Abstract
The Wnt/β-catenin signaling pathway is critical in both cellular proliferation and organismal development. However, how the β-catenin degradation complex is inhibited upon Wnt activation remains unclear. Using a directed RNAi screen we find that protein phosphatase 1 (PP1), a ubiquitous serine/threonine phosphatase, is a novel potent positive physiologic regulator of the Wnt/β-catenin signaling pathway. PP1 expression synergistically activates, and inhibition of PP1 inhibits, Wnt/β-catenin signaling in Drosophila and mammalian cells as well as in Xenopus embryos. The data suggest that PP1 controls Wnt signaling through interaction with, and regulated dephosphorylation of, axin. Inhibition of PP1 leads to enhanced phosphorylation of specific sites on axin by casein kinase I. Axin phosphorylation markedly enhances the binding of glycogen synthase kinase 3, leading to a more active β-catenin destruction complex. Wnt-regulated changes in axin phosphorylation, mediated by PP1, may therefore determine β-catenin transcriptional activity. Specific inhibition of PP1 in this pathway may offer therapeutic approaches to disorders with increased β-catenin signaling.
Keywords: Axin, CKI, GSK3; PP1, Wnt/β-catenin
Introduction
Wg/Wnt signaling is a highly conserved pathway that is critical for metazoan development. Aberrant regulation of this pathway leads to developmental defects in the early embryo and contributes to oncogenesis (Polakis, 2000; Logan and Nusse, 2004; Reya and Clevers, 2005). The molecular events that cause an increase in β-catenin stability upon Wnt ligand binding are intensively studied but not fully understood. In the absence of Wnt, β-catenin interacts with a cytosolic complex containing axin, casein kinase 1 (CKI)α, and APC and is then phosphorylated on Ser 45 by axin-bound CKIα (Liu et al, 2002). With β-catenin primed by CKIα, axin-bound glycogen synthase kinase 3 (GSK3) then phosphorylates a series of upstream residues in a stepwise manner, culminating in the creation of an E3 ubiquitin ligase-binding site, polyubiquitination of β-catenin, and its proteasome-mediated degradation. After specific Wnt molecules bind to membrane receptors of the Fz and LRP5/6 families, a signaling cascade is initiated that results in specific phosphorylation of the LRP5/6 cytoplasmic domain (Mao et al, 2001; Tamai et al, 2004; Davidson et al, 2005; Zeng et al, 2005). This enhances the affinity of LRP5/6 for axin. Subsequently, while CKIα phosphorylation of β-catenin does not change, β-catenin is stabilized through a decrease in the phosphorylation of β-catenin by GSK3 (Liu et al, 2002). Several mechanisms for the decreased phosphorylation of β-catenin by GSK3 have been proposed. The recruitment of Dvl and the GSK3 inhibitor FRAT to axin can inhibit axin-bound GSK3 kinase activity in overexpression studies (Yost et al, 1998; Li et al, 1999). However, the physiologic relevance of FRAT1 in Wnt signaling is unclear, as FRAT-deficient mice are developmentally normal and Wnt/β-catenin signaling is normal in FRAT-null mouse embryo fibroblasts (van Amerongen and Berns, 2005). Alternatively, it has been proposed that Wnt signals lead to axin degradation, thereby removing the β-catenin degradation complex (Lee et al, 2003; Tolwinski et al, 2003). However, recent studies suggest that axin degradation may be a secondary, long-term, positive feedback mechanism rather than the primary event that initiates β-catenin stabilization (Liu et al, 2005). Finally, GSK3 itself can be inhibited by phosphorylation by Akt, but most studies have found no acute regulation of GSK3 via Akt phosphorylation during Wnt signaling (Chen et al, 2000; Ding et al, 2000). Therefore, how β-catenin phosphorylation and degradation is inhibited upon Wnt stimulation is still unclear.
Wnt ligand binding results in significant changes in phosphorylation of multiple intracellular signaling components, including LRP5/6, axin, Dvl, CKIɛ, β-catenin, and TCF-4 (Jho et al, 1999; Ikeda et al, 2000; Lee et al, 2001; Amit et al, 2002; Gao et al, 2002; Davidson et al, 2005; Zeng et al, 2005). While several kinases (CKIα, CKIɛ, CKIγ, PAR-1, GSK3, CK2, and others) have been implicated in regulating Wnt signaling, the role of cellular phosphatases that oppose these kinases is less clear. Protein phosphatase 2A (PP2A) interacts with axin and APC and negatively regulates signaling (Hsu et al, 1999; Seeling et al, 1999; Li et al, 2001), but its direct targets are unknown. One report indicates PP2C interacts with axin and acts as a positive regulator of β-catenin signaling by dephosphorylating and decreasing the stability of axin (Strovel et al, 2000). PP1 is an abundant serine/threonine phosphatase that regulates multiple cellular functions including cell cycle progression and intermediary metabolism through interactions with a diverse array of targeting and regulatory subunits (Ceulemans and Bollen, 2004). PP1 is found in all eukaryotes from fungi to mammals. The mammalian genome encodes four PP1 catalytic (PP1c) subunits, PP1α, PP1β, PP1γ1, and PP1γ2 (Okano et al, 1997), while in Drosophila, three PP1α (96A, 87B, 13C) and one PP1β (FLW or 9C) genes have been identified (Dombradi et al, 1990, 1993). As the number of PP1c isoforms is small and they have nearly 90% amino-acid sequence identity, partial redundancy of the different PP1c isoforms has made it difficult to fully analyze their developmental role.
Here we report that PP1 plays a significant role in regulating β-catenin stability. In an RNAi screen for phosphatases regulating Wg signaling in Drosophila S2 cells, we found that the PP1 family plays a positive role in this pathway. PP1 physically interacts with axin, and both pharmacologic and genetic inhibition of PP1 increased axin phosphorylation, increased the binding of GSK3 to axin, and inhibited downstream signaling in insect and human cells and in Xenopus embryos. CKI was identified as the kinase that enhances axin–GSK3 binding. Four CKI phosphorylation sites in axin that influence the axin–GSK3 interaction were mapped and shown to be important in PP1-regulated binding of GSK3. Our data indicate that PP1 and CKI can reciprocally regulate the interaction of GSK3 with axin. Thus, Wnt may regulate the activity of the β-catenin destruction complex via PP1-mediated changes in Axin–GSK3 binding. Targeted inhibition of PP1 may provide a novel approach to inhibit Wnt/β-catenin signaling.
Results
PP1 is a conserved positive regulator of Wg/Wnt signaling upstream of Armadillo/-catenin
To identify potential cellular serine–threonine phosphatases that regulate Wg signaling, the catalytic subunits of known intracellular protein phosphatases were knocked down in Drosophila S2 cells using double-stranded RNA (dsRNA) and the effect on Wg signaling was assessed. This screen differs from that of DasGupta et al (2005) in that we specifically targeted potentially redundant genes with sets of related dsRNA. In our initial screen, knockdown of the PP1 catalytic subunit family (PP1c) inhibited Wg/Armadillo (Drosophila β-catenin) signaling (Figure 1A). Simultaneous knockdown of three PP1c isoforms (96A, 87B, and FLW) significantly inhibited LEF-1-mediated transcription induced by partial knockdown of dAxin or by coexpression of Wg and Fz2, but did not inhibit transcription activated by ArmS10 expression (Figure 1B). ArmS10 is a mutant of Armadillo that lacks the domain required for phosphorylation-regulated degradation. These data indicates that PP1 is required for Wg signaling upstream of Armadillo.
Figure 1.
