Abstract
Neonatal marmosets express an adrenal fetal zone comparable to humans. While adult males fail to express a functional ZR, with barely detectable blood DHEA levels, females produce higher levels of DHEA than males in adulthood. We investigated the presence of a putative functional ZR in adult female marmosets. In contrast to males, immunohistochemical analysis showed the ZR marker cytochrome b5 was elevated in the innermost zone in cycling females (compared to testis-intact males), further elevated in the adrenals from anovulatory females, and substantially elevated and continuous in ovariectomized females. As a functional test in vivo, following overnight dexamethasone treatment, cycling and anovulatory females showed higher levels of DHEA relative to males, but DHEA failed to increase in response to ACTH. In direct contrast, while ovariectomized females exhibited lower initial DHEA levels, clear increases were detectable after ACTH administration (p<0.05), suggesting an adrenal origin. The apparent differences in cytochrome b5 expression between groups were also further verified by western blotting of adrenal microsomes, and compared to 17,20-lyase activity; the two parameters were positively correlated (p<0.01) across multiple treatment groups. We conclude that the cycling female marmoset expresses a rudimentary ZR with at least a capacity for DHEA production that becomes significantly ACTH-responsive after anovulation. Expression of cytochrome b5 in this region may be directly or indirectly controlled by gonadal function, and is, at least in part, a critical determinant in the development of an adrenal ZR that is more defined and significantly ACTH-responsive.
Keywords: Adrenal, Marmoset, CYP17, Cytochrome b5, Zona Reticularis, Female, DHEA
Introduction
The human adrenal cortex is delineated into three zones, the zona glomerulosa (ZG), zona fasciculata (ZF), and zona reticularis (ZR). Aldosterone production in the ZG is regulated primarily by angiotensin II and marginally by adrenocorticotropin (ACTH) (Lebel & Gross 1976; Young et al. 2003). Cortisol secretion in the ZF occurs primarily in response to ACTH, rather than angiotensin II (Bird et al. 1998; Lebel & Gross 1976; Young et al. 2003). In the ZR, ACTH stimulates production of C19 steroids, namely dehydroepiandrosterone (DHEA) in sulphated form (DHEAS; reviewed in Conley & Bird 1997; Hyatt et al. 1983). Other factors, however, are also involved in the regulation of C19 steroid production by the ZR, which declines steadily with advancing age (reviewed in Conley et al. 2004).
Discrete adrenal zonation results from the differential expression of key steroidogenic enzymes and accessory proteins. Aldosterone production in the ZG occurs because these cells lack 17α-hydroxylase/17,20-lyase cytochrome P450 (CYP17) (Fig. 1). Cortisol synthesis in the ZF requires co-expression of both CYP17 and 3β-hydroxysteroid dehydrogenase (3β-HSD), with the ratio of the two proteins determining the amount of cortisol produced without significant amounts of DHEA (reviewed in Conley & Bird 1997). In the ZR, DHEA is produced by CYP17 under conditions of relatively low co-expression of 3β-HSD and high expression of cytochrome b5 (cytb5) (Auchus 2004, Conley & Bird 1997, Conley et al. 2004). Additionally, CYP17, and all other microsomal cytochromes P450, require an obligate electron donor in the form of NADPH cytochrome P450 oxido-reductase (POR) (Conley & Bird 1997). Therefore, high DHEA production in the adrenal ZR by CYP17 also requires sufficient POR expression in addition to high expression of cytb5 and low expression of 3β-HSD. Finally, a number of studies suggest cross-talk occurs between the gonads and the adrenal, indicating a possible role for gonadal hormones in control of adrenal function (reviewed in Conley & Bird 1997; Bielinska et al. 2006).
Figure 1. The adrenocortical steroid biosynthesis pathway in human and nonhuman primates.
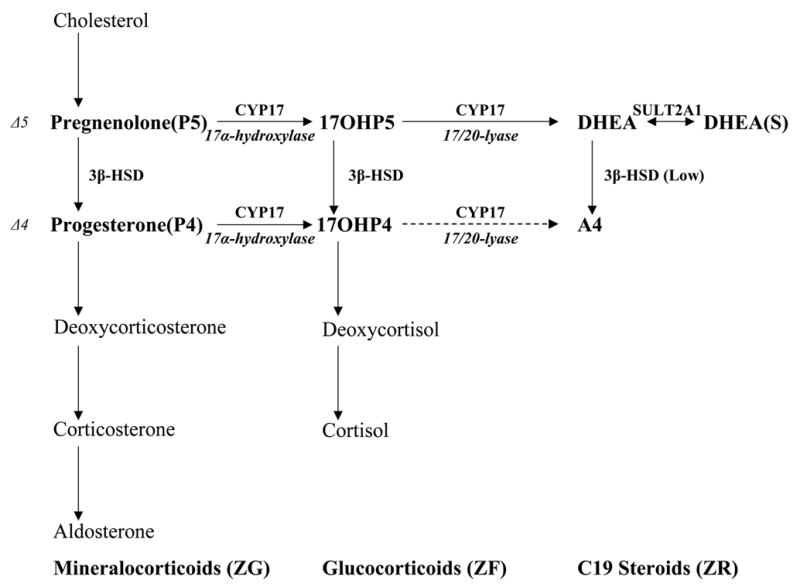
Cortisol is predominantly synthesized through the Δ-5 pathway [pregnenolone (P5) converted to 17alpha-hydroxypregnenolone (17OHP5) converted to 17alpha-hydroxyprogesterone (17OHP4) converted to 11-deoxycortisol converted to cortisol]. High dehydroepiandrosterone (DHEA) production requires metabolism of P5 through the Δ-5 pathway, in the face of low 3beta-hydroxysteroid dehydrogenase (3β-HSD) expression and high cytochrome b5 (cytb5) and cytochrome P450 oxido-reductase (POR) expression. 17alpha-hydroxylase/17,20-lyase cytochrome P450 (CYP17) and 3β-HSD are critically positioned for the biosynthesis of 17OHP5, DHEA, 17OHP4, and androstenedione (A4). These same enzymes are also critically positioned for the biosynthesis of cortisol. Sulfotransferase (SULT2A1).
