Abstract
The purpose of this study was to examine the in vivo efficacies of meropenem and ertapenem against extended-spectrum-β-lactamase (ESBL)-producing isolates with a wide range of MICs. Human-simulated dosing regimens in mice were designed to approximate the free drug percent time above the MIC (fT>MIC) observed for humans following meropenem at 1 g every 8 h and ertapenem at 1 g every 24 h. An in vivo neutropenic mouse thigh infection model was used to examine the bactericidal effects against 31 clinical ESBL Escherichia coli and Klebsiella pneumoniae isolates and 2 non-ESBL isolates included for comparison at a standard 105 inoculum. Three isolates were examined at a high 107 inoculum as well. Meropenem displayed greater in vitro potency, with a median MIC (range) (μg/ml) of 0.125 (0.03 to 32), than did ertapenem, with 0.5 (0.012 to 128). Seven of the 31 ESBL isolates were removed from the efficacy analysis due to their inability to establish infection in the mouse model. When MICs were ≤1.5 μg/ml for ertapenem (≤0.5 μg/ml for meropenem), similar reductions in CFU (≈ 2-log kill) were observed for both ertapenem (fT>MIC ≥ 23%) and meropenem (fT>MIC ≥ 75%). Ertapenem showed bacterial regrowth for seven of eight isolates, with MICs of ≥2 μg/ml (fT>MIC ≤ 20%), while meropenem displayed antibacterial potency that varied from a static effect to a 1-log bacterial reduction in these isolates (fT>MIC = 30 to 65%). At a 107 inoculum, both agents eradicated bacteria due to adequate exposures (fT>MIC = 20 to 45%). Due to low MICs, no difference in bacterial kill was noted for the majority of ESBL isolates tested. However, for isolates with raised ertapenem MICs of ≥2 μg/ml, meropenem displayed sustained efficacy due to its greater in vitro potency and higher resultant fT>MIC.
The increase in antimicrobial resistance threatens the utility of many currently available antimicrobial agents to treat infection. One clinically important mechanism of resistance in bacteria is the production of extended-spectrum β-lactamase (ESBL). Currently, the highest prevalence of ESBL production occurs in Escherichia coli and Klebsiella pneumoniae. A surveillance study conducted across the United States indicated that 15% of E. coli isolates and 24% of K. pneumoniae isolates had elevated MICs of 2 μg/ml or more to ceftazidime, consistent with the presence of an ESBL phenotype (14). More recently, results from the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) surveillance program in 2004 have found the overall prevalence of ESBL-producing Enterobacteriaceae in Europe and the United States to be between 1.4 to 13.6% (10). Interestingly, the prevalence of ESBL-producing E. coli isolates has increased from 2.1% to 10.8% from 1997 to 2004 (10). These pathogens challenge the treatment of a variety of infections, including pneumonia, bacteremia, cystitis, and intra-abdominal infection.
To date, agreement on the significance of ESBL-producing organisms on clinical outcomes has not been reached (28). Some studies have reported an increased risk of mortality, length of hospital stay, and hospital charges resulting from infection due to ESBL-producing pathogens, especially when patients are treated with inadequate empirical antibiotics (17, 27, 30). Currently, the carbapenems (imipenem, meropenem) are recommended for the empirical treatment of ESBL isolates since they are stable to hydrolysis by these enzymes. Other agents potentially beneficial for treatment include fluoroquinolones, aminoglycosides, sulfamethoxazole-trimethoprim, and cefepime; however, the potential for cross-resistance due to plasmid transfer and/or heightened expression of the AmpC gene makes the empirical use of these agents worrisome without documented in vitro susceptibilities (5).
Despite the advocacy for carbapenems as a first-line treatment for ESBL-producing organisms, differences in pharmacokinetics (PKs) and in vitro potencies and the lack of comparative human efficacy data make the selection of the optimal carbapenem difficult. Meropenem and imipenem have been traditionally used to treat these multidrug-resistant pathogens. However, the newer agent ertapenem is expected to be a viable treatment option as well. Based principally on in vitro data, no difference in efficacy was expected for the different carbapenems (imipenem, meropenem, and ertapenem) against ESBL-producing E. coli and Klebsiella spp. (16, 32). Surveillance studies have determined that carbapenems remain >99% susceptible against these organisms, with clinically achievable MIC90 values as follow: for meropenem, 0.25 to 1 μg/ml; for imipenem, 0.5 μg/ml; and for ertapenem, 0.06 μg/ml (12, 13, 16, 18). However, the results of a pharmacodynamic analysis using Monte Carlo simulation to predict the clinical efficacy of the carbapenems against 39 ESBL-producing isolates dispute this fact (23). With PK data from healthy human subjects and a target time above the MIC of 40%, imipenem and meropenem had an increased likelihood of achieving the pharmacodynamic targets compared with ertapenem. The low target attainment rate (78%) of ertapenem was principally due to the elevated MIC90 of 4 μg/ml, which was fourfold higher than that previously reported (23). In addition, more-recent in vitro data have noted an eightfold increase in ertapenem MICs (i.e., ≤0.03 to 0.25 μg/ml) between wild-type and ESBL-producing E. coli and K. pneumoniae isolates, which was not observed with the older agents meropenem and imipenem. These data suggest that the older carbapenems have enzyme stability greater than that of ertapenem (15). Therefore, the objective of this study was to compare the efficacies of meropenem and ertapenem against ESBL-producing E. coli and K. pneumoniae isolates, at various MICs, in a murine neutropenic thigh infection model.
