matrix-free chondrocytes. 21
The adhesion of NIH:OVCAR5 cells to mesothelial cells was dependent on hyaluronic acid as enzymatic digestion of mesothelial-associated hyaluronic acid with hyaluronidase resulted in significantly decreased levels of adhesion, regardless of whether the mesothelial cells were fixed in 2% formaldehyde to prevent re-expression of hyaluronic acid or not. However, when the mesothelial cells were not fixed with formaldehyde, hyaluronidase treatment was not as effective at inhibiting NIH:OVCAR5 adhesion to mesothelial cells, suggesting that hyaluronic acid has a rapid turnover time on these cells. Furthermore, the treatment of mesothelial cells with the fixative agent 2% formaldehyde resulted in a dramatic decrease (∼50%) in the adhesion of NIH:OVCAR5 cells to the mesothelial cells, which was seen throughout all of the hyaluronidase enzyme concentrations that were tested. This may be due to the destruction of a multitude of epitopes on the mesothelial cells after their treatment with the fixative agent. However, it seems that most, if not all, of the epitopes involved in the hyaluronic acid-CD44 interactions had been preserved after fixation as these interactions were significantly decreased after enzymatic digestion with hyaluronidase.
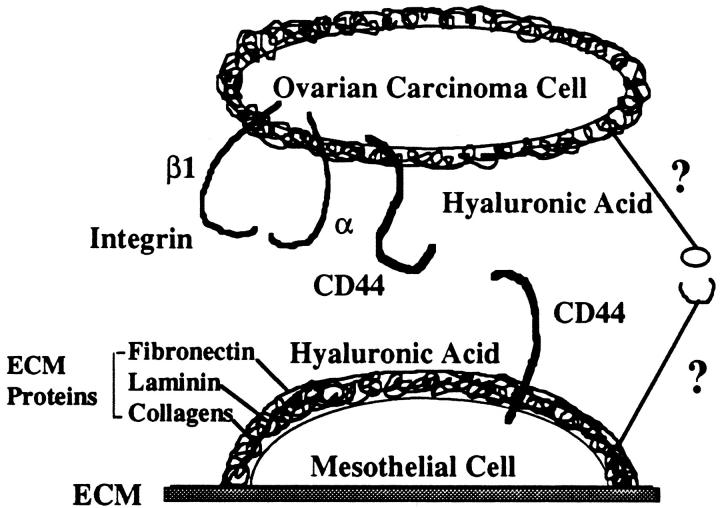
The presence of hyaluronic acid on NIH:OVCAR5 cells, and the high levels of expression of CD44 by mesothelial cells, suggests that a second CD44-hyaluronic acid interaction may be possible in our assay system. As our adhesion assays were performed in the continuous presence of the CD44 MAb, it is possible that excess CD44 MAb may have adhered to CD44 on the mesothelial cells as well as the NIH:OVCAR5 cells. Thus, we propose a new model for the interaction of ovarian carcinoma cells with mesothelial cells, in which CD44 and hyaluronic acid may be present on both the mesothelial cells and the NIH:OVCAR5 cells (Figure 9) ▶ .
Figure 9.
Model for the interaction of ovarian carcinoma cells with mesothelial cells. Based on our findings in this study, mesothelial cells are depicted as synthesizing a pericellular matrix composed of hyaluronic acid, proteoglycans, and the ECM molecules fibronectin, laminin, and collagens. In addition, mesothelial cells express high levels of CD44 on their surface, which would permit them to adhere to hyaluronic acid on ovarian carcinoma cells. The ovarian carcinoma cells may also have a pericellular matrix composed of various amounts of hyaluronic acid and proteoglycans. The ovarian carcinoma cells express high levels of the β1 integrin subunit and CD44, which facilitates their interaction with ECM proteins and hyaluronic acid, respectively, which are present on mesothelial cells. Other ligand-receptor molecules involved in this interaction have yet to be identified (indicated by question marks).