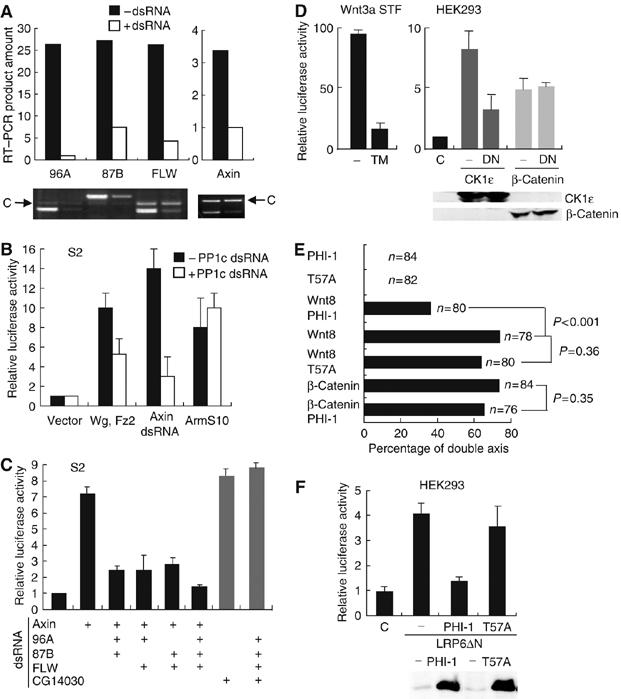
PP1 regulates Wg/Wnt signaling upstream of Arm/β-catenin. (A) RNAi-mediated knockdown of gene expression in S2 cells. Total RNA was extracted from S2 cells 72 h after RNAi, and analyzed by RT–PCR. An unrelated RNA fragment indicated by the arrow was amplified and run on the same gel as a control (marked C) for RNA recovery. The bar graph shows the relative quantity of RT–PCR product before (black) and after (white) RNAi treatment. Of note, dAxin mRNA knockdown was partial (∼70%). (B) Knockdown of PP1c genes inhibits signaling activity induced by expressing Wg and Fz2 or by partial depletion of axin, but not by stable Armadillo (ArmS10). S2 cells were transfected with 100 ng of empty vector or Wg/Fz2 or ArmS10 together with 100 ng of each of pPac-PL-LEF-1, TOPFLASH, and SV40-Renilla luc 3 days after RNAi of 96A, 87B, and FLW. At 24 h post-transfection, cells were lysed and luciferase activity was measured as described. The results shown are mean±s.e. of three independent experiments and were repeated. (C) PP1c isoforms redundantly regulate Wg signaling. dsRNA targeting PP1c 96A, 87B, and FLW were added to S2 cells as indicated together with dsRNA targeting axin or CG14030 as indicated. pPac-PL-LEF-1, TOPFLASH, and SV40-Renilla luc plasmids were transfected 3 days later. At 24 h after transfection, cells were lysed and luciferase activity was determined. The results shown are the mean±s.d. of three independent experiments and were repeated. (D) Inhibition of PP1 by tautomycin (TM) or DN-PP1 (DN) abolished LEF-1-mediated transcription induced by Wnt3a or CKIɛ, but not stabilized β-catenin in mammalian cells. Left, STF cells (293 cells with integrated TOPFLASH reporter) (Xu et al, 2004) expressing Wnt3a were treated with or without 1 μM TM for 7 h before assay. Right, HEK293 cells were cotransfected with 100 ng of CKIɛ or stabilized β-catenin expression plasmids and 200 ng of DN-PP1 expression plasmid together with 100 ng each of pEV3S-LEF-1, TOPFLASH, and SV40-Renilla luc. Luciferase activity was measured at 24 h post-transfection as described. Results shown are averages±s.e. of three experiments and was repeated. (E) The PP1 inhibitor PHI-1, but not inactive PHI-1(T57A), blocks secondary axis formation induced by Wnt8 in Xenopus embryos. Xenopus embryos were injected ventrally at the four-cell stage with 300 pg of PHI-1 or PHI-1(T57A) RNA and/or 500 pg of Wnt8 or 200 pg of β-catenin S45A RNA. RNA was adjusted to 800 pg per injection by adding GFP RNA when necessary. (F) PHI-1 but not PHI-1(T57A) inhibits LEF-1-mediated transcription induced by LRP6ΔN. HEK293 cells were transfected with 100 ng of LRP6ΔN expression plasmid together with 100 ng of each of pEV3S-LEF-1, TOPFLASH, and SV40-Renilla luc in the absence or presence of 200 ng of PHI-1 or PHI-1(T57A) plasmid. Lower panel: Equal expression of PHI-1 or PHI-1(T57A) was validated by immunoblotting.
PP1c subunits exhibit functional redundancy in several systems, presumably due to high similarity in their amino-acid sequence (Doonan et al, 1991; Komeili and O'Shea, 1999). We found that Wg signaling activated by dAxin RNAi was totally inhibited only when three PP1c genes were simultaneously knocked down (Figure 1C). This inhibition is not due to nonspecific toxicity, as triple knockdown of PP1c did not reverse the signal induced either by expression of ArmS10 (Figure 1B) or by knockdown of CG14030, a recently identified downstream negative regulator of Wg/Armadillo signaling (DasGupta et al, 2005) (Figure 1C). These data indicate that PP1c isoforms redundantly regulate Wg signaling in S2 cells and epistatically position it upstream of both CG14030 and Armadillo.
Multiple elements of the Wg/Wnt signaling pathway are conserved between invertebrates and vertebrates. We next tested whether PP1 is a conserved regulator of Wnt signaling in mammalian cells. Tautomycin (TM), a small molecule semiselective inhibitor of PP1, markedly reduced the activity of an integrated β-catenin-responsive reporter in human embryonic kidney (HEK293) cells expressing Wnt3A (Figure 1D). To achieve more specific inhibition of PP1, a dominant negative form of PP1 (PP1(D95N), DN-PP1) (Furnari et al, 1997) was expressed in HEK293 cells. DN-PP1 expression inhibited pathway activation caused by the Wnt signaling activator CKIɛ, but it had no effect on transcription driven by stabilized β-catenin (Figure 1D). These findings suggest that the role of PP1 in Wnt signaling is conserved between invertebrates and mammals.
This result was next extended to a physiologic/developmental setting. We asked whether inhibition of PP1 activity by PHI-1, a specific PP1 inhibitor that requires phosphorylation at T57 for activity (Eto et al, 1999; Deng et al, 2002), could reduce the secondary axis formation that is a typical phenotype of ectopic Wnt signaling in early vertebrate development. Ventral injection of XWnt8 RNA into four cell Xenopus embryos caused secondary axis formation in about 75% of the injected embryos (Figure 1E). Neither PHI-1 nor PHI-1(T57A) RNA ventral injection alone induced an obvious phenotype, as expected, given the absence of Wnt/β-catenin signaling in ventral cells. The ability of XWnt8 to induce a secondary axis was dependent on PP1 function, since coexpression of PHI-1 but not inactive PHI-1(T57A) with XWnt8 significantly reduced the number of embryos with ectopic secondary axis (P<0.001). However, PHI-1 coexpression did not inhibit the secondary axis formation induced by stabilized β-catenin, confirming that PP1 acts upstream of β-catenin (Figure 1E). The effect of PHI-1 is not unique to Xenopus, because PHI-1 but not PHI-1(T57A) expression also reduced Wnt/β-catenin signaling in HEK293 cells (Figure 1F). Because inhibition of Xenopus secondary axis formation is a highly sensitized assay, we also tested if inhibition of PP1 could block primary axis formation, which is dependent on an endogenous Wnt signal. Dorsal injection of PHI-1 RNA induced ventralized embryos (data not shown). Thus, PP1 appears to regulate primary and ectopic secondary axes, supporting its physiological significance.
