Abstract
Rat cytomegalovirus (RCMV) is a β-herpesvirus with a 230-kbp genome containing over 167 open reading frames (ORFs). RCMV gene expression is tightly regulated in cultured cells, occurring in three distinct kinetic classes (immediate early, early, and late). However, the extent of viral-gene expression in vivo and its relationship to the in vitro expression are unknown. In this study, we used RCMV-specific DNA microarrays to investigate the viral transcriptional profiles in cultured, RCMV-infected endothelial cells, fibroblasts, and aortic smooth muscle cells and to compare these profiles to those found in tissues from RCMV-infected rat heart transplant recipients. In cultured cells, RCMV expresses approximately 95% of the known viral ORFs with few differences between cell types. By contrast, in vivo viral-gene expression in tissues from rat heart allograft recipients is highly restricted. In the tissues studied, a total of 80 viral genes expressing levels twice above background (5,000 to 10,000 copies per μg total RNA) were detected. In each tissue type, there were a number of genes expressed exclusively in that tissue. Although viral mRNA and genomic DNA levels were lower in the spleen than in submandibular glands, the number of individual viral genes expressed was higher in the spleen (60 versus 41). This finding suggests that the number of viral genes expressed is specific to a given tissue and is not dependent upon the viral load or viral mRNA levels. Our results demonstrate that the profiles, as well as the amplitude, of viral-gene expression are tissue specific and are dramatically different from those in infected cultured cells, indicating that RCMV gene expression in vitro does not reflect viral-gene expression in vivo.
Cytomegaloviruses (CMV) are ubiquitous β-herpesviruses that establish lifelong latent infections following primary infection. Although antiviral therapy has significantly reduced disease in transplant and AIDS patients, human CMV (HCMV) still is a significant problem in congenital disease and bone marrow transplant patients (23). In these infected individuals, HCMV can manifest itself in a number of CMV-related acute diseases, including retinitis, encephalitis, gastritis, and mononucleosis. In addition, HCMV has also been associated with long-term diseases, such as atherosclerosis, chronic rejection following solid-organ transplantation, and, more recently, malignancies (9). CMV is one of the largest human viruses known, with over 200 open reading frames (ORFs), and a number of viral genes have been implicated in the development of CMV-related diseases. However, the in vivo expression profiles of these and other viral genes are unknown. Therefore, a comprehensive profiling of CMV gene expression during infection in the host is warranted. In addition, it is important to determine whether one can use the viral-gene expression patterns seen in in vitro models of virus-infected cells to predict those patterns seen in vivo.
In cultured cells, CMV gene expression occurs in three kinetic phases designated immediate early (IE), early (E), and late (L) (21). These phases were defined through the use of drugs that target either cellular translation or the viral DNA polymerase. Transcription of IE genes begins shortly following virus penetration and uncoating and does not require de novo protein synthesis. The IE proteins enhance transcription from both host and viral promoters, leading to the expression of the E genes, which include genes involved in viral DNA replication, such as the viral DNA polymerase gene (UL54). L viral genes are expressed following viral DNA synthesis, thus making expression of this class of genes sensitive to inhibitors of the viral DNA polymerase. The L viral-gene kinetic class is generally involved in viral DNA packaging, assembly, and egress. Recently, virus-specific DNA microarray analysis has been utilized to classify global viral gene transcription of herpes simplex virus type 1 and HCMV (8, 11, 12, 25). Goodrum et al. used HCMV-specific microarrays to examine HCMV gene expression in latently infected CD34+ hematopoietic progenitors (11, 12). A subset of HCMV genes representing IE, E, and L stages of infection were detected in these latently infected cell cultures at 1, 5, and 8 days postinfection. However, in these experiments, the investigators did not identify an obvious pattern of viral-gene expression that represented a potential kinetic class of latency genes. The HCMV genes expressed during the establishment of latency in this system may represent an initial burst of expression followed by viral quiescence in the cell. Interestingly, the extent of viral-gene expression was dependent upon the status of cellular differentiation. An important question related to these studies concerns the relevance of HCMV gene expression prior to the development of latency and how this relates to viral-gene expression during viral persistence in vivo.
The RCMV-rat model has proven to be an important tool for studying mechanisms involved in CMV-related diseases, including the effect of the virus on accelerated solid-organ transplant rejection. Infection of immunocompetent rats leads to a limited subclinical infection that typically persists in the columnar epithelial cells of the submandibular glands (SMG) for up to 180 days postinfection (p.i.) (2, 3). RCMV infection of immunocompromised rats causes a widespread infection of most tissues, infecting a number of different cell types, including endothelial cells (EC), epithelial cells, macrophages, polymorphonuclear cells, and fibroblasts. In this report, we profiled viral-gene expression utilizing microarrays containing DNA oligonucleotides specific for the 167 known RCMV ORFs (19). We compared viral-gene expression in cultured rat fibroblasts, vascular smooth muscle cells (SMC), and aortic EC to the expression profiles observed in the tissues from RCMV-infected allograft recipients, including SMG (the site of virus persistence), allograft heart, lung, liver, kidney, and spleen. We observed that the pattern of RCMV gene expression in vivo differs dramatically from that in RCMV-infected cultured cells. Our data suggest that RCMV gene expression is highly tissue specific. Importantly, the majority of the RCMV genes expressed at high levels in tissues are not known to be involved in virus replication but may represent the profile of immune modulator genes required for CMV persistence.
MATERIALS AND METHODS
RCMV.
SMG-derived stocks of the Maastricht strain of RCMV were titered using rat embryo fibroblasts (REFs) (3, 7). Plaque assays were performed in confluent 24-well plates by infection with an appropriate serial virus dilution in 0.2 ml of medium and then incubated at 37°C for 90 min. Following incubation, the infected cells were rinsed with phosphate-buffered saline (PBS) and overlaid with 1 ml Eagle minimal essestial medium supplemented with 10% fetal calf serum, nonessential amino acids, penicillin-streptomycin, and 20 mM l-glutamine with a final concentration of 0.6% agarose (Sigma, St. Louis, MO). After 7 days, the cells were fixed in 10% formalin in PBS and stained with 0.05% aqueous methylene blue (Sigma). The plaques were counted by light microscopy.
RCMV infection of tissue culture cells.
Primary aortic SMC, aortic EC, and REFs were isolated from F344 rats and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and penicillin-streptomycin-glutamine (20, 26). The EC phenotype was confirmed by staining these cells with antibodies directed against von Willebrand factor. These cells exhibited characteristics and morphology typical of their origin when maintained in culture beyond passage 15 (data not shown). REFs, SMC, and EC were plated on 60-mm dishes (Costar) and infected with RCMV upon confluence at a multiplicity of infection equal to 0.1. After 2 hours, the cells were washed three times with PBS. The infected cells were harvested at 4, 8, 16, 24, 36, and 48 h p.i. by first washing them once with PBS and then adding 1 ml of Trizol reagent. The reagent was allowed to lyse the cells for 5 min. Subsequently, the samples were stored frozen at −80°C.
Preparation of rat tissues.
In order to determine viral-gene expression in vivo, we isolated RNA from the SMG, lungs, liver, kidney, spleen, and graft hearts from allograft recipients (26). For these studies, adult male F344 rats (Harlan Sprague-Dawley, Indianapolis, IN) served as allogeneic heart donors, while Lewis rats (Harlan Sprague-Dawley) served as solid-organ transplant recipients (26). To prevent acute rejection, Lewis recipients were treated with low-dose cyclosporine for 10 days (5 mg/kg of body weight/day; Sandoz Inc., East Hanover, NJ). In all animals, the native heart remained intact. Acute RCMV infection was accomplished by injecting 1 × 105 PFU of RCMV intraperitoneally on day 1 following the heart transplant operation. The tissues were harvested from allograft recipients at 7, 14, 21, and 28 days posttransplantation. Total RNA was prepared from 0.25 g of rat tissues using the Trizol method. All animals were housed in the Portland VA Medical Center animal facilities in a specific-pathogen-free room. This facility is AAALAC accredited and complies with the requirements for animal care as stipulated by the U.S. Department of Agriculture and HHS.