The fetal zone of the human and nonhuman primate fetal adrenal gland contains comparatively low 3β-HSD and substantial cytb5 (Dharia et al. 2004, Narasaka et al. 2001). Circulating DHEAS levels are correspondingly high (2000–7000 nmol/L; Havelock et al. 2004) and fetal DHEA/S is a necessary precursor for placental estrogen production (Conley et al. 2004, Rainey et al. 2004). DHEAS levels peak at birth (~7000 nmol/L; Havelock et al. 2004) and decline within a few months, due to the regression of the fetal zone (Conley et al. 2004, Havelock et al. 2004, Levine et al. 1982, Rainey et al. 2004). At around 5–7 years of age in the human, DHEAS levels begin to rise (~50 μg/dL) in a process known as adrenarche, which marks the development of a functional ZR (Havelock et al. 2004). Recent evidence suggests that adrenarche is a continuous process beginning in utero or at birth (Remer et al. 2005). Once again, ZR development of DHEA production by CYP17 in the adrenal cortex is associated with both a reticularis-specific increase in cytb5 expression while 3β-HSD expression decreases (Endoh et al. 1996, Gell et al. 1996, 1998, Suzuki et al. 2000). Adrenarche precedes puberty, and the two processes are thought to be independent. Nonetheless, throughout puberty and into the third decade of life, the size of the ZR increases. After the age of 25 in humans, DHEAS levels begin a gradual, continuous decline (peak at 250–350 μg/dL) that is associated with a decrease in ZR thickness (Conley et al. 2004).
The common marmoset (Callithrix jacchus), a New World monkey, is increasingly used as a model in studies of stress, reproductive biology, and the hypothalamic-pituitary-gonad/adrenal axes (Abbott et al. 2003). Marmosets differ from most other primates in that they form social groups, both in the wild and in captivity, in which, typically, only a single, socially dominant male and female breed (Abbott et al. 1997, Saltzman 2003). Subordinate animals of both sexes generally do not reproduce, but rather help in rearing the offspring of their dominant groupmates. Reproductive suppression in subordinate males is mediated largely by inhibition of sexual behavior (Abbott 1993, Baker et al. 1999), whereas subordinate females become anovulatory and hypoestrogenemic in response to social cues (Abbott et al. 1997). In addition, anovulatory subordinate females in laboratory groups have markedly lower basal cortisol levels than ovary-intact dominants or ovariectomized females (Baker et al. 1999, Johnson et al. 1996, Saltzman et al. 1994, 1998, 2004).
In spite of the increasing biomedical use of marmosets, until recently there has been little characterization of the marmoset adrenal gland. Levine and colleagues performed in vivo analyses on male and female marmosets from the neonatal period to puberty (18 months) (Levine et al. 1982) and found evidence indicating that marmosets have a large, functional fetal zone at birth (DHEA 1300–3100 ng/dL) that regresses within 3 months (DHEA 40 ng/dL), but fail to develop a prominent ZR upon reaching sexual maturity (DHEA 40 ng/dL in males). Immunohistochemical procedures were not widely available at the time of Levine’s study, and identification of a fetal zone and ZR was based on hematoxylin & eosin staining. Since then, marmosets have been shown by immunohistochemistry for CYP17, cytb5 and 3β-HSD to express a clearly delineated fetal zone with prominent cytb5 expression. Nonetheless, inner zone cytb5 protein expression is virtually undetectable in adult male marmosets. Combined with in vivo analysis of ACTH action on adrenal steroid production, this confirmed the lack of a prominent ZR (Pattison et al. 2005) as Levine first reported. Levine et al (1982) also reported that ovary-intact female marmosets had higher circulating DHEA levels than males upon reaching maturity (800 ng/dL), and attempts to use traditional stains to identify a morphological ZR gave inconclusive results. In this study, we show a distinct sex difference in expression of an adrenal ZR in adult marmosets: ovary-intact females exhibit a rudimentary ZR by cytb5 expression, in contrast to our recent findings that testis-intact males do not (Pattison et al. 2005). Herein we further show that social subordination accompanied by ovulation suppression, or alternatively ovariectomy, results in an increase in cytb5 expression in the ZR, and gonadectomy reveals significant ACTH-responsive DHEA production in female marmosets. Thus we report for the first time, to our knowledge, gonadal regulation of adreno-cortical differentiation and function in this nonhuman primate.
Materials and Methods
Materials
All materials and reagents were obtained from Sigma Aldrich (St. Louis, MO) or Fisher Scientific International (Pittsburgh, PA) unless otherwise noted.
Animals
This research was conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act and its subsequent amendments. All animal procedures were reviewed and approved by the Graduate School Animal Care and Use Committee of the University of Wisconsin–Madison. The National Primate Research Center at the University of Wisconsin–Madison (WPRC) is accredited by AAALAC as part of the UW-Madison Graduate School.
Establishment of social groups
Gonadectomized male and female marmosets were pair-housed with an opposite-sexed adult. Dominant and subordinate female marmosets were housed in groups containing 2–3 unrelated females and 1–2 gonadally intact adult males. Social groups were formed as described previously (Saltzman et al. 1998) at least 2 months prior to data collection. Dominance hierarchies in such groups are usually established within 1–2 weeks and may remain stable for several years or more (Abbott et al. 1998). Characterization of dominant and subordinate status, based on directionality of submissive behavior (Saltzman et al. 1996), was confirmed by the occurrence of ovulatory cycles in dominant females and anovulation in subordinate females, based on plasma progesterone levels in blood samples collected twice weekly (Saltzman et al. 1994). Subordinates had not ovulated for at least 15 weeks prior to data collection and had not exhibited elevated plasma progesterone concentrations (>10 ng/ml), characteristic of the luteal phase of the ovarian cycle, for at least 60 days.
Marmosets were housed indoors at WPRC, with lights on from 06h30 to 18h30, ambient temperature maintained at approximately 27ºC, and humidity at approximately 50%. Most of the animals occupied aluminum and wire mesh cages measuring 61 x 91 x 183 cm, 122 x 61 x 183 cm, or 61 x 61 x 183 cm; however, some were housed in larger rooms measuring 235 x 370 x 225 cm or 310 x 141 x 250 cm. Marmosets were fed Mazuri Callitrichid High Fiber Diet 5M16 (Purina Mills, St. Louis, MO) supplemented with fruit, cereal, nuts and miniature marshmallows. Animals were fed once daily between 12h30 and 14h30, and water was available ad lib. Additional information on marmoset housing and husbandry has been provided by Saltzman et al (1998).
Gonadectomy of female marmoset monkeys
Ovariectomy was performed by midline incision under Saffan anesthesia (8.1 mg alphaxalone:2.7 mg alphadolone acetate, IM; Pitman-Moore, Harefield, Uxbridge, Middlesex, UK), at least 6 months (range 7–16 months) prior to data collection.
