Abstract
Hsp22/HspB8 is a member of the small heat-shock protein family, whose function is not yet completely understood. Our immunolocalization studies in a human neuroblastoma cell line, SK-N-SH, using confocal microscopy show that a significant fraction of Hsp22 is localized to the plasma membrane. We therefore investigated its interactions with lipid vesicles in vitro. Intrinsic tryptophan fluorescence is quenched in the presence of lipid vesicles derived from either bovine brain lipid extract or purified lipids. Time-resolved fluorescence studies show a decrease in the lifetimes of the tryptophan residues. Both of these results indicate burial of some tryptophan residues of Hsp22 upon interaction with lipid vesicles. Membrane interactions also lead to increase in fluorescence polarization of Hsp22. Gel-filtration chromatography shows that Hsp22 binds stably with lipid vesicles; the extent of binding depends on the nature of the lipid. Hsp22 binds more strongly to vesicles made of lipids containing a phosphatidic acid, phosphatidylinositol or phosphatidylserine headgroup (known to be present in the inner leaflet of plasma membrane) compared with lipid vesicles made of a phosphatidylcholine head-group alone. Far-UV CD spectra reveal conformational changes upon binding to the lipid vesicles or in membrane-mimetic solvent, trifluoroethanol. Thus our fluorescence, CD and gel-filtration studies show that Hsp22 interacts with membrane and this interaction leads to stable binding and conformational changes. The present study therefore clearly demonstrates that Hsp22 exhibits potential membrane interaction that may play an important role in its cellular functions.
Keywords: heat-shock protein, Hsp22, HspB8, lipid headgroup, plasma membrane
Abbreviations: BLE FrI, bovine brain lipid extract Folch fraction I; BLE FrIII, bovine brain lipid extract Folch fraction III; DAPI, 4′,6-diamidino-2-phenylindole; DiIC16, 1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DPH, diphenylhexatriene; Hsp, heat-shock protein; PA, L-α-phosphatidic acid sodium salt; PC, 1-palmitoyl-2-oleoyl phosphatidylcholine; PI, L-α-phosphatidylinositol sodium salt; PS, L-α-phosphatidyl-L-serine; sHsp, small Hsp; SUV, small unilamellar vesicle
INTRODUCTION
Physiological stress, such as elevated temperatures, oxidative insult, toxic chemicals or extremes of pH, leads to selective overexpression of a group of proteins called the Hsps (heat-shock proteins). In addition to providing tolerance to stress, Hsps play an important role in folding nascent polypeptides, intracellular localization and degradation of cellular proteins, as well as regulation of other cellular functions such as maintaining cytoskeletal integrity, apoptosis, differentiation, signal transduction and cell cycle (see [1–3] for reviews). sHsps (small Hsps) form a distinct subset of the Hsp family with monomer molecular masses generally in the range 12–43 kDa. A characteristic feature of the sHsps is the presence of a highly conserved 80–100-amino-acid-long sequence, called the ‘α-crystallin domain’, in their C-terminal domains. Ten mammalian sHsps have been identified so far [4].
Hsp22, also called as HspB8 [4], H11 kinase [5] or E2IG1 [6], is a relatively new member of the family of mammalian sHsps [7]. It is expressed in all muscle-related tissues and in brain tissues [8,9]. High levels of expression of Hsp22 have been reported in the spinal cord, specifically in motor and sensory neurons; it plays a crucial role in neuronal cell survival and metabolism [10]. sHsps, apart from providing stress tolerance, take part in varied cellular functions. Studies from our laboratory [11] and those of Gober et al. [12] have shown that Hsp22 is up-regulated upon heat stress; Hsp22 was also up-regulated upon ischaemic stress [13], indicating that its primary role in vivo is in stress tolerance. Recent studies have shown that Hsp22 can reverse the phenotypic inclusions of disease-causing polyglutamine repeats [14] and the R120G mutant αB-crystallin [15]. Overexpression of the K141N or K141E mutants of Hsp22 (which are associated with distal motor neuropathy type II disease) in N2a mouse neuroblast cells leads to cell mortality [10], implying a crucial role for Hsp22 in neuronal cell survival. Hsp22 is shown to play a role in apoptosis, although the exact mechanism of action is not clear. Proteasomal inhibition-induced apoptosis in neuronal cells leads to up-regulation of Hsp22 [16]. When overexpressed in a cardiac myocyte model, Hsp22 is shown to modulate the activity of a signal transduction pathway protein, Akt, which gets activated at the inner membrane surface [17], in a dose-dependent manner [18]. A recent study using a transgenic mouse model overexpressing Hsp22/H11 kinase showed that H11 kinase could interact with cell survival kinases such as Akt, AMPK (AMP-activated protein kinase) and protect the myocardium against ischaemia and infarction [19]. Hsp22 exists as a phosphoprotein in vivo [5,7] and purified Hsp22 shows mild Mn2+-dependent autokinase activity [5,11]. Our earlier study showed that, unlike other sHps, which are oligomeric in nature and exhibit β-sheet structure, the purified recombinant Hsp22 exists as a monomer and shows predominantly randomly coiled secondary structure [11]. However, like the other sHsps, such as αB-crystallin and Hsp27, Hsp22 also exhibits chaperone-like activity in vitro in preventing the aggregation of other proteins [11,20].
Yu et al. [8], following studies in SK-MEL-2 cells, a melanoma cell line, have suggested that Hsp22 may associate with the plasma membrane or with membrane-anchored proteins. These studies suggest that Hsp22 may have an important role in the events occurring at the membrane surface such as signal transduction. In the present study, using confocal microscopy, we have investigated the localization of Hsp22 in SK-N-SH human neuroblastoma cells. Our study shows that endogenous as well as ectopically overexpressed Hsp22 localizes to the plasma membrane, but not the cytoplasm and nucleus. Prompted by our observation of its localization with membrane regions, we investigated the possible interaction of Hsp22 with lipid membranes in vitro. We have demonstrated that Hsp22 binds stably with the lipid vesicles, and the headgroups of the lipids have significant influence on the binding. The interaction of Hsp22 with lipids is accompanied by a subtle conformational change that may influence its cellular functions.
EXPERIMENTAL
Materials
BLE FrI and BLE FrIII (bovine brain phospholipid extract Folch fractions I and III), PI (L-α-phosphatidylinositol sodium salt), PS (L-α-phosphatidyl-L-serine), PA (L-α-phosphatidic acid sodium salt), anti-FLAG M2 monoclonal antibody, tetramethylpentadecane (pristane) and β-lactoglobulin were from Sigma Chemical Company. Incomplete Freund's adjuvant was obtained from Bangalore Genei. VectaShield medium was from Vector Laboratories. PC (1-palmitoyl-2-oleoyl phosphatidylcholine) was obtained from Avanti Polar Lipids. All other chemicals used were of analytical grade.
Cell culture methods
SK-N-SH, a human neuroblastoma cell line, was obtained from the American Type Culture Collection (Manassas, VA, U.S.A.). Cells were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with non-essential amino acids and 10% foetal calf serum. Sodium bicarbonate was added to the medium at 1.5 gm/l.
Hsp22 constructs
For fusion of FLAG tag to Hsp22 and ectopic overexpression of the fusion protein in SK-N-SH cells, the coding sequence of Hsp22 was cloned in-frame, downstream of the FLAG-coding sequence in pCDNA3 vector using EcoRI and XhoI restriction sites. Clones were confirmed by sequencing.