In summary, RNA interference, small molecule and dominant negative and physiologic inhibitors of PP1 in Drosophila, and mammalian cells and Xenopus embryos all indicate that PP1 functions downstream of the membrane and upstream of activated β-catenin as a conserved positive regulator of Wnt/β-catenin signaling.
PP1 is a synergistic regulator of Wg/Wnt signaling
Overexpression of PP1 alone is not sufficient to ectopically activate Wg/Wnt signaling. None of three PP1c isoforms overexpressed individually in S2 cells stimulated Wg/Arm signaling (Figure 2A). However, when any one of the PP1c genes was coexpressed in combination with Wg and Fz2, transcription from an LEF-1/TCF-dependent promoter was synergistically increased compared to the transcription induced by Wg and Fz2 alone. Differences in synergy between PP1 isoforms were consistently observed and may be related to sequence differences but were not further studied. Similar to the result in insect cells, synergistic coactivation of TCF-dependent transcription was seen when mammalian PP1α was coexpressed with Wnt3a in HEK293 cells (Figure 2B). To test whether PP1 also synergizes with Wnt signaling in intact animals, XWnt8 and/or PP1 RNA was ventrally injected into Xenopus embryos. To better detect an interaction, an amount of XWnt8 RNA was chosen that induces secondary axes in about 20% of the animals. As shown in Figure 2C, injection of PP1 RNA alone did not cause axis duplication in embryos. However, coinjection of XWnt8 with PP1 RNA significantly (P=0.027) increased secondary axis formation, confirming PP1 is a conserved synergistic positive regulator of Wnt signaling in cells as well as in embryos.
Figure 2.
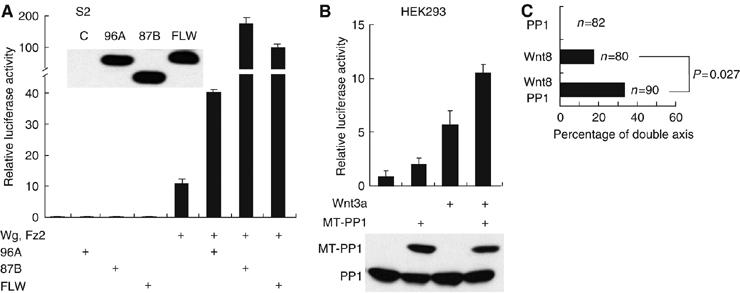
PP1 synergistically activates Wg/Wnt signaling. (A) Individual wild-type PP1 genes activate signaling in the presence of Wg and Fz2 in Drosophila cells. In total, 100–200 ng of pAFW-PP1c 96A or 87B or FLW plasmids (adjusted to achieve equal protein expression) as indicated was cotransfected with or without 100 ng of each of Wg and Fz2 plasmid, together with 100 ng of each of pPac-PL-LEF-1, TOPFLASH, and SV40-Renilla luc plasmids in S2 cells. Cell lysates were subjected to luciferase assays and immunoblotting. (B) PP1α activates β-catenin signaling induced by Wnt3a in human cells. HEK293 cells were transfected with 100 ng of Myc-epitope-tagged-(MT)-PP1α and/or 100 ng of pCS2-Wnt3a expression plasmids together with 100 ng of each of pEV3S-LEF-1, TOPFLASH, and SV40-Renilla luc plasmids. Luciferase activities were measured as described. The lower panel shows the PP1α protein abundance determined by immunoblotting with anti-PP1 antibody in total cell lysates. Results shown are the average±s.e. of three replicates and repeated on a separate occasion. (C) PP1 RNA coinjection increases secondary axis formation induced by Wnt8 expression in Xenopus embryos. Embryos were injected at the four-cell stage in ventral blastomere with 100 pg of Wnt8 RNA and/or 500 pg of PP1 RNA. Total RNA amounts were adjusted using GFP RNA when necessary. Significance was calculated as described in Figure 1E.
PP1 regulates Armadillo/β-catenin abundance
As epistasis experiments placed PP1 upstream of β-catenin, we asked if PP1 regulates the abundance of β-catenin. Partial dAxin knockdown induced an increase in free armadillo abundance, which was inhibited by knockdown of PP1, but not of the unrelated Protein Phosphatase 2A regulator B55 (PP2A B55) (Figure 3A). Thus, PP1 activity contributes to the accumulation of armadillo. To test if PP1 also regulates the abundance of β-catenin in mammalian cells, two different phosphatase inhibitors, TM or okadaic acid (OA), with differential effects on PP1 and PP2A (Favre et al, 1997) were added to Wnt3a-treated mouse L cells. TM, relatively selective for PP1, decreased the abundance of β-catenin in a dose-dependent manner (Figure 3B, left). OA, relatively selective for PP2A, did not decrease β-catenin abundance. The specific PP1 inhibitors DN-PP1, I-2, and PHI-1 also reduced the abundance of epitope-tagged β-catenin in HEK293 cells (Figure 3B, right). Thus, PP1 appears to regulate β-catenin protein abundance in both insect and mammalian cells.
Figure 3.
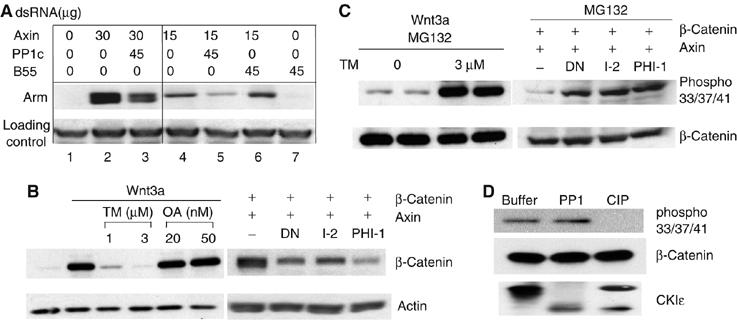
PP1 regulates Arm/β-catenin abundance. (A) The abundance of armadillo is reduced by PP1 knockdown. Drosophila S2 cells were treated with dsRNA targeting axin and either PP1c (96A, 87B, and FLW together) or PP2A B55 as indicated for 3 days. Cells were then lysed in hypotonic buffer and free Armadillo was extracted and analyzed by SDS–PAGE and immunoblotting. A nonspecific band was used as a loading control. (B) The abundance of β-catenin is reduced by PP1 inhibition. Left, mouse L cells were treated with control or Wnt3a-conditioned medium together with or without different concentrations of TM or OA as indicated for 7 h. Right, HEK293 cells were transfected with 1 μg of myc-β-catenin and 1 μg of DN-PP1 or I-2 or PHI-1 together with 1 μg of HA-axin expression plasmid. Cells were lysed with hypotonic buffer 24 h post-transfection. In both cases, cell lysates were analyzed by immunoblotting using anti-β-catenin and anti-actin antibodies. OA activity was verified by its ability to stabilize overexpressed β-catenin as previously described (data not shown)(Seeling et al, 1999). (C) β-Catenin phosphorylation was increased in the presence of PP1 inhibitors. Left, L cells were incubated with Wnt3a-conditioned medium with or without 3 μM of tautomycin in the presence of 30 μM of MG132 for 3 h. Right, β-catenin and HA-axin were expressed in HEK293 cells in the absence or presence of DN-PP1, I-2, or PHI-1. After 24 h, cells were treated with 30 μM of MG132 for 3 h before lysing with hypotonic lysis buffer and analysis by SDS–PAGE and immunoblotting. (D) β-Catenin was not dephosphorylated by PP1 in vitro. β-Catenin was immunoprecipitated from cells extracts of L cells treated with 50 nM of Calyculin A and 30 μM of MG132 for 3 h. Immunoprecipitates were incubated with phosphatase buffer only, PP1, or CIP for 30 min at 37°C. Autophosphorylated recombinant CKIɛ served as a control substrate for phosphatases.