Immunohistochemistry
Adrenals from adult female marmosets (n=6 dominant, aged 48–84 months; n=6 ovariectomized, aged 24–48 months; n=3 subordinate, aged 30–60 months) were fixed in 10% formalin, routinely processed, and paraffin embedded. Tissues were cut into 5μm sections and mounted on glass slides. All sections were prepared for antibody incubation as previously described (Pattison et al. 2005). The Vectastain Elite ABC Rabbit IgG Kit (Vector Laboratories, Inc., Burlingame, CA) was used for all antibodies detected. All sections were incubated with normal goat serum (1:200) for 60 min at room temperature to block nonspecific binding. They were then incubated with primary antibody diluted in buffer at room temperature for 60 min. The sections were incubated first in diluted secondary antibody (1:200) and then avidin-biotin-conjugated peroxidase for 40 min each at room temperature before exposure to 3,3’-diaminobenzidine (Vector Laboratories, Inc.) for 5 min, also at room temperature. Nuclei were counterstained blue with Harris modified hematoxylin (neat, 1 sec, rinsed 5 min in tap water); sections were then dehydrated in graded alcohols and clearing solution, and coverslipped with permanent mounting media. Antigens were detected using antisera raised against bovine CYP17 (1:750, rabbit polyclonal, Dr. A. J. Conley, Department of Population Health and Reproduction, University of California-Davis), human Type II 3β-HSD (1:400, rabbit polyclonal, Dr. J. I. Mason, Department of Clinical Biochemistry, University of Edinburgh, Edinburgh, Scotland, UK), rat POR (1:3000, rabbit polyclonal, Dr. A. J. Conley), and human cytb5 (1:3000, rabbit polyclonal, Dr. A. J. Conley). Anti-rabbit IgG serum (1:750–3000, Cell Signaling, Beverly, MA) was used as a control. Images were captured at 10X magnification and analyzed as previously described (Pattison et al. 2005).
Microsomal preparation and immunoblotting
Whole frozen adrenals from 6 adult testis-intact male marmosets (aged 60–132 months), 6 dominant, ovary-intact female marmosets (aged 24–132 months), 6 ovariectomized female marmosets (aged 72–132 months), and 4 ovary-intact, immature female rhesus monkeys (aged 0.5–13 months) were homogenized and microsomal fractions isolated as described previously (Pattison et al. 2005). Briefly, adrenals were homogenized directly in buffer [0.1 M KPO4 (pH 7.4), 20% glycerol, 5 mM β-mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride] at a ratio of 1 ml/0.1 g tissue. Samples were centrifuged at 1000 x g for 10 min at 4ºC to remove debris, and the supernatant was then centrifuged at 16000 x g for 10 min at 4ºC to isolate the mitochondrial pellet. The supernatant was further centrifuged at 100000 x g for 60 min at 4ºC under vacuum to isolate the microsomal fraction. Microsomal pellets were resuspended in lysis buffer containing 1 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate detergent and stored at −80ºC. Protein concentration was assessed by a bichinonic assay. Ten (10) micrograms of total microsomal protein were size-separated by lane on 16% SDS-PAGE (Bio-Rad Laboratories, Inc., Hercules, CA; 150 V constant, 1.5 h) and protein was transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc., 100 V, 1 h). Proteins were then immunoblotted with chicken antihuman CYP17 (1:10000, chicken polyclonal, Dr. A. J. Conley; chicken horseradish peroxidase-linked IgY secondary antibody 1:5000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and antihuman cytb5 (1:2000; donkey antirabbit horseradish peroxidase-linked IgG secondary antibody 1:10000, Amersham Biosciences, Piscataway, NJ), and detected by chemiluminescent reagents (ECL, Amersham Biosciences) as previously described (Pattison et al. 2005).
Marmoset adrenal microsomal 17,20-lyase activity assay
Aliquots of 50 μg of microsomal protein from the 6 testis-intact male, 6 dominant, ovary-intact female, and 6 ovariectomized female marmoset adrenals described above were tested for 17,20-lyase activity using a radiometric assay modified from Grigoryev et al (1999) as previously described (Moran et al. 2002). Infant female rhesus tissue was used as a positive control. Microsomal protein was added to a generating system and buffer, plus trilostane (10 μM), and 3.5 μM [3H]C21-17OHP5 (100,000 DPM) (generously provided by Drs. V.C. Njar and A.M. Brodie, University of Maryland-Baltimore) and 7μM cold 17OHP5 (Steraloids) in a final volume of 1 ml. To calculate extraction efficiency, the same volumes of [14C]acetic acid (50,000 cpm) (Perkin Elmer, Boston, MA) and [3H]C21-17OHP5 were added to assay tubes with buffer (as extraction controls to be analyzed with the assay) and compared to 5μl of [14C]acetic acid directly added to two scintillation vials (totals for calculations). All assay tubes were incubated for two hours in a 37ºC water bath. The reaction was stopped using ice-cold trichloroacetic acid (30%; 500 μl) and then vortexed. To extract the unmetabolized steroid substrate and organic metabolite, chloroform (2 ml) was added to each assay tube and then centrifuged at 2000 x g for 5 min at room temperature. One (1) ml of the aqueous phase was pipetted off and added to a 8.5%:0.85% charcoal:dextran mixture, then centrifuged at 2000 x g for 30 minutes at 4ºC. One (1) ml of each assay was counted in 10 ml of scintillation fluid. 17,20-Lyase activities were determined from [3H]acetic acid, converted to amount of substrate metabolized, and expressed as nmol [3H]17OHP5 metabolized per mg of microsomal protein per hour.
Steroid responses in vivo
Adrenocortical responses to a combined dexamethasone (dex) suppression/adrenocorticotropin (ACTH1-39) challenge were performed as previously described (Pattison et al. 2005). Briefly, female marmosets that were either socially dominant and undergoing ovulatory cycles (n=6; 34.9±2.5 months, 389±12 g; mean±SEM), socially subordinate and anovulatory (n=7, 27.2±1.3 months, 396±13 g), or ovariectomized and neither dominant nor subordinate to other females (n=6, 38.1±4.4 months, 412±13 g), as well as three males (45.9±9.7 months, 410±11 g) were injected with dex (5 mg/kg, i.m.; American Regent Laboratories, Shirley, NJ) at 1600h. The following morning the animals were injected at 0900–1000h with human ACTH1-39 (10 μg/kg, i.v.). Blood samples (0.2–0.6 ml) were taken at ~08h40 (20 min pre-ACTH1-39) and 60 min post-ACTH1-39 treatment. Samples were immediately placed on ice and centrifuged at 2000 rpm for 10 min, and the plasma fractions were aspirated and frozen at −20ºC until assayed.
Hormone assays
All plasma hormone concentrations were determined by RIAs that had been validated for use with marmoset plasma at the WPRC Assay Services laboratories. The cortisol, corticosterone, aldosterone, and DHEA assays were described previously (Pattison et al. 2005, Satlzman et al. 1994). The intra- and interassay coefficients of variation (CVs) for cortisol were 8.67% and 15.05%, respectively. Intra- and interassay CVs were 5.6% and 20.8% (low pool) and 2.2% and 14.7%, respectively, for aldosterone, 2.16% and 13.9%, respectively, for corticosterone, and 8.75% and 17.32% (low pool) and 4.28% and 11.59%, respectively, for DHEA. Corticosterone and DHEA assays were performed after celite chromatography. Assay detection limits, were DHEA, 0.5 ng/ml; corticosterone, 0.35 ng/ml; cortisol, 1ng/ml; aldosterone, 25 pg/ml.