MATERIALS AND METHODS
Antimicrobial test agents.
Standard analytical-grade meropenem (Astra Zeneca, Wilmington, DE) and ertapenem (Merck, West Point, PA) were used for in vitro testing. For in vivo analysis, commercially available meropenem and ertapenem for injection were obtained from their respective drug manufacturers. One gram of meropenem powder (Astra Zeneca) and 1 g of ertapenem powder (Merck) were reconstituted with 20 ml and 10 ml, respectively, of sterile water for injection as per the manufacturer's instructions. Final concentrations were diluted to achieve the desired milligram-per-kilogram of body weight doses administered immediately prior to each experiment. These final solutions were kept under refrigeration when not in use and were discarded 24 h after reconstitution.
Bacterial isolates.
Thirty-one clinical isolates of ESBL-producing E. coli or K. pneumoniae were included. These isolates were either collected from the Hartford Hospital microbiology department or collected as part of the MYSTIC database in both North and South America from 2004 and 2005 (29). Additionally, two non-ESBL E. coli isolates were tested for comparison.
Susceptibilities.
The MICs of meropenem and ertapenem were determined using either Etest methodology (AB Biodisk, Solna, Sweden) or the microdilution method according to CLSI guidelines with cation-adjusted Mueller-Hinton broth (20 to 25 mg/liter calcium, 10 to 12.5 mg/liter magnesium) (2a). The MICs of three randomly selected isolates were also tested at a greater starting inoculum of 107 CFU/ml by use of broth microdilution methods. The specific ESBL enzyme was not known for the majority of these clinical isolates.
Thigh infection model.
Specific-pathogen-free, female ICR mice weighing approximately 25 g each were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and utilized throughout these experiments. The animals were maintained and utilized in accordance to National Research Council recommendations and provided food and water ad libitum. Mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide at doses of 150 and 100 mg/kg of body weight at 4 days and 1 day before inoculation, respectively (11). Three days prior to inoculation, the mice received a single intraperitoneal injection of 5 mg/kg of uranyl nitrate to induce a predictable degree of renal impairment (2).
A suspension of each isolate was prepared from a fresh subculture of organism that had been incubated for less than 20 h and diluted to achieve a final inoculum of 105 or 107 CFU/ml for the standard- and high-inoculum bacterial density studies, respectively. Thigh infection was produced by a single intramuscular injection of 0.1 ml of inoculum into each mouse thigh 2 h prior to the initiation of antimicrobial therapy.
PK studies and dosing regimen determination.
PK studies were performed to find a meropenem regimen in mice that simulated the PK profile observed for healthy adults receiving meropenem at 1 g every 8 h. These regimens were designed to mimic the free drug percent time above the MIC (fT>MIC) observed for humans over a wide range of MICs. The free drug concentration-time profile in humans was derived from the work of Dreetz et al., and the protein binding of 8% in this study was considered to be equivalent for both humans and mice (7, 21). Previously defined meropenem PK parameters for mice (26) were entered into WinNonLin (version 3.3; Pharsight, Mountain View, CA) to determine the approximate dosing frequency to best approximate the human PK profile. Based on these PK parameters, a resultant dosing regimen of 65, 30, 20, and 5 mg/kg of body weight at 0, 1.5, 3, and 5 h in three 8-h intervals over 24 h best approximated the human fT>MIC over a wide MIC range. Prior to the efficacy studies, confirmation of these exposures was performed with a separate group of infected mice.
Meropenem serum samples were analyzed by a validated high-performance liquid chromatography (HPLC) method as previously described (9). The meropenem HPLC assay was linear (r = 0.998) over a concentration range of 0.25 to 40.0 μg/ml. The intraday quality control samples (n = 10) of 0.5 and 30.0 μg/ml had percents coefficient of variation of 4.39% and 3.77%, respectively. The interday quality control samples (n = 6) of 0.5 and 30.0 μg/ml had percents coefficient of variation of 3.75% and 7.44%, respectively.