RCMV microarray techniques.
The Spotted Microarray Core at the Vaccine and Gene Therapy Institute, Oregon Health and Science University (http://www.ohsu.edu/gmsr/smc/), printed the RCMV microarray used for this study. Each slide contained two unique 70-mer antisense oligonucleotides for each of the 167 predicted viral ORFs (29) and an additional 2,925 rat cellular genes. The RCMV gene slides were printed on aminosilane-coated glass slides using the Cartesian PixSys 5500 XL microarray printer (Genomic Solutions; located at the Spotted Microarray Core), and each cDNA was spotted twice to account for intrachip variation. The oligonucleotides were chosen with a 3′ bias and compared against the NCBI database for alignment to the genome sequence of the RCMV Maastricht strain and for possible cross-hybridization to cellular sequences.
First-strand cDNA was synthesized from 2 μg of total RNA using 1.0 μM oligo(dT)-T7 primer [GGCCAGTGAATTGTAATACGACTCACTATAGGG (A)24] and 200 U Superscript III reverse transcriptase (Invitrogen). Doubled-stranded cDNA was generated by the addition of second-strand buffer (Invitrogen) according to the manufacturer's protocol and purified by phenol-chloroform-isoamyl alcohol extraction. The cDNA was amplified for one round using the T7 Megascript Kit (Ambion) to produce amplified RNA (aRNA), which was purified using the RNeasy Mini kit (QIAGEN). Five micrograms of aRNA was reverse transcribed with 300 U Superscript III reverse transcriptase in the presence of 9 μg of random hexamers; 0.5 mM (each) dATP, dGTP, and dCTP; 0.35 mM dTTP; and 0.15 mM aminoallyl dUTP. aRNA was hydrolyzed with 0.3 N NaOH (70°C for 10 min) and then neutralized with 0.625 M HEPES (pH 7.5). Aminoallyl-labeled cDNA was purified using the Cyscribe GFX Purification Kit (Amersham) and coupled to Cy3 or Cy5 fluorescent dye (Amersham) through the incorporated aminoallyl UTP. The labeled cDNA was purified using the Cyscribe GFX Purification Kit (Amersham) and then hybridized to microarray slides in the presence of Slide Hybe no. 2 (Ambion) at 65°C for 18 h. The slides were washed consecutively with 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.1% sodium dodecyl sulfate, 0.06× SSC-0.1% sodium dodecyl sulfate, and 0.06× SSC for 5 min at room temperature. All slides were scanned using a Bioscience GeneScan Lite laser scanner and analyzed using Imagene digital processing software and Genesight data analysis software (Biodiscovery). Local background values were subtracted using Imagene 3.5.1 software, and genes determined to be “on” had values twice that of the background.
Quantitative RT-PCR detection of RCMV gene expression.
Real-time reverse transcriptase (RT) PCR was used to confirm and quantify the RCMV gene expression results obtained using microarray analysis. cDNA was generated as described above using Superscript III RT (Invitrogen) and analyzed by real-time PCR techniques using primer sets recognizing RCMV gene sequences (Table 1). The primer sets were identified using Primer Express software (Applied Biosystems). RT-PCRs were performed using the SYBR Green PCR Master Mix (Applied Biosystems), except for PCRs for R78, r119.1, r119.2, r151, and r152.4, which were performed using specific TaqMan probes and Master Mix. Following thermal activation of AmpliTaq Gold (10 min at 95°C), a total of 40 cycles were performed (15 s at 95°C and 1 min at 58°C) using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Plasmid clones containing each gene fragment were used as positive controls and quantification standards. PCR results were analyzed using ABI Prism 7700 Sequence Detection Software. The sensitivity of detection of this assay was <100 plasmid copies for all of the tested RCMV genes.
TABLE 1.
Quantitative PCR primer sequences
| RCMV ORF | Primer sequence |
|---|---|
| R32 Forward | 5′-ATC CGG TCC ATG AGG TCG A |
| R32 Reverse | 5′-GTG ATC AAT GAA TGT CGC GG |
| R33 Forward | 5′-ACC CTG ACG TTC GTG ACG AC |
| R33 Reverse | 5′-TGA TCG GCC AGT TCA GCA C |
| R35 Forward | 5′-CCC TGA CCG TGT TCA AGA GG |
| R35 Reverse | 5′-GAC TTT CGC ATG GCG ATC A |
| R49 Forward | 5′-CGC GGT ATC GTT ATG GGT G |
| R49 Reverse | 5′-ATG GGC AAG GAC AAA CTC GA |
| R75 Forward | 5′-CTT CCG CAG AAC TCG CAG TG |
| R75 Reverse | 5′-CCT GCG TCT ACA GCA CCT CCT A |
| R78 Forward | 5′-CTT CTA CGC CCT GCA CTT CG |
| R78 Reverse | 5′-GCC AGC TCG TAG TAC CCG AC |
| R114 Forward | 5′-ACC TTT ACG GAA CCG GAG TTG |
| R114 Reverse | 5′-ACG GAC AAG GTC GAT AGG GA |
| R116 Forward | 5′-TCC GGC TGA ATA AGA CCT CG |
| R116 Reverse | 5′-CCC ATC CTC AAC AGC ACA CA |
| r119.1 Forward | 5′-TTC GGA ATC GAT GGT GAC AAG |
| r119.1 Reverse | 5′-CAC TCA TCG CCG TCA ACA GA |
| r119.2 Forward | 5′-GTT CCA TCG GCA TCA TGT AAG A |
| r119.2 Reverse | 5′-ACG ACG CTA ATG AAA CTG GCA |
| r119.4 Forward | 5′-GTT CCA CTG AGA CTG CTT GCG |
| r119.4 Reverse | 5′-CAG GAT TAT TTG GCG GCA AC |
| r123 Forward | 5′-CCA CTA TCT TGG GCA CGG A |
| r123 Reverse | 5′-ACC GAA ACC TTC AGA CAA CCA |
| r148 Forward | 5′-TAC GAC CCA CGT CAA AAG TTG A |
| r148 Reverse | 5′-ATC TCT ATG TCG TTT ACT GCG A |
| r149 Forward | 5′-GAA CCG CGG ATT CGT AGT CTC |
| r149 Reverse | 5′-ATT CCA GTG ACA CCG AGG GA |
| r151 Forward | 5′-AAT CCA TTT TGT GGT CCA AGG A |
| r151 Reverse | 5′-TCG ATG ACC GTT GGA GGA AC |
| r152.4 Forward | 5′-ACC TTC GAG CCA ATG TGA ATG |
| r152.4 Reverse | 5′-CAA CGT CTC AGA TGC GGA GA |
Statistical analysis.
Quantitative PCR data were analyzed by analysis of variance and Student's t test. P values of <0.05 were considered significant.
RESULTS
Profiling RCMV gene expression in vitro.
RCMV infects a number of different cell types both in vivo and in vitro, including EC, SMC, epithelial cells, fibroblasts, and macrophages (17, 28). Similar to HCMV, the RCMV genome is about 230 kbp and includes 167 predicted ORFs (29). Whether CMV gene expression differs in various cell types or how in vivo viral-gene expression compares to that observed in cultured cells has not been reported. In order to answer these important questions, we generated RCMV microarrays to profile viral-gene expression both in vivo and in vitro. The RCMV microarray slides contained two unique, nonoverlapping 70-mer antisense oligonucleotides for each of the 167 predicted viral ORFs (29) and an additional 2,925 rat cellular genes used to normalize the data. The oligonucleotides were chosen with a 3′ bias and compared against the NCBI database for alignment to the RCMV Maastricht strain and for possible cross-hybridization to cellular sequences. The specificity of the RMCV microarray was confirmed by hybridization of labeled RCMV genomic DNA, which bound each of the viral oligonucleotides on the chip but did not display binding activity toward the cellular control oligonucleotides (data not shown).
