Abstract
Although stress and methamphetamine (Meth) can independently and acutely affect glutamate transmission in the hippocampus, no studies have examined how chronic unpredictable stress modulates glutamate function and alters glutamate responsiveness to Meth. Therefore, the effects of chronic unpredictable stress on markers of glutamate function and subsequent Meth-induced increases in extracellular glutamate in the dorsal hippocampus were examined. Ten days of chronic unpredictable stress increased the plasmalemmal glial-glutamate transporter (EAAT2) and increased vesicular glutamate transporter-1 (VGLUT1) immunoreactivity in a vesicle associated fraction. In addition, a 2-fold increase in vesicular glutamate content was observed. Chronic stress also enhanced Meth-induced increases in extracellular glutamate in the dorsal hippocampus in a TTX dependent manner. Overall, the finding that chronic stress resulted in an upregulation of glutamate function and an enhanced glutamate response to Meth may have implications for glutamate responsiveness in chronically stressed animals exposed to other challenges or stressors.
Keywords: stress, glutamate, hippocampus, methamphetamine, vesicular glutamate transporter, excitatory amino acid transporter
1. Introduction
The hippocampus is vulnerable to the effects of both chronic stress and the widespread drug of abuse, methamphetamine (Meth). The vulnerability to chronic stress is due in part to the high density of glucocorticoid receptors that are activated by elevated concentrations of corticosterone during stress (Aronsson et al., 1988;Munck et al., 1984;Reul and de Kloet 1985) and is evidenced by atrophy to apical dendrites of the hippocampus (Magarinos and McEwen 1995). In comparison to chronic stress, the vulnerability of the hippocampus to Meth is manifested as a long lasting, widespread toxicity to serotonin (5HT) neurons as evidenced by loss of 5HT neurons, decreases in 5HT uptake sites, and decreases in tryptophan hydroxylase activity (Guilarte et al., 2003;Hotchkiss and Gibb 1980;Ricaurte et al., 1980). Despite differences in how this vulnerability is manifested, there are similarities between the acute effects of stress and Meth that may mediate their long-term consequences. Specifically, both acute restraint stress and Meth increase extracellular glutamate in the dorsal and ventral hippocampus, respectively (Lowy et al., 1993;Rocher and Gardier 2001). Furthermore, stress-induced dendritic atrophy and Meth-induced decreases in 5HT in the hippocampus are attenuated by glutamate receptor antagonism (Farfel et al., 1992;Magarinos and McEwen 1995). Despite these parallels, no studies have examined the effects of Meth alone on glutamate in the dorsal hippocampus and how prior stress exposure might modify the glutamate response to Meth. With regard to behavior, the dorsal hippocampus is involved in spatial memory and stress decreases spatial learning performance in a manner that is dependent upon excitatory amino acids (Luine et al., 1994;Sousa et al., 2000). Similarly, Meth alone also produces decrements in spatial learning (Friedman et al., 1998). Therefore, it is important to determine if Meth in combination with chronic stress affects glutamate transmission in the dorsal hippocampus that in turn, could have behavioral implications with regard to possible cognitive deficits produced by the combined effects of stress and Meth.
The regulation of glutamate is mediated primarily be re-uptake and packaging of glutamate into vesicles for release. In the hippocampus, these processes are regulated largely by EAAT2 (the plasmalemmal glial-glutamate transporter) and VGLUT1 (the vesicular glutamate transporter-1), respectively (Hisano 2003;Rothstein et al., 1994). In fact, EAAT2 is increased in the CA3 region of the hippocampus following 21 days of chronic restraint stress (Reagan et al., 2004). In contrast, the effects of stress on VGLUT1 are unknown; however, glutamate receptor activation with NMDA in primary culture leads to an upregulation of VGLUT1 (referred to as the brain-specific Na+-dependent inorganic phosphate cotransporter (BNPI); (Ni et al., 1994)). Previous studies have demonstrated that VGLUT1 regulates quantal size, synaptic vesicle volume, and the amount of glutamate loaded into vesicles for release (Daniels et al., 2004;Wojcik et al., 2004). Therefore, elevated concentrations of glutamate during chronic stress may alter EAAT2 and VGLUT1 immunoreactivity as well as vesicular glutamate content and Meth-induced glutamate release. The evaluation of these putative mechanisms may help to explain how glutamate mediates the deleterious effects of stress (McEwen 2001) and may uncover novel targets for therapeutic intervention that can counteract the deleterious effects of stress and Meth.
