Abstract
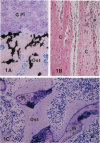
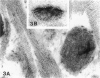
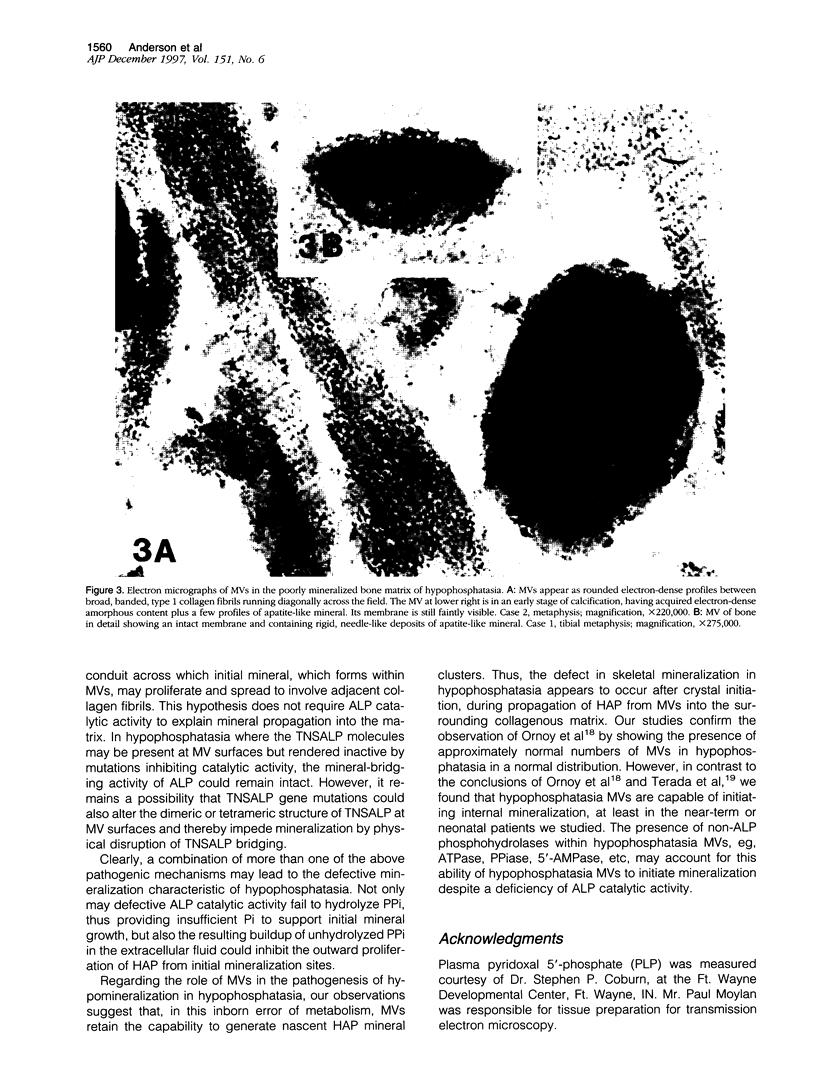
Hypophosphatasia, a heritable disease characterized by deficient activity of the tissue nonspecific isoenzyme of alkaline phosphatase (TNSALP), results in rickets and osteomalacia. Although identification of TNSALP gene defects in hypophosphatasia establishes a role of ALP in skeletal mineralization, the precise function remains unclear. The initial site of mineralization (primary mineralization) normally occurs within the lumen of TNSALP-rich matrix vesicles (MVs) of growth cartilage, bone, and dentin. We investigated whether defective calcification in hypophosphatasia is due to a paucity and/or a functional failure of MVs secondary to TNSALP deficiency. Nondecalcified autopsy bone and growth plate cartilage from five patients with perinatal (lethal) hypophosphatasia were studied by nondecalcified light and electron microscopy to assess MV numbers, size, shape, and ultrastructure and whether hypophosphatasia MVs contain apatite-like mineral, as would be the case if these MVs retained their ability to concentrate calcium and phosphate internally despite a paucity of TNSALP in their investing membranes. We found that hypophosphatasia MVs are present in approximately normal numbers and distribution and that they are capable of initiating internal mineralization. There is retarded extravesicular crystal propagation. Thus, in hypophosphatasia the failure of bones to calcify appears to involve a block of the vectorial spread of mineral from initial nuclei within MVs, outwards, into the matrix. We conclude that hypophosphatasia MVs can concentrate calcium and phosphate internally despite a deficiency of TNSALP activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akisaka T., Gay C. V. Ultrastructural localization of calcium-activated adenosine triphosphatase (Ca2+-ATPase) in growth-plate cartilage. J Histochem Cytochem. 1985 Sep;33(9):925–932. doi: 10.1177/33.9.3160764. [DOI] [PubMed] [Google Scholar]
- Ali S. Y., Evans L. The uptake of [Ca]calcium ions by matrix vesicles isolated from calcifying cartilage (Short Communication). Biochem J. 1973 Jun;134(2):647–650. doi: 10.1042/bj1340647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y., Sajdera S. W., Anderson H. C. Isolation and characterization of calcifying matrix vesicles from epiphyseal cartilage. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1513–1520. doi: 10.1073/pnas.67.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. C., Sajdera S. W. Calcification of rachitic cartilage to study matrix vesicle function. Fed Proc. 1976 Feb;35(2):148–153. [PubMed] [Google Scholar]