Transient transfections of Hsp22
SK-N-SH cells were grown on coverslips in a six-well dish to reach 70–80% confluence. Transfections were carried out using Lipofectamine™ 2000 (Invitrogen). Cells were processed for immunostaining 24 h after transfection.
Purification of rat Hsp22
Rat Hsp22 protein was overexpressed from Hsp22 pET21a vector, a prokaryotic expression vector, in the BL21(DE)3 strain of Escherichia coli and purified as described previously [11]. The purified rat Hsp22 was used in all in vitro lipid-vesicle-binding experiments.
Preparation of anti—Hsp22 antibodies
Polyclonal antibodies against Hsp22 were generated in mice following the method described by Lacy and Voss [21]. BALB/c mice (12 weeks old) were primed to induce ascites by injecting 0.5 ml of tetramethylpentadecane (pristane) intraperitoneally before immunizing with Hsp22 protein. A 1:1 emulsion of Hsp22 protein (250 μg) and Freund's incomplete adjuvant was injected intraperitoneally into each mouse 15 days after pristane injection. After three immunizations and a final intravenous booster, ascites fluid was collected and clarified by centrifugation at 8000 g for 30 min. Immunoglobulins were precipitated from the ascites fluid by addition of 50% saturated ammonium sulphate. Antibody titre and specificity were assessed by Western immunoblotting using purified recombinant Hsp22 or SK-N-SH total cell lysate as antigen.
Immunostaining for Hsp22
SK-N-SH cells grown on coverslips to 70% confluence were fixed with 3.7% (w/v) formaldehyde and permeabilized with either ice-cold acetone or 0.1% Triton X-100 treatment for 10 min. Samples were incubated with 2% (w/v) BSA to avoid non-specific adsorption of antibodies. Samples were developed further with either anti-Hsp22 mouse polyclonal antibodies or anti-FLAG M2 monoclonal antibody (wherever transfected with pCDNA3-FLAG-Hsp22) followed by anti-(mouse IgG) goat antibody conjugated to Alexa Fluor® 488 dye (Molecular Probes). Samples were then incubated with 1 mM DiIC16 (1,1′-dihexadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) dye in PBS for 40 min and washed with PBS before mounting them with VectaShield medium containing 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole) as a nuclear counterstain.
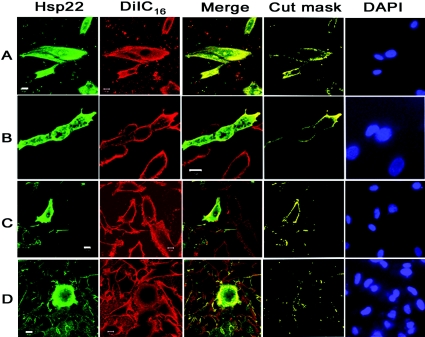
Samples were scanned using a LSM 510Meta confocal laser-scanning microscope (Carl Zeiss) using a 60× oil-immersion Plan Apochromat objective. Images were analysed using Zeiss software supplied with the machine. The merged images of the three bottom-most optical sections (0.3 μm each) are represented in Figure 1.
Figure 1. Immunolocalization of Hsp22 in SK-N-SH cells.
(A) Immunolocalization of endogenous Hsp22. (B and C) Immunolocalization of ectopically expressed FLAG-tagged Hsp22. Cells were permeabilized either by acetone treatment (B) or by 0.1% Triton X-100 treatment (C). (D) The transiently transfected cells were treated with 1% Triton X-100 for 20 min after fixation to determine the effect of membrane disruption on the localization of Hsp22. Cells were probed with either polyclonal antibody against Hsp22 (A) or monoclonal antibodies against the FLAG epitope of the transiently expressed FLAG-tagged Hsp22 (B–D) and DiIC16, a membrane-specific dye (all panels). Nuclei were stained with DAPI. Regions of membrane of the cells exhibiting co-localization of Hsp22 and DiIC16 are shown in the merge panel. The cut mask panel shows the co-localized areas masking the rest of the image. This cut mask image is created using the manufacturer-provided (LSM 510 meta, Zeiss) software. All of the images were processed under identical conditions.
In another experiment cells were treated for 20 min after fixation with high concentrations of Triton X-100 (0.5–2%) in PBS to disrupt the membrane. Immunostaining of these samples was performed as described above.
Preparation of lipid vesicle and protein mixtures
Hsp22–membrane interaction was studied using lipid SUVs (small unilamellar vesicles) as a model system. SUVs are preferred, as these are more stable and homogeneously sized (∼25 nm) and pose least interference in spectroscopic studies when compared with LUVs (large unilamellar vesicles). The samples were prepared by mixing purified Hsp22 and lipid SUVs at different w/w ratios and incubated at room temperature (∼25 °C) for 1 h before starting the experiment. We have also used β-lactoglobulin as a negative control in our fluorescence and gel-filtration experiments, as it is known to interact with anionic lipid vesicles under extremely acidic conditions, but not at neutral pH or with neutral lipid vesicles [22].
Preparation of lipid SUVs
Freeze-dried powders of BLE FrI, BLE FrIII and purified lipids were dissolved in spectroscopy-grade chloroform to make 25 mg/ml stocks and were stored under nitrogen gas at −20 °C. SUVs were prepared using the sonication technique of Barenholz et al. [23]. For preparing SUVs with BLE FrI and BLE FrIII, 5 mg of each lipid was taken in a glass vial and chloroform was evaporated under a flow of nitrogen gas to make a thin layer of lipid on the walls of the vial. Lipids were rehydrated overnight at room temperature in TNES (100 mM Tris/HCl buffer, pH 8.0, containing 100 mM NaCl, 2 mM EDTA and 0.2% sorbitol). SUVs of different lipid compositions were prepared by mixing PC with PS (PC/PS), PA (PC/PA) or PI (PC/PI) in a 1:1 (w/w) ratio to achieve a final lipid concentration of 5 mg/ml.
Fluorescence spectroscopy
Intrinsic fluorescence spectra of Hsp22 alone or lipid SUV–Hsp22 complexes were recorded at room temperature using a F4500 fluorescence spectrophotometer (Hitachi). The excitation wavelength was set at 295 nm and the emission spectra were recorded in corrected spectrum mode. Excitation and emission band passes were set at 5 nm. Fluorescence spectra of 0.1 mg/ml β-lactoglobulin in either the absence or the presence of 0.5 mg/ml BLE FrI were also recorded. Spectra recorded in the presence of lipid SUVs were corrected for inner filter effect.
Quenching of Hsp22 intrinsic fluorescence in the presence of the lipids was measured at 340 nm. The extent of quenching was calculated by using the formula (F0−F)/F0, where F0 is the fluorescence intensity of Hsp22 alone at 340 nm and F is the fluorescence of Hsp22 in the presence of appropriate concentrations of lipid.
We have performed the fluorescence experiment at different temperatures ranging from 25 to 50 °C and under different pH conditions ranging from 5.5 to 8.0. Since we did not find significant variation in the results, we performed all other experiments at room temperature (∼25 °C) and in pH 8.0 buffer.