In order to model chronic stressful experiences, several different paradigms using rats have been employed (e.g. social aggression, maternal separation, footshock, tail pinch, and restraint). One paradigm that has been used widely to mimic repeated but mild stressful events is chronic unpredictable stress (CUS). In the CUS paradigm, the type and time of stress exposures are varied (Katz et al., 1981; Willner et al., 1992; Ortiz et al., 1996). The advantage of this paradigm over other chronic stress models is that the unpredictable nature of exposure to varied stressors mimics the exposure to unexpected stressful life events. Moreover, the paradigm is not confounded with learning and adaptation and avoids the stimulus-specific aspects of a given stressor (Herman et al., 1995). This paradigm of CUS has also been shown to enhance some of the acute and long-term toxic effects of Meth on striatal dopamine function (Matuszewich and Yamamoto 2004). Although this enhancement of dopamine function in the striatum is known, it is unknown whether CUS enhances the acute effects of Meth on glutamate function in the hippocampus. Based on the important role of glutamate in mediating the effects of stress and Meth, the current study investigated the effects of ten days of CUS on Meth-induced glutamate release, EAAT2 and VGLUT1 immunoreactivity, and vesicular glutamate content in the dorsal hippocampus. It was hypothesized that ten days of CUS would enhance EAAT2 and VGLUT1 immunoreactivity, vesicular glutamate content, and Meth-induced glutamate release.
2. Results
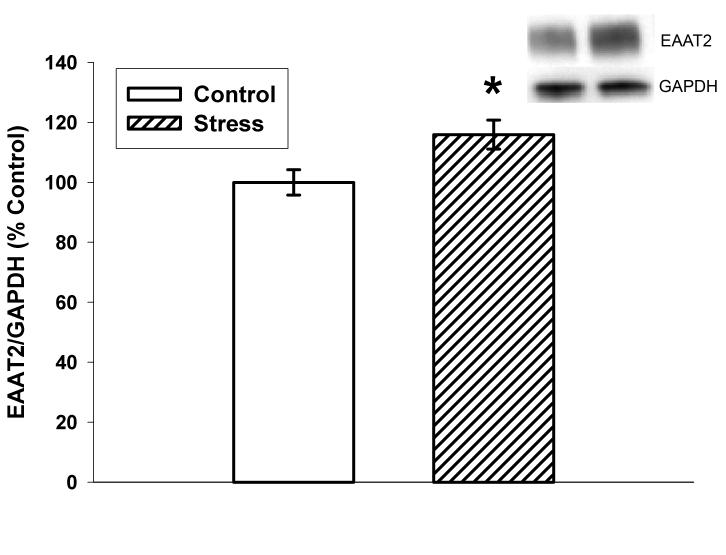
Figure 1 illustrates EAAT2/GAPDH immunoreactivity as percent saline control optical density. Ten days of CUS significantly increased EAAT2/GAPDH immunoreactivity (115.9±4.9%; p=0.02) in the dorsal hippocampus relative to controls (100±4.2). Preliminary data indicated that ten days of CUS does not alter GAPDH immunoreactivity which has been used as a loading control for western blotting in previous stress-related studies (Gerges et al. 2003;Rosenbrock et al. 2005).
Figure 1.
Effects of ten days of CUS on EAAT2 immunoreactivity in the dorsal hippocampus. Stress significantly increased EAAT2 when compared to non-stressed controls (p=0.02). n=15 for Control and n=13 for Stress. *p<0.05 vs. control. Data are presented as mean ± SEM.