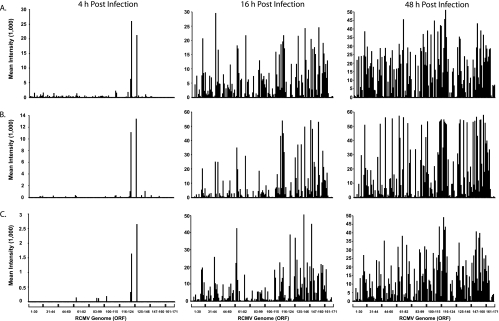
In order to determine whether RCMV gene expression is cell type specific, rat fibroblasts, aortic SMC, and aortic EC were infected with RCMV and harvested at 4, 8, 16, 24, 36, and 48 h p.i. Then, RNA was purified from the cells and reverse transcribed into doubled-stranded cDNA. The cDNA was labeled and hybridized to RCMV microarray slides for 18 h. The slides were washed, scanned, and analyzed. A typical microarray analysis of RCMV gene expression in infected REFs is shown in Fig. 1. RCMV gene expression profiles for infected REFs, SMCs, and ECs at 4, 16, and 48 h p.i. are depicted in Fig. 2 as the average intensity above background. At 4 h p.i., only two genes, r123Exon4 (IE1) and r128, were highly expressed in all cell types. However, by 16 h p.i., nearly 80% of RCMV genes were expressed at detectable levels. Ninety-six viral genes (60% of the predicted viral ORFs) were expressed with early kinetics in RCMV-infected REFs treated with phosphonoacetic acid and harvested at 48 h p.i. (Table 2). Maximal RCMV gene expression occurs at 36 and 48 h p.i., when over 87% of the viral genes are expressed (Fig. 2 and Table 2). Since labeled RCMV DNA bound to all of the viral oligonucleotides on the chip (data not shown), it is likely that the 10 to 13% of RCMV genes that failed to be detected represent either genes with unknown splicing properties or genes that are expressed at very low levels. These genes were also not detected in RNA samples isolated from in vivo-infected tissues, as described below. A positive signal on our RCMV DNA microarray required that any given gene express 5,000 to 10,000 copies of mRNA in 2 μg of starting total RNA (data not shown). This was determined by comparing quantitative real-time RT-PCR (QRT-PCR) evaluation of the expression of at least 15 RCMV genes to the microarray results from 10 different samples in which gene expression varied for the particular gene of interest from negative to positive. Interestingly, according to our microarray results, there are at least seven genes expressed exclusively in ECs, one in SMCs, and three in REFs. Our findings suggest that, other than a small subset of genes, RCMV gene expression is not cell type specific in vitro.
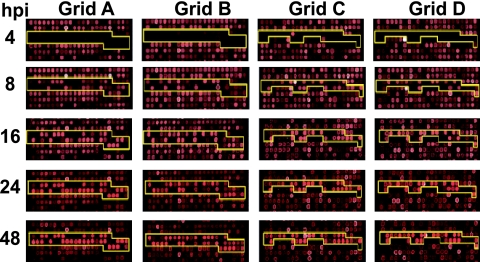
FIG. 1.
Microarray analysis of RCMV gene expression. Each chip contained two unique oligonucleotides for each viral gene, and these oligonucleotides were spotted in duplicate. The RCMV oligonucleotides were distributed over four positions on each chip depicted in grids A to D. The yellow lines outline the positions of the RCMV-specific oligonucleotides. The data shown represent microarray analysis of RNA extracted from RCMV-infected REFs at 4, 8, 16, 24, 36, and 48 h p.i.
FIG. 2.
RCMV in vitro gene expression. Fluorescence intensities from microarray analysis of RCMV RNAs isolated from infected REFs (A), SMC (B), and EC (C) harvested at 4, 16, and 48 h p.i. The RNA was amplified, and cDNA samples were labeled and hybridized to the RCMV microarray chips containing oligonucleotides specific for all of the known viral genes.
TABLE 2.
Classification of RCMV gene expression in cultured rat fibroblasts
| IE (4 h) | E (PAA; 48 h)a | L (48 h) |
|---|---|---|
| IE r123ex4 | r2 | r2.1 |
| r128 | r4 | r3 |
| r5 | R33 | |
| R23 | R46 | |
| R24 | R47 | |
| r25.1 | R48 | |
| R25.3 | R52 | |
| R25 | R53 | |
| R26 | r70.1 | |
| R27 | R73 | |
| R28 | r74 | |
| R29 | R75 | |
| R31 | R76 | |
| R32 | R84 | |
| R34 | R85 | |
| R35 | r90 | |
| R36 | R100 | |
| R37 | R104 | |
| R38 | r106 | |
| r39 | r107 | |
| r40 | r110 | |
| r41 | r111.2 | |
| r42 | r121.2 | |
| r43.1 | R121 | |
| R43 | R122ex5 | |
| R44 | r124 | |
| R45 | r127 | |
| R49 | r128 | |
| R50 | r131 | |
| R51 | r133 | |
| R54 | r135 | |
| R55 | r136 | |
| R56 | r137 | |
| R57 | r138 | |
| R69 | r139 | |
| r70.2 | r140 | |
| r70.3 | r151.2 | |
| r70.4 | r152 | |
| r70.5 | r155 | |
| R72 | r168 | |
| R77 | ||
| R78 | ||
| R80 | ||
| R82 | ||
| R83 | ||
| R87 | ||
| R88 | ||
| R91 | ||
| R92 | ||
| R93 | ||
| R94 | ||
| R95 | ||
| R96 | ||
| R97 | ||
| R98 | ||
| R99 | ||
| R102 | ||
| R105 | ||
| r109 | ||
| R112ex1 | ||
| R113 | ||
| R114 | ||
| R115 | ||
| R116 | ||
| R117 | ||
| R118 | ||
| r119.1 | ||
| r119.2 | ||
| r119.3 | ||
| r119.4 | ||
| r119.5 | ||
| r119.6 | ||
| r142 | ||
| r143 | ||
| r144 | ||
| r145 | ||
| r146 | ||
| r147 | ||
| r149 | ||
| r150 | ||
| r151.1 | ||
| r151.3 | ||
| r151 | ||
| r152.1 | ||
| r152.3 | ||
| r152.4 | ||
| r152.5 | ||
| r157 | ||
| r158 | ||
| r160 | ||
| r161 | ||
| r162 | ||
| r164 | ||
| r166 | ||
| r167 | ||
| r171.1 |
PAA, phosphonoacetic acid.
Profiling RCMV gene expression in vivo.