In support of a role for CD44 and hyaluronan in cancer, there is increasing evidence that CD44-hyaluronan interactions may enhance tumor growth and metastasis. A wide range of malignancies of epithelial and mesenchymal origin express high levels of CD44H (the standard CD44 isoform, which does not contain variant exon products) and a variety of variant isoforms of CD44. 27 CD44 variant expression has been shown to be a common feature in epithelial ovarian cancer, 10 and tumor-cell-associated hyaluronan has been shown to be an unfavorable prognostic factor in colorectal cancer. 11 In some experimental systems, an increase in CD44 expression correlates with increased adhesion to hyaluronan, which then has been postulated to cause an increase in metastatic potential. Murine studies with melanoma and lymphoma cell lines have shown the marked effect on tumor growth that transfection with CD44, 28,29 interaction with soluble CD44-Ig fusion protein, 28-30 or subcutaneous injection of hyaluronan oligomers 27 can have. However, other studies have shown that invasiveness of certain tumors coincides with down-regulation of CD44 expression. 31 In other studies, hyaluronan does not appear to be involved in tumor progression, 32 suggesting that the growth and/or metastasis of some but not all tumors may be promoted by CD44-hyaluronan interactions. For example, one immunohistochemical study using formalin-fixed, paraffin-embedded samples of primary ovarian tumors and peritoneal metastasis reported that CD44 expression was detected in only 7.1% or 10.7% of the samples, respectively. 33 The authors concluded that CD44 is not involved in the metastatic spread of ovarian cancer. 33
Interestingly, when mesothelial cells were pretreated with hyaluronidase to remove hyaluronic acid, and then either SKOV3 or NIH:OVCAR5 cells that had been preincubated with the MAb against the β1 integrin subunit were added, a decrease in cell adhesion was observed (data not shown). These data suggest that removal of hyaluronic acid from the pericellular matrix of mesothelial cells may expose the ECM molecules that are expressed by the mesothelial cells and permit the ovarian carcinoma cells to adhere, primarily via their β1 integrin heterodimers, to the mesothelial monolayers (Figure 9) ▶ .
A previous report did not provide evidence that the β1 integrin heterodimers were responsible for the adhesion of ovarian carcinoma cells to mesothelial cells, 15 even though the ovarian carcinoma cells expressed the β1 integrin subunit on their surface and the mesothelial cells synthesized fibronectin. Recently, however, this same group has provided preliminary evidence that the β1 integrin subunit on ovarian carcinoma cells may be involved in the adhesion of ovarian carcinoma cells to mesothelial cells, 34 similar to our findings. This discrepancy with their earlier studies was attributed to the choice of antibodies that they had used to functionally block the β1 integrin heterodimers.
The differences that we observed in the cell adhesion properties and pericellular matrix phenotypes of the two ovarian carcinoma cell lines suggests a heterogeneity in ovarian carcinoma cells. In a similar study, 35 two ovarian carcinoma cell lines, NIH:OVCAR-3 and NIH:OVCAR-5, were found to exhibit distinct preferences in their binding to ECM proteins, which were mediated primarily through β1 integrin interactions. Furthermore, it was found that focal adhesion kinase expression was greater in NIH:OVCAR-5 cells whereas mitogen-activated protein kinase activity was higher in NIH:OVCAR-3 cells. The data suggest that these two ovarian cancer cell lines exhibit specific ECM-binding preferences and distinct differences in their profiles of expression of phosphotyrosine, focal adhesion kinase, and mitogen-activated protein kinase. Tumor heterogeneity has already been shown in ovarian cancer at both genetic 36 and cellular levels. 37 Recently, fresh human ovarian tumors were shown to respond differently to chemotherapeutic agents in an in vitro chemosensitivity assay. 38 It may become possible to modify therapy by using accurate quantitative methods to identify target antigens or peptides for individual patient needs. 39
In conclusion, we have shown that two different human ovarian carcinoma cell lines use primarily two different families of receptors for binding to mesothelial cells. The adhesion of ovarian cancer cells to the peritoneal mesothelium may therefore be a stepwise process whereby different populations of cancer cells display different receptor-ligand specificities during the initial adhesion stage. The involvement of other receptors during this process, as well as the exact mechanisms of ovarian carcinoma cell adhesion, migration, and invasion, will require further elucidation.
Acknowledgments
We thank Dr. James B. McCarthy for providing antibodies and fibronectin, Dr. Leo T. Furcht for providing laminin and the MAb P5D2 against the β1 integrin subunit, Dr. Robert C. Bast, Jr. for providing the SKOV3 cell line, and Dr. Thomas Hamilton for providing the NIH:OVCAR5 cell line. We also appreciate the helpful suggestions and advice of Drs. Keith Skubitz and James McCarthy as well as the technical assistance of Judith Kahm.
Footnotes
Address reprint requests to Dr. Amy P. N. Skubitz, Department of Laboratory Medicine and Pathology, University of Minnesota, Box 609 UMHC, Minneapolis, MN 55455-0315. E-mail: skubi002@tc.umn.edu.
Supported by a grant from the National Institutes of Health/National Cancer Institute (CA60658) and the Minnesota Medical Foundation.