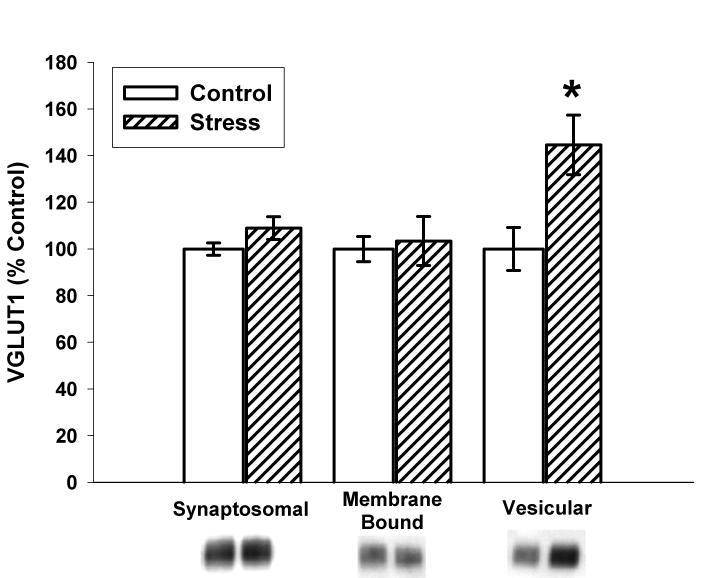
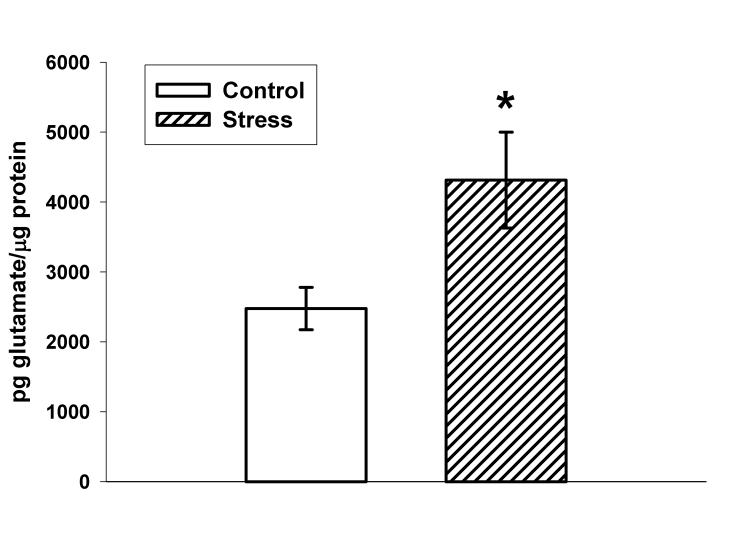
As illustrated in Figure 2, ten days of CUS also significantly increased VGLUT1 immunoreactivity in the vesicular fraction (144.6±12.8% versus 100±9.2% for control; p=0.01) with a non-significant trend towards an increase in the synaptosomal fraction (109.0±4.9% versus 100±2.7 for control; p=0.13). No changes in VGLUT1 immunoreactivity were observed in the membrane bound fraction (103.5±10.5% versus 100±5.4% for control; p=0.77). Figure 3 illustrates that vesicular glutamate concentrations in stressed rats were significantly increased when compared to control (4316±685 versus 2477±303 pg glutamate/μg protein; p=0.04).
Figure 2.
Effects of ten days of CUS on VGLUT1 immunoreactivity in the synaptosomal, membrane bound, and vesicular fractions. Stress trended towards an increase in VGLUT1 in the synaptosomal fraction (p=0.13), did not alter VGLUT1 in the membrane bound fraction (p=0.77), and significantly increased VGLUT1 in the vesicular fraction (p=001). n=8 for Control and Stress. *p<0.05 vs. control. Data are presented as mean ± SEM.
Figure 3.
Effects of ten days of CUS on vesicular glutamate content in the dorsal hippocampus. Stress significantly increased vesicular concentrations of glutamate when compared to control animals (p=0.04). n=10 for Control and n=14 for Stress. *p<0.05 vs. control. Data are presented as mean ± SEM.