Statistics
All values are expressed as mean±SEM, unless otherwise stated. Analyses of in vivo steroid levels were performed on values corrected for baseline steroid measurements. In vivo steroid levels were compared by one-way ANOVA followed by post-hoc univariate F tests, and both unpaired and paired Student’s t-test, where appropriate. Densitometry on immunoblot bands, as well as regression analysis between cytb5 protein expression by immunoblot and 17,20-lyase activity by radiometric assay, was performed by one-way ANOVA followed by post-hoc univariate F tests. The significance level was set at p<0.05.
Results
Immunohistochemistry localization of steroidogenic enzymes in female marmoset adrenals
While CYP17 was undetectable in the ZG, as expected (Figure 2), it was clearly present throughout the ZF and “zona reticularis”, up to the cortico-medullary junction, in dominant, ovariectomized and subordinate female adrenal sections. Cytochrome P450 oxido-reductase (POR) was present throughout all regions of the adrenal cortex. Expression of 3β-HSD in dominant females (Fig. 2A) was observed throughout the ZF and “zona reticularis”, up to the cortico-medullary junction, but in subordinate (Fig. 2B) and ovariectomized females (Fig. 2C) 3β-HSD expression was often diminished in the innermost “zona reticularis” region. Cytochrome b5 staining revealed immunopositive cells in all three groups of females in the “zona reticularis” region, but the intensity and width of staining was clearly more pronounced in ovariectomized females than in ovary-intact subordinate females, and staining in both ovariectomized and subordinate females was more pronounced than in ovary-intact dominant females. The cytb5 staining pattern in all three female groups contrasted with that previously shown in testis-intact adult males, in which cytb5 was not detectable in any region of the adrenal cortex (Pattison et al. 2005).
Figure 2. Immunohistochemical analysis of marmoset adrenal sections.
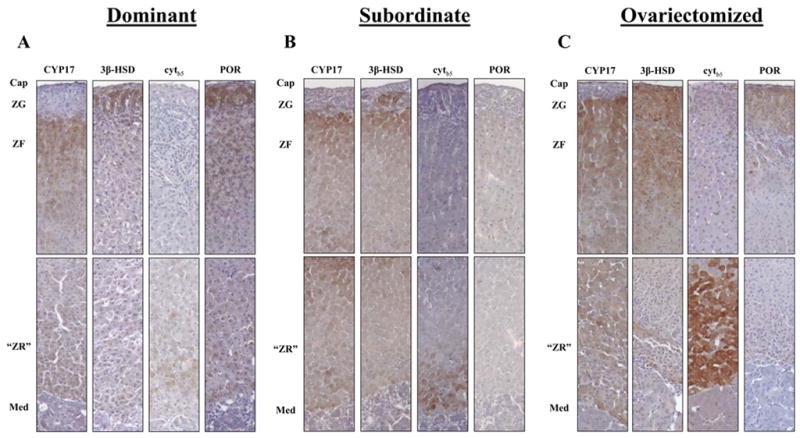
Primary antibody specificities are as labeled in each panel. Immunodetectable protein is indicated by brown stain while counterstain color is blue. (A) Dominant female (n=6; aged 24–48 months), (B) Subordinate female (n=3; aged 30–60 months), (C) Ovariectomized female (n=6; aged 48–84 months). Cap (capsule), med (medulla). 10X Magnification.
Plasma DHEA
In contrast to the previously reported barely detectable levels in intact males following dexamethasone suppression, circulating DHEA levels were clearly detectable in dominant females and similar to that in subordinate females. Administration of intravenous ACTH1-39 failed to yield a significant increase in circulating DHEA in dominant females or in subordinate females. While basal circulating DHEA levels were reduced by ovariectomy, consistent with an ovarian source suggested by Levine et al (1982), circulating DHEA was increased significantly by ACTH1-39 stimulation in ovariectomized females (p<0.05; Table 1). With regard to gender differences, our previous findings in testis-intact, adult males showed DHEA levels were not above the detection limit of the assay after dex treatment, either before or after ACTH1-39 injection (Pattison et al. 2005).
Table 1.
In vivo circulating DHEA response to a combined dex/ACTH1-39 challenge in marmosets.
Data were obtained from 6 dominant female (DomF; 34.9±2.5 months), 7 subordinate female (SubF; 27.2±1.3 months), 6 ovariectomized female (OvxF; 38.1±4.4 months), and 3 testis intact males (IM) (45.9±9.7 months) marmosets. Dex was administered at 17h00, blood was taken at 08h40 (pre-ACTH) the next morning, ACTH administered at 09h00, and blood taken at 10h00 (ACTH). Detection limit for DHEA was 0.50 ng/ml. Note the mean changes were calculated from individual animals, rather than from the mean pre- and post-ACTH values shown in the table.
| DHEA ng/ml
|
|||
|---|---|---|---|
| Pre-ACTH | ACTH | Mean Change | |
| DomF | 6.84±1.87 | 11.65±3.80 | 4.81±2.76 |
| SubF | 10.52±8.17 | 14.14±7.91 | 3.61±1.35 |
| OvxF | 2.12±0.31b | 5.36±1.40 a | 3.23±1.25 |
| IM | 0.50±0.00b,c,d | 0.50±0.00c,d | 0.00±0.00 |
, effect of ACTH (p<0.05)
, significant difference from dominant females (p<0.05)
, significant difference from ovariectomized females (p<0.05)
, significant difference from subordinate females (p<0.05)
Immunoblotting of adrenal microsomes
To enable quantification of the proteins shown in figure 2 and correlation to activity, Western blot analysis of CYP17 and cytb5 was also performed in marmoset adrenal microsomes (Fig. 3). Figure 3A shows immunoblots for CYP17 and cytb5. While CYP17 expression was remarkably consistent across male, dominant and ovariectomized female microsomes, cytb5 showed considerable changes between groups. Quantification of the immunoblots (Fig. 3B) confirmed that cytb5 expression was highest in ovariectomized females (p<0.05), followed by dominant females, and then intact males. These results are fully consistent with the immunohistochemistry observations in male (Pattison et al. 2005) and female (Fig. 2A,C) marmoset adrenals.
Figure 3. Marmoset adrenal microsomal protein expression as detected by Western blot.