With regards to ertapenem, previous work from our laboratory determined that 50 mg/kg of body weight every 6 h for mice achieved a concentration-time profile and fT>MIC similar to those observed following ertapenem at 1 g every 24 h for humans (33). Similar to meropenem, a more complex regimen, which closely mimicked the concentration-time profile following the administration of ertapenem at 1 g every 24 h for humans, was investigated. However, since no difference in efficacy was observed between the simplified and complex regimens, the regimen of 50 mg/kg of body weight every 6 h was utilized in this analysis (6). The ertapenem free drug concentration-time profile was derived from the work of Musson et al. (24) and was summarized by Nix et al. (25). An ertapenem protein binding of 95.5% in mice, determined by Xuan et al. (33), was used to determine fT>MICs at various MICs.
Treatment regimens.
Human PK-simulated regimens of meropenem and ertapenem in mice were administered as 0.2-ml subcutaneous injections and studied for each pathogen in groups of three mice over a 24-h period. Control animals received sterile normal saline subcutaneously at the same volume (0.2 ml) and schedule used in the active drug regimen. Untreated control mice (three per group) were sacrificed just prior to antibiotic initiation (0 h) and after 24 h. After the 24-h treatment period, all animals were euthanized by CO2 exposure followed by cervical dislocation.
Efficacy as assessed by bacterial density.
After sacrifice, the thighs were removed and individually homogenized in normal saline. Serial dilutions were plated on Trypticase soy agar with 5% sheep blood for CFU determination. Efficacy (change in bacterial density) was calculated as the log10 change in bacterial CFU/thigh obtained for the meropenem- or ertapenem-treated mice after 24 h from the preantibiotic CFU/thigh measured for the 0-h control animals.
RESULTS
In vitro susceptibility testing.
Meropenem and ertapenem MICs for the study isolates are displayed in Table 1. Against the 31 ESBL isolates, meropenem displayed greater in vitro potency, with a median MIC of 0.125 μg/ml, than did ertapenem, with a median MIC of 0.5 μg/ml. The MICs for the three isolates that were tested at a higher inoculum of 107, E. coli isolates 315, 321, and 322, were ≥64-fold higher than those at the standard inoculum. Meropenem MICs rose from 0.032, 0.032, and 0.094 μg/ml to 4, 4, and 8 μg/ml, respectively, for these three isolates. Likewise, ertapenem MICs rose from 0.012, 0.012, and 0.032 μg/ml to 1, 1, and 2 μg/ml, respectively.
TABLE 1.
MICs of ESBL and non-ESBL test isolates at standard inoculum with corresponding fT>MICs
| Isolate | Value for:
|
|||
|---|---|---|---|---|
| Meropenem
|
Ertapenem
|
|||
| MIC (μg/ml)a | fT>MIC (%)b | MIC (μg/ml)a | fT>MIC (%)b | |
| ATCC 25922c | 0.03 | 93 | 0.008 | 93 |
| E. coli 120c | 0.03 | 93 | 0.03 | 77 |
| E. coli 320 | 0.03 | 93 | 0.5 | 40 |
| E. coli 321 | 0.032 | 93 | 0.012 | 90 |
| E. coli 322 | 0.032 | 93 | 0.012 | 90 |
| E. coli 310 | 0.047 | 90 | 0.064 | 67 |
| E. coli 312 | 0.047 | 90 | 0.19 | 53 |
| E. coli 118 | 0.047 | 90 | 0.012 | 90 |
| E. coli 243d | 0.047 | 90 | 0.064 | 67 |
| E. coli 285d | 0.047 | 90 | 0.023 | 80 |
| K. pneumoniae 225 | 0.047 | 90 | 0.016 | 87 |
| K. pneumoniae 60 | 0.064 | 88 | 0.012 | 90 |
| E. coli 315 | 0.094 | 85 | 0.032 | 77 |
| E. coli 133 | 0.094 | 85 | 1.5 | 23 |
| K. pneumoniae 101 | 0.094 | 85 | 0.094 | 60 |
| K. pneumoniae 230 | 0.094 | 85 | 0.094 | 60 |
| E. coli 130 | 0.125 | 85 | 1 | 30 |
| K. pneumoniae 265 | 0.125 | 85 | 0.5 | 40 |
| K. pneumoniae 268d | 0.125 | 85 | 0.25 | 47 |
| K. pneumoniae 270 | 0.25 | 80 | 0.5 | 40 |
| K. pneumoniae 260 | 0.5 | 75 | 1 | 30 |
| K. pneumoniae 264 | 0.5 | 75 | 4 | 10 |
| E. coli 311d | 0.75 | 70 | 4 | 10 |
| K. pneumoniae 255e | 1 | 65 | 2 | 20 |
| K. pneumoniae 254d | 2 | 58 | 16 | 0 |
| K. pneumoniae 259e | 2 | 58 | 16 | 0 |
| K. pneumoniae 263e | 2 | 58 | 8 | 0 |
| K. pneumoniae 266e | 2 | 58 | 8 | 0 |
| K. pneumoniae 257e | 4 | 45 | 16 | 0 |
| K. pneumoniae 258e | 4 | 45 | 16 | 0 |
| K. pneumoniae 262e | 8 | 30 | 64 | 0 |
| K. pneumoniae 256d | 16 | 18 | 32 | 0 |
| K. pneumoniae 261d | 32 | 5 | 128 | 0 |
Median of ≥3 separate MIC experiments.