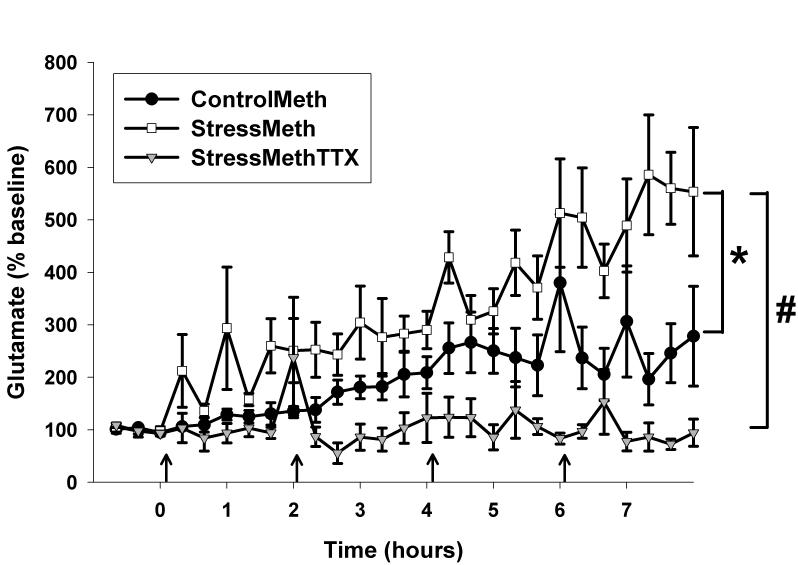
For in vivo microdialysis experiments, average baseline concentrations of glutamate were as follows: 896±192 pg glutamate/20μL for unstressed rats administered Meth (ControlMeth), 1057±223 pg glutamate/20μL for stressed rats administered Meth (StressMeth), and 676±295 pg glutamate/20μL for stressed rats administered Meth and TTX (StressMethTTX). No significant differences were observed between any of the groups with regards to baseline values of glutamate (F(2,20)=0.646, p=0.536). Analysis of data as % baseline following Meth administration illustrated a significant treatment (F(2,515)=19.977, p<0.001), time (F(26,515)=5.591, p<0.001) and treatment by time interaction. (F(52,515)=2.798, p<0.001). A significant difference between ControlMeth and StressMeth treatments was observed (q=0.015, p<0.05) as well a significant difference between StressMeth and StressMethTTX (q<0.001, p0.05). When compared to baseline, both ControlMeth (M18 versus B1, q=5.376, p=0.035) and StressMeth (M18 versus B1, q=9.047, p<0.001) treatments produced a significant increase in glutamate at specific time points, whereas glutamate did not differ from baseline in StressMethTTX rats.
3. Discussion
This study examined the effects of CUS on markers of glutamate function and Meth-induced increases in extracellular glutamate in the dorsal hippocampus. Ten days of CUS increased EAAT2 and vesicular VGLUT1 immunoreactivity in the dorsal hippocampus and increased vesicular glutamate content. Stress also potentiated the Meth-induced increases in extracellular concentrations of glutamate in a TTX-dependent manner.
The finding that chronic stress increased EAAT2 immunoreactivity in the dorsal hippocampus (Figure 1) is consistent with previous studies illustrating increases in both EAAT2 (GLT-1) mRNA and protein expression following 21 days of chronic restraint stress (Reagan et al., 2004). The current study however, employed a mild unpredictable chronic stress regimen of varied stressors for a shorter duration than the more protracted repeated restraint stress regimen of 6 hr/day over 21 days. Despite this procedural difference, increases in EAAT2 within the hippocampus can be observed even with mild stressors for a shorter period of time. Based on the findings that stress activates excitatory projections in the hippocampus and increases the synaptic concentrations of glutamate (Lowy et al., 1993), the increase in EAAT-2 may be a homeostatic response to modulate or buffer elevated extracellular concentrations of glutamate.
CUS also increased VGLUT1 in the dorsal hippocampus (Figure 2). This effect of stress on VGLUT1 was specific to the vesicular fraction. No significant changes were observed in the membrane bound or synaptosomal fractions. To our knowledge, this is the first report of increased VGLUT1 protein in response to an environmental manipulation. Although the mechanisms responsible for these CUS-induced increases in VGLUT1 and EAAT2 are unknown, it is known that acute restraint stress increases glutamate in the dorsal hippocampus (Lowy et al., 1993). Therefore, the upregulation of VGLUT1 and EAAT2 expression may occur as a compensatory response to stress-induced elevations in glutamate. In support of this hypothesis, increases in VGLUT-1 occur in response to application of the glutamate receptor agonist N-methyl-D-aspartate (NMDA) (Hisano 2003;Ni et al., 1994). Similarly, application of glutamate to primary glial cultures dose-dependently increases in EAAT2 (GLT-1) mRNA (Thorlin et al., 1998). Therefore, the overall expression levels of both VGLUT1 and EAAT appear to be regulated by glutamate.