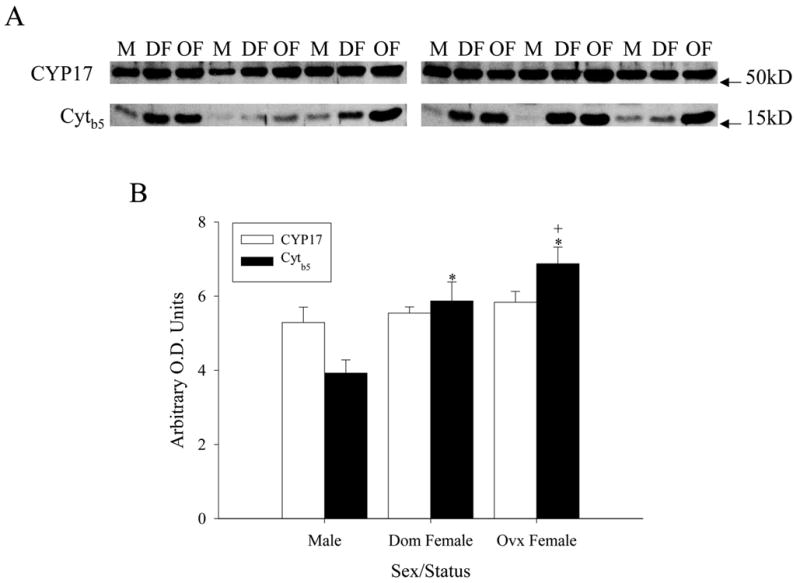
(A) Stripblots for CYP17 and cytb5 are shown, with molecular weights (right). Abbreviations are: adult testis-intact male (M), dominant female (DF), and ovariectomoized female (OF). (B) Quantified protein expression in adrenal microsomes from adult testis-intact males (n=6, aged 60–132 months), ovary-intact, dominant females (DomF; n=6, aged 24–132 months), and ovariectomized females (OvxF; n=6, aged 72–132 months). * significant difference from male (p<0.01), + significant difference from dominant female (p<0.01).
17,20-Lyase activity in marmoset adrenal microsomes
Microsomal 17,20-Lyase activity was detectable by a sensitive radiometric assay, but did not differ significantly between individual groups (data not shown). Nonetheless, testis-intact males tended to have the lowest activity (10.07±0.79 nmol/mg/h), followed by dominant females (10.20±1.87 nmol/mg/h) with ovariectomized females having the highest activity (11.28±1.30 nmol/mg/h). Furthermore, plotting cytb5 expression (data obtained from Fig. 3B) against 17,20-lyase activity for all three marmoset groups revealed a positive correlation (r2 value of 0.355, p<0.01; Fig. 4). Additionally, 17,20-lyase activity from any one of the three groups could be predicted from the cytb5 values from all three groups.
Figure 4. Regression of marmoset adrenal microsomal cytb5 expression vs. 17,20-lyase activity.
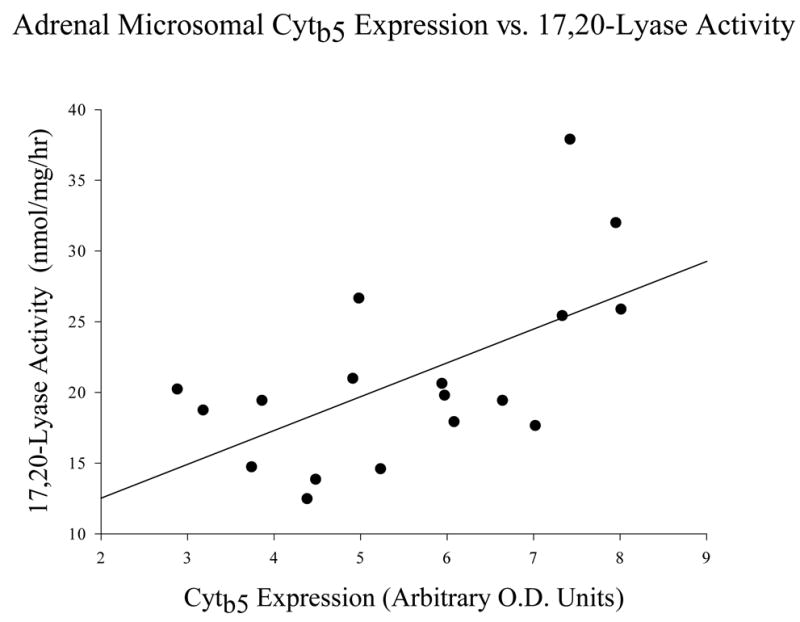
Cytochrome b5 expression (from Fig. 4) vs. 17,20-lyase activity (values in text) data were compared for microsomal preparations from the following groups: Open circles: testis-intact males, black squares: ovary-intact, dominant females, black triangles: ovariectomized females. Regression analysis revealed a correlation of r2=0.355, (p<0.01).
Plasma steroids of ZG and ZF origin
In order to gain more insight into whether effects of gender and gonadectomy were unique to a putative “ZR” or were zonally specific, we further examined changes in steroids characteristic of the zona glomerulosa and fasciculata following dexamethasone suppression and ACTH challenge. We have shown previously that intravenous injection of ACTH1-39 into dex-suppressed, testis-intact adult male marmosets yielded increases in aldosterone, corticosterone and cortisol levels, but not DHEA (Pattison et al. 2005). Dominant, cycling adult female marmosets exposed to the same combined dex/ACTH1-39 challenge yielded increases in corticosterone as well as the expected increases in cortisol levels (p<0.05; Table 2). Application of the combined dex/ACTH1-39 challenge in ovary-intact, anovulatory socially subordinate female marmosets yielded increases in circulating levels of aldosterone, corticosterone and cortisol (p<0.05, Table 2).
Table 2.
In vivo steroid responses to a combined dex/ACTH1-39 challenge in marmosets.
Data were obtained from 6 dominant female (DomF; 34.9±2.5 months), 7 subordinate female (SubF; 27.2±1.3 months), 6 ovariectomized female (OvxF; 38.1±4.4 months), and 3 testis intact males (IM) (45.9±9.7 months) marmosets. Dex was administered at 17h00, blood was taken at 08h40 (pre-ACTH) the next morning, ACTH administered at 09h00, and blood taken at 10h00 (ACTH). Detection limits for aldosterone, corticosterone, and cortisol were 25 pg/ml, 0.35 ng/ml, and 1 ng/ml, respectively. Note the mean changes were calculated from individual animals, rather than from the mean pre- and post-ACTH values shown in the table.
| Cortisol ng/ml | Corticosterone ng/ml | Aldo pg/ml | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-ACTH | ACTH | Mean Change | Pre-ACTH | ACTH | Mean Change | Pre-ACTH | ACTH | Mean Change | |
| DomF | 309.1±39.1 | 1535.2±23.6a | 1226.1±52.0 | 0.88±0.36 | 46.9±4.2a | 46.1±4.2 | 528.9±321.5 | 1707.2±584.9 | 1178.4±544.3 |
| SubF | 244.5±64.5 | 1361.4±120.3a | 1116.8±95.1 | 1.19±0.38 | 26.8±5.8a,b | 25.6±5.7b | 629.9±288.8 | 1834.0±550.3a | 1204.1±313.7c |
| OvxF | 394.5±108.1 | 1365.0±160.4a | 990.5±132.5 | 0.54±0.12 | 30.3±7.9a | 29.7±7.9 | 281.4±91.3 | 695.0±216.7 | 414.3±171.4 |
| IM | 107.7±23.6b,c | 1002.0±53.7a,b | 894.3±48.3b | 0.35±0.00 | 12.3±4.7a,b | 11.9±4.7b | N.A. | N.A. | N.A. |
, effect of ACTH (p<0.05)
, significant difference from dominant females (p<0.05)
, significant difference from ovariectomized females (p<0.05)
, significant difference from subordinate females (p<0.05)
Amongst the female groups, ovary-intact, dominant females had the highest ACTH1-39-stimulated corticosterone levels, followed by ovariectomized females, with subordinates having the lowest levels. The ACTH1-39-stimulated increases in corticosterone in subordinates were significantly lower than in dominant females (p<0.05, Table 2), suggesting altered ZF function.