Degradation of β-catenin is dependent on its phosphorylation by GSK3 and CKIα, while bound to axin (Liu et al, 2002). We therefore tested if inhibition of PP1 increased the phosphorylation of β-catenin. To block degradation of phosphorylated β-catenin, the proteasome inhibitor MG132 was used. Phosphorylation of β-catenin at Ser33/Ser37/Thr41 (GSK3 sites) was markedly increased when PP1 was inhibited by TM (Figure 3C, left), or other PP1 inhibitors (DN-PP1, I-2, PHI-1) (Figure 3C, right). Taken together, these results suggest that PP1 regulates Wnt/β-catenin signaling by regulating the rate of phosphorylation of β-catenin.
One potential mechanism by which PP1 could stabilize β-catenin is by direct dephosphorylation. To test if β-catenin is in fact a substrate of PP1, phospho-β-catenin was immunopurified from HEK293 cells and incubated with purified phosphatases. As Figure 3D shows, phospho-β-catenin can be dephosphorylated by calf intestinal phosphatase but not by PP1. The PP1 was active, as it was still able to dephosphorylate CKIɛ. Thus, PP1 is unlikely to stabilize β-catenin by direct dephosphorylation.
PP1 physically interacts with and dephosphorylates axin
β-Catenin phosphorylation is regulated by an axin–kinase complex. As PP1 acts upstream of β-catenin, and PP1 does not target β-catenin directly, we hypothesized that PP1 might regulate β-catenin phosphorylation via the degradation complex. We first tested whether PP1 interacts with axin. As shown in Figure 4A, axin co-precipitates with endogenous as well as overexpressed wild-type PP1 and DN-PP1 (lanes 2–4). In parallel experiments, PP1 did not interact with β-catenin (data not shown). We also found that endogenous PP1 interacts with endogenous axin in untransfected HEK293 cells (Figure 4B). The domain in axin required for PP1 binding was mapped using three distinct HA-tagged axin constructs. Both full-length and the carboxyl-terminal half of axin co-immunoprecipitated PP1, while the amino-terminal half of axin did not, indicating that the carboxyl-terminal region of axin is required for interaction with PP1 (Figure 4C).
Figure 4.
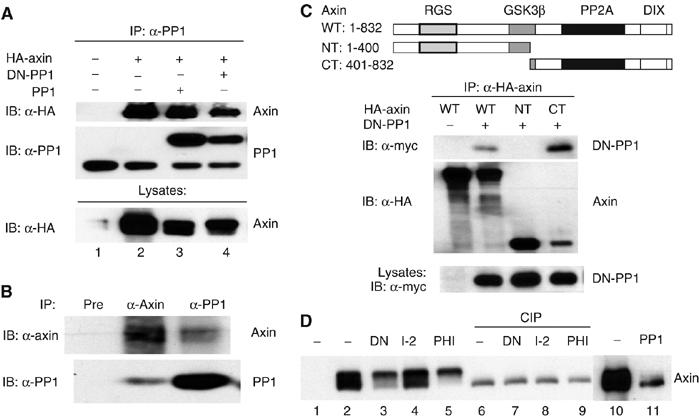
PP1 associates with and dephosphorylates axin. (A) PP1 interacts with axin in vitro. HA-axin expression plasmid (1 μg) was transfected with or without 1 μg of wild-type PP1 or DN-PP1 into HEK293 cells. PP1 proteins were immunoprecipitated from 300 μg of extract. Immunoprecipitates and cell lysates were analyzed by immunoblotting. (B) Endogenous PP1 associates with axin. Endogenous axin and PP1 were immunoprecipitated from 5 mg of total cell lysates from untransfected HEK293 cells. Axin preimmune serum was used as negative control in immunoprecipitation (pre, lane 1). (C) Identification of the PP1 and axin interaction domain in axin. Top, schematic diagram indicating the axin deletion constructs used in this experiment. HEK293 cells were transfected with 1 μg of each of the HA-axin expression constructs and 1 μg of DN-PP1 and immunoprecipitated with antibodies to the HA epitope before immunoblotting. (D) Inhibition of PP1 leads to axin hyperphosphorylation, while PP1α overexpression decreases axin phosphorylation. HEK293 cells were cotransfected with 1 μg of HA-axin and 1 μg of DN-PP1, PP1 I-2, PHI-1, or PP1α plasmids. Cells were lysed 24 h after transfection in the absence of phosphatase inhibitors. Lanes 6–9, 50 μl of cell lysate was treated with or without 1 μl of alkaline phosphatase before immunoblotting.
Axin electrophoretic mobility appears to decrease when PP1 activity is inhibited by diverse means (Figure 4A and D). This mobility shift is due to phosphorylation, as it is eliminated by treatment with calf intestinal alkaline phosphatase (CIP). Thus, axin phosphorylation in cells is regulated by PP1. PP1 overexpression also causes an increase in axin electrophoretic mobility (Figure 4D, lanes 10 and 11), consistent with the hypothesis that axin is a substrate for PP1 in cells.
Inhibition of PP1 results in increased association of axin and GSK3β
Wnt signaling results in dephosphorylation of axin (Willert et al, 1999), dissociation of the β-catenin degradation complex (Gao et al, 2002; Liu et al, 2005), and subsequent stabilization of β-catenin. We therefore tested whether binding of PP1 to axin and dephosphorylation of axin by PP1 regulates the assembly of the degradation complex. Interestingly, we found the interaction of axin and endogenous GSK3 (Figure 5A, lanes 1–3), but not that of axin and APC or axin and β-catenin (Supplementary Figure 2), was markedly increased when we coexpressed DN-PP1 with axin. Coexpression of other PP1 inhibitors (I–2 and PHI-1) gave a similar result (Figure 5A, lanes 4–7). Furthermore, TM but not OA significantly increased the binding of axin and endogenous GSK3, further demonstrating the role of PP1 but not PP2A in regulating the axin–GSK3 interaction. As PP1 inhibition results in an increase both in axin phosphorylation and GSK3 binding, this suggests that PP1 regulates a phosphorylation-dependent interaction of GSK3 with axin.
Figure 5.
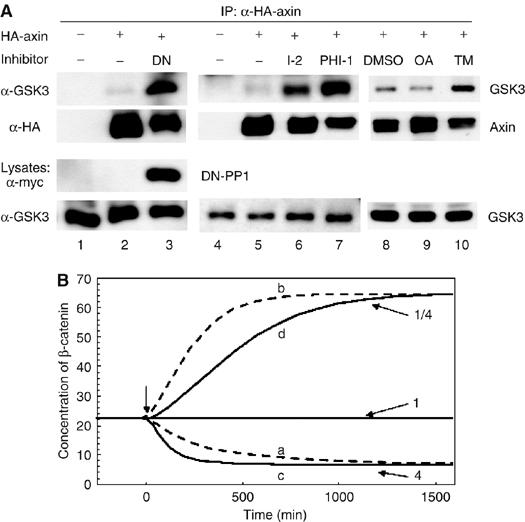
PP1 inhibition results in increased association of axin and GSK3. (A) HA-axin was expressed alone (lanes 8–10) or with vector, DN-PP1, I-2, or PHI-1 (lanes 1–7) (1 μg plasmid each) in HEK293 cells. For lanes 8–10, 24 h after transfection, cells were treated with 20 nM of OA or 1 μM of TM for 7 h. Cells were lysed and axin was immunoprecipitated from 300 μg of extract using an anti-HA antibody. Co-immunoprecipitation of endogenous GSK3 was analyzed by immunoblotting using anti-GSK3 antibody. (B) Equivalent changes in β-catenin concentrations resulting from changes in GSK3–axin binding and changes in axin synthesis. The curves were calculated using a model of the Wnt signaling pathway based on data from Xenopus oocyte extracts (Lee et al, 2003) and Supplementary Figure 1. In curve a and b, the constant (K6) of GSK3 binding to the APC/axin complex is increased (curve a) and decreased (curve b) by a factor of 4 (t<0:K6=0.1 nM−1, t⩾0:K6=0.4 nM−1 (curve a), K6=0.025 nM−1 (curve b)). Curves c and d are obtained by fourfold changes in the rate of axin synthesis t<0: ν14=8.22 × 10−5, for t⩾0 fourfold increase or fourfold decrease of that value).