fT>MIC in mice.
Non-ESBL isolate.
Removed from analysis due to inability to establish infection.
For this isolate, there was a difference between meropenem and ertapenem efficacies.
PK determination.
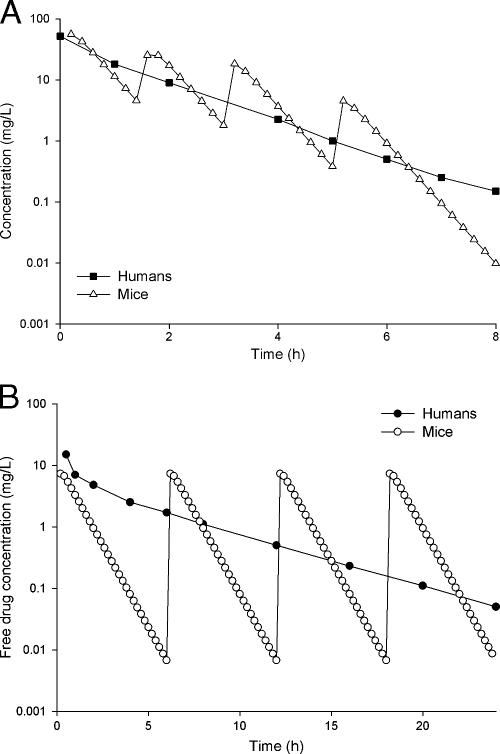
A comparison of the resultant concentration-time profiles achieved with mice for meropenem and ertapenem to those observed for humans is shown in Fig. 1. The meropenem free area under the curve (fAUC) obtained for mice was higher than that for humans; these values were 61.1 μg·hr/ml and 47.5 μg·hr/ml, respectively. However, as displayed in Table 2, the human and animal fT>MIC values agreed to within 8% over an MIC range of 0.5 to 8 μg/ml. Emphasis was placed on obtaining similar fT>MICs at MICs near the susceptibility breakpoint (i.e., 0.5 to 2 μg/ml for ertapenem and 1 to 4 μg/ml for meropenem). For ertapenem, the fAUC obtained for mice was 27.7 μg·hr/ml, which closely approximated the fAUC of 33.2 μg·hr/ml obtained for humans. The fT>MICs for the mouse and human dosing regimens agreed to within 10% at MICs of ≥0.5 μg/ml (Table 2).
FIG. 1.
Comparison of concentration-time profiles of meropenem and ertapenem in healthy human volunteers and mice. (A) Meropenem dosing in mice involved three cycles of 60, 30, 20, and 5 mg/kg administered at 0, 1.5, 3, and 5 h every 8 h, and the human regimen represents meropenem 1 g every 8 h. (B) Ertapenem dosing in mice was 50 mg/kg every 6 h, and the human regimen represents 1 g every 24 h.
TABLE 2.
Comparison of fT>MICs for humans and mice
Efficacy as assessed by bacterial density.