Cortisol levels did not differ significantly among the three groups of females either before or after ACTH1-39 treatment, but it was noteworthy that both basal and the ACTH1-39- stimulated increase in cortisol in dominant females was greater than in intact males.
Testis-intact males also responded to ACTH1-39 with increases in corticosterone (p<0.05; Table 2) and again this ACTH1-39- stimulated increase was less than observed in dominant females. These results were consistent with the data we previously published (Pattison et al. 2005).
While these data certainly suggests gender and gonadectomy sensitive differences in adrenal function may occur beyond a putative ZR to include other adrenocortical zones, greater insight into the change in adrenal function was revealed when we calculated the corticosterone:cortisol ratio (as a percent) from the change between dex-suppressed and ACTH1-39- stimulated levels to reflect the % “inefficiency” of the ZF. We previously measured this ratio in testis-intact adult male marmosets to determine the percentage of cortisol that was made via the Δ-5 pathway (P5-to-17OHP5-to-17OHP4-to-cortisol) vs. the Δ-4 pathway (P5-to-P4-to-17OHP4-to-cortisol) (Pattison et al. 2005) (see Fig. 1 for diagram). Higher percentages of corticosterone:cortisol reflect a less efficient ZF [i.e. a larger proportion of P5 is converted to P4 by 3β-HSD before 17α-hydroxylation and in view of the very high affinity of 21-hydroxylase cytochrome P450 (P450c21) for P4, most becomes irreversibly committed to corticosterone biosynthesis]. This measure in female marmosets revealed that more P5 “leaked” from the Δ-5 pathway in cycling, dominant and ovariectomized females than in anovulatory subordinate females and testis-intact males (p<0.05; Fig. 5).
Figure 5. Inefficiency of the adrenal zona fasciculata in marmosets.
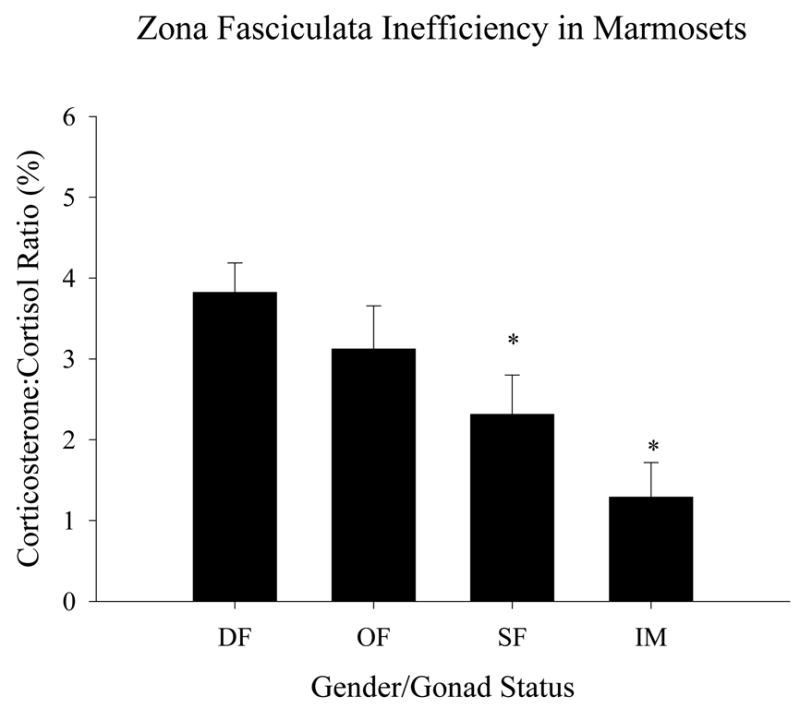
Corticosterone production, as a fraction of cortisol production, was calculated as percentages from the data obtained in Table 1. Abbreviations are: dominant female (DF), ovariectomized female (OF), subordinate female (SF), testis-intact male (IM). * significant difference from dominant (p<0.05).
Discussion
Cytochrome b5 staining defines the ZR in mature human adrenals comprising the inner zone of the cortex and is a consistently immunoreactive area (Dharia et al. 2004, 2005). We have previously shown that adult male marmosets lack a functional ZR by demonstrating the absence of an adrenal DHEA response to ACTH and low cytb5 expression (Pattison et al. 2005). Nonetheless female marmosets are known to produce more DHEA than males, particularly with increasing age (Levine et al. 1982). Our current studies were designed to ask whether female marmosets could possess a functional ZR, and further whether development of a ZR can be influenced by social and gonadal status. While the ZR in humans can be easily defined, physiological and biochemical regulation of ZR developent and regression remains a mystery.
Most striking in the initial histochemical analysis of female marmoset adrenals were the dramatic differences in cytb5 staining in the innermost adrenocortical region of adult females, which were in stark contrast to that previously reported in adult males. In ovary-intact cycling dominant and anovulatory subordinate female marmosets, cytb5 expression was detectable, though not uniformly expressed, and DHEA levels failed to rise significantly in response to ACTH challenge. In contrast, gonadectomized females exhibited the strongest and most uniformly solid staining for cytb5 and we also observed a corresponding significant increase in circulating DHEA following ACTH administration. Thus our data from ovarectomized females strongly suggest both a gender difference and further that gonadal function clearly affects adrenal steroid production in marmoset females.
From both the studies of Levine et al (1982) and our observations of DHEA levels in a dexamethasone-suppressed state without ACTH challenge we hypothesize that a gonadally intact female marmoset derives its circulating DHEA from the ovaries. However, when this source is depleted, either surgically (ovariectomy) or naturally (social subordination), a more defined adrenal ZR develops to a degree necessary for minimum DHEA compensation. Shideler et al noted that there was a progressive increase in circulating DHEAS concentrations in rhesus monkeys as they transitioned into anovulatory menopause (2001). A similar observation was made in women (Lasley et al. 2002), suggesting the existence of regulatory links between the ovaries and ZR which influence adrenal androgen output that may be of particular importance in the menopausal transition.