- Anderson H. C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol. 1969 Apr;41(1):59–72. doi: 10.1083/jcb.41.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT A. M., FAIRWEATHER D. V., MCCANCE R. A., MORRISON A. B. Genetic, clinical, biochemical, and pathological features of hypophosphatasia; based on the study of a family. Q J Med. 1956 Oct;25(100):523–537. [PubMed] [Google Scholar]
- Bellows C. G., Aubin J. E., Heersche J. N. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 1991 Jul;14(1):27–40. doi: 10.1016/0169-6009(91)90100-e. [DOI] [PubMed] [Google Scholar]
- Caswell A. M., Whyte M. P., Russell R. G. Hypophosphatasia and the extracellular metabolism of inorganic pyrophosphate: clinical and laboratory aspects. Crit Rev Clin Lab Sci. 1991;28(3):175–232. doi: 10.3109/10408369109106862. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Mechanism of calcification: inhibitory role of pyrophosphate. Nature. 1962 Sep 1;195:911–911. doi: 10.1038/195911a0. [DOI] [PubMed] [Google Scholar]
- Fallon M. D., Whyte M. P., Teitelbaum S. L. Stereospecific inhibition of alkaline phosphatase by L-tetramisole prevents in vitro cartilage calcification. Lab Invest. 1980 Dec;43(6):489–494. [PubMed] [Google Scholar]
- Fedde K. N., Michell M. P., Henthorn P. S., Whyte M. P. Aberrant properties of alkaline phosphatase in patient fibroblasts correlate with clinical expressivity in severe forms of hypophosphatasia. J Clin Endocrinol Metab. 1996 Jul;81(7):2587–2594. doi: 10.1210/jcem.81.7.8675582. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Raducha M., Fedde K. N., Lafferty M. A., Whyte M. P. Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9924–9928. doi: 10.1073/pnas.89.20.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. H., Anderson H. C. A role for ATPase in the mechanisms of ATP-dependent Ca and phosphate deposition by isolated rachitic matrix vesicles. Int J Biochem Cell Biol. 1995 Dec;27(12):1349–1356. doi: 10.1016/1357-2725(95)00103-v. [DOI] [PubMed] [Google Scholar]
- Hsu H. H., Anderson H. C. A simple and defined method to study calcification by isolated matrix vesicles. Effect of ATP and vesicle phosphatase. Biochim Biophys Acta. 1977 Nov 7;500(1):162–172. doi: 10.1016/0304-4165(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T., Anderson H. C. Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem. 1971 Dec;19(12):801–808. doi: 10.1177/19.12.801. [DOI] [PubMed] [Google Scholar]
- Ornoy A., Adomian G. E., Rimoin D. L. Histologic and ultrastructural studies on the mineralization process in hypophosphatasia. Am J Med Genet. 1985 Dec;22(4):743–758. doi: 10.1002/ajmg.1320220410. [DOI] [PubMed] [Google Scholar]
- Ozono K., Yamagata M., Michigami T., Nakajima S., Sakai N., Cai G., Satomura K., Yasui N., Okada S., Nakayama M. Identification of novel missense mutations (Phe310Leu and Gly439Arg) in a neonatal case of hypophosphatasia. J Clin Endocrinol Metab. 1996 Dec;81(12):4458–4461. doi: 10.1210/jcem.81.12.8954059. [DOI] [PubMed] [Google Scholar]