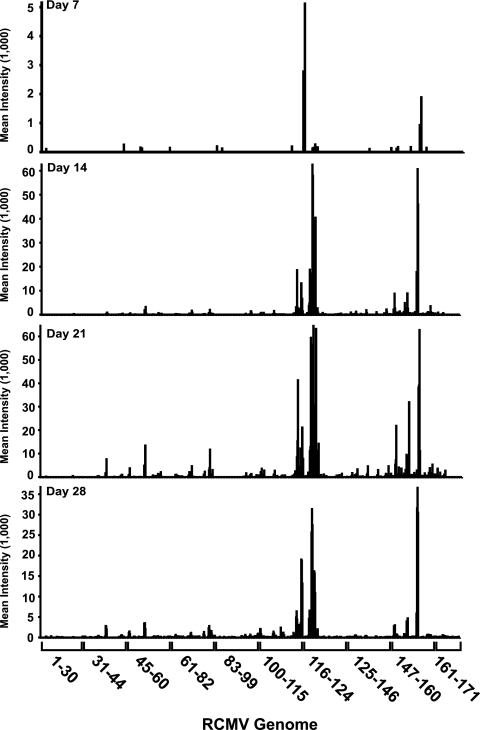
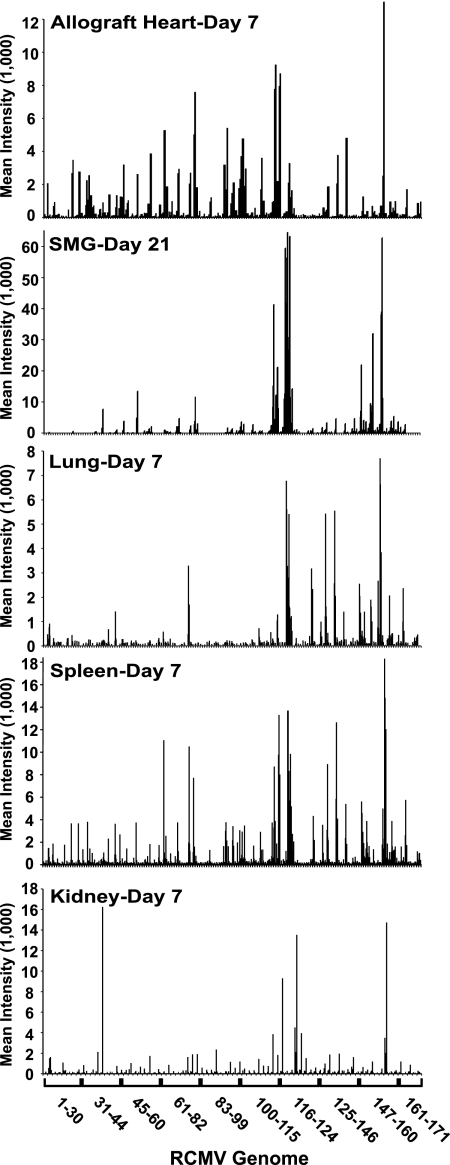
In order to explore viral-gene expression in vivo, RCMV microarray slides were utilized to initially analyze RNA isolated from SMG from RCMV-infected heterotopic heart allograft recipients. For these studies, adult F344 rats served as allogeneic heart donors, while Lewis rats served as transplant recipients (22, 26). RNA was isolated from heart allograft recipient tissues harvested at 7, 14, 21, and 28 days posttransplantation. The RNA was converted to cDNA, labeled, and hybridized to the RCMV microarray chips as described above. Interestingly, on day 7, in RNA samples from each of the four rats tested, only two RCMV genes (R116 and r152.4) were expressed to at least twice the background level. These genes constitute approximately 1% of the known RCMV genes (Fig. 3). This finding is not surprising, since previous studies had demonstrated that RCMV takes approximately 14 days to establish infection in SMG (5, 6, 17, 26). Approximately 11% of the RCMV genes are expressed at 14 days p.i., and this number increases to nearly 25% by day 21, which includes genes expressed with IE, E, and L kinetics. The number of RCMV genes expressed decreased to 15% on day 28 (Fig. 3). Compared to the 87% transcriptionally active genes observed in the RCMV-infected tissue culture cells, the number of transcripts detected in vivo was quite low. This difference was not due to lower signal intensity. Rather, there was a clear bias in gene expression. For instance, even at day 21, when the greatest numbers of RCMV genes are expressed, only a few of these genes are known to be involved in virus replication. In fact, most of these highly expressed genes are in clusters on the right half of the RCMV genome, which is the region that contains genes thought to be involved in host cell manipulation and/or immune evasion (19). For example, r151 was highly expressed in the SMG of infected rats. The r151 gene is a homolog of the murine CMV m152 gene, which blocks cell surface expression of major histocompatibility complex class I in infected cells through retention of the protein in the cis-Golgi compartment (10, 16, 18).
FIG. 3.
RCMV in vivo gene expression. Fluorescence intensities from microarray analysis of RCMV mRNA isolated from infected rat SMG that were harvested at days 7, 14, 21, and 28 days p.i. At each time point, SMG samples were isolated from four individual infected rats and analyzed on separate microarray chips. The data shown include gene intensities acquired from each of these four separate arrays.
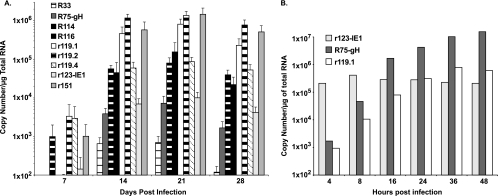
To rule out the possibility that technical reasons were the cause of differences in expression patterns observed in vitro versus in vivo, we used QRT-PCR to quantify the RCMV transcript levels. Shown in Fig. 4A are the normalized average copy numbers of R33 (vGPCR), R75 (gH), R114, R116, r119.1, r119.2, r119.4, r123 (IE1), and r151 as detected by QRT-PCR in SMG tissue from four RCMV-infected rats. The levels of IE1 (r123) and other essential genes, such as gH (R75), were more than 100-fold lower than the expression levels of r119.1, r119.2, and r151. By contrast, in cultured RCMV-infected fibroblasts, the expression levels of IE1 were much higher than those of r119.1 at early time points (4 and 8 h p.i.), whereas at later time points, the expression levels of these genes were similar (Fig. 4B). Additionally, by 16 h p.i. the expression levels of gH in the RCMV-infected cultured cells were 10- to-100-fold higher than those of r119.1. These findings confirm the trend observed by microarray analysis and indicate that in vitro, RMCV gene expression profiles do not accurately predict the levels of gene expression found in vivo.
FIG. 4.
Confirmation of in vivo RCMV gene expression. RT-PCR TaqMan analysis was used to confirm and quantify viral-gene expression in RNA samples from (A) in vivo-infected SMG at 7, 14, 21, and 28 days p.i. and (B) in vitro-infected rat fibroblasts. Primers specific for the RCMV genes R75 (gH), R114, R116, r119.1, r119.2, r119.4, R123 (IE1), and r151 were used to quantify viral-gene expression. RNA samples were normalized to expression of L32 (cellular ribosomal protein), and the relative copy numbers were determined using plasmids containing each viral gene. The error bars indicate standard deviations.
RCMV gene expression is tissue specific.
To determine whether the RCMV gene expression pattern of SMG is also observed in other tissues, we profiled viral transcription in lungs, liver, kidney, spleen, and native and allograft hearts harvested from the transplant recipients (n = 4). The tissues harvested at days 7, 14, 21, and 28 were prescreened for viral DNA and mRNA by quantitative PCR in order to identify tissues containing appropriate levels of viral message to be detected by microarray analysis. Previous studies have indicated that wild-type RCMV DNA levels directly reflect the viral load in rat tissues (14, 15). At 7 days p.i., viral-DNA levels in the allograft heart, spleen, lung, liver, SMG, and kidney ranged from 5 × 104 to 2 × 105 RCMV genomic equivalents per μg of input DNA (Table 3). The level of viral DNA in the SMG at 14 days p.i. was approximately 1 × 107 genomic equivalents per μg of input DNA, which increased at 21 and 28 days p.i. to approximately 1 × 108 to 3 × 108. All of the tissues, except the native heart, tested positive for viral mRNAs (r119.2 and r151) at 7 days p.i. (data not shown), which was consistent with the detection of viral DNA (Table 3). As expected due to normal viral clearance, none of the samples collected from these tissues at 14, 21, or 28 days p.i. contained sufficient viral mRNA to be used for microarray analysis, except for the SMG.
TABLE 3.