Fluorescence polarization changes of Hsp22 protein in the absence and the presence of lipid SUVs were measured using excitation and emission polarizers. The measurements were made at a lipid/protein ratio of 5:1 (w/w), where Hsp22 binding to lipid shows saturation. The excitation wavelength was set to 295 nm, and fluorescence intensity at 340 nm was recorded in photometry mode, with the excitation and emission band passes set at 5 nm.
Time-resolved fluorescence measurements
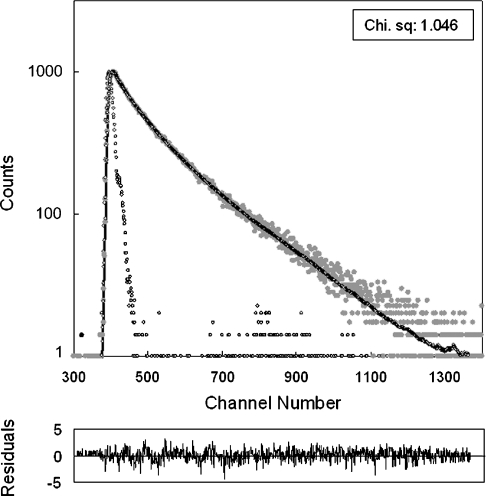
Fluorescence lifetime measurements of Hsp22 were made in a Model 5000 U-TCSPC Picosecond Lifetime measurement system (HORIBA Jobin Yvon) in TCSPC (time-correlated single-photon counting) mode. The excitation source was a 294 nm wavelength NanoLED pulsed laser with a repetition rate of 200 kHz. The instrument response function (prompt) was obtained at 294 nm using Ludox™ suspension. The emission decay (at 340 nm) data were analysed using the software, DAS6, provided with the instrument. The analysis used the iterative least-squares reconvolution statistical method, assuming three exponential decay function [A+B1·exp(−i/τ1)+B2·exp(−i/τ2)+B3·exp (−i/τ3)]. The best fit was assessed based on the parameter χ2, which was close to 1.0 for all the samples, and the distribution of weighted residual along the zero line (as shown in Figure 3).
Figure 3. Time-resolved tryptophan fluorescence intensity decay of Hsp22.
Excitation source was a 294 nm NanoLED pulsed laser, and emission was monitored at 340 nm. Grey circles, fluorescence decay; open black circles, prompt. The experimental fluorescence decay data were fitted assuming a three-exponential decay. Chi. sq, χ2. The lower panel shows the weighted residuals. The calculated fluorescence lifetimes are listed in Table 1.
Gel-filtration chromatography
Gel-filtration chromatography of Hsp22 alone and lipid/protein mixtures was performed on a Sephacryl S-300 column (30 cm×1.0 cm). Hsp22 alone (50 μg) or lipid/Hsp22 mixture (5:1, w/w) was loaded on the column. The column was eluted with TNES buffer, pH 8.0, at a flow rate of 23 ml/h, and fractions (0.5 ml) were collected. Elution of Hsp22 was monitored by recording the intrinsic fluorescence intensity (excitation at 295 nm, emission at 340 nm). To monitor the elution of the lipid, DPH (diphenylhexatriene), a dye that fluoresces intensely when bound to lipid, was added to each fraction to a final concentration of 10 μM, and the emission intensity at 430 nm measured with the excitation wavelength set at 358 nm. The fraction of Hsp22 bound to lipid SUVs in each case was determined from the amount of Hsp22 that was eluted at the position corresponding to lipid elution peak.
CD spectroscopy
Far-UV CD spectra of Hsp22 (0.1 mg/ml) in TNES buffer, pH 8.0, in either the presence or the absence of lipid or in increasing concentrations of trifluoroethanol were recorded in a 1 mm path-length quartz cuvette using a Jasco J-715 spectropolarimeter at room temperature. The spectra of lipid/protein mixtures were recorded at a lipid/protein ratio of 5:1 (w/w). Spectra shown are cumulative averages of four scans and have been corrected for respective blanks. Mean residue ellipticity values were plotted against wavelength in each case.
RESULTS
Immunolocalization of Hsp22 in a human neuroblastoma cell line
To understand the functional role of Hsp22, we have carried out immunolocalization studies of endogenously expressed Hsp22 as well as ectopically expressed FLAG-tagged Hsp22 in SK-N-SH cells, a human neuroblastoma cell line (Figure 1). The staining pattern of both endogenous Hsp22 as well as ectopically expressed FLAG-tagged Hsp22, when probed using a polyclonal antibody against Hsp22 and a monoclonal antibody against the FLAG tag respectively, shows a widespread localization of the protein in the cytoplasm and nucleus, in addition to some distinct localization along the cell boundary, suggesting a possible localization of the protein to the plasma membrane. We have probed the membrane using the membrane-specific lipophilic dye, DiIC16. Co-localization of the membrane-specific dye with Hsp22 in some regions, shown in the merge and cut mask panels of Figures 1(A) and 1(B), indicates that Hsp22 can associate with the membrane. However, since we did not find co-localization all along the membrane, and as only a fraction of the total Hsp22 was found to co-localize with the membrane-specific dye, we presume that the membrane interaction of Hsp22 in vivo might be restricted to certain regions of the membrane or membrane-associated proteins.
We also carried out an experiment to find out whether disruption of membrane by treating with a high concentration of detergent would alter the localization of Hsp22. It is evident from Figure 1(D) that treatment of cells with 1% Triton X-100 disrupts the membrane as seen by internalization and diffused staining of DiIC16 accompanied by disruption of the membrane localization of Hsp22. This result further indicates the interaction of Hsp22 with membrane. We have used both acetone permeabilization and permeabilization by a low concentration of detergent (0.1% Triton X-100) in our sample processing. In both cases (Figures 1B and 1C), the membrane localization of Hsp22 is seen, indicating that the observed membrane localization is not due to artefacts associated with cell permeabilization. Moreover, the observed membrane localization of both the endogenous Hsp22 and ectopically expressed Hsp22 also rules out any possible artefacts associated with overexpression of the protein.
Yu et al. [8] have also suggested that Hsp22 may interact with the membrane or proteins associated with the inner surface of the membrane. We have therefore studied the interaction of purified recombinant rat Hsp22 with lipid vesicles prepared either from brain lipid extracts or from purified lipids (PC, PC/PA, PC/PS and PC/PI). BLE FrI and BLE FrIII are enriched in phosphatidylinositol or phosphatidylserine respectively. Although phosphatidylcholine is known to be present in the outer leaflet of the plasma membrane, phosphatidylinositol is known to be distributed in the inner as well as the outer leaflet of the plasma membrane, and phosphatidylserine and phosphatidic acid are known to be present in the inner leaflet of the plasma membrane [24].