VGLUT1 also plays in important role in the regulation of glutamate by modulating the amount of glutamate loaded into synaptic vesicles. As illustrated in Figure 3, the amount of glutamate in vesicles from a purified vesicular preparation is enhanced following ten days of CUS. Based on the finding that no changes in VGLUT1 were observed in the membrane bound fraction and that non-neuronal extraneous sources of glutamate exist in membranes, the glutamate content of the membrane bound vesicle fraction was not measured.
The increases in glutamate concentrations observed in the vesicular fraction may by secondary to enhanced levels of VGLUT1 observed specifically in the vesicular fraction (Figure 2). Overexpression of VGLUT1 has been reported to increase quantal size and the synaptic vesicle volume of glutamate (Daniels et al. 2004;Wojcik et al. 2004) that in turn, could result in enhanced glutamate release (Wojcik et al., 2004). Furthermore, the greater relative increase in VGLUT-1 (40%) compared to EAAT-2 (15%) suggests that the increase in glutamate release is not sufficiently counterbalanced by the uptake and removal of glutamate and consequently results in the accumulation of glutamate in the extracellular space.
Although no change in basal concentrations of extracellular glutamate were observed following CUS, a subsequent challenge with Meth potentiated the increase in the extracellular glutamate in a TTX-dependent manner (Figure 4). This potentiation of Meth-induced glutamate release may be dependent upon increased glutamate content per vesicle, an increase in the number of vesicles, or an enhancement in the proportion of vesicles readily available for release by prior exposure to CUS. As illustrated in Figure 3, an overall increase in vesicular glutamate content per vesicle protein was observed, which would indicate an increase in the amount of glutamate per vesicle. In fact, the overexpression of VGLUT-1 increases quantal size and the amount of glutamate per vesicle (Wojcik et al., 2004). Alternatively, the number of vesicles may also be increased but this possibility is unlikely since chronic stress decreases the area occupied by vesicles (Magarinos et al., 1997). In addition, overexpression of VGLUT1 occurs independently of changes in the number of synaptic vesicles (Wilson et al., 2005). Another possibility is that chronic stress could increase in the density of vesicles near active synaptic zones (Magarinos et al., 1997) and thus provide a more readily releasable pool of vesicles. This enhanced releasable pool of vesicles is supported by an increase in the expression of proteins involved in synaptic vesicle exocytosis after chronic stress (Gao et al., 2006;Thome et al., 2001). Therefore, the most parsimonious explanation for the enhancement of glutamate transmission by Meth in CUS exposed rats may be a combination of a stress-induced increase in vesicle volume and a vesicle pool primed for release.
Figure 4.
Effects of CUS on Meth-induced increases in glutamate in the dorsal hippocampus. A significant treatment (F(2,515)=19.977, p<0.001), time (F(26,515)=5.591, p<0.001) and treatment by time interaction (F(52,515)=2.798, p<0.001) was observed. n=6 for ControlMeth, n=8 for StressMeth, and n=7 for StressMethTTX. *p<0.05 StressMeth vs. ControlMeth, #p<0.05 StressMeth vs. StressMethTTX. Data are presented as mean ± SEM. Arrows represent Meth injections.
The novel finding that chronic unpredictable stress increases markers of glutamate transmission and augments the glutamate response to Meth has broader significance. Both EAAT2 and VGLUT1 are regulated via glutamate (Thorlin et al., 1998; Ni et al., 1994) and acute stress-induced increases in glutamate in the hippocampus are dependent upon CORT (Lowy et al., 1993). Therefore, the increases in EAAT2 and VGLUT1 may be regulated indirectly via CORT through a glutamate-dependent mechanism. Although CORT may initiate the stress-induced increase in glutamate, the longer-term upregulation of VGLUT1 by glutamate may contribute to a persistent elevation of glutamate and stress-induced neuroadaptations in the face of diminished CORT responses observed during the course of chronic stress exposure (Magarinos and McEwen 1995).