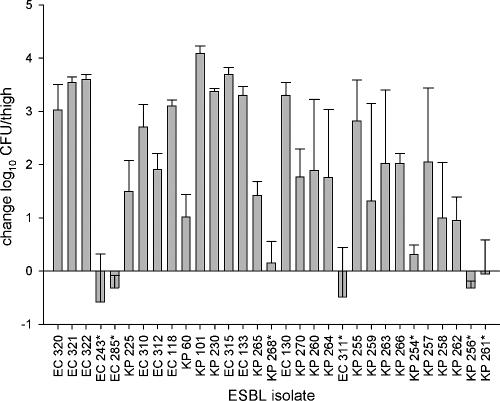
The numbers of organisms recovered from the thighs of infected animals serving as 0-h controls (immediately prior to antibiotic treatment) ranged from 4.52 to 5.98 log10 CFU/thigh with a mean ± standard deviation of 5.42 ± 0.29 log10 CFU/thigh. The bacterial densities in the 24-h-growth control animals are displayed in Fig. 2. In 7 of the 31 ESBL isolates tested, infection did not establish well, as evidenced by either a slight mean growth (≤0.31 log10 CFU/thigh) or a decrease in log10 CFU/thigh at 24 h despite the administration of saline instead of antibiotic. Therefore, these nonvirulent isolates were removed from the efficacy analysis. These isolates had a wide MIC range of 0.047 to 32 μg/ml for meropenem and 0.023 to 128 μg/ml for ertapenem. In general, a 1- to 4-log increase in CFU/thigh was observed at 24 h for the remaining 24 ESBL isolates (Fig. 2). The numbers of organisms recovered at 24 h ranged from 6.20 to 9.76 log10 CFU/thigh, with a mean ± standard deviation of 7.87 ± 0.52 log10 CFU/thigh. For isolates ATCC 25922 and E. coli 118, animals in the 24-h-growth control groups were harvested at 8 and 18 h, respectively, because the virulence of the established infection precluded their survival over the entire 24-h period. Animals infected with these organisms that received either meropenem or ertapenem survived to the completion of the study.
FIG. 2.
Bacterial growth (measured as density) for the change in log10 CFU/thigh for 24-h-growth control mice compared to that for 0-h control mice for each ESBL isolate tested (n = 31). Each bar represents the mean ± standard deviation of between three and six mice. Isolates marked with asterisks represent inadequately established infections. Mice infected with E. coli 118 were processed at 18 h. EC, E. coli; KP, K. pneumoniae.
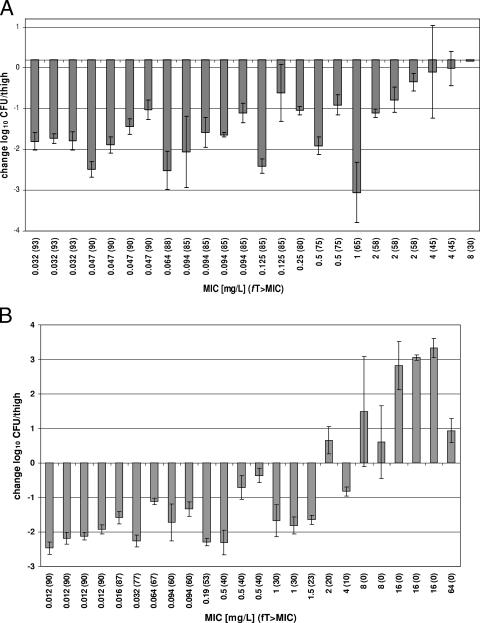
The abilities of meropenem and ertapenem to eradicate ESBL-producing E. coli and K. pneumoniae strains are displayed in Fig. 3. In general, both antibiotics display decreases in organisms at 24 h of between 1 and 3 log10 for the majority of the isolates tested. For each ESBL isolate examined, encompassing a wide MIC range, meropenem displayed either decreased or static activity. Bacteriostasis (−0.04-log kill) was observed even for K. pneumoniae 262 (MIC = 8 μg/ml), which is considered intermediate according to CLSI-defined breakpoints. On the other hand, ertapenem resulted in bacterial kill up to an MIC of 1.5 μg/ml, above which bacteria grew between approximately 0.5 to 3.5 logs. Ertapenem, however, did show efficacy against K. pneumoniae 264, with an MIC of 4 μg/ml, which is defined as intermediate by CLSI susceptibility breakpoints.
FIG. 3.
Change in log10 CFU/thigh measured in density for meropenem (A) and ertapenem (B) at 24 h compared to 0-h controls.
No difference in bacterial kill was observed for either carbapenem for the majority of the ESBL isolates tested, because the MICs were low (≤0.5 μg/ml for meropenem; ≤1.5 μg/ml for ertapenem). This corresponded to fT>MICs of ≥75% and ≥23% for meropenem and ertapenem, respectively. For seven of the eight ESBL isolates with ertapenem MICs of ≥2 μg/ml, greater bacterial kill was observed in the mice given meropenem. This is most likely due to the two- to eightfold-greater in vitro potency of meropenem, which resulted in an fT>MIC of 30 to 65%, compared with the ertapenem fT>MIC of ≤20%.
For the two non-ESBL isolates tested, ATCC 25922 and E. coli 120, an approximate 2-log decrease in CFU/thigh was observed for both meropenem and ertapenem. Because of the low MICs of these organisms, the fT>MIC was high (77 to 93%). These isolates showed kills similar to those of ESBL isolates with similar MICs.