Interaction of Hsp22 with lipid vesicles
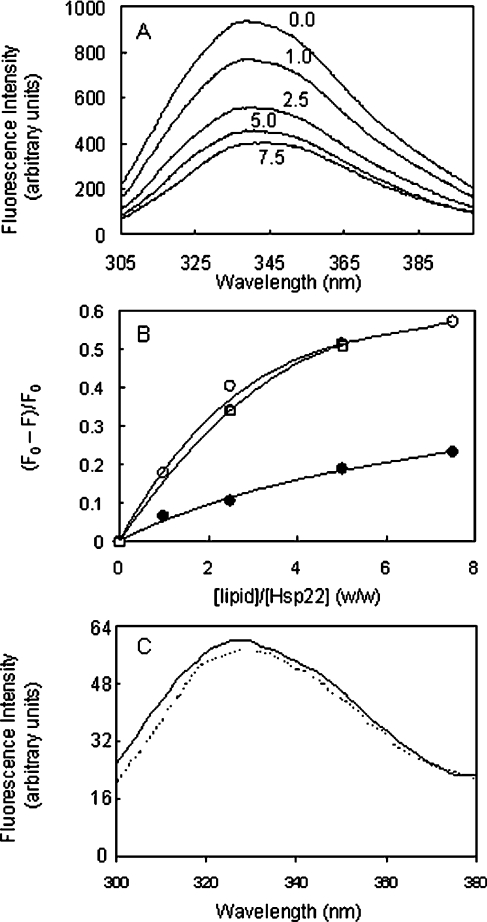
We studied the interaction between lipid vesicles and Hsp22 by its intrinsic fluorescence. Hsp22 has four tryptophan residues in its sequence, three of which are located in the N-terminal region and the fourth is in the α-crystallin domain. Figure 2(A) shows the intrinsic tryptophan fluorescence spectra of Hsp22 in buffer alone and in the presence of increasing concentrations of SUVs prepared from BLE FrI. In buffer alone, Hsp22 shows an emission maximum at ∼340 nm, indicating that some tryptophan residues are in a polar environment. The fluorescence intensity decreases upon addition of the lipid vesicles (Figure 2A) and the extent of quenching tends to saturate at a lipid/protein ratio of 5:1 (w/w) (Figure 2B). The emission maximum did not change significantly in the presence of lipid vesicles. There are two possible reasons for the observed fluorescence quenching: (i) since we used vesicles prepared from the brain lipid extract, some unknown fluorescence-quencher molecules present in the vesicle preparation led to dynamic quenching of the fluorescence, or (ii) interaction of the protein with the vesicle leads to changes in the microenvironment of the tryptophan residues of the protein. The following results support the second possibility, while ruling out the first possibility. (i) We observed similar quenching of Hsp22 fluorescence by vesicles prepared from other purified lipids. Interestingly, the extent of decrease in the fluorescence intensity depends on the nature of lipid. For instance, at a lipid/protein ratio of 5:1 (w/w), the extent of fluorescence quenching was 0.509, 0.449 and 0.319 with SUVs made of PC/PA, PC/PI and PC/PS respectively. On the other hand, at the same lipid/protein ratio with SUVs made of PC, a neutral or uncharged lipid, alone, the extent of quenching was minimal (0.203). (ii) We used β-lactoglobulin, a protein known to interact with anionic lipid vesicles only under extremely acidic conditions, but not under neutral pH conditions [22], as a negative control and found that its fluorescence is not quenched by lipid vesicles under our experimental conditions (Figure 2C). (iii) When we tested other sHsps that are known to interact with membranes such as human αB-crystallin and Hsp27 [25–29], we observed quenching of their tryptophan residues: the extent of quenching at the lipid/protein ratio of 5:1 (w/w) was found to be 0.46 and 0.76 respectively. (iv) We also found that the fluorescence of N-acetyltryptophan amide is not quenched upon addition of vesicles of brain lipid fractions (results not shown). Thus these results indicate that Hsp22 can interact with lipid vesicles and such interaction leads to changes in the microenvironment of the tryptophan residues. We have also studied the changes in the microenvironment of tryptophan residues of Hsp22 upon interacting with lipid vesicles by fluorescence lifetime measurements as described below.
Figure 2. Quenching of the tryptophan fluorescence of Hsp22 by lipid vesicles.
(A) Intrinsic fluorescence of Hsp22 in the absence and the presence of increasing concentrations of SUVs prepared from BLE FrI. Fluorescence spectra of Hsp22 (0.2 mg/ml) incubated with lipid SUVs at different indicated SUV/protein ratios (w/w) are shown. Excitation wavelength was 295 nm, and excitation and emission band passes were set at 5 nm. (B) Extent of quenching [(F0−F)/F0, where F0 and F are the fluorescence intensities at 340 nm in the absence and in the presence of lipid vesicles respectively] of Hsp22 fluorescence upon interaction with lipid SUVs of BLE FrI (○), BLE FrIII (●) and PC/PA (□) is plotted against different lipid/Hsp22 ratios (w/w). (C) Intrinsic fluorescence of β-lactoglobulin (0.1 mg/ml) in the absence (solid line) and in the presence (broken line) of SUVs (0.5 mg/ml) prepared from BLE FrI. Excitation wavelength was 295 nm, and excitation and emission band passes were set at 5 nm.
We have also performed experiments under different pH conditions. The extents of fluorescence quenching of Hsp22 in the presence of BLE FrI vesicles at pH 5.5, 6.0, 6.5, 7.0 and 7.5 were found to be 0.51, 0.53, 0.52, 0.53 respectively, indicating that the interaction of Hsp22 with the lipid vesicles is not altered significantly in the pH range. Since Hsp22 is relatively more stable at pH 8.0 [11], we carried out other experiments at this pH condition. The fluorescence experiment was also performed at different temperatures ranging from 25 to 50 °C. The extent of fluorescence quenching was not significantly altered in this temperature range (results not shown). Thus our results show that under the physiologically relevant pH and temperature conditions, Hsp22 exhibits membrane interaction.
Figure 3 shows the time-resolved fluorescence decay profile of Hsp22 in buffer. The decay data can be best fitted assuming a three-exponential decay model. This was borne out by symmetric distribution of weighted residuals as well as the χ2 value close to 1.0. As shown in Table 1, the tryptophan residues of Hsp22 exhibit fluorescence lifetimes of 2.716, 6.023 and 0.580 ns, indicating that the tryptophan residues of the protein are in different microenvironments. Longer fluorescence lifetimes for tryptophan residues in proteins exposed to the solvent and shorter lifetimes for buried tryptophan residues have been reported [30–34]. Of the four tryptophan residues, some appear to be exposed to the solvent, while the others may be partially buried. Table 1 also shows that all three fluorescence lifetimes of the tryptophan residues of Hsp22 decrease significantly in the presence of lipid vesicles of BLE FrI and BLE FrIII; the decrease is more pronounced in the case of BLE FrI. We have also determined fluorescence lifetime of N-acetyltryptophan amide in the absence and the presence of SUVs of BLE FrI, and found that the lifetime is not significantly affected in the presence of the vesicles, hence ruling out the possibility of the involvement of dynamic quenching by any unknown quencher in the brain lipid extract (results not shown). The decrease in fluorescence lifetimes of tryptophan residues of Hsp22 suggests conformational changes in the protein molecule upon interaction with the lipid vesicles, leading to significant changes in the solvent accessibility (buried). Thus our time-resolved fluorescence measurements further confirm the interaction of Hsp22 with lipid vesicles.