Portions of this manuscript appear in abstract form elsewhere (FASEB J 1998, 12:A377)
References
- 1.Daly M, Obrams GI: Epidemiology and risk assessment for ovarian cancer. Semin Oncol 1998, 25:255-264 [PubMed] [Google Scholar]
- 2.Coukos G, Rubin SC: Chemotherapy resistance in ovarian cancer: new molecular perspectives. Obstet Gynecol 1998, 91:783-792 [DOI] [PubMed] [Google Scholar]
- 3.Sood AK, Buller RE: Drug resistance in ovarian cancer: from the laboratory to the clinic. Obstet Gynecol 1998, 92:312-319 [DOI] [PubMed] [Google Scholar]
- 4.Catterall JB, Gardner MJ, Jones LM, Thompson GA, Turner GA: A precise, rapid, and sensitive in vitro assay to measure the adhesion of ovarian tumour cells to peritoneal mesothelial cells. Cancer Lett 1994, 87:199-203 [DOI] [PubMed] [Google Scholar]
- 5.Jones LM, Gardner MJ, Catterall JB, Turner GA: Hyaluronic acid secreted by mesothelial cells: a natural barrier to ovarian cancer cell adhesion. Clin Exp Metastasis 1995, 13:373-380 [DOI] [PubMed] [Google Scholar]
- 6.Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C: Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res 1993, 53:3830-3838 [PubMed] [Google Scholar]
- 7.Gardner MJ, Catterall JB, Jones LM, Turner GA: Human ovarian tumour cells can bind hyaluronic acid via membrane CD44: a possible step in peritoneal metastasis. Clin Exp Metastasis 1996, 14:325-334 [DOI] [PubMed] [Google Scholar]
- 8.Strobel T, Swanson L, Cannistra SA: In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res 1997, 57:1228-1232 [PubMed] [Google Scholar]
- 9.Catterall JB, Gardner MJ, Jones LM, Turner GA: Binding of ovarian cancer cells to immobilized hyaluronic acid. Glyco J 1997, 14:647-649 [DOI] [PubMed] [Google Scholar]
- 10.Cannistra SA, Abu-Jawdeh G, Niloff J, Strobel T, Swanson L, Andersen J, Ottensmeier C: CD44 variant expression is a common feature of epithelial ovarian cancer: lack of association with standard prognostic factors. J Clin Oncol 1995, 13:1912-1921 [DOI] [PubMed] [Google Scholar]
- 11.Ropponen K, Tammi M, Parkkinen J, Eskelinen M, Tammi R, Lipponen P, Agren U, Alhava E, Kosma VM: Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 1998, 58:342-347 [PubMed] [Google Scholar]
- 12.Newham P, Humphries MJ: Integrin adhesion receptors: structure, function and implications for biomedicine. Mol Med Today 1996, 2:304-313 [DOI] [PubMed] [Google Scholar]
- 13.Sheppard D: Epithelial integrins. Bioessays 1996, 18:655-660 [DOI] [PubMed] [Google Scholar]
- 14.Tozer EC, Hughes PE, Loftus JC: Ligand binding and affinity modulation of integrins. Biochem Cell Biol 1996, 74:785-798 [DOI] [PubMed] [Google Scholar]
- 15.Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J: Expression and function of β1 and αvβ3 integrins in ovarian cancer. Gynecol Oncol 1995, 58:216-225 [DOI] [PubMed] [Google Scholar]
- 16.Trempe G, Fogh J: New human tumor cell lines. Fogh J eds. Human Tumor Cells in Vitro. 1975, :pp 115-159 Plenum Press, New York [Google Scholar]
- 17.Hamilton TC, Young RC, Ozols RF: Experimental model systems of ovarian cancer: applications to the design and evaluation of new treatment approaches. Semin Oncol 1984, 11:285-298 [PubMed] [Google Scholar]
- 18.McCarthy JB, Skubitz AP, Palm SL, Furcht LT: Metastasis inhibition of different tumor types by purified laminin fragments and a heparin-binding fragment of fibronectin. J Natl Cancer Inst 1988, 80:108-116 [DOI] [PubMed] [Google Scholar]
- 19.Smith DE, Mosher DF, Johnson RB, Furcht LT: Immunological identification of two sulfhydryl-containing fragments of human plasma fibronectin. J Biol Chem 1982, 257:5831-5838 [PubMed] [Google Scholar]
- 20.Pattaramalai S, Skubitz KM, Skubitz AP: A novel recognition site on laminin for the α3β1 integrin. Exp Cell Res 1996, 222:281-290 [DOI] [PubMed] [Google Scholar]