The enhanced glutamate responsiveness to Meth following stress suggests that chronically stressed animals are more vulnerable to hippocampal damage produced by excitotoxicity. In fact, the magnitude of increase in glutamate observed in chronically stressed rats in response to a challenge administration regimen of Meth is similar to that observed in the striatum following higher Meth doses (Mark et al., 2004) known to produce excitotoxic damage evidenced by spectrin proteolysis (Staszewski and Yamamoto, 2006). In addition, chronically stressed animals may be more sensitive to Meth-induced toxicity to monoaminergic systems as glutamate has also been implicated in mediating Meth-induced 5HT depletions in the hippocampus (Farfel et al., 1992). Based on the exacerbation of Meth-induced glutamate release in the hippocampus by prior exposure to chronic stress, it is possible that the combination of stress and Meth may augment hippocampal damage and cognitive deficits associated with excitotoxic levels of glutamate. Furthermore, based on the observations that stress is a precipitating factor for drug abuse (Sinha 2001), drug abusers exposed to stress may be vulnerable to the toxic effects of drugs of abuse and events that markedly increase glutamate.
4. Experimental Procedure
Animals and Chronic Stress
Male Sprague-Dawley rats were purchased from Harlan Sprague Dawley (Indianapolis, IN) and weighed 175-200g upon arrival. Rats were housed 3 per cage in a temperature (21-23°C) and humidity controlled room, on a 12 hour light/dark cycle (lights on at 7:00 off at 19:00), with food and water available ad libitum. Upon intracranial surgery, rats were singly housed. All procedures were performed in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Stressed rats were exposed to 10 days of chronic stress that varied in both type and time (Matuszewich and Yamamoto 2004). For experiments not requiring intracranial surgery the following stress paradigm was used: Day 1 10:00 50 min cold room (4°C), 13:00 60 min cage rotation; Day 2 10:00 60 min restraint stress, 18:00 lights on overnight (12h); Day 3 10:00 3 h lights off, 15:00 50 min cold room (4°C); Day 4 11:00 50 min cage rotation, 18:00 food and water deprivation overnight (14 h); Day 5 9:00 60 min restraint stress, 18:00 lights on overnight; Day 6 15:00 15 min cold room isolation, 16:00 isolation overnight; Day 7 10:00 60 min restraint stress, 18:00 food and water deprivation overnight (14 h); Day 8 11:00 30 min cage rotation, 15:00 isolation overnight; Day 9 9:00 15 min cold room, 18:00 lights on overnight; Day 10 10:00 3 h lights off, 1:00 20 min cage rotation. For rats in the microdialysis studies, intracranial surgery replaced a stress episode on Day 7.
Western Blot Analysis and Vesicular Glutamate Content
For EAAT2, VGLUT1, and vesicular glutamate content studies, rats were sacrificed via rapid decapitation at 7:00 on day 11. Brains were quickly removed and frozen on dry ice. The dorsal hippocampus (−3.2mm posterior to bregma) was free-hand dissected from a 400μm slice and stored at −80°C until further analysis.
In EAAT2 studies, hippocampal tissue was homogenized in 500μL of ice cold 0.32M sucrose. For VGLUT1 and vesicular glutamate content studies, synaptasomal, membrane-bound, and vesicular fractions were prepared via differential centrifugation. Dorsal hippocampal tissue was homogenized in 1mL of ice cold 0.32M sucrose and centrifuged at 800g for 12 min at 4°C. The resulting supernatant (S1) was removed and centrifuged at 22,000g for 17 min at 4°C. The synaptosomal fraction was the resulting pellet (P2) which was resuspended in 150μL ice cold dH2O. An aliquot of this fraction was saved for Western blot analysis and the remainder centrifuged at 22,000g for 17 min at 4°C. The resulting supernatant (S3) yielded the vesicular fraction and the pellet (P3) resuspended in 60μL dH2O yielding the membrane bound fraction. For vesicular glutamate content studies, a further purified vesicular preparation was used. The S3 fraction was added to 5mL of dH2O and centrifuged at 100,000g for 60 min at 4°C. The pellet containing the purified vesicular preparation was resuspended in 100μL of Tissue Assay Buffer (0.05M Na2HPO4, 0.03M Citric Acid, 15% MeOH, pH 2.5). Samples were sonicated at 4°C and centrifuged at 22,000g for 25 min at 4°C. The supernatant was analyzed for glutamate via HPLC and the pellet dissolved in 25μL 1M NaOH. Protein values of hippocampal tissues for western blot analysis of EAAT2 and VGLUT-1 as well as pellets from the purified vesicular preparation were determined via the method of Bradford.