Table 1. Fluorescence lifetime measurements of Hsp22.
| Sample | τ1 (ns) | α1 (%) | τ2 (ns) | α2 (%) | τ3 (ns) | α3 (%) |
|---|---|---|---|---|---|---|
| Hsp22 | 2.716 | 49.89 | 0.580 | 9.85 | 6.023 | 40.26 |
| Hsp22+BLE FrI | 2.061 | 43.23 | 0.322 | 17.26 | 5.288 | 39.51 |
| Hsp22+BLE FrIII | 2.289 | 45.63 | 0.327 | 14.85 | 5.610 | 39.51 |
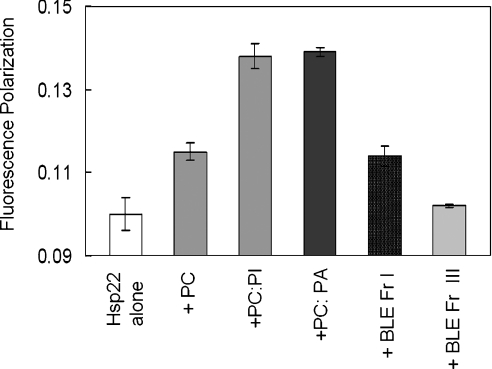
In order to probe further the interaction of Hsp22 with the lipid vesicles, we performed fluorescence polarization measurements. Figure 4 shows the fluorescence polarization of Hsp22 in the absence and the presence of various SUVs (at a lipid/protein ratio of 5:1, w/w). As is evident from Figure 4, the polarization value increases in the presence of lipid vesicles; the values vary significantly depending on the nature of the lipid. The observed increase in the fluorescence polarization may be due to slower tumbling of the SUV–Hsp22 complex or binding-induced changes in the Hsp22. Upon interacting with lipid vesicles, the protein molecule might become more ordered, with decrease in the segmental mobility around the tryptophan residues. The observed increase in the polarization does not correlate well with the extent of fluorescence quenching with different lipid vesicles, perhaps due to the heterogeneity in the interfacial regions of protein–vesicle contacts arising from the intrinsic properties of component lipids. However, our results of gross increase in fluorescence polarization, fluorescence quenching and decrease in the lifetime in the presence of lipid vesicles clearly show that Hsp22 interacts with lipid membrane. We used gel-filtration chromatography to investigate whether such interactions represent transient reversible interactions or stable binding.
Figure 4. Effect of lipid association on fluorescence polarization of Hsp22.
Fluorescence polarization values of the samples of Hsp22 (0.1 mg/ml) in the absence and the presence of various SUVs at a lipid/protein ratio of 5:1 (w/w) are shown. Results are means±S.E.M. for three independent measurements.
Hsp22 binds stably to lipid vesicles
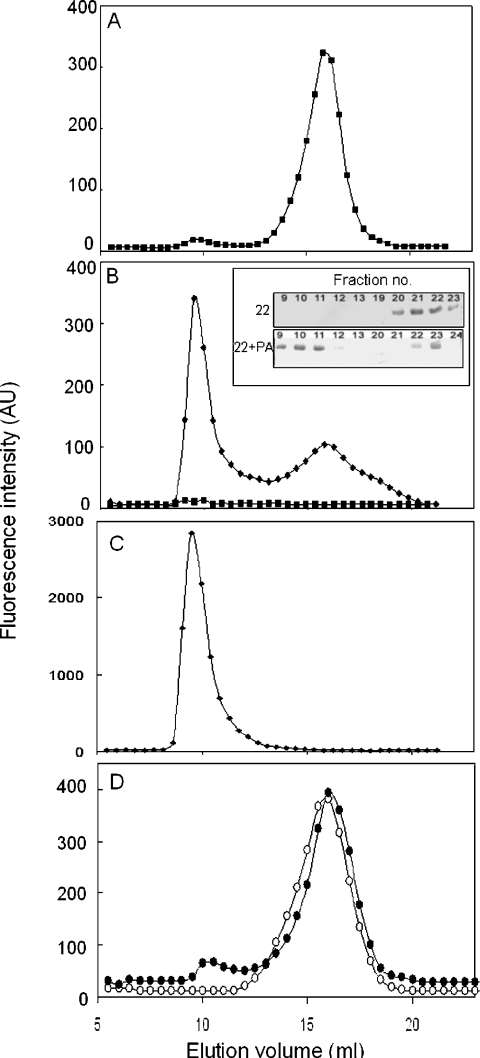
We have used Sephacryl S300 gel-filtration chromatography to investigate Hsp22–lipid vesicle interaction. Lipid SUVs with average diameter of ∼25 nm are expected to be eluted in the void volume, whereas Hsp22, a monomeric sHsp with molecular mass of 22 kDa, should be eluted in the fractionation range of the column (10–1500 kDa). The elution profile of Hsp22 is shown in Figure 5(A). Hsp22 is eluted in the fractionation range as expected, as a single peak, with an elution volume of ∼16 ml. The elution profile of the same amount of Hsp22 in the presence of PC/PA vesicles shows two peaks as monitored by tryptophan fluorescence of the protein (Figure 5B) as well as SDS/PAGE of the fractions (inset in Figure 5B). A major portion of the protein is eluted in the void volume, with a concomitant decrease in the peak corresponding to free Hsp22. In order to investigate whether the protein that was eluted in the void volume is complexed with the lipid vesicles, we have probed for the lipid vesicles using DPH. A plot of the fluorescence intensity of DPH against the elution volume of the lipid vesicle (Figure 5C) shows that the DPH fluorescence peak position overlaps exactly with that of the Hsp22 peak eluted in the void volume (Figure 5B), indicating that Hsp22 is stably complexed with the lipid vesicles. β-Lactoglobulin is not eluted as a complex with lipid vesicles (Figure 5D). We found that αB-crystallin, which is known to interact with lipid vesicles [26–28], is also eluted along with lipid vesicles, suggesting a stable interaction with lipid vesicles (results not shown).
Figure 5. Binding of Hsp22 to lipid SUVs shown by gel-filtration chromatography.
(A) Elution profile of Hsp22 alone monitored by fluorescence intensity at 340 nm. (B) Elution profile of a mixture of Hsp22 and PC/PA (1:5, w/w) (◆). The elution of protein was detected by its fluorescence at 340 nm upon excitation at 295 nm. Under this excitation and emission wavelength, the elution of SUVs alone (■) could not be detected, indicating that the fluorescence seen in the mixture is due to protein alone. Inset: Coomassie Blue R250-stained SDS/polyacrylamide gel showing the band corresponding to Hsp22 in the eluted fractions. Upper panel: Hsp22 alone; lower panel: Hsp22+PC/PA. (C) Elution profiles of SUVs in the mixture of Hsp22 and PC/PA (1:5, w/w) monitored using the extrinsic fluorescence probe, DPH (10 μM), added to the fractions. DPH partitions into the membrane bilayer, leading to increase in its fluorescence intensity. It does not yield significant fluorescence in the presence of protein alone. Samples were excited at 358 nm and emission was monitored at 430 nm. (D) Elution profiles of β-lactoglobulin (0.5 mg/ml) in the absence (○) and in the presence of SUVs (2.5 mg/ml) (●). AU, arbitrary units.
To assess the extent of association of Hsp22 with SUVs of different lipid composition, we have estimated the percentage of total protein that is eluted along with the different types of lipid SUVs in the gel-filtration chromatography using the fluorescence intensities of Hsp22 alone and those in the presence of lipid SUVs. However, since the SUVs quench the fluorescence of the protein (Figure 2), we have taken into account the extent of quenching at a lipid/protein ratio of 5:1 (w/w), while estimating the protein eluted in the complex. At the same lipid to protein ratio, the percentage of Hsp22 eluted along with vesicles made of PC alone was the least (8.8%), while it was very high in the SUVs made up of PC/PS (70.6%), BLE FrI (65.84%) and PC/PA (62.7%). The percentage of protein eluted with SUVs made of PC/PI and BLE FrIII was 48.9% and 40.3% respectively. Thus the extent of binding of Hsp22 to the lipid membrane is significantly more to anionic lipid vesicles compared with that to neutral lipid vesicles.