Could simply altering the affinity of GSK3 for axin be sufficient to account for the observed changes in β-catenin stability? To model this, the effects of changing the strength of axin–GSK3 binding on β-catenin stabilization were assessed using a mathematical model developed for Wnt signaling (Lee et al, 2003; Heinrich, 2005 and Supplementary Figure 1). The model predicts that changes in the strength of GSK3–axin binding can cause a significant decrease or increase of β-catenin concentration (Figure 5B and Supplementary Figure 1). Thus, consistent with the concept that the activity of the axin complex is the rate-limiting step in Wnt/β-catenin signaling, changes in the affinity of GSK3 for axin appears sufficient to explain the observed effect on β-catenin abundance.
PP1 dephosphorylation of axin in CKI phosphorylation sites renders axin unfavorable for GSK3β binding
Axin can be phosphorylated by GSK3 and CKI (Jho et al, 1999; Yamamoto et al, 1999; Gao et al, 2002). Our data suggest that phosphorylation of a PP1-sensitive site in axin enhances GSK3 binding. To investigate which kinase might augment GSK3 binding, we inhibited GSK3 or CKI (Naerum et al, 2002; Rena et al, 2004), and found that inhibition of CKI with D4476 blocked the PHI-1-induced increase in axin–GSK3 binding, while inhibition of GSK3 by GSK3 Inh-2 (2-Thio(3-iodobenzyl)-5-(1-pyridyl)-[1,3,4]-oxadiazole) did not (Figure 6A). We noted an increase in basal axin–GSK3 binding in the presence of GSK3 Inh-2 and speculate that this is due to the well-documented regulation of PP1 activity through GSK3 phosphorylation of endogenous PP1 inhibitor-2 (when GSK3 is inhibited, PP1 is further inhibited by dephospho-inhibitor-2) (Resink et al, 1983). However, in the case of GSK3 Inh-2, PHI-1 expression results in a further increase in the axin–GSK3 association. In contrast, CKI inhibition by D4476 blocks the PHI-1-induced increase in axin–GSK3 interaction (Figure 6A). These results suggest that CKI phosphorylation of axin enhances GSK3 binding.
Figure 6.
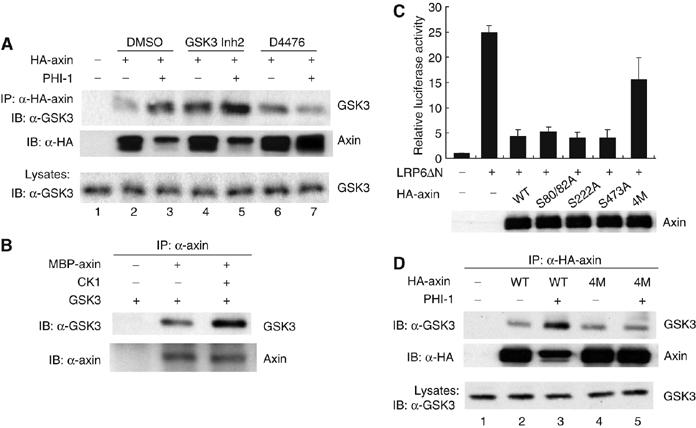
CKI phosphorylation of axin enhances GSK3 binding. (A) CKI inhibition blocks the PHI-1-stimulated increase in GSK3–axin interaction. HA-axin was expressed in HEK293 cells without or with PHI-1 (1 μg plasmid each). At 24 h after transfection, cells were treated with DMSO or 1 μM of GSK3 Inh2 for 2 h or 100 μM of D4476 for 1 h. After treatment, cells were lysed and axin–GSK3 co-immunoprecipitation was analyzed as above. (B) Axin phosphorylation by CKI increases axin–GSK3 interaction in vitro. Recombinant MBP-axin (0.3 μg) was incubated with GSK3 (0.3 μg) after incubation without or with recombinant CKIɛΔC (0.05 μg) and ATP (0.5 mM) at 37°C for 15 min. Axin protein was pulled down by anti-axin protein A beads. Co-precipitation of GSK3 was detected by immunoblotting. (C) Mutation of CKI phosphorylation sites in axin decreases axin activity. Cells were transfected with 100 ng of LRP6ΔN plasmid to stimulate β-catenin signaling, and 100 ng of each of pEV3S-LEF-1, TOPFLASH, and SV40-Renilla luc. Where indicated, 100 ng of each of the axin constructs was included. Cells were lysed 24 h after transfection and luciferase activity was assayed. (D) Mutation of CKI phosphorylation sites blocked the phosphorylation-activated axin–GSK3 binding. Cells were transfected with 1 μg of HA-axin or HA-axin(4M) in the absence or presence of 1 μg of PHI-1. The interaction of axin and GSK3 was assessed as above.
The effect of CKI on the axin–GSK3 interaction in cells could be direct or indirect. To test if direct phosphorylation of axin regulates the binding of GSK3, we utilized bacterially expressed recombinant proteins. Axin is phosphorylated in vitro by CKI on multiple sites, while GSK3 is not a CKI substrate (Gao et al, 2002; L Klimowski, BG, DH, and DMV, data not shown). The presence of active CKI significantly enhanced the binding of purified GSK3 to axin (Figure 6B), demonstrating that direct phosphorylation of axin regulates the interaction of axin and GSK3. To identify the sites in axin that are regulated by CKI and PP1, a mass spectrometric analysis of axin phosphorylated in vivo before and after inhibition of PP1 was carried out. Four sites, S80, S82, S222, and S473, were identified to be PP1 regulated (Supplementary Figure 3). Three of them (S80, S82, and S473) were also phosphorylated in vitro by CKI and are conserved between axin1 and axin2/conductin.
The contribution of these phosphorylation sites to axin's ability to repress Wnt signaling was assessed. Axin with the individual S80/82A, S222A, or S473A mutations suppressed transcription induced by LRP6ΔN as effectively as wild-type axin. In contrast, the quadruple mutant S80/82/222/473A, axin(4M), expressed normally but was markedly hindered in its repressing activity (Figure 6C). This suggests that cumulative phosphorylation of axin is required for it to fully downregulate Wnt/β-catenin signaling.
The data suggest that PP1 stabilizes β-catenin through site-specific dephosphorylation of axin, leading to decreased binding of GSK3. If this is true, then inhibition of PP1 should not further increase the GSK3–axin(4M) interaction. Indeed, while axin(4M) still interacted with GSK3, this binding was no longer enhanced by PHI-1 coexpression (Figure 6D, lanes 4 and 5). Taken together, these results indicate that PP1 dephosphorylation of axin at CKI sites weakens the association of axin and GSK3, leading to the stabilization of β-catenin and upregulation of β-catenin/TCF signaling.