For the three isolates (E. coli 315, E. coli 321, and E. coli 322) that were tested at a 100-fold-higher inoculum (107), the bacterial density for the 0-h control group was 7.40 ± 0.08 log10 CFU/thigh. Unlike at the standard 105 inoculum, the 24-h control group did not grow well, with a mean difference of −0.08 ± 0.33 log10 CFU/thigh. Despite the higher MICs at this inoculum, which led to corresponding fT>MICs of 30 to 45% and 20 to 30% for meropenem and ertapenem, respectively, approximately 2- to 3.5-log decreases in bacteria were observed at 24 h. No differences in efficacy were observed between meropenem and ertapenem.
DISCUSSION
Due to the multidrug-resistant nature of ESBL-producing organisms, carbapenems have remained the drugs of choice for the treatment of infections with these organisms. However, at present, it is not clear if there are inherent differences in efficacy among the different carbapenems. The addition of ertapenem to the antibiotic armamentarium presents an interesting alterative to the older agents, meropenem and imipenem, because of its once-daily dosing compared to the three- to four-times-daily dosing of the older agents. Original in vitro studies showed that there might be a potential benefit to ertapenem against ESBL-producing organisms because of a lower MIC90 (12, 13, 16, 18). However, more-recent in vitro work has demonstrated that ertapenem may be less stable to hydrolysis than meropenem and imipenem, as the MIC90 rose from ≤0.016 to 0.25 μg/ml when ESBLs were present (15).
For the β-lactams, the pharmacodynamic parameter that correlates best with antimicrobial efficacy is the fT>MIC (3). For carbapenems, the breakpoint that correlates best with bacteriostasis is an fT>MIC of 20%, where maximal bactericidal activity is ≥40% (8, 22, 31, 31a). Recent animal work has demonstrated that no alternative pharmacodynamic breakpoints are needed to ensure efficacy against ESBL-producing isolates (1, 19). In other words, even if the MICs increase for ESBL-producing isolates, as long as the antimicrobial regimen is able to exceed the critical fT>MIC, efficacy remains.
The results of this analysis are in agreement with what has been observed previously. First, the in vitro potency observed for meropenem was approximately fourfold greater than that for ertapenem for the same 31 ESBL test isolates. Even though 7 isolates were removed from the efficacy analysis because of their inability to establish infection, these isolates had a wide range of MICs, and the median MIC of the 24 remaining isolates did not change. Second, since the majority of the ESBL isolates tested had low MICs and correspondingly high fT>MICs, bacterial kill was observed for both meropenem and ertapenem. Third, differences were not observed between these two agents until isolates had an ertapenem MIC of ≥2 μg/ml, which corresponds to an fT>MIC of ≤20%. An MIC of 2 μg/ml is the highest value deemed susceptible according to the CLSI-defined susceptibility breakpoints. The greater meropenem in vitro potency for these isolates yielded much higher fT>MICs of 30 to 65% and appears to explain its increased efficacy. It should be noted that one exception did exist with ESBL isolate K. pneumoniae 264, which demonstrated similar efficacies for meropenem and ertapenem, even though the fT>MICs were 75% and 10%, respectively. Fourth, regrowth of bacteria was observed in animals treated with ertapenem at higher MICs; however, meropenem showed continued efficacy against all isolates. This may simply be a result of the greater in vitro potency and higher fT>MICs observed for meropenem. Even for isolate K. pneumoniae 262, which had the highest MIC to meropenem, 8 μg/ml, the resultant fT>MIC of 30% would predict efficacy, and bacteriostasis was observed. Because of the difficulty in finding ESBL isolates with high MICs to meropenem, a more adequate examination of the efficacy of meropenem at lower fT>MICs could not be performed. Fifth, bacterial kill was still observed among the three ESBL isolates tested at the higher 107 inoculum, although the MICs rose ≥64-fold. The achievement of adequate fT>MICs of 30 to 45% for meropenem and 20 to 30% for ertapenem, respectively, is the most likely explanation for these findings. Alternatively, since the raised MICs at a higher inoculum have been described as an artifact of in vitro susceptibility testing, they may not be clinically relevant (4, 19, 20).
In conclusion, as a result of the high potencies of meropenem and ertapenem, these carbapenems demonstrated comparable efficacies against the majority of the ESBL-producing E. coli and K. pneumoniae isolates tested. Meropenem appears to yield greater efficacy against isolates with ertapenem MICs of ≥2 μg/ml. This is most likely due to the two- to eightfold-greater in vitro potency and higher fT>MIC of meropenem for these isolates. Further analysis using patients will be necessary to determine if differences in outcomes exist between these carbapenems for ESBL isolates with MICs of ≥2 μg/ml.