Conformational changes in Hsp22 upon interaction with lipid vesicles
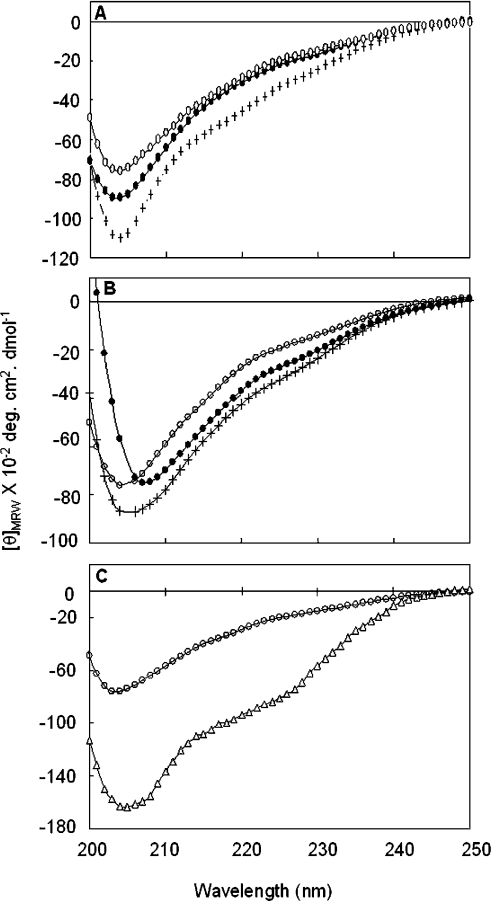
We studied the effect of the membrane interaction of Hsp22 on its structure using CD. The far-UV CD spectrum of Hsp22 is devoid of characteristic features of α-helical or β-sheet conformation, suggesting that the protein has predominantly randomly coiled conformation. sHsps contain a conserved α-crystallin domain that exhibits β-sheet and has an immunoglobulin fold [35]. Although some strand conformation in the corresponding conserved α-crystallin domain of Hsp22 is possible, both secondary-structure prediction and far-UV CD spectra indicate that major parts of the sequence exist in a randomly coiled conformation [11], unlike in other sHsps [36–38]. Upon addition of lipid vesicles, ellipticity increase was observed in the far-UV CD spectrum of Hsp22 in the case of SUVs of both bovine brain lipid extracts (Figure 6A) and purified lipids (Figure 6B). While the increase in the ellipticity indicates that conformational change of some regions of Hsp22 molecule occurs upon interaction with lipid membrane, considering the extent of increase in ellipticity and the shape of the spectra, it appears that membrane interaction does not lead to extensive folding of the unfolded regions of Hsp22. Our CD study shows subtle conformational changes or rearrangement of Hsp22 upon interacting with lipid membrane, depending on the nature of the lipid components. Alcohol/water mixtures are commonly used as membrane-environment-mimicking models to understand the membrane–protein interactions. We have studied the secondary structure of Hsp22 in the presence of increasing concentrations of trifluoroethanol. Far-UV CD spectra of Hsp22 show increases in ellipticity as the concentration of trifluoroethanol increases, saturating at 40% trifluoroethanol. Figure 6(C) compares the far-UV CD spectra of Hsp22 in buffer alone and in the presence of 40% trifluoroethanol, indicating a significant conformational change.
Figure 6. Far-UV CD spectra of Hsp22 in the absence or the presence of lipid SUVs.
(A) Far-UV CD spectra of Hsp22 alone (○), or in the presence of lipid/protein (5:1, w/w), BLE FrI (+) or BLE FrIII (●). (B) Far-UV CD spectra of Hsp22 alone (○), or in the presence of lipid/protein (5:1, w/w) of PC/PA (+) or PC/PI (●). (C) Far-UV CD spectra of Hsp22 in buffer alone (○) and in 40% trifluoroethanol (△).
DISCUSSION
Soluble Hsps can associate with membranes and may play an ‘amphitropic’ role in cells. Such amphitropisms have been proposed to make them suitable for membrane protection under stress conditions. Some proteins of the sHsp family, such as α-crystallin and Hsp17, have been reported to interact with the membrane and modulate its physical properties in vitro [26]. αA-crystallin is known to associate with lens fibre cell membranes [27]. α-Crystallin physiologically bound to membrane has been suggested to be required for correct lens refraction [28]. Hsp27 is shown to co-localize at the focal adhesion points (the membrane adhesion points to the substratum), probably through its association with membrane-associated proteins or with the membrane itself [29]. However, the functional significance of sHsp–membrane interaction is not yet understood.
Hsp22 is a member of the sHsp family whose function is not completely understood. Based on sequence similarity with sHsps, it has been included in the sHsp family [7]. Our earlier study showed that its expression is heat-inducible [11] and, indeed, it exhibits molecular-chaperone-like activity in preventing the aggregation of other proteins [11,20]. Growing evidence shows that sHsps, in general, also take part in other cellular functions besides their role in thermotolerance and chaperone properties (see [1–3] for reviews). For example, their role is becoming increasingly evident in various cellular processes such as apoptosis [39,40], proteasome-mediated protein degradation [41,42] and cell cycle and differentiation [2]. It is possible that Hsp22 may also participate in several diversified functions, which have not yet been identified. It was identified previously as a kinase and is believed to be involved in signal transduction and to play a role in apoptosis [5,18]. Hsp22 shows mild Mn2+-dependent serine/threonine autokinase activity in vitro. The significance of this kinase activity in the cellular context is debated [43]. Overexpression of Hsp22 results in increased phosphorylation and activation of Akt, a serine/threonine kinase that is involved in important signal transduction events [18]. Our present immuno-localization study in human neuroblastoma cells (SK-N-SH cell line) showed that Hsp22 is predominantly present in the cytoplasm and the nucleus; however, a fraction of the protein was also found to localize to the membrane. This in vivo result suggests that Hsp22 might interact with the membrane at specific regions or under specific conditions.
A recent study by Maddala and Rao [25] showed that distribution of αA- and αB-crystallin in confluent primary lens epithelial cells is predominantly cytoplasmic, whereas, only in migrating cells, a significant fraction of αA- and αB-crystallin localizes to the membrane in leading edges (lamellipodia), suggesting a possible role of αB-crystallin in actin dynamics and cell motility. Interestingly, α-synuclein, which exists predominantly as a cytosolic protein, has been shown to rapidly exchange a fraction of the protein between the membrane surface and the cytosol [44]. While it binds stably to membranes in vitro [44], a recent study has proposed that its interaction with biological membrane is transient in vivo [45]. Similarly, in response to different stimuli, inactive cytoplasmic Akt migrates to the inner plasma membrane surface, where it binds to PtdIns(3,4,5)P3, undergoes phosphorylation, and becomes activated [17]. As our in vivo localization studies in SK-N-SH cells show a significant localization of Hsp22 to the plasma membrane, we have investigated its membrane interaction in vitro. In the present study, we have demonstrated that Hsp22 binds to lipid vesicles and that the lipid composition also dictates its membrane binding. Hsp22 exhibits strong tendency to bind to vesicles that contain PA, PI or PS, which are known to be enriched in the inner leaflet of the plasma membrane [24]. It is interesting to note that α-synuclein binds exclusively to small unilamellar phospholipid vesicles containing acidic phospholipids, but not to vesicles with net neutral charge [44].