Discussion
The Wnt/β-catenin signaling pathway has been the object of intense study due to its role in development, proliferation, and stem cell maintenance. One key consequence of Wnt action is stabilization of β-catenin and the resultant alteration of cellular transcription. Here we identify a new conserved component in the Wnt signaling pathway. We find that PP1 is a positive regulator of β-catenin signaling, and that it controls the effective concentration of the Axin–GSK3 complex through a cycle of phosphorylation and dephosphorylation of axin, a scaffold protein in the β-catenin degradation complex (see model, Figure 7) (Luo and Lin, 2004). Phosphorylation of axin by CKI on multiple sites enhances GSK3 binding, potentially through a cumulative conformational change, while dephosphorylation of axin via PP1 releases GSK3, leaving β-catenin phosphorylated by CKIα alone. PP1-dependent dephosphorylation of axin therefore results in accumulation of β-catenin, and stimulation of Wnt/β-catenin/LEF-1-dependent transcription.
Figure 7.
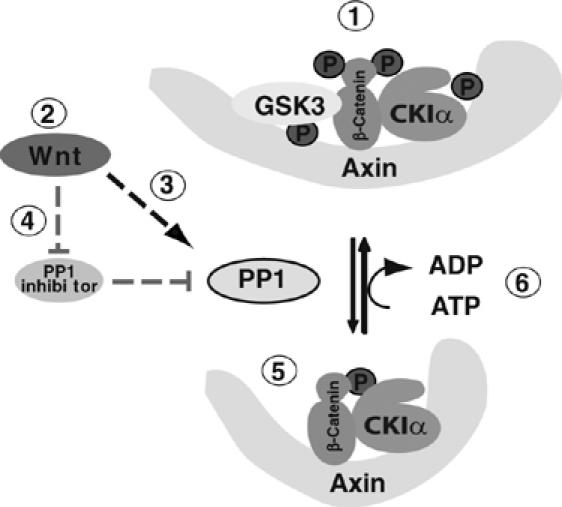
Model: How PP1 regulates the Wnt signaling pathway. In the absence of Wnt (1), the axin/CKIα/GSK3 β-catenin degradation complex phosphorylates β-catenin, leading to its degradation. (2), in the presence of Wnt, PP1 activity on axin increases, either by (3) increased phosphatase activity, or by (4) inhibition of a PP1 inhibitor. Dephospho-axin assumes a conformation unfavorable for GSK3 binding (5), and hence only CKIα remains to phosphorylate β-catenin on ser 45. When PP1 activity decreases, axin is rephosphorylated (6), allowing GSK3 to bind, and degradation of β-catenin to resume.
A detailed quantitative model of the Wnt/β-catenin pathway predicts that small changes in the abundance of the axin–GSK3 complex can have large effects on β-catenin stability. Changes in the abundance of this complex can be achieved in at least two different ways. First, the abundance of axin can be decreased by Wnt signaling, an effect that has been considered previously (Lee et al, 2003). However, a decrease in axin abundance upon Wnt signaling may be a long-term feedback effect, rather than an acute event (Liu et al, 2005). Changes in the axin–GSK3 complex can also be achieved by decreasing the amount of GSK3 bound to axin (see models, Figure 7 and Supplementary Figure 1). Importantly, the interaction of GSK3 with the axin scaffold enhances phosphorylation of β-catenin by >20 000-fold (Dajani et al, 2003). The mathematical modeling is, therefore, consistent with our data that small decreases in the amount of GSK3 bound to axin produces significant increases in free β-catenin and downstream signaling.
During the initial RNAi screen using dAxin knockdown, it was not known that dAxin was a target of PP1. Subsequent studies lead us to Axin as the relevant target of PP1, raising the question of how PP1 worked under conditions of axin depletion. In our RNAi assays, the knockdown of dAxin was partial and the remaining dAxin in the cells can have its activity significantly increased by inhibition or knockdown of PP1. When the remaining dAxin is more highly phosphorylated, it is more able to recruit GSK3. In fact, we found that the amount of axin-bound GSK3 increased several fold upon PP1 inhibition, as demonstrated in Figure 5A. This indicates that the specific activity of the β-catenin destruction complex increases markedly despite partial axin depletion. This explains why PP1 inhibition produces a decrease in β-catenin abundance under conditions of axin depletion.
There are several potential reasons why PP1 has not been implicated in the Wg/Wnt pathway earlier. As there are four highly homologous PP1 genes, it is likely that there is functional redundancy between the isoforms in the Wg/Wnt pathway. In our studies, simultaneous knockdown of two or three PP1c genes is required to block Wg signaling in Drosophila S2 cells. Second, due to the high conservation of PP1, it is possible that dominant negative PP1 phenotypes might be lethal before the appearance of a Wg/Wnt phenotype. Furthermore, overexpression of PP1 alone does not produce a Wg phenotype, suggesting that PP1 catalytic subunit abundance is not rate limiting for axin dephosphorylation. Notably, however, PP1 expression synergizes with activators of Wg/Wnt signaling in Drosophila and HEK293 cells and in Xenopus embryogenesis, indicating that PP1 might require activation by Wg/Wnt so it can dephosphorylate its downstream target(s) (see model, Figure 7).
Reversible protein phosphorylation is arguably the most important controlling event in the Wnt/β-catenin signaling pathway. A remarkable functional pleiotrophy is seen in the multiple roles GSK3 and members of the casein kinase I family play in the Wnt pathway. GSK3 and CKI are each involved in multiple positive and negative steps in Wnt signaling. CKIα primes β-catenin for GSK3 phosphorylation, while CKIγ1 has recently been shown to phosphorylate the cytoplasmic domain of LRP6 after priming by GSK3 (Davidson et al, 2005; Zeng et al, 2005). CKIɛ and GSK3 also phosphorylate Dvl and APC (Salic et al, 2000; Gao et al, 2002). Our study adds another intimately paired role for CKI and GSK3. We identified multiple novel CKI phosphorylation sites in axin that stimulate GSK3 binding of axin, leading to the enhanced phosphorylation and subsequent degradation of β-catenin. It is of note that a large-scale Drosophila siRNA screen for regulators of the Wg/Armadillo pathway uncovered an additional CKIα-related protein, CG2577, as a negative regulator (DasGupta et al, 2005). It is therefore possible that four distinct CKI genes (CKIα, CG2577, CKIγ1, and CKIɛ) positively and negatively regulate the Wnt/β-catenin pathway.
PP1 is also a complex family of enzymes, with its four related catalytic subunits interacting with a large variety of targeting subunits that direct PP1 to substrates as diverse as myosin light chain, glycogen, and p53 (Ceulemans and Bollen, 2004). In addition, PP1 is regulated by a large number of small phosphoproteins such as DARPP-32, NIPP-1, I-2, and PHI-1 that are regulated by signaling-mediated phosphorylation. Further study is needed to determine the role of these PP1 targeting and regulatory subunits in the Wnt/β-catenin pathway. Based on the multiple roles for CKI, GSK3, and PP2A in the Wnt pathway, we anticipate further complexities and pleiotropy for PP1 as its function in Wnt signaling is studied in greater depth. Of note, it was reported recently that Gα proteins may also mediate the disruption of axin–GSK3 interactions in response to prostaglandin E2 (PGE2) and Wnt3A (Castellone et al, 2005; Liu et al, 2005). Whether PGE2 and Gα play roles in the regulation of PP1 is the subject of ongoing study.