Protein samples for EAAT2 and VGLUT1 analysis (5 μg) were mixed with 2X TRIS-glycine loading buffer (Invitrogen) and subjected to SDS-PAGE on a 10% TRIS-glycine gel (running buffer: 25mM TRIS; 192mM Glycine; 0.02% SDS). Samples were then electroblotted onto PVDF membranes in 1x transfer buffer (25mM TRIS; 192mM Glycine; 20% Methanol; 0.02% SDS). Following transfer, membranes were blocked with a TBST-milk solution (10mM TRIS; 150mM NaCl; pH 8.0; 5% non-fat dry milk and 0.05% Tween-20) for 1 hour at room temp. Membranes were then incubated overnight at 4°C (EAAT2 1:2500, Santa Cruz sc-7760; GAPDH 1:1000, Santa Cruz sc-20357) or for 1 hour at room temperature (VGLUT-1 1:5000, MAb Technologies VGT1-3) in primary antibody diluted in 5% milk solution. Membranes were washed 4X in 1X TBST for three minutes each then incubated with a HRP conjugated rabbit anti-goat secondary antibody (1:2500 Santa Cruz sc-2768) for EAAT2/GAPDH or HRP conjugated goat anti-rabbit secondary antibody (1:2500 Chemicon AP307P) for VGLUT-1 in TBST-milk solution for 1 hour at room temperature. Membranes were then washed as described above and treated with ECL detection reagents (GE Healthcare). Band intensities were analyzed using Kodak image analysis software (Kodak Inc.) and GAPDH used as a loading control for EAAT2 studies.
Intracranial Surgery and Microdialysis
Rats were anesthetized with ketamine/xylazine (70 mg/kg, 6 mg/kg) and placed into a stereotaxic frame. The skull was exposed and a hole drilled over the dorsal hippocampus (3.5mm posterior and 2.0mm lateral to bregma). A 21 gauge guide cannula 11mm in length was stereotaxically placed over the hole with a stylet obturator and secured with cranioplastic cement along with 3 set screws. Following 3 days of recovery, the obturator was removed, the guide cannula reamed with a 26 gauge needle that extended 0.5mm past the end of the guide cannula in order to puncture dura, and a microdialysis probe inserted. Probes were constructed as previously described with a 2mm active membrane (Yamamoto and Pehek 1990). Probes were perfused overnight with Dulbecco's phosphate-buffered saline (Sigma-Aldrich) at a flow rate of 0.5 μL/min. The following morning, the infusion pump (Harvard Apparatus) was increased to 1.5 μL/min. Following 2 hours of pre-baseline, 1 hour of baseline samples were collected every 20 minutes. Meth (Sigma-Aldrich) was dissolved in saline and injected intraperitoneally (7.5 mg/kg) once every two hours over a period of 6 hrs. Dialysate samples were collected every 20 minutes for 8 hours. For TTX studies, TTX (Sigma-Aldrich) was dissolved in water and added to the Dulbecco's phosphate-buffered saline. TTX (1μM) was reverse dialysed throughout the entire dialysis collection period. Rats were killed at the end of the experiment and their brains frozen on dry ice. Brains were sectioned using a cryostat microtome and probe placement verified. Only rats with probes in the dorsal hippocampus were included in the data analysis.
Biochemical Measurement of Glutamate
Concentrations of glutamate in microdialysis samples as well as vesicular glutamate content were analyzed via HPLC coupled to fluorescence detection as previously described (Donzanti and Yamamoto 1988). Glutamate was derivatized with O-phthaldialdehyde (OPA; Sigma-Aldrich) prepared by dissolving 27mg OPA in 9mL of 0.1M sodium tetraborate pH 9.4 and 1 mL of 100% methanol to which 15μL of β-mercaptoethanol was added. This solution was then diluted 1:3 with sodium tetraborate and 10μL added to 20μL of dialysate sample using an ESA Model 542 autosampler. The sample and derivatization solution reacted for 90 seconds before being injected onto a C18 column (100×2.0mm, 3μm particle size, Phenomenex). Glutamate was eluted using a mobile phase containing 0.1M sodium phosphate and 0.1mM EDTA in 10% methanol at pH 6.7. Glutamate was detected using a Waters 474 Scanning Fluorescence Detector with excitation and emission wavelengths set at 340 and 440 nm respectively. Data were analyzed using EZChrom Elite software (Scientific Software Inc)
Statistical Analysis
Western blot data was analyzed using t-tests and significance set at p<0.05. Two-way repeated measures ANOVA followed by Tukey's post hoc test was used to determine significant differences between groups in the microdialysis experiment. Glutamate is expressed as % baseline and all data are represented as mean±SEM.