It is tempting to speculate that changes in cytb5 expression underlie changes in 17,20-lyase activity, but quantification of histochemical data is not as accurate as that by Western blot. To further test our hypothesis we therefore compared measurement of 17,20-lyase activity in microsomal preparations from each group to cytb5 and CYP17 expression. Using this approach we were able to show clearly that while CYP17 expression was essentially constant, regardless of the animal subgroup, there was a significant positive correlation between cytb5 expression and 17,20-lyase activity across multiple treatment groups. Our data are consistent with the notion that the capacity for DHEA production is indeed increased in vivo by adrenal cytb5 expression in a newly emerging ZR in the adrenal cortex and its role becomes more significant.
One final question which remains is what possible effects may there be in other zones of the adrenal cortex with the appearance of a ZR? Since adrenal weights do not differ between groups (not shown) it may be argued the appearance of this new zone would have to occur at the expense of the ZF. Does this require altered adrenal ZF function? While subtle differences were seen among the four groups with respect to the other ACTH-sensitive and zone-specific steroids, the most interesting finding was the ratio of corticosterone:cortisol under ACTH challenge. We have previously established (Pattison et al. 2005) that the corticosterone:cortisol ratio provides an estimate of the amount of P5 substrate leaking from the Δ-4, rather than the preferred Δ-5, pathway of the ZF (Fig. 1). While the very small % value of this corticosterone:cortisol ratio may be of modest physiological significance at best, a change in this value does provide a useful index of adrenal zonal efficiency in vivo without removing the organ. While all three of the female groups exhibited similar cortisol levels on ACTH stimulation (Table 2) it is interesting to note that corticosterone:cortisol ratios varied between groups. Indeed, the subordinate female ratios were significantly lower than in dominant females or ovariectomized females. Likewise, the ratio for intact males was less than any other group. This does not directly correlate with measures of ZR function (either DHEA production in vivo or cytb5 expression or 17,20-lyase activity in microsomal preparations) so it appears that the physical presence of a ZR alone does not require an automatic change in ZF efficiency with reduced size. Nonetheless, these findings do suggest that both gender and gonadal function are affecting more than just the ZR in the marmoset adrenal cortex in a manner that is yet to be defined.
In summary, we have shown that, in contrast to males, female marmosets have a rudimentary ZR by cytb5 staining that is more apparent than that observed in males, but that is marginally unresponsive to ACTH in reproductively active, ovary-intact females. As ovarian hormone levels decline (such as in anovulatory subordinate females or particularly in ovariectomized females), progressive induction of cytb5 occurs in the innermost region of the adrenal cortex and significant DHEA secretion becomes evident on ACTH-challenge. While this is clearly apparent in females, further studies will be necessary to establish any similar effect of gonadectomy in males. Nonetheless, elucidating the basis for this gonadal control of cytb5 expression in marmoset adrenals could be important to understanding in humans how we may counteract phases of life associated with adrenal androgen secretion, either DHEA production (i.e. menopause and aging) (Conley et al. 2004), or associated female endocrinopathies involving an overproduction of DHEA (i.e. polycystic ovary syndrome) (Azziz et al. 1998, Yildiz et al. 2004). Likewise, deciphering the differences between Old World and New World primate adrenal and general biology provides greater insight into the appropriate usage of these animals as models for human disease.
Acknowledgments
We thank Brian Horman for technical assistance, Dan Wittwer and Fritz Wegner in WPRC Assay Services for performing hormone assays, WPRC Animal Care and Veterinary Services for care and maintenance of the marmosets, and Amy Usborne, DVM, in Pathology Services at WPRC for providing marmoset adrenal tissue. At the University of California-Davis we thank Samantha Mapes, Jo Corbin, and Francisco Moran, PhD, in Population Health and Reproduction for assistance in microsomal preparation and assays, and Alice Tarantal, PhD, and staff at the California National Primate Research Center for help in collecting rhesus adrenal tissue. This work was supported by National Institutes of Health Grants MH060728 (to W.S., D.H.A., and I.M.B.), HL064601 (to I.M.B.), HD036913 (to A.J.C.), HD041921 (to J.C.P.), and RR000167 (to WPRC, a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01) and National Science Foundation Grant IBN-9604321 (to D.H.A. and W.S.). JCP was the recipient of support from T32 Training award HD041921, and this work is part of her studies in the Endocrinology-Reproductive Physiology Program at the University of Wisconsin - Madison. The authors acknowledge there is no conflict of interest that would prejudice this work’s impartiality.
Abbreviations
- DHEA
Dehydroepiandrosterone
- P5
Pregnenolone
- 17OHP5
17alpha-hydroxypregnenolone
- 3β-HSD
3beta-hydroxysteroid dehydrogenase
- CYP17
17alpha-hydroxylase/17,20-lyase cytochrome P450
- cytb5
cytochrome b5
- POR
NADPH cytochrome P450 oxido-reductase
- dom
dominant
- ovx
ovariectomized
- sub
subordinate
- casx
castrated
Footnotes
This work was supported by NIH grants MH060728, HL064601, HD036913, HD041921 and RR000167, and NSF grant IBN-9604321.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH. Social conflict and reproductive suppression in marmoset and tamarin monkeys. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. Albany: State University of New York Press; 1993. pp. 331–72. [Google Scholar]
- Abbott DH, Saltzman W, Schultz-Darken NJ, Smith TE. Specific neuroendocrine mechanisms not involving generalized stress mediate social regulation of female reproduction in cooperatively breeding marmoset monkeys. Annals of the New York Academy of Sciences. 1997;807:219–23. doi: 10.1111/j.1749-6632.1997.tb51923.x. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Saltzman W, Schultz-Darken NJ, Tannenbaum PL. Adaptations to subordinate status in female marmoset monkeys. Comparative Biochemistry and Physiology. 1998;119:261–274. doi: 10.1016/s0742-8413(98)00015-2. [DOI] [PubMed] [Google Scholar]
- Abbott DH, Barnett DK, Colman RJ, Yamamoto ME, Schultz-Darken NJ. Aspects of common marmoset basic biology and life history important for biomedical research. Comparative Medicine. 2003;53:339–50. [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. Molecular evolution of adrenarche: structural and functional analysis of P450c17 from four primate species. Endocrinology. 2002;143:4665–72. doi: 10.1210/en.2002-220456. [DOI] [PubMed] [Google Scholar]
- Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in Reproductive Medicine. 2004;22:281–8. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. Journal of Clinical Endocrinology and Metabolism. 1998;83:2317–2323. doi: 10.1210/jcem.83.7.4948. [DOI] [PubMed] [Google Scholar]