Detection of RCMV DNA
| Day | Tissue | No. of copies for animala:
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Avg | ||
| 7 | Graft heart | 2.5 × 105 | 9.8 × 103 | 6.6 × 105 | 5.0 × 103 | 2.3 × 105 ± 3.1 × 105 |
| Spleen | 0 | 5.2 × 105 | 0 | 0 | 1.3 × 105 ± 2.6 × 105 | |
| Lung | 3.6 × 104 | 8.8 × 104 | 8.4 × 104 | 6.9 × 103 | 5.4 × 104 ± 3.9 × 104 | |
| Liver | 2.5 × 105 | 5.1 × 104 | 1.6 × 105 | 1.4 × 104 | 1.2 × 105 ± 1.1 × 105 | |
| Kidney | 9.0 × 104 | 3.3 × 104 | 1.6 × 105 | 0 | 7.2 × 104 ± 7.1 × 104 | |
| Salivary gland | 2.6 × 105 | 9.2 × 104 | 2.0 × 104 | 1.5 × 105 | 1.3 × 105 ± 1.0 × 105 | |
| 14 | Salivary gland | 1.0 × 107 | 4.6 × 106 | 3.3 × 107 | 2.3 × 107 | 1.8 × 107 ± 1.3 × 107 |
| 21 | Salivary gland | 5.9 × 108 | 8.0 × 108 | 1.9 × 107 | 6.5 × 107 | 3.7 × 108 ± 3.9 × 108 |
| 28 | Salivary gland | 2.3 × 108 | 2.2 × 107 | 2.6 × 107 | 4.5 × 107 | 1.4 × 108 ± 1.2 × 108 |
Genomic copies per 1 μg of total DNA determined by quantitative PCR (TaqMan).
Comparisons of the viral-gene expression profiles for lung, liver, kidney, spleen, allograft heart, and SMG are shown in Fig. 5 and Table 4. As observed for SMG, fewer viral transcripts were detected in infected tissues than in those expressed in vitro. Moreover, the RCMV gene expression profiles appear to be unique to each tissue type. Most of the tissues expressed genes from each kinetic class (IE, E, and L), making it difficult to classify in vivo virus replication based on traditional in vitro replication kinetics. Interestingly, while viral-gene expression levels were consistently highest in the SMG, there were more RCMV genes expressed above background in both spleen and liver than in the SMG. In fact, a total of 60 viral genes were expressed at levels twofold above background in the spleen (38% of the RCMV genome) compared to 41 in the SMG (25% of the genome). These findings indicate that the number of viral genes expressed at detectable levels in vivo is not dependent upon the viral-DNA copy number and that the pattern of expression of RCMV genes is tissue specific. As shown in Table 4, compared to the spleen, a reduced number of viral genes were detected in the other organs; 44 viral genes were detected in the liver, 39 in the allograft heart, 26 in the kidney, and 17 in the lung. A total of nine RCMV genes were detected in all of the tissues tested: R78 (vGPCR), R116, r119.2, r119.3, r133, r138 (Fc receptor homolog), r142 (m142 homolog; macrophage tropism determinant), r149, and r152.4. Apart from r133, all of these genes are expressed with early kinetics in vitro. Five viral genes (R73 [gN], R114, r119.4, r151, and r166) were expressed at detectable levels in at least five out of six tissues. Eleven RCMV genes were detected in four out of six tissues (R28, r41, R80 [assembly protein], R94 [tegument protein], R96 [viral kinase], R115, r124, r151.1, r152.3, r157 [homolog of murine CMV m157, which binds Ly49H], and r158), most of which are on the right half of the viral genome. There were 18 genes uniquely detected in a single tissue type, and 10 of these genes were specific for the SMG. As shown in Table 4, a subset of genes expressed in each tissue were not detectable; some of the weakly expressed genes scored negative because their intensities were below the detection limit of the microarray assay (0.5 × 104 to 1 × 104 copies/2 μg of total RNA). We attribute differences in detection to the level of virus infection, which varies between individual rats (Table 3). In general, the genes that are consistently detected in vivo are regarded as dispensable for virus replication in vitro and may be involved in immune evasion, cell cycle manipulation, antiapoptosis, and/or cell survival. These regulatory genes potentially include nine members of the m145 gene family and seven of the US22 gene family.
FIG. 5.
Microarray analysis of RCMV gene expression in infected rat tissues. Total RNA was extracted from RCMV-infected spleen, lung, kidney, and heart allograft at 7 days p.i.; SMG at 21 days p.i. The RNA was processed and analyzed using our RCMV microarray chips (n = 4). The mean gene intensity from each of the four biological replicates is plotted on the y axis, and RCMV gene number on the x axis.
TABLE 4.
RCMV gene expression in rat tissues
| RCMV/ORF | Intensitya
|
Comments | |||||
|---|---|---|---|---|---|---|---|
| Spleen | Lung | Liver | Kidney | Salivary gland | Allograft heart | ||
| r2 | 0 | 0 | 0 | 0 | 0 | 690 | |
| r2.1 | 809 | 0 | 4,420 | 1,239 | 0 | 0 | |
| r4 | 1,009 | 0 | 1,122 | 0 | 0 | 0 | |
| r5 | 0 | 0 | 754 | 0 | 0 | 0 | |
| r6 | 0 | 0 | 0 | 548 | 0 | 0 | |
| R23 | 594 | 0 | 744 | 0 | 0 | 0 | US22 family homolog |
| r25.1 | 1,462 | 0 | 777 | 0 | 0 | 2,319 | US22 family homolog |
| R25 | 1,158 | 0 | 504 | 0 | 0 | 1,917 | UL25 family homolog |
| R27 | 583 | 0 | 0 | 0 | 0 | 1,024 | |
| R28 | 1,434 | 0 | 632 | 823 | 0 | 1,160 | |
| R29 | 647 | 0 | 0 | 5,504 | 0 | 949 | |
| R31 | 455 | 0 | 0 | 0 | 0 | 0 | |
| R35 | 0 | 0 | 0 | 0 | 3,058 | 0 | UL25 family homolog |
| R36 | 686 | 0 | 903 | 0 | 0 | 0 | US22 family homolog |
| R38 | 901 | 0 | 982 | 0 | 0 | 942 | |
| r41 | 1,650 | 602 | 1,403 | 0 | 0 | 705 | |
| r42 | 0 | 0 | 0 | 496 | 0 | 0 | |
| R43 | 976 | 0 | 0 | 0 | 1,135 | 1,568 | US22 family homolog |
| r43.1 | 0 | 0 | 1,109 | 0 | 0 | 844 | |
| R45 | 636 | 0 | 895 | 0 | 0 | 639 | Homolog of HCMV gene encoding RRL |
| R49 | 1,356 | 0 | 0 | 628 | 0 | 905 | |
| R54 | 0 | 0 | 0 | 0 | 767 | 0 | DNA polymerase |
| R55 | 683 | 0 | 0 | 0 | 754 | 2,929 | glycoprotein B |
| R69 | 861 | 0 | 827 | 0 | 0 | 0 | Transactivator of gene expression |
| r70.2 | 4,172 | 0 | 555 | 0 | 0 | 4,452 | m145 gene family member |
| r70.3 | 1,175 | 0 | 0 | 0 | 0 | 1,534 | m145 gene family member |