Our gel-filtration data presented in Figure 5 demonstrate that Hsp22 binds stably to lipid vesicles. CD studies show that membrane interaction leads to an increase in the negative ellipticity of Hsp22 in the 230–200 nm region, but does not lead to extensive folding of the unfolded regions of Hsp22 (Figure 6), as seen when α-synuclein interacts with the membrane [44]. Various secondary-structure prediction programs predict a predominantly random coil content (ranging from 73 to 80%), with a very low propensity to form helices (<10%) for the Hsp22 sequence [11]. The observed changes in the far-UV CD spectrum of Hsp22 may be due to subtle changes in local folding of Hsp22 upon interaction with the membrane. However, it is important to note that subtle changes in conformation may be sufficient to bring about changes in the functional properties of a protein. Milburn et al. [46] studied the binding of Akt to PtdIns(3,4,5)P3 using far-UV CD and X-ray crystallography. Their studies showed that addition of PtdIns(3,4,5)P3 brought about a relatively small systematic change in the far-UV CD spectrum of Akt. The maximum change in ellipticity over the range 230–200 nm observed was only 8%, which cannot conclusively indicate a significant change in the conformation of the protein on binding to the ligand. However, X-ray crystallographic data showed a conformational change within the PH (pleckstrin homology) domain, which resulted in the phosphorylation and activation of the protein [46]. Subtle conformational changes in Hsp22 observed upon interaction with membrane, like those observed in Akt, could modulate its cellular functions. Since Hsp22 binds to lipid vesicles, we tested whether membrane interaction affects its autokinase activity and found that it is not affected significantly (results not shown). Autokinase activity of Hsp22 might not have any functional significance [43]. Further investigations are required to understand its function in vivo.
In conclusion, our study clearly shows that Hsp22 localizes to the plasma membrane. Its stable interaction with lipid membrane leads to the burial of the tryptophan residues and observable conformational change. In addition to its already demonstrated chaperone-like activity, the interaction of Hsp22 with the plasma membrane may be of functional significance in other cellular activities such as signal transduction and apoptosis.
Acknowledgments
We thank Mrs Nandini Rangaraj for her help with the confocal microscopy and analysis, and Mr T. S. Udaya Kumar for his help in cloning work. T. K. C. acknowledges the Council of Scientific and Industrial Research (New Delhi, India) for the grant of a Senior Research Fellowship.
References
- 1.Parcellier A., Gurbuxani S., Schmitt E., Solary E., Garrido C. Heat shock proteins, cellular chaperones that modulate mitochondrial cell death pathways. Biochem. Biophys. Res. Commun. 2003;304:505–512. doi: 10.1016/s0006-291x(03)00623-5. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo P. In search of the molecular mechanism by which small stress proteins counteract apoptosis during cellular differentiation. J. Cell. Biochem. 2005;94:241–246. doi: 10.1002/jcb.20349. [DOI] [PubMed] [Google Scholar]
- 3.Garrido C., Gurbuxani S., Ravagnan L., Kroemer G. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem. Biophys. Res Commun. 2001;286:433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- 4.Kappe G., Franck E., Verschuure P., Boelens W. C., Leunissen J. A., de Jong W. W. The human genome encodes 10 α-crystallin-related small heat shock proteins: HspB1–10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C. C., Yu Y. X., Kulka M., Aurelian L. A novel human gene similar to the protein kinase (PK) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) codes for a serine-threonine PK and is expressed in melanoma cells. J. Biol. Chem. 2000;275:25690–25699. doi: 10.1074/jbc.M002140200. [DOI] [PubMed] [Google Scholar]
- 6.Charpentier A. H., Bednarek A. K., Daniel R. L., Hawkins K. A., Laflin K. J., Gaddis S., MacLeod M. C., Aldaz C. M. Effects of estrogen on global gene expression: identification of novel targets of estrogen action. Cancer Res. 2000;60:5977–5983. [PubMed] [Google Scholar]
- 7.Benndorf R., Sun X., Gilmont R. R., Biederman K. J., Molloy M. P., Goodmurphy C. W., Cheng H., Andrews P. C., Welsh M. J. HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 (3DHSP27) J. Biol. Chem. 2001;276:26753–26761. doi: 10.1074/jbc.M103001200. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y. X., Heller A., Liehr T., Smith C. C., Aurelian L. Expression analysis and chromosome location of a novel gene (H11) associated with the growth of human melanoma cells. Int. J. Oncol. 2001;18:905–911. doi: 10.3892/ijo.18.5.905. [DOI] [PubMed] [Google Scholar]
- 9.Verschuure P., Tatard C., Boelens W. C., Grongnet J. F., David J. C. Expression of small heat shock proteins HspB2, HspB8, Hsp20 and cvHsp in different tissues of the perinatal developing pig. Eur. J. Cell Biol. 2003;82:523–530. doi: 10.1078/0171-9335-00337. [DOI] [PubMed] [Google Scholar]
- 10.Irobi J., Van Impe K., Seeman P., Jordanova A., Dierick I., Verpoorten N., Michalik A., De Vriendt E., Jacobs A., Van Gerwen V., et al. Hot-spot residue in small heat-shock protein 22 causes distal motor neuropathy. Nat. Genet. 2004;36:597–601. doi: 10.1038/ng1328. [DOI] [PubMed] [Google Scholar]
- 11.Chowdary T. K., Raman B., Ramakrishna T., Rao C. M. Mammalian Hsp22 is a heat-inducible small heat-shock protein with chaperone-like activity. Biochem. J. 2004;381:379–387. doi: 10.1042/BJ20031958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gober D., Smith C. C., Ueda K., Toretsky J. A., Aurelian L. Forced expression of the H11 heat shock protein can be regulated by DNA methylation and trigger apoptosis in human cells. J. Biol. Chem. 2003;278:37600–37609. doi: 10.1074/jbc.M303834200. [DOI] [PubMed] [Google Scholar]
- 13.Depre C., Tomlinson J. E., Kudej R. K., Gaussin V., Thompson E., Kim S. J., Vatner D. E., Topper J. N., Vatner S. F. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carra S., Sivilotti M., Chavez Zobel A. T., Lambert H., Landry J. HspB8, a small heat shock protein mutated in human neuromuscular disorders, has in vivo chaperone activity in cultured cells. Hum. Mol. Genet. 2005;14:1659–1669. doi: 10.1093/hmg/ddi174. [DOI] [PubMed] [Google Scholar]
- 15.Chavez Zobel A. T., Loranger A., Marceau N., Theriault J. R., Lambert H., Landry J. Distinct chaperone mechanisms can delay the formation of aggresomes by the myopathy-causing R120G αB-crystallin mutant. Hum. Mol. Genet. 2003;12:1609–1620. doi: 10.1093/hmg/ddg173. [DOI] [PubMed] [Google Scholar]