- Baker JV, Abbott DH, Saltzman W. Social determinants of reproductive failure in male common marmosets housed with their natal family. Animal Behaviour. 1999;58:501–13. doi: 10.1006/anbe.1999.1200. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. Veterinary Pathology. 2006;43:97–117. doi: 10.1354/vp.43-2-97. [DOI] [PubMed] [Google Scholar]
- Bird IM, Mason JI, Rainey WE. Battle of the kinases: integration of adrenal responses to cAMP, DG, and Ca2+ at the level of steroidogenic cytochromes P450 and 3betaHSD expression in H295R cells. Endocrine Research. 1998;24:345–54. doi: 10.3109/07435809809032614. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Bird IM. The role of cytochrome P450 17a-hydroxylase and 3B-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta5 and delta4 pathways of steroidogenesis in mammals. Biology of Reproduction. 1997;56:789–99. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Seminars in Reproductive Medicine. 2004;22:311–26. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues. Biology of Reproduction. 2004;71:83–8. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, Brissie RM, Parker CR., Jr Effects of aging on cytochrome b5 expression in the human adrenal gland. Journal of Clinical Endocrinology and Metabolism. 2005;90:4357–61. doi: 10.1210/jc.2005-0017. [DOI] [PubMed] [Google Scholar]
- Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 beta-hydroxysteroid dehydrogenase. Journal of Clinical Endocrinology and Metabolism. 1996;81:3558–65. doi: 10.1210/jcem.81.10.8855801. [DOI] [PubMed] [Google Scholar]
- Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR. Adrenarche is associated with decreased 3 beta-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocrine Research. 1996;22:723–8. doi: 10.1080/07435809609043768. [DOI] [PubMed] [Google Scholar]
- Gell JS, Carr BR, Sasano H, Atkins B, Margraf L, Mason JI, Rainey WE. Adrenarche results from development of a 3b-hydroxysteroid dehydrogenase-deficient adrenal reticularis. Journal of Clinical Endocrinology and Metabolism. 1998;83:3695–3701. doi: 10.1210/jcem.83.10.5070. [DOI] [PubMed] [Google Scholar]
- Grigoryev DN, Kato K, Njar VC, Long BJ, Ling YZ, Wang X, Mohler J, Brodie AM. Cytochrome P450c17-expressing Escherichia coli as a first-step screening system for 17alpha-hydroxylase-C17,20-lyase inhibitors. Analytical Biochemistry. 1999;267:319–30. doi: 10.1006/abio.1998.2993. [DOI] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Seminars in Reproductive Medicine. 2004;22:337–47. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Hyatt PJ, Bhatt K, Tait JF. Steroid biosynthesis by zona fasciculata and zona reticularis cells purified from the mammalian adrenal cortex. Journal of Steroid Biochemistry. 1983;19:953–9. doi: 10.1016/0022-4731(83)90039-0. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, Chrousos GP. The biobehavioral consequences of psychogenic stress in a small, social primate (callithrix jacchus) Biological Psychiatry. 1996;40:317–37. doi: 10.1016/0006-3223(95)00397-5. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. Journal of Clinical Endocrinology and Metabolism. 2002;87:3760–7. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- Lebel M, Grose JH. Selective hypoaldosteronism; a study of steroid biosynthetic pathways under adrenocorticotrophin and angiotensin II infusion. Clincal Science and Molecular Medicine Supplement. 1976;3:335s–337s. doi: 10.1042/cs051335s. [DOI] [PubMed] [Google Scholar]
- Levine J, Wolfe LG, Schiebinger RJ, Loriaux DL, Cutler GB., Jr Rapid regression of fetal adrenal zone and absence of adrenal reticular zone in the marmoset. Endocrinology. 1982;111:1797–802. doi: 10.1210/endo-111-6-1797. [DOI] [PubMed] [Google Scholar]
- Moran FM, Ford JJ, Corbin CJ, Mapes SM, Njar VC, Brodie AM, Conley AJ. Regulation of microsomal P450, redox partner proteins, and steroidogenesis in the developing testes of the neonatal pig. Endocrinology. 2002;143:3361–9. doi: 10.1210/en.2002-220329. [DOI] [PubMed] [Google Scholar]
- Narasaka T, Suzuki T, Moriya T, Sasano H. Temporal and spatial distribution of corticosteroidogenic enzymes immunoreactivity in developing human adrenal. Molecular and Cellular Endocrinology. 2001;174:111–20. doi: 10.1016/s0303-7207(00)00445-7. [DOI] [PubMed] [Google Scholar]
- Pandey AV, Miller WL. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. Journal of Biological Chemistry. 2005;280:13265–71. doi: 10.1074/jbc.M414673200. [DOI] [PubMed] [Google Scholar]
- Pattison JC, Abbott DH, Saltzman W, Nguyen AD, Henderson G, Jing H, Pryce CR, Allen AJ, Conley AJ, Bird IM. Male marmoset monkeys express an adrenal fetal zone at birth, but not a zona reticularis in adulthood. Endocrinology. 2005;146:365–74. doi: 10.1210/en.2004-0689. [DOI] [PubMed] [Google Scholar]
- Rainey WE, Rheman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Seminars in Reproductive Medicine. 2004;22:327–36. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- Remer T, Boye KR, Hartmann MF, Wudy SA. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. Journal of Clinical Endocrinology and Metabolism. 2005;90:2015–21. doi: 10.1210/jc.2004-1571. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Scheffler G, Wegner FH, Abbott DH. Social and reproductive influences on plasma cortisol in female marmoset monkeys. Physiology and Behavior. 1994;56:801–10. doi: 10.1016/0031-9384(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Abbott DH. Behavioural and endocrine predictors of dominance and tolerance in female common marmosets, Callithrix jacchus. Animal Behaviour. 1996;51:657–674. [Google Scholar]
- Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. Suppression of cortisol levels in subordinate female marmosets: reproductive and social contributions. Hormones and Behavior. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- Saltzman W. Reproductive competition among female common marmosets (Callithrix jacchus): Proximate and ultimate causes. In: Jones CB, editor. Sexual Selection and Reproductive Competition in Primates: New Perspectives and Directions. Norman, OK: American Society of Primatologists; 2003. pp. 197–229. [Google Scholar]
- Saltzman W, Prudom SL, Schultz-Darken NJ, Wittwer DJ, Abbott DH. Social suppression of cortisol in female marmoset monkeys: role of circulating ACTH levels and glucocorticoid negative feedback. Psychoneuroendocrinology. 2004;29:141–61. doi: 10.1016/s0306-4530(02)00159-2. [DOI] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Lasley BL. Estrogen and progesterone metabolites and follicle-stimulating hormone in the aged macaque female. Biology of Reproduction. 2001;65:1718–25. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, Rainey WE. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clinical Endocrinology. 2000;53:739–47. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- Yildiz BO, Woods KS, Stanczyk F, Bartolucci A, Azziz R. Stability of adrenocortical steroidogenesis over time in healthy women and women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 2004;89:5558–5562. doi: 10.1210/jc.2004-0934. [DOI] [PubMed] [Google Scholar]
- Young LS, Murphy G, Kelly SN, Smith TP, Cunningham SK, McKenna JT. Differential production of adrenal steroids by purified cells of the human adrenal cortex is relative rather than absolute. European Journal of Endocrinology. 2003;148:139–45. doi: 10.1530/eje.0.1480139. [DOI] [PubMed] [Google Scholar]
- Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10619–23. doi: 10.1073/pnas.92.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]