| r70.5 | 0 | 0 | 884 | 0 | 0 | 0 | m145 gene family member |
| R72 | 0 | 0 | 0 | 0 | 1,034 | 0 | UTPase |
| R73 | 1,779 | 0 | 454 | 658 | 2,286 | 2,143 | gN |
| R78 | 4,519 | 1,812 | 7,293 | 777 | 1,274 | 1,819 | GPCR gene homolog |
| R80 | 3,347 | 0 | 0 | 725 | 6,118 | 4,571 | Assembly protein |
| R82 | 0 | 0 | 809 | 0 | 1,747 | 1,244 | Upper matrix protein homolog |
| R88 | 636 | 0 | 0 | 0 | 0 | 740 | |
| R93 | 687 | 0 | 0 | 0 | 0 | 2,272 | |
| R94 | 2,938 | 0 | 637 | 918 | 0 | 2,436 | Tegument protein |
| R95 | 666 | 0 | 0 | 0 | 0 | 796 | |
| R96 | 2,382 | 0 | 0 | 464 | 969 | 1,514 | |
| R98 | 738 | 0 | 0 | 0 | 0 | 0 | Exonuclease (DNase) |
| R99 | 1,276 | 0 | 0 | 474 | 0 | 2,106 | Tegument phosphoprotein |
| R100 | 1,416 | 0 | 0 | 0 | 1,592 | 3,542 | Glycoprotein M |
| R102 | 1,526 | 0 | 0 | 0 | 1,405 | 1,955 | HP complex component |
| r106 | 734 | 0 | 607 | 0 | 0 | 0 | |
| r109 | 1,576 | 0 | 0 | 523 | 0 | 1,859 | |
| r110 | 544 | 0 | 0 | 0 | 0 | 0 | |
| R113 | 1,677 | 0 | 789 | 0 | 1,083 | 0 | |
| R114 | 5,259 | 0 | 736 | 1,396 | 21,637 | 6,256 | Uracil DNA glycosylase |
| R115 | 849 | 0 | 0 | 665 | 4,678 | 1,518 | Glycoprotein L |
| R116 | 10,322 | 865 | 1,434 | 3,268 | 10,578 | 6,189 | |
| R118 | 0 | 0 | 0 | 0 | 665 | 0 | |
| r119.1 | 556 | 0 | 0 | 0 | 27,712 | 0 | |
| r119.2 | 11,846 | 5,237 | 18,217 | 6,721 | 52,110 | 1,885 | |
| r119.3 | 6,881 | 3,255 | 9,417 | 1,577 | 38,222 | 976 | |
| r119.4 | 1,974 | 972 | 2,064 | 691 | 6,389 | 0 | |
| r119.6 | 0 | 0 | 0 | 0 | 420 | 0 | |
| r124 | 2,862 | 2,517 | 2,825 | 0 | 765 | 0 | |
| r128 | 1,364 | 0 | 2,001 | 0 | 675 | 0 | US22 family homolog |
| r133 | 4,697 | 2,610 | 7,068 | 567 | 1,748 | 1,362 | Homolog of murine CMV SGG1 |
| r138 | 7,498 | 3,389 | 9,199 | 856 | 1,681 | 2,048 | Fc receptor |
| r139 | 0 | 0 | 849 | 0 | 0 | 0 | US22 family homolog |
| r142 | 3,164 | 597 | 1,954 | 814 | 1,464 | 3,625 | US22 family homolog |
| r146 | 0 | 0 | 0 | 0 | 1,084 | 0 | |
| r147 | 0 | 0 | 0 | 0 | 720 | 0 | |
| r149 | 4,337 | 1,976 | 4,075 | 922 | 5,653 | 593 | |
| r150 | 1,068 | 0 | 1,462 | 0 | 2,042 | 0 | m145 gene family member |
| r151.1 | 958 | 644 | 1,652 | 0 | 1,787 | 0 | |
| r151.3 | 599 | 0 | 0 | 0 | 3,071 | 0 | |
| r151 | 2,077 | 1,487 | 1,837 | 570 | 13,367 | 0 | m145 gene family member |
| r152.2 | 0 | 0 | 0 | 0 | 829 | 0 | m145 gene family member |
| r152.3 | 599 | 1,289 | 2,350 | 0 | 729 | 0 | m145 gene family member |
| r152.4 | 15,024 | 6,077 | 13,520 | 6,759 | 35,768 | 5,592 | m145 gene family member |
| r152.5 | 955 | 0 | 792 | 0 | 0 | 0 | |
| r157 | 2,044 | 959 | 1,828 | 0 | 1,453 | 0 | m145 gene family member |
| r158 | 1,375 | 0 | 631 | 509 | 2,805 | 0 | |
| r161 | 0 | 0 | 0 | 0 | 435 | 0 | |
| r166 | 3,333 | 1,312 | 5,271 | 0 | 564 | 792 | |
| r171.1 | 461 | 0 | 854 | 0 | 0 | 0 | |
| r171 | 780 | 0 | 865 | 0 | 0 | 0 | |
| Total viral genes detected per tissue | 60 | 17 | 44 | 26 | 41 | 39 | |
| % of RCMV genome | 36 | 10 | 27 | 16 | 25 | 24 | |
The average fluorescence intensity for each viral gene (n = 3).
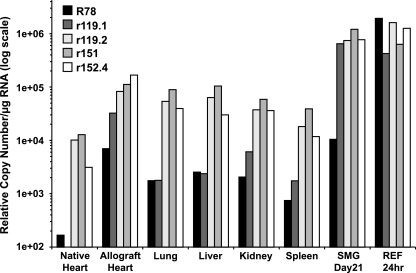
In order to confirm the tissue-specific expression pattern of RCMV genes, QRT-PCR was performed in triplicate on mRNAs isolated from the tissues of three individual rats. TaqMan probes and primers specific for R78, r119.1, r119.2, r151, and r152.4 were generated. Average copy numbers were normalized to expression of the cellular housekeeping gene L32, and the ratio between each pair of genes was determined (Fig. 6). Three of the genes, r119.2, r151, and r152.4, were highly expressed at similar levels in all of the tissues that were tested. The expression of these genes was at most threefold different (r119.2/r152.4 in the native heart samples), which was not statistically significant (P = 0.15). R78 and r119.1 were two of a number of genes that, according to our microarray analysis, appeared to be expressed in a tissue-specific manner. In all tissues tested, R78 and R119.1 were expressed at similar levels, except the SMG, where r119.1 was expressed at a considerably higher level (approximately 60-fold) than was R78. By contrast, R78 expression in RCMV-infected REFs at 24 h p.i. was significantly higher than that of both r119.1 and r119.2. Expression levels for r119.1 in the allograft heart and SMG tissues was increased relative to R78, whereas the expression levels of these two genes were similar in the liver, lung, kidney, and spleen. Compared to expression levels of r119.2, r151, and r152.4, r1191.1 was dramatically lower in these tissues. This is an interesting finding, since these infected tissues express a unique viral transcriptome. For example, in the spleen, 61 genes are expressed to detectable levels, whereas 44 and 17 genes are expressed in the liver and lung, respectively. The expression levels of r119.1 in allograft hearts and SMG were similar to those of r119.2, r151, and r152.4 (Fig. 4A and 6), suggesting that expression of these genes may be similarly controlled.
FIG. 6.
RCMV gene expression is tissue specific. RT-PCR TaqMan analysis was used to confirm and quantify viral-gene expression in RNA samples from in vivo-infected native heart, allograft heart, lung, liver, kidney, spleen, and SMG. All tissues were harvested at 7 days p.i., except SMG (21 days p.i.). RCMV-infected REFs harvested at 24 h p.i. were used for in vitro-in vivo comparisons. Primers specific for the RCMV genes R78, r119.1, r119.2, r151, and r152.4 were used to quantify viral-gene expression. RNA samples were normalized to expression of L32 (cellular ribosomal protein), and the relative copy numbers were determined using plasmids containing each viral gene. The limit of detection for this assay was 100 copies.