Materials and methods
Plasmids, cells, and reagents
Anti-armadillo antibody was from Developmental Studies Hybridoma Bank. Anti-β-catenin and anti-GSK3β antibodies were from BD Transduction Laboratories; phospho-β-catenin (Ser33/37/Thr41) antibody was from Cell signaling; anti-PHI-1 antibody was a gift from Masumi Eto and Dr D Brautigan (University of Virginia). GSK3β inhibitor 2 and tautomycin were from Calbiochem; CKI inhibitor D4476 was a gift from Drs G Rena and P Cohen. Drosophila PP1c 96A, 87B, and FLW were PCR-amplified from a Drosophila cDNA library and cloned into Gateway vector pAFW (Drosophila Genomics Resource Center, Bloomington, IN) by Gateway technology (Invitrogen). Constitutively activated Armadillo expression construct pAFW-ArmS10 (Pai et al, 1997) was generated by Gateway cloning as described above followed by deletion of aa 34–87 using QuikChange mutagenesis (Stratagene). Constructs expressing Drosophila Wg and Fz2 pMK-Wg and Fz2 were gifts from Dr S Yanagawa (Kyoto University, Japan) and used as described previously (Yanagawa et al, 1998). pCS2-XWnt8, pcDNA3-Wnt3a, pCS2-VSVG-LRP6ΔN, pcDNA3-myc-PP1α, pcDNA3-myc-PP1α D95N, pKVYFP-PHI-1, and pCMV2-I-2 were described previously (Swiatek et al, 2004; Tamai et al, 2004; Tountas and Brautigan, 2004). To construct pPac-PL-LEF-1 that expresses LEF-1 in Drosophila cells, pEV3S-LEF-1 was digested with NheI, blunted with Klenow fragment, and digested with BamHI. The released LEF-1 DNA was inserted into pPac-PL vector digested with XbaI, blunted with Klenow fragment, and digested with BamHI. To create pCS2-PHI-1 to make RNA for Xenopus embryo injections, PHI-1 DNA fragment was released from pKVYFP-PHI-1 with BamHI and EcoRI digestion; and was inserted into pCS2 vector also digested with BamHI and EcoRI. Axin constructs pCMV5-HA-AxinWT, Axin NT, and Axin CT were gifts from Dr SC Lin (Xiamen University, PR China). Culture and transfection of S2 and HEK293 cells was described previously (Li et al, 2002) (Swiatek et al, 2004). Preparation of dsRNA and knockdown of target genes in S2 cells were performed as previously described (Li et al, 2002).
Luciferase assays
Luciferase assays were performed using the Dual-luciferase reporter assay kit (Promega, Alam and Cook, 1990). In all, 100 ng each of pEV3S-LEF-1 (the generous gift of Marian Waterman), TOPFLASH reporter (Korinek et al, 1997), and SV40-Renilla-luc were cotransfected in the presence of 20 ng of Wg/Wnt activator and/or 20–100 ng of PP1 or PP1 inhibitors or axin constructs and luciferase activity was measured 24–48 h later. For luciferase assays in RNAi-treated cells, transfection was carried out 3 days after RNAi and luciferase activity was measured as described above.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously (Swiatek et al, 2004).
mRNA injection in Xenopus embryos
Genes of interest in pCS2 were linearized and used as templates for synthesis of RNA using MEGAscript (Ambion). RNA was then purified using MEGAclear (Ambion). Xenopus embryos were obtained by in vitro fertilization and raised in a standard amphibian saline solution (12% modified Marc's Ringer, MMR) at 23°C. Embryos were injected at the four-cell stage in ventral or dorsal blastomeres as indicated. After injection, embryos were kept in 12% MMR at room temperature until reached the required stage and scored. Significance was calculated by the z-test using SigmaStat software.
Mass spectrometry
SDS–PAGE gel bands of immunoprecipitated HA-axin were in-gel digested with trypsin according to standard protocols (Shevchenko et al, 1996). The extracted peptides were then concentrated to a volume of about 1.0 μl and brought up to 20 μl with 0.1% acetic acid for LC-MS/MS. Samples was loaded onto a 360 μm o.d. × 75 μm i.d. microcapillary-fused silica tubing packed with C18 irregular 5–20 μm-sized resin (Polymicro Technologies, Phoenix, AZ). After sample loading, the pre-column was washed with 0.1% acetic acid for 15 min to remove any buffer salts. The pre-column was then connected to a 360 μm o.d. × 50 μm i.d. analytical column (Polymicro Technologies, Phoenix, AZ) packed with C18 regular 5 μm-sized resin constructed with an integrated electrospray emitter tip (Martin et al, 2000). Samples were then gradient eluted (Agilent 1100 Series, Santa Clara, CA) directly into a Finnigan LTQ quadrupole ion trap mass spectrometer (Thermo Electron, San Jose, CA) at a flow rate of 60 nl/min operated in the MS/MS data-dependent mode. The nano-flow HPLC gradient used was 0–60% acetonitrile in 0.1% acetic acid in 60 min. All MS/MS data were searched using the Sequest™ program and validated manually.
RT–PCR
Total RNA from S2 cells was isolated using RNeasy minikit (Qiagen) according to the manufacturer's instructions. cDNA was obtained by using Superscript III first-strand synthesis system for RT–PCR (Invitrogen). The primers used for PCR were
dAxin Fwd, 5′-GTCAGCGATGGCGCCATG; Rev, 5′-CTCGCAGTGCGTCTTGAAG;
96A Fwd, 5′-CCAGCATCAATCGCATCTACG; Rev, 5′-CAGGTCAAATTCGTGCTTCTG;
87B Fwd, 5′-CGATCTGTTGCGTCTGTTC; Rev, 5′-CTTGTGGATTTGGACTCGC; and
FLW Fwd, 5′-CGGCAACCACGAGTGCGCCAG; Rev, 5′-CCGAAGGTGAAGCTCACACCG;
PCR products were analyzed by agarose gel electrophoresis.
Mutagenesis
PCR-based site-directed mutagenesis was performed by using QuikChange mutagenesis kit (Stratagene).
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Acknowledgments
Reinhart Heinrich (1946–2006), former professor at Humboldt University, Berlin, died October 23, 2006 while this manuscript was being reviewed. His tragic death leaves a void in the scientific community of systems biology, and his far-reaching theoretical work on metabolism, signal transduction, and other cellular processes will be greatly missed in the future. We thank Dr David Brautigan for providing the PHI-1 plasmid and antibody; and Dr Anthea Letsou for the Drosophila cDNA clones; Drs G Rena and P Cohen for the CKI inhibitor D4476; Dr Carl S Thummel for pPac-PL vector; Dr Shin-ichi Yanagawa for Drosophila Wg and Fz2 plasmids; Marian Waterman for pEV3S-Lef-1, and Dr Sheng-Cai Lin for Axin constructs. The expert assistance of Kathleen Clark, JX Shen and Phil Gray is gratefully acknowledged. The anti-Armadillo antibody developed by Eric Wieschaus was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. We thank Drs David Jones and Nadeem Moghal for thoughtful reading of the manuscript. This study was supported by Grants P01CA073992 (DMV), P30CA42014, GM 37537 (DFH), 5R01HL057840 (HJY) and the Huntsman Cancer Foundation.