Acknowledgments
We thank Ron Jones (The Jones Group/JMI Laboratories, Iowa) and Carlos Kiffer (Fleury - Centro de Medicina Diagnostica, Sao Paulo, Brazil) for sending us many of the isolates tested in this analysis. We also thank Christina Sutherland, Lindsay Tuttle, Hank Christensen, Debora Santini, Ioannis Kioumis, and Pamela Tessier for their assistance with the animal studies and HPLC analysis.
This work was supported by an unrestricted educational grant from Astra Zeneca Pharmaceuticals, Wilmington, DE.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2001. Impact of extended-spectrum beta-lactamase production on the activity of cefepime in a murine-thigh infection model, abstr. 1099. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 2.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A., S. M. Bhavnani, and P. G. Ambrose. 2004. The inoculum effect: fact or artifact? Diagn. Microbiol. Infect. Dis. 50:229-230. [DOI] [PubMed] [Google Scholar]
- 5.Dandekar, P. K., R. Quintiliani, C. H. Nightingale, and D. P. Nicolau. 2002. Extended-spectrum beta-lactamases (ESBL). Conn. Med. 66:13-15. [PubMed] [Google Scholar]
- 6.DeRyke, C. A., and D. P. Nicolau. 2007. Is all free time above the minimum inhibitory concentration the same: implications for beta-lactam in vivo modeling. Int. J. Antimicrob. Agents 29:341-343. [DOI] [PubMed] [Google Scholar]
- 7.Dreetz, M., J. Hamacher, J. Eller, K. Borner, P. Koeppe, T. Schaberg, and H. Lode. 1996. Serum bactericidal activities and comparative pharmacokinetics of meropenem and imipenem-cilastatin. Antimicrob. Agents Chemother. 40:105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drusano, G. L. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36:S42-S50. [DOI] [PubMed] [Google Scholar]
- 9.Elkhaili, H., S. Niedergang, D. Pompei, L. Linger, D. Leveque, and F. Jehl. 1996. High-performance liquid chromatographic assay for meropenem in serum. J. Chromatogr. B 686:19-26. [DOI] [PubMed] [Google Scholar]
- 10.Goossens, H., and B. Grabein. 2005. Prevalence and antimicrobial susceptibility data for extended-spectrum beta-lactamase- and AmpC-producing Enterobacteriaceae from the MYSTIC program in Europe and the United States (1997-2004). Diagn. Microbiol. Infect. Dis. 53:257-264. [DOI] [PubMed] [Google Scholar]
- 11.Joly-Guillou, M. L., M. Wolff, J. J. Pocidalo, F. Walker, and C. Carbon. 1997. Use of a new mouse model of Acinetobacter baumannii pneumonia to evaluate the postantibiotic effect of imipenem. Antimicrob. Agents Chemother. 41:345-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, R. N., D. J. Biedenbach, and A. C. Gales. 2003. Sustained activity and spectrum of selected extended-spectrum beta-lactams (carbapenems and cefepime) against Enterobacter spp. and ESBL-producing Klebsiella spp.: report from the SENTRY antimicrobial surveillance program (USA, 1997-2000). Int. J. Antimicrob. Agents 21:1-7. [DOI] [PubMed] [Google Scholar]
- 13.Jones, R. N., and M. A. Pfaller. 2003. Antimicrobial activity against strains of Escherichia coli and Klebsiella spp. with resistance phenotypes consistent with an extended-spectrum beta-lactamase in Europe. Clin. Microbiol. Infect. 9:708-712. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., M. A. Pfaller, G. V. Doern, M. E. Erwin, R. J. Hollis, et al. 1998. Antimicrobial activity and spectrum investigation of eight broad-spectrum beta-lactam drugs: a 1997 surveillance trial in 102 medical centers in the United States. Diagn. Microbiol. Infect. Dis. 30:215-228. [DOI] [PubMed] [Google Scholar]
- 15.Jones, R. N., H. S. Sader, and T. R. Fritsche. 2005. Comparative activity of doripenem and three other carbapenems tested against Gram-negative bacilli with various beta-lactamase resistance mechanisms. Diagn. Microbiol. Infect. Dis. 52:71-74. [DOI] [PubMed] [Google Scholar]
- 16.Kohler, J., K. L. Dorso, K. Young, G. G. Hammond, H. Rosen, H. Kropp, and L. L. Silver. 1999. In vitro activities of the potent, broad-spectrum carbapenem MK-0826 (L-749,345) against broad-spectrum β-lactamase- and extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 43:1170-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lautenbach, E., J. B. Patel, W. B. Bilker, P. H. Edelstein, and N. O. Fishman. 2001. Extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin. Infect. Dis. 32:1162-1171. [DOI] [PubMed] [Google Scholar]