- Robison R., Rosenheim A. H. Calcification of hypertrophic cartilage in vitro. Biochem J. 1934;28(2):684–698.1. doi: 10.1042/bj0280684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow D. M., Povey S., Parkar M., Andrews P. W., Harris H., Pym B., Goodfellow P. Mapping of the gene coding for the human liver/bone/kidney isozyme of alkaline phosphatase to chromosome 1. Ann Hum Genet. 1986 Jul;50(Pt 3):229–235. doi: 10.1111/j.1469-1809.1986.tb01043.x. [DOI] [PubMed] [Google Scholar]
- Terada S., Suzuki N., Ueno H., Uchide K., Kohama T. A congenital lethal form of hypophosphatasia: histologic and ultrastructural study. Acta Obstet Gynecol Scand. 1996 May;75(5):502–505. doi: 10.3109/00016349609033363. [DOI] [PubMed] [Google Scholar]
- Termine J. D., Conn K. M. Inhibition of apatite formation by phosphorylated metabolites and macromolecules. Calcif Tissue Res. 1976 Dec 22;22(2):149–157. doi: 10.1007/BF02010354. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Cole D. E., Ray K., Whyte M. P., Lafferty M. A., Mulivor R. A., Harris H. A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a lethal form of hypophosphatasia. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7666–7669. doi: 10.1073/pnas.85.20.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Henthorn P. S., Lafferty M. A., Slaughter C., Raducha M., Harris H. Isolation and characterization of a cDNA encoding a human liver/bone/kidney-type alkaline phosphatase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7182–7186. doi: 10.1073/pnas.83.19.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte M. P. Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev. 1994 Aug;15(4):439–461. doi: 10.1210/edrv-15-4-439. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Magill H. L., Fallon M. D., Herrod H. G. Infantile hypophosphatasia: normalization of circulating bone alkaline phosphatase activity followed by skeletal remineralization. Evidence for an intact structural gene for tissue nonspecific alkaline phosphatase. J Pediatr. 1986 Jan;108(1):82–88. doi: 10.1016/s0022-3476(86)80773-9. [DOI] [PubMed] [Google Scholar]
- Whyte M. P., Walkenhorst D. A., Fedde K. N., Henthorn P. S., Hill C. S. Hypophosphatasia: levels of bone alkaline phosphatase immunoreactivity in serum reflect disease severity. J Clin Endocrinol Metab. 1996 Jun;81(6):2142–2148. doi: 10.1210/jcem.81.6.8964842. [DOI] [PubMed] [Google Scholar]
- Wu L. N., Genge B. R., Lloyd G. C., Wuthier R. E. Collagen-binding proteins in collagenase-released matrix vesicles from cartilage. Interaction between matrix vesicle proteins and different types of collagen. J Biol Chem. 1991 Jan 15;266(2):1195–1203. [PubMed] [Google Scholar]