Acknowledgements
This work was supported by DA020310
Abbreviations
- Meth
methamphetamine
- EAAT2
excitatory amino acid transporter 2
- VGLUT1
vesicular glutamate transporter 1
- 5HT
serotonin
- CUS
chronic unpredictable stress
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronsson M, Fuxe K, Dong Y, Agnati LF, Okret S, Gustafsson JA. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc Natl Acad Sci U S A. 1988;85:9331–9335. doi: 10.1073/pnas.85.23.9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. A rapid and simple HPLC microassay for biogenic amines in discrete brain regions. Pharmacol Biochem Behav. 1988;30:795–799. doi: 10.1016/0091-3057(88)90102-5. [DOI] [PubMed] [Google Scholar]
- Farfel GM, Vosmer GL, Seiden LS. The N-methyl-D-aspartate antagonist MK-801 protects against serotonin depletions induced by methamphetamine, 3,4-methylenedioxymethamphetamine and p-chloroamphetamine. Brain Res. 1992;595:121–127. doi: 10.1016/0006-8993(92)91460-v. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol Biochem Behav. 1998;61:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Gao Y, Bezchlibnyk YB, Sun X, Wang JF, McEwen BS, Young LT. Effects of restraint stress on the expression of proteins involved in synaptic vesicle exocytosis in the hippocampus. Neuroscience. 2006;141:1139–1148. doi: 10.1016/j.neuroscience.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Aleisa AM, Schwarz LA, Alkadhi KA. Chronic psychosocial stress decreases calcineurin in the dentate gyrus: a possible mechanism for preservation of early ltp. Neuroscience. 2003;117:869–874. doi: 10.1016/s0306-4522(02)00766-2. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hisano S. Vesicular glutamate transporters in the brain. Anat Sci Int. 2003;78:191–204. doi: 10.1046/j.0022-7722.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Chronic stress augments the long-term and acute effects of methamphetamine. Neuroscience. 2004;124:637–646. doi: 10.1016/j.neuroscience.2003.12.007. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Ni B, Rosteck PR, Jr., Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci U S A. 1994;91:5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ. Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology. 1996;14:443–452. doi: 10.1016/0893-133X(95)00152-4. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, McEwen BS. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. Proc Natl Acad Sci U S A. 2004;101:2179–2184. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-Fenfluramine on serotonin and glutamate release in rat ventral hippocampus: comparison with methamphetamine using in vivo microdialysis. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:422–428. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Staszewski RD, Yamamoto BK. Methamphetamine-induced spectrin proteolysis in the rat striatum. J Neurochem. 2006;96:1267–1276. doi: 10.1111/j.1471-4159.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS, Henn FA. Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry. 2001;50:809–812. doi: 10.1016/s0006-3223(01)01229-x. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Roginski RS, Choudhury K, Nilsson M, Ronnback L, Hansson E, Eriksson PS. Regulation of the glial glutamate transporter GLT-1 by glutamate and delta-opioid receptor stimulation. FEBS Lett. 1998;425:453–459. doi: 10.1016/s0014-5793(98)00288-9. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik SM, Rhee JS, Herzog E, Sigler A, Jahn R, Takamori S, Brose N, Rosenmund C. An essential role for vesicular glutamate transporter 1 (VGLUT1) in postnatal development and control of quantal size. Proc Natl Acad Sci U S A. 2004;101:7158–7163. doi: 10.1073/pnas.0401764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Pehek EA. A neurochemical heterogeneity of the rat striatum as measured by in vivo electrochemistry and microdialysis. Brain Res. 1990;506:236–242. doi: 10.1016/0006-8993(90)91256-g. [DOI] [PubMed] [Google Scholar]