- 21.Knudson CB: Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol 1993, 120:825-834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanazashi SI, Sharma CP, Arnaout MA: Integrin-ligand interactions: scratching the surface. Curr Opin Hematol 1997, 4:67-74 [DOI] [PubMed] [Google Scholar]
- 23.Kumano K, Schiller B, Hjelle JT, Moran J: Effects of osmotic solutes on fibronectin mRNA expression in rat peritoneal mesothelial cells. Blood Purif 1996, 14:165-169 [DOI] [PubMed] [Google Scholar]
- 24.Yen CJ, Fang CC, Chen YM, Lin RH, Wu KD, Lee PH, Tsai TJ: Extracellular matrix proteins modulate human peritoneal mesothelial cell behavior. Nephron 1997, 75:188-195 [DOI] [PubMed] [Google Scholar]
- 25.Harvey W, Amlot PL: Collagen production by human mesothelial cells in vitro. J Pathol 1983, 139:337-347 [DOI] [PubMed] [Google Scholar]
- 26.Perfumo F, Altieri P, Degl’Innocenti ML, Ghiggeri GM, Caridi G, Trivelli A, Gusmano R: Effects of peritoneal effluents on mesothelial cells in culture: cell proliferation and extracellular matrix regulation. Nephrol Dial Transplant 1996, 11:1803-1809 [PubMed] [Google Scholar]
- 27.Zeng C, Toole BP, Kinney SD, Kuo JW, Stamenkovic I: Inhibition of tumor growth in vivo by hyaluronan oligomers. Int J Cancer 1998, 77:396-401 [DOI] [PubMed] [Google Scholar]
- 28.Sy MS, Guo YJ, Stamenkovic I: Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med 1992, 176:623-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartolazzi A, Peach R, Aruffo A, Stamenkovic I: Interaction between CD44 and hyaluronate is directly implicated in the regulation of tumor development. J Exp Med 1994, 180:53-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahalka MA, Okon E, Gosslar U, Holzmann B, Naor D: Lymph node (but not spleen) invasion by murine lymphoma is both CD44- and hyaluronate-dependent. J Immunol 1995, 154:5345-5355 [PubMed] [Google Scholar]
- 31.Salmi M, Gron-Virta K, Sointu P, Grenman R, Kalimo H, Jalkanen S: Regulated expression of exon v6 containing isoforms of CD44 in man: downregulation during malignant transformation of tumors of squamocellular origin. J Cell Biol 1993, 122:431-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sleeman JP, Arming S, Moll JF, Hekele A, Rudy W, Sherman LS, Kreil G, Ponta H, Herrlich P: Hyaluronate-independent metastatic behavior of CD44 variant-expressing pancreatic carcinoma cells. Cancer Res 1996, 56:3134-3141 [PubMed] [Google Scholar]
- 33.Speiser P, Wanner C, Breitenecker G, Kohlberger P, Kainz C: CD-44 is not involved in the metastatic spread of ovarian cancer in vivo. Anticancer Res 1995, 15:2767-2769 [PubMed] [Google Scholar]
- 34.Strobel T, Tai YT, Cannistra SA: Binding of ovarian cancer cell to peritoneal mesothelium in vitro is partly mediated by β-1 integrins. Proc Am Assoc Cancer Res 1998, 39:497 [Google Scholar]
- 35.Buczek-Thomas JA, Chen N, Hasan T: Integrin-mediated adhesion and signalling in ovarian cancer cells. Cell Signal 1998, 10:55-63 [DOI] [PubMed] [Google Scholar]
- 36.Narod SA, Ford D, Devilee P, Barkardottir RB, Lynch HT, Smith SA, Ponder BA, Weber BL, Garber JE, Birch JM, et al: An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families: Breast Cancer Linkage Consortium. Am J Hum Genet 1995, 56:254-264 [PMC free article] [PubMed] [Google Scholar]
- 37.Aabo K: Cellular heterogeneity in malignant tumors: the importance of clonal interaction on tumor evolution, response to chemotherapy and metastatic ability evaluated in experimental systems. Danish Med Bull 1996, 43:336-349 [PubMed] [Google Scholar]
- 38.Sevin BU, Perras JP: Tumor heterogeneity and in vitro chemosensitivity testing in ovarian cancer. Am J Obstet Gynecol 1997, 176:759-768 [DOI] [PubMed] [Google Scholar]
- 39.Fleuren GJ, Gorter A, Kuppen PJ, Litvinov S, Warnaar SO: Tumor heterogeneity and immunotherapy of cancer. Immunol Rev 1995, 145:91-122 [DOI] [PubMed] [Google Scholar]