- 18.Livermore, D. M., K. J. Oakton, M. W. Carter, and M. Warner. 2001. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent beta-lactamases. Antimicrob. Agents Chemother. 45:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maglio, D., M. A. Banevicius, C. Sutherland, C. Babalola, C. H. Nightingale, and D. P. Nicolau. 2005. Pharmacodynamic profile of ertapenem against Klebsiella pneumoniae and Escherichia coli in a murine thigh model. Antimicrob. Agents Chemother. 49:276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maglio, D., C. Ong, M. A. Banevicius, Q. Geng, C. H. Nightingale, and D. P. Nicolau. 2004. Determination of the in vivo pharmacodynamic profile of cefepime against extended-spectrum-beta-lactamase-producing Escherichia coli at various inocula. Antimicrob. Agents Chemother. 48:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattie, H., L. C. Zhang, E. van Strijen, B. R. Sekh, and A. E. Douwes-Idema. 1997. Pharmacokinetic and pharmacodynamic models of the antistaphylococcal effects of meropenem and cloxacillin in vitro and in experimental infection. Antimicrob. Agents Chemother. 41:2083-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattoes, H. M., J. L. Kuti, G. L. Drusano, and D. P. Nicolau. 2004. Optimizing antimicrobial pharmacodynamics: dosage strategies for meropenem. Clin. Ther. 26:1187-1198. [DOI] [PubMed] [Google Scholar]
- 23.Moczygemba, L. R., C. R. Frei, and D. S. Burgess. 2004. Pharmacodynamic modeling of carbapenems and fluoroquinolones against bacteria that produce extended-spectrum beta-lactamases. Clin. Ther. 26:1800-1807. [DOI] [PubMed] [Google Scholar]
- 24.Musson, D. G., A. Majumdar, S. Holland, K. Birk, L. Xi, G. Mistry, D. Sciberras, J. Muckow, P. Deutsch, and J. D. Rogers. 2004. Pharmacokinetics of total and unbound ertapenem in healthy elderly subjects. Antimicrob. Agents Chemother. 48:521-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nix, D. E., A. K. Majumdar, and M. J. DiNubile. 2004. Pharmacokinetics and pharmacodynamics of ertapenem: an overview for clinicians. J. Antimicrob. Chemother. 53(Suppl. 2):ii23-ii28. [DOI] [PubMed] [Google Scholar]
- 26.Ong, C. T., P. R. Tessier, C. Li, C. H. Nightingale, and D. P. Nicolau. 2007. Comparative in vivo efficacy of meropenem, imipenem, and cefepime against Pseudomonas aeruginosa expressing MexA-MexB-OprM efflux pumps. Diagn. Microbiol. Infect. Dis. 57:153-161. [DOI] [PubMed] [Google Scholar]
- 27.Paterson, D. L., W. C. Ko, A. Von Gottberg, S. Mohapatra, J. M. Casellas, H. Goossens, L. Mulazimoglu, G. Trenholme, K. P. Klugman, R. A. Bonomo, L. B. Rice, M. M. Wagener, J. G. McCormack, and V. L. Yu. 2004. Antibiotic therapy for Klebsiella pneumoniae bacteremia: implications of production of extended-spectrum beta-lactamases. Clin. Infect. Dis. 39:31-37. [DOI] [PubMed] [Google Scholar]
- 28.Ramphal, R., and P. G. Ambrose. 2006. Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin. Infect. Dis. 42(Suppl. 4):S164-S172. [DOI] [PubMed] [Google Scholar]
- 29.Rhomberg, P. R., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2005. Comparative antimicrobial potency of meropenem tested against Gram-negative bacilli: report from the MYSTIC surveillance program in the United States (2004). J. Chemother. 17:459-469. [DOI] [PubMed] [Google Scholar]
- 30.Schwaber, M. J., S. Navon-Venezia, K. S. Kaye, R. Ben-Ami, D. Schwartz, and Y. Carmeli. 2006. Clinical and economic impact of bacteremia with extended-spectrum-beta-lactamase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 50:1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 31a.Walker, R., D. Andes, J. Conklin, S. Ebert, and W. A. Craig. 1994. Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A91.
- 32.Wexler, H. M. 2004. In vitro activity of ertapenem: review of recent studies. J. Antimicrob. Chemother. 53(Suppl. 2):ii11-ii21. [DOI] [PubMed] [Google Scholar]
- 33.Xuan, D., M. Banevicius, B. Capitano, M. K. Kim, C. Nightingale, and D. Nicolau. 2002. Pharmacodynamic assessment of ertapenem (MK-0826) against Streptococcus pneumoniae in a murine neutropenic thigh infection model. Antimicrob. Agents Chemother. 46:2990-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]