DISCUSSION
Originally, our plan for these studies was to compare RCMV gene expression in only allograft versus native hearts to determine the viral mechanisms associated with CMV-accelerated allograft rejection and vascular disease. However, after profiling other tissues from the infected transplant recipients, it became evident that there were major differences in viral-gene expression between the various tissues. Because of these significant findings, we expanded our study to include all major tissues in the transplant recipient. In the current report, we used a global microarray approach to examine RCMV gene expression in infected cultured cells and in tissues from infected rats. By studying CMV gene expression at the viral-transcriptome level, we avoided biasing this study toward a limited number of viral genes. This process has enabled us to identify the viral-gene expression profiles during acute infection of heart transplant recipients. In cultured cells, RCMV expresses approximately 95% of the known viral ORFs, and we observed very little difference in the expression patterns between infected SMC, EC, and fibroblasts. However, viral-gene expression in tissues from infected rat heart allograft recipients was very different. The results from the in vivo transcription analysis allow the following conclusions to be drawn. (i) The profiles and amplitude of viral-gene expression differed dramatically compared to infected cultured cells, indicating that CMV gene expression in vitro does not accurately reflect viral-gene expression in vivo. (ii) Viral-gene expression in tissues is skewed toward genes that are not known to have direct roles in virus replication or assembly but most likely represent genes involved in immune evasion or host cell survival. Other viral genes are expressed, as detected by RT-PCR; however, the expression levels are below the detection threshold of the microarray assay. (iii) The viral-DNA load and viral-mRNA levels do not correlate with profiles of gene expression. (iv) Viral-gene expression is tissue specific, suggesting either that the virus actively adapts to its environment in order to control gene expression or that CMV merely passively responds to transcriptional controls implemented by the host environment. Our findings emphasize the importance of in vivo analysis using animal models in translational research.
The first global analysis of CMV gene expression was performed by Chambers et al. using microarray chips designed to 151 of the 200 known HCMV ORFs (8). This study demonstrated the feasibility of microarray technology to monitor the gene expression profiles of large complex viruses, such as the herpesviruses. They determined the temporal kinetic class of each of the HCMV ORFs, including those of many uncharacterized ORFs. An interesting finding of this study was that many of the genes in a specific temporal class contain common potential regulatory motifs within their promoter regions, suggesting that HCMV can express its genes with specific transcriptional programs, much like what is observed in tissues during development. Our results demonstrate that the in vivo gene profiles for RCMV are different from those observed in cultured cells and that the viral-gene expression pattern is tissue specific. An explanation for our findings of limited in vivo viral-gene expression is that the multiplicity of infection in the tissues is low or that clearance of the productively infected cells leaves only abortively infected cells that express fewer genes to high levels. Our data do not support or directly refute these scenarios. On the contrary, we detected the highest viral titers in the SMG between days 21 and 28, during the time of limited viral-gene expression. Thus, another explanation may involve the number of different infected cell types per tissue; the more types of infected cells, the wider the viral-gene profile. Interestingly, infection in the SMG is limited to the striated duct cells (15), and the number of highly expressed genes is limited to 41 in this tissue. Other tissues, like the spleen, display a wider viral-gene profile, and multiple cell types may be infected. However, the fact that RCMV gene expression occurs in a tissue-specific manner suggests that there exists a level of transcriptional control at the tissue level. This tissue specificity suggests that the virus may have multiple transcriptional programs, depending upon the cellular environment, or that other posttranscriptional events, such as mRNA stability, may be important to elicit this effect. What we do not yet know is whether the virus and/or the host cell determines this level of control. The ability of the virus to control gene expression in a tissue-specific manner may be regulated by specific motifs within the viral promoters that are active in some tissues but not in others. This level of transcriptional control does not exist in vitro for RCMV-infected cells, where eventually almost all of the viral ORFs are expressed to a detectable level. We were unable to detect any obvious regulatory-motif pattern in the promoters of the RCMV genes that are highly expressed in vivo. However, a more intensive study is warranted to identify these regions in order to determine the selective mechanisms of in vivo gene expression. An interesting parallel in the ability of CMV to regulate viral-gene expression is observed during natural human papillomavirus infections of the skin. Papillomaviruses contain only two promoters, early and late. The early promoter is active in many different cell types but appears to be most active in keratinocytes, suggesting that the promoter is cell/tissue type specific (2, 19, 30). The late promoter is differentiation dependent and is activated only during terminal differentiation of the epithelial cells of the granular layer (19). An important question that arises from virus gene expression studies with papillomavirus and those we present here is what are the cell-type-specific factors that promote the activation of some viral genes and repression of others?
Adamo et al., Bresnahan et al., Goodrum et al., and Heider et al. utilized similar HCMV microarrays, containing 191 viral ORFs, to monitor viral-gene expression on a global level (1, 4, 11-13). Utilization of these HCMV microarrays to identify the viral-gene expression patterns in specific subsets of CD34+ hematopoietic stem cells demonstrated that HCMV infection of these cells produced an initial transient burst of gene expression, followed by a period of viral transcriptional quiescence and genome maintenance. The infected CD34+ cells were capable of reactivating virus when cocultured with fibroblasts. The HCMV expression profiles in CD34+ cells parallel our in vivo RCMV gene expression profiles on a number of levels. First, gene expression in the CD34+ cells was significantly different from that in HCMV-infected fibroblasts. Second, viral expression in CD34+ cells was very limited and did not correspond to conventional IE, E, and L viral-gene expression profiles. Finally, many of the viral genes expressed in the CD34+ stem cells are not directly involved in virus replication. The significance of this early burst of viral-gene expression is not understood. However, it might play a role in the establishment of latency. Clearly many, if not all, of the herpesviruses can regulate their gene expression patterns during persistence and latency. For example, Epstein-Barr virus infection of B cells results in one of five transcriptional programs, depending upon the activation and differentiation statuses of the host cell (reviewed in reference 27). Most of these transcriptional programs are involved in maintaining persistence of the viral genome during periods of cell quiescence and during cellular replication. In addition, herpes simplex virus type 1 expresses the viral latency-associated transcript during all stages of replication, but it is exclusively expressed during latent infections of neurons (reviewed in reference 24). Expression of the latency-associated transcript is required for cell survival during reactivation. Similarly, CMV expression in the SMG (a major site of virus persistence), even in the presence of high levels of viral genome, is tightly regulated and highly skewed. The viral genes expressed in the SMG may be involved in preserving the infected host cell, allowing immune escape, and/or promoting transmission to a new host. Thus, a common theme among these viruses is the ability to control viral-gene expression as a method of persistence in the infected host, whether it is during latency or during smoldering infection states, both of which occur in a CMV-infected host.
Our in vivo observations indicate that gene expression of the immune modulators occurs at higher levels than previously predicted based upon in vitro studies. It may be that this differential level of in vivo viral-gene expression between the two gene groups responsible for immune evasion and viral replication allows the virus to persist by turning over small amounts of infectious virus while remaining relatively undetected by the host immune system. A relevant question that surfaces from these findings is why the virus specifically overexpresses certain viral genes and not others. For example, is there relevance to the fact that in our study the RCMV-encoded Fc receptor, r138, was highly expressed in the spleen, liver, and lung? In these tissues, r138 is expressed at nearly the same level as r119.2, whereas in the SMG, r138 is expressed at 30-fold lower levels than r119.2 (Fig. 5 and Table 4). Is the virus more prone to antibody-mediated immune pressures in the lung, liver, and spleen than in SMG tissues? Our findings suggest that the virus does not produce its full arsenal of immune modulator genes in every infected cell type in vivo but rather selectively regulates their respective gene expressions. The host response to viral infection is probably not the same in every tissue. Thus, the ability of CMV immune evasion genes involved in preventing immune recognition would similarly need to be specific for each of these types of responses. Consequently, the virus would need to express multiple transcriptional programs appropriate for the specific host cell type and the type of immune response prompted by the infected cell and/or by its juxtaposition to the immune system.