References
- Alam J, Cook JL (1990) Reporter genes: application to the study of mammalian gene transcription. Anal Biochem 188: 245–254 [DOI] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I (2002) Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS (2005) Prostaglandin E2 promotes colon cancer cell growth through a Gs–axin–beta-catenin signaling axis. Science 310: 1504–1510 [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Bollen M (2004) Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84: 1–39 [DOI] [PubMed] [Google Scholar]
- Chen RH, Ding WV, McCormick F (2000) Wnt signaling to beta-catenin involves two interactive components. Glycogen synthase kinase-3beta inhibition and activation of protein kinase C. J Biol Chem 275: 17894–17899 [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH (2003) Structural basis for recruitment of glycogen synthase kinase 3beta to the axin–APC scaffold complex. EMBO J 22: 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Kaykas A, Moon RT, Perrimon N (2005) Functional genomic analysis of the Wnt-wingless signaling pathway. Science 308: 826–833 [DOI] [PubMed] [Google Scholar]
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C (2005) Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872 [DOI] [PubMed] [Google Scholar]
- Deng JT, Sutherland C, Brautigan DL, Eto M, Walsh MP (2002) Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI-1, by integrin-linked kinase. Biochem J 367: 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F (2000) Differential regulation of glycogen synthase kinase 3beta by insulin and wnt signaling. J Biol Chem 275: 32475–32481 [DOI] [PubMed] [Google Scholar]
- Dombradi V, Axton JM, Brewis ND, da Cruz e Silva EF, Alphey L, Cohen PT (1990) Drosophila contains three genes that encode distinct isoforms of protein phosphatase 1. Eur J Biochem 194: 739–745 [DOI] [PubMed] [Google Scholar]
- Dombradi V, Mann DJ, Saunders RD, Cohen PT (1993) Cloning of the fourth functional gene for protein phosphatase 1 in Drosophila melanogaster from its chromosomal location. Eur J Biochem 212: 177–183 [DOI] [PubMed] [Google Scholar]
- Doonan JH, MacKintosh C, Osmani S, Cohen P, Bai G, Lee EY, Morris NR (1991) A cDNA encoding rabbit muscle protein phosphatase 1 alpha complements the Aspergillus cell cycle mutation, bimG11. J Biol Chem 266: 18889–18894 [PubMed] [Google Scholar]
- Eto M, Karginov A, Brautigan DL (1999) A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry 38: 16952–16957 [DOI] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem 272: 13856–13863 [DOI] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK (1997) Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proc Natl Acad Sci USA 94: 12479–12484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z-H, Seeling JM, Hill V, Yochum A, Virshup DM (2002) Casein kinase I phosphorylates and destabilizes the b-catenin degradation complex. Proc Natl Acad Sci USA 99: 1182–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich R (2005) Mathematical modelling of the Wnt-pathway. In Systems Biology: Definitions and Perspectives, Alberghina L, Westerhoff H (eds), p 408 Berlin Heidelberg: Springer [Google Scholar]
- Hsu W, Zeng L, Costantini F (1999) Identification of a domain of axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem 274: 3439–3445 [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A (2000) GSK-3beta-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by beta-catenin and protein phosphatase 2A complexed with Axin. Oncogene 19: 537–545 [DOI] [PubMed] [Google Scholar]
- Jho E, Lomvardas S, Costantini F (1999) A GSK3beta phosphorylation site in axin modulates interaction with beta-catenin and Tcf-mediated gene expression. Biochem Biophys Res Commun 266: 28–35 [DOI] [PubMed] [Google Scholar]
- Komeili A, O'Shea EK (1999) Roles of phosphorylation sites in regulating activity of the transcription factor Pho4. Science 284: 977–980 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kirschner MW (2001) Physiological regulation of b-catenin stability by Tcf3 and CK1epsilon. J Cell Biol 154: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW (2003) The Roles of APC and axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1: E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Weaver CD, Mao J, Farr GH III, Sussman DJ, Jonkers J, Kimelman D, Wu D (1999) Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J 18: 4233–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Scuderi A, Letsou A, Virshup DM (2002) B56-associated protein phosphatase 2A is required for survival and protects from apoptosis in Drosophila melanogaster. Mol Cell Biol 22: 3674–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yost HJ, Virshup DM, Seeling JM (2001) Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J 20: 4122–4131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X (2002) Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Liu X, Rubin JS, Kimmel AR (2005) Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol 15: 1989–1997 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Luo W, Lin S-C (2004) Axin: a master scaffold for multiple signaling pathways. Neurosignals 13: 99–113 [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH III, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D (2001) Low-density lipoprotein receptor-related protein-5 binds to axin and regulates the canonical Wnt signaling pathway. Mol Cell 7: 801–809 [DOI] [PubMed] [Google Scholar]
- Martin SE, Shabanowitz J, Hunt DF, Marto JA (2000) Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI Fourier transform ion cyclotron resonance mass spectrometry. Anal Chem 72: 4266–4274 [DOI] [PubMed] [Google Scholar]
- Naerum L, Norskov-Lauritsen L, Olesen PH (2002) Scaffold hopping and optimization towards libraries of glycogen synthase kinase-3 inhibitors. Bioorg Med Chem Lett 12: 1525–1528 [DOI] [PubMed] [Google Scholar]
- Okano K, Heng H, Trevisanato S, Tyers M, Varmuza S (1997) Genomic organization and functional analysis of the murine protein phosphatase 1c gamma (Ppp1cc) gene. Genomics 45: 211–215 [DOI] [PubMed] [Google Scholar]
- Pai LM, Orsulic S, Bejsovec A, Peifer M (1997) Negative regulation of Armadillo, a Wingless effector in Drosophila. Development 124: 2255–2266 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Rena G, Bain J, Elliott M, Cohen P (2004) D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep 5: 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resink TJ, Hemmings BA, Tung HYL, Cohen P (1983) Characterisation of a reconstituted Mg-ATP-dependent protein phosphatase. Eur J Biohem 133: 455–461 [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H (2005) Wnt signalling in stem cells and cancer. Nature 434: 843–850 [DOI] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW (2000) Control of β-catenin stability: reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell 5: 523–532 [DOI] [PubMed] [Google Scholar]
- Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM (1999) Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science 283: 2089–2091 [DOI] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M (1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858 [DOI] [PubMed] [Google Scholar]
- Strovel ET, Wu D, Sussman DJ (2000) Protein phosphatase 2Calpha dephosphorylates axin and activates LEF-1-dependent transcription. J Biol Chem 275: 2399–2403 [DOI] [PubMed] [Google Scholar]
- Swiatek W, Tsai IC, Klimowski L, Pepler A, Barnette J, Yost HJ, Virshup DM (2004) Regulation of casein kinase I epsilon activity by Wnt signaling. J Biol Chem 279: 13011–13017 [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X (2004) A mechanism for Wnt coreceptor activation. Mol Cell 13: 149–156 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E (2003) Wg/Wnt signal can be transmitted through arrow/LRP5, 6 and axin independently of Zw3/Gsk3beta activity. Dev Cell 4: 407–418 [DOI] [PubMed] [Google Scholar]
- Tountas NA, Brautigan DL (2004) Migration and retraction of endothelial and epithelial cells require PHI-1, a specific protein-phosphatase-1 inhibitor protein. J Cell Sci 117: 5905–5912 [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A (2005) Re-evaluating the role of Frat in Wnt-signal transduction. Cell Cycle 4: 1065–1072 [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R (1999) Wnt-induced dephosphorylation of axin releases beta-catenin from the axin complex. Genes Dev 13: 1768–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J (2004) Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 116: 883–895 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A (1999) Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem 274: 10681–10684 [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Lee JS, Ishimoto A (1998) Identification and characterization of a novel line of Drosophila Schneider S2 cells that respond to wingless signaling. J Biol Chem 273: 32353–32359 [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH III, Pierce SB, Ferkey DM, Chen MM, Kimelman D (1998) GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93: 1031–1041 [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X (2005) A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3