- 16.Yew E. H., Cheung N. S., Choy M. S., Qi R. Z., Lee A. Y., Peng Z. F., Melendez A. J., Manikandan J., Koay E. S., Chiu L. L., et al. Proteasome inhibition by lactacystin in primary neuronal cells induces both potentially neuroprotective and pro-apoptotic transcriptional responses: a microarray analysis. J. Neurochem. 2005;94:943–956. doi: 10.1111/j.1471-4159.2005.03220.x. [DOI] [PubMed] [Google Scholar]
- 17.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 18.Hase M., Depre C., Vatner S. F., Sadoshima J. H11 has dose-dependent and dual hypertrophic and proapoptotic functions in cardiac myocytes. Biochem. J. 2005;388:475–483. doi: 10.1042/BJ20041314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Depre C., Wang L., Sui X., Qiu H., Hong C., Hedhli N., Ginion A., Shah A., Pelat M., Bertrand L., et al. H11 kinase prevents myocardial infarction by preemptive preconditioning of the heart. Circ. Res. 2006;98:280–288. doi: 10.1161/01.RES.0000201284.45482.e8. [DOI] [PubMed] [Google Scholar]
- 20.Kim M. V., Seit-Nebi A. S., Marston S. B., Gusev N. B. Some properties of human small heat shock protein Hsp22 (H11 or HspB8) Biochem. Biophys. Res. Commun. 2004;315:796–801. doi: 10.1016/j.bbrc.2004.01.130. [DOI] [PubMed] [Google Scholar]
- 21.Lacy M. J., Voss E. W., Jr A modified method to induce immune polyclonal ascites fluid in BALB/c mice using Sp2/0-Ag14 cells. J. Immunol. Methods. 1986;87:169–177. doi: 10.1016/0022-1759(86)90527-2. [DOI] [PubMed] [Google Scholar]
- 22.Lefevre T., Subirade M. Interaction of β-lactoglobulin in phospholipid bilayers: a molecular level elucidation as revealed by infrared spectroscopy. Int. J. Biol. Macromol. 2000;28:59–67. doi: 10.1016/s0141-8130(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 23.Barenholz Y., Gibbes D., Litman B. J., Goll J., Thompson T. E., Carlson F. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977;16:2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 24.Op den Kamp J. A. Lipid asymmetry in membranes. Annu. Rev. Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 25.Maddala R., Rao V. P. α-Crystallin localizes to the leading edges of migrating lens epithelial cells. Exp. Cell Res. 2005;306:203–215. doi: 10.1016/j.yexcr.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Tsvetkova N. M., Horvath I., Torok Z., Wolkers W. F., Balogi Z., Shigapova N., Crowe L. M., Tablin F., Vierling E., Crowe J. H., Vigh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleschner C. R., Cenedella R. J. Examination of a lens ‘native’ plasma membrane fraction and its associated crystallins. Curr. Eye Res. 1992;11:739–752. doi: 10.3109/02713689209000748. [DOI] [PubMed] [Google Scholar]
- 28.Bloemendal H., Zweers A., Vermorken F., Dunia I., Benedetti E. L. The plasma membranes of eye lens fibres: biochemical and structural characterization. Cell Differ. 1972;1:91–106. doi: 10.1016/0045-6039(72)90032-2. [DOI] [PubMed] [Google Scholar]
- 29.Huot J., Houle F., Rousseau S., Deschesnes R. G., Shah G. M., Landry J. SAPK2/p38-dependent F-actin reorganization regulates early membrane blebbing during stress-induced apoptosis. J. Cell Biol. 1998;143:1361–1373. doi: 10.1083/jcb.143.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross J. B., Schmidt C. J., Brand L. Time-resolved fluorescence of the two tryptophans in horse liver alcohol dehydrogenase. Biochemistry. 1981;20:4369–4377. doi: 10.1021/bi00518a021. [DOI] [PubMed] [Google Scholar]
- 31.Brochon J. C., Wahl P., Charlier M., Maurizot J. C., Helene C. Time resolved spectroscopy of the tryptophyl fluorescence of the E. coli LAC repressor. Biochem. Biophys. Res. Commun. 1977;79:1261–1271. doi: 10.1016/0006-291x(77)91142-1. [DOI] [PubMed] [Google Scholar]
- 32.Privat J. P., Wahl P., Auchet J. C., Pain R. H. Time resolved spectroscopy of tryptophyl fluorescence of yeast 3-phosphoglycerate kinase. Biophys. Chem. 1980;11:239–248. doi: 10.1016/0301-4622(80)80026-3. [DOI] [PubMed] [Google Scholar]
- 33.Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973;18:263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- 34.Lakowicz J. R. Principles of Fluorescence Spectroscopy. 1st edn. New York: Plenum Press; 1983. Protein fluorescence; pp. 342–381. [Google Scholar]
- 35.van Montfort R. L., Basha E., Friedrich K. L., Slingsby C., Vierling E. Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 2001;8:1025–1030. doi: 10.1038/nsb722. [DOI] [PubMed] [Google Scholar]
- 36.de Jong W. W., Caspers G. J., Leunissen J. A. Genealogy of the α-crystallin small heat shock protein superfamily. Int. J. Biol. Macromol. 1998;22:151–162. doi: 10.1016/s0141-8130(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 37.Li L. K., Spector A. Circular dichroism and optical rotatory dispersion of the aggregates of purified polypeptides of α-crystallin. Exp. Eye Res. 1974;19:49–57. doi: 10.1016/0014-4835(74)90071-2. [DOI] [PubMed] [Google Scholar]
- 38.Koteiche H. A., Mchaourab H. S. Folding pattern of the α-crystallin domain in αA-crystallin determined by site-directed spin labeling. J. Mol. Biol. 1999;294:561–577. doi: 10.1006/jmbi.1999.3242. [DOI] [PubMed] [Google Scholar]
- 39.Kamradt M. C., Chen F., Cryns V. L. The small heat shock protein αB-crystallin negatively regulates cytochrome C- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 40.Charette S. J., Lavoie J. N., Lambert H., Landry J. Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 2000;20:7602–7612. doi: 10.1128/mcb.20.20.7602-7612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ito H., Kameic K., Iwamoto I., Inaguma Y., Garcia-Mata R., Sztul E., Kato K. Inhibition of proteasomes induces accumulation, phosphorylation, and recruitment of HSP27 and αB-crystallin to aggresomes. J. Biochem. (Tokyo) 2002;131:593–603. doi: 10.1093/oxfordjournals.jbchem.a003139. [DOI] [PubMed] [Google Scholar]
- 42.den Engelsman J., Keijsers V., de Jong W. W., Boelens W. C. The small heat-shock protein αB-crystallin promotes FBX4-dependent ubiquitination. J. Biol. Chem. 2003;278:4699–4704. doi: 10.1074/jbc.M211403200. [DOI] [PubMed] [Google Scholar]
- 43.Kim M. V., Seit-Nebi A. S., Gusev N. B. The problem of protein kinase activity of small heat shock protein Hsp22 (H11 or HspB8) Biochem. Biophys. Res. Commun. 2004;325:649–652. doi: 10.1016/j.bbrc.2004.10.074. [DOI] [PubMed] [Google Scholar]
- 44.Davidson W. S., Jonas A., Clayton D. F., George J. M. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y. S., Laurine E., Woods W., Lee S. J. A novel mechanism of interaction between α-synuclein and biological membranes. J. Mol. Biol. 2006;360:386–397. doi: 10.1016/j.jmb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Milburn C. C., Deak M., Kelly S. M., Price N. C., Alessi D. R., van Aalten D. M. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]