In conclusion, we explored the characteristics of RCMV gene expression in cultured cells and in tissues from a rat cardiac transplant model. Combined, our findings indicate that viral-gene expression is highly complex. One cannot predict in vivo viral-gene expression solely based upon the in vitro viral-transcriptome analysis. In fact, most studies aimed at identifying viral expression during latency and reactivation have focused on the IE proteins, which we found to be expressed at very low levels compared to the other viral genes likely involved in immune modulation. The most efficient and effective way for CMV to remain undetected by the host during persistence is by regulating viral-gene expression in such a way as to overexpress the immune evasion genes while repressing viral genes involved in replication. One of the reasons that CMV carries so many genes might be that different gene expression programs are used in different cell and tissue types. This is also highly relevant for the analysis of immune modulators. If the wrong tissues and organs are studied, the effect might be missed. Identifying the viral transcriptomes of different tissues and at different times associated with virus-induced disease, such as accelerated allograft rejection, will aid in the design of novel targeted therapeutics.
Acknowledgments
This work was supported by research grants to S. L. Orloff from the Department of Veterans Affairs and from the National Institutes of Health (HL 66238-01) and grants from the National Institutes of Health to D. N. Streblow (HL083194) and J. A. Nelson (AI21640, HL65754, and HL71695). D.N. Streblow is also supported by an AHA Scientist Development Grant. C. Vink is supported by a grant from the Royal Netherlands Academy of Arts and Sciences.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Adamo, J. E., J. Schroer, and T. Shenk. 2004. Human cytomegalovirus TRS1 protein is required for efficient assembly of DNA-containing capsids. J. Virol. 78:10221-10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apt, D., T. Chong, Y. Liu, and H. U. Bernard. 1993. Nuclear factor I and epithelial cell-specific transcription of human papillomavirus type 16. J. Virol. 67:4455-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beisser, P. S., C. Vink, J. G. Van Dam, G. Grauls, S. J. Vanherle, and C. A. Bruggeman. 1998. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J. Virol. 72:2352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., and T. Shenk. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373-2376. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman, C. A., W. M. Debie, G. Grauls, G. Majoor, and C. P. van Boven. 1983. Infection of laboratory rats with a new cytomegalo-like virus. Arch. Virol. 76:189-199. [DOI] [PubMed] [Google Scholar]
- 6.Bruggeman, C. A., H. Meijer, F. Bosman, and C. P. van Boven. 1985. Biology of rat cytomegalovirus infection. Intervirology 24:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Bruggeman, C. A., H. Schellekens, G. Grauls, W. M. Debie, and C. P. van Boven. 1983. Rat cytomegalovirus: induction of and sensitivity to interferon. Antivir. Res. 3:315-324. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, J., A. Angulo, D. Amaratunga, H. Guo, Y. Jiang, J. S. Wan, A. Bittner, K. Frueh, M. R. Jackson, P. A. Peterson, M. G. Erlander, and P. Ghazal. 1999. DNA microarrays of the complex human cytomegalovirus genome: profiling kinetic class with drug sensitivity of viral gene expression. J. Virol. 73:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cobbs, C. S., L. Harkins, M. Samanta, G. Y. Gillespie, S. Bharara, P. H. King, L. B. Nabors, C. G. Cobbs, and W. J. Britt. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62:3347-3350. [PubMed] [Google Scholar]
- 10.Gold, M. C., M. W. Munks, M. Wagner, U. H. Koszinowski, A. B. Hill, and S. P. Fling. 2002. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 169:359-365. [DOI] [PubMed] [Google Scholar]
- 11.Goodrum, F., C. T. Jordan, S. S. Terhune, K. High, and T. Shenk. 2004. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood 104:687-695. [DOI] [PubMed] [Google Scholar]
- 12.Goodrum, F. D., C. T. Jordan, K. High, and T. Shenk. 2002. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc. Natl. Acad. Sci. USA 99:16255-16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heider, J. A., W. A. Bresnahan, and T. E. Shenk. 2002. Construction of a rationally designed human cytomegalovirus variant encoding a temperature-sensitive immediate-early 2 protein. Proc. Natl. Acad. Sci. USA 99:3141-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaptein, S. J., P. S. Beisser, Y. K. Gruijthuijsen, K. G. Savelkouls, K. W. van Cleef, E. Beuken, G. E. Grauls, C. A. Bruggeman, and C. Vink. 2003. The rat cytomegalovirus R78 G protein-coupled receptor gene is required for production of infectious virus in the spleen. J. Gen. Virol. 84:2517-2530. [DOI] [PubMed] [Google Scholar]
- 15.Kaptein, S. J., K. W. van Cleef, Y. K. Gruijthuijsen, E. V. Beuken, L. van Buggenhout, P. S. Beisser, F. R. Stassen, C. A. Bruggeman, and C. Vink. 2004. The r131 gene of rat cytomegalovirus encodes a proinflammatory CC chemokine homolog which is essential for the production of infectious virus in the salivary glands. Virus Genes 29:43-61. [DOI] [PubMed] [Google Scholar]
- 16.Kavanagh, D. G., M. C. Gold, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2001. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloover, J. S., J. L. Hillebrands, G. de Wit, G. Grauls, J. Rozing, C. A. Bruggeman, and P. Nieuwenhuis. 2000. Rat cytomegalovirus replication in the salivary glands is exclusively confined to striated duct cells. Virchows Arch. 437:413-421. [DOI] [PubMed] [Google Scholar]
- 18.Krmpotic, A., M. Messerle, I. Crnkovic-Mertens, B. Polic, S. Jonjic, and U. H. Koszinowski. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 190:1285-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCance, D. J. 2005. Transcriptional regulation by human papillomaviruses. Curr. Opin. Genet. Dev. 15:515-519. [DOI] [PubMed] [Google Scholar]
- 20.Melnychuk, R. M., D. N. Streblow, P. P. Smith, A. J. Hirsch, D. Pancheva, and J. A. Nelson. 2004. Human cytomegalovirus-encoded G protein-coupled receptor US28 mediates smooth muscle cell migration through Gα12. J. Virol. 78:8382-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mocarski, E. S. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In B. N. Fields and D. M. Knipe (ed.), Fields Virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 22.Orloff, S. L., D. N. Streblow, C. Soderberg-Naucler, Q. Yin, C. Kreklywich, C. L. Corless, P. A. Smith, C. B. Loomis, L. K. Mills, J. W. Cook, C. A. Bruggeman, J. A. Nelson, and C. R. Wagner. 2002. Elimination of donor-specific alloreactivity prevents cytomegalovirus-accelerated chronic rejection in rat small bowel and heart transplants. Transplantation 73:679-688. [DOI] [PubMed] [Google Scholar]
- 23.Pass, R. F. 2001. Cytomegalovirus, p. 2675-2705. In B. N. Fields and D. M. Knipe (ed.), Fields Virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 24.Rajcani, J., V. Andrea, and R. Ingeborg. 2004. Peculiarities of herpes simplex virus (HSV) transcription: an overview. Virus Genes 28:293-310. [DOI] [PubMed] [Google Scholar]
- 25.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streblow, D. N., C. Kreklywich, Q. Yin, V. T. De La Melena, C. L. Corless, P. A. Smith, C. Brakebill, J. W. Cook, C. Vink, C. A. Bruggeman, J. A. Nelson, and S. L. Orloff. 2003. Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J. Virol. 77:2182-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorley-Lawson, D. A., and A. Gross. 2004. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N. Engl. J. Med. 350:1328-1337. [DOI] [PubMed] [Google Scholar]
- 28.van der Strate, B. W., J. L. Hillebrands, S. S. Lycklama a Nijeholt, L. Beljaars, C. A. Bruggeman, M. J. Van Luyn, J. Rozing, T. H. The, D. K. Meijer, G. Molema, and M. C. Harmsen. 2003. Dissemination of rat cytomegalovirus through infected granulocytes and monocytes in vitro and in vivo. J. Virol. 77:11274-11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 74:7656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yukawa, K., K. Butz, T. Yasui, H. Kikutani, and F. Hoppe-Seyler. 1996. Regulation of human papillomavirus transcription by the differentiation-dependent epithelial factor Epoc-1/skn-1a. J. Virol. 70:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]