Abstract
The protein storage vacuole (PSV) is a plant-specific organelle that accumulates reserve proteins, one of the main agricultural products obtained from crops. Despite the importance of this process, the cellular machinery required for transport and accumulation of storage proteins remains largely unknown. Interfering with transport to PSVs has been shown to result in secretion of cargo. Therefore, secretion of a suitable marker could be used as an assay to identify mutants in this pathway. CLV3, a negative regulator of shoot stem cell proliferation, is an extracellular ligand that is rendered inactive when targeted to vacuoles. We devised an assay where trafficking mutants secrete engineered vacuolar CLV3 and show reduced meristems, a phenotype easily detected by visual inspection of plants. We tested this scheme in plants expressing VAC2, a fusion of CLV3 to the vacuolar sorting signal from the storage protein barley lectin. In this way, we determined that trafficking of VAC2 requires the SNARE VTI12 but not its close homologue, the conditionally redundant VTI11 protein. Furthermore, a vti12 mutant is specifically altered in transport of storage proteins, whereas a vti11 mutant is affected in transport of a lytic vacuole marker. These results demonstrate the specialization of VTI12 and VTI11 in mediating trafficking to storage and lytic vacuoles, respectively. Moreover, they validate the VAC2 secretion assay as a simple method to isolate genes that mediate trafficking to the PSV.
Keywords: protein storage vacuole, protein trafficking, SNARE
Among the unique characteristics of the plant secretory pathway is the ability to store proteins in specialized organelles, such as the protein storage vacuoles (PSVs) present in many plant species or the endoplasmic reticulum (ER)-derived protein bodies present in certain cereals (1). These storage organelles provide nutrients to the germinating seedling, and, in addition, they constitute the principal source of proteins for human and animal nutrition. PSVs are most prevalent in the storage tissues of seeds, but they are also present in the vegetative tissues of adult plants, including Arabidopsis (2). Plant cells also have a prototypical lytic vacuole (LV), which contains a different complement of membrane and luminal proteins, has an acidic pH, and high hydrolytic activity and shares certain functions with yeast vacuoles and mammalian lysosomes.
It has been shown that PSVs and LVs coexist in some cells (2, 3). Moreover, trafficking to these organelles can be selectively and independently inhibited (4, 5), indicating that separate transport pathways to these vacuoles exist in plant cells. It is thought that proteins targeted to LVs are sorted in the Golgi complex by vacuolar sorting receptors that recruit cargo proteins into clathrin-coated vesicles for transport to the vacuole via a prevacuolar compartment (6, 7), a trafficking pathway similar to those present in other eukaryotes. In contrast, storage proteins are sorted in the Golgi complex into plant-specific dense vesicles that are devoid of a recognizable protein coat, and are then transported to PSVs (8, 9). In addition, some storage proteins may travel directly from the ER to PSVs (10).
Very few components of the vacuolar trafficking machinery have been identified in plants, and only some of them have been associated with transport to PSVs (5, 11, 12). Genetic evidence linking genes to functions in trafficking to PSVs is virtually absent. The only example is a mutant in the putative vacuolar sorting receptor VSR1, which was shown to secrete seed vacuolar storage proteins (12); however, definitive proof for its function in sorting storage proteins is still lacking (13). In yeast, >100 genes required for trafficking to vacuoles have been identified, most of them through genetic screens for mutants that secrete vacuolar proteins (14). The main hurdle in developing similar screens in multicellular organisms is that disruption of vacuolar trafficking may cause fatal defects early in development (15, 16), precluding the isolation of viable mutants. However, PSVs, although important for the overall success of seed germination, may be dispensable for seedling survival or for the development of the adult plant. We reasoned, then, that genetic dissection of the PSV transport pathway was feasible. The assays developed to find vacuolar trafficking mutants in yeast were based on detecting vacuolar enzyme activities in the growth media of mutant cells. In plants, perturbation of trafficking to PSVs also leads to secretion of storage cargo (5, 12, 17, 18), so the rationale for the mutant screen would be similar to the yeast assays. Still, secretion to the apoplasm in multicellular organism is not as readily measured as secretion to growth media in yeast. Also, because of their role as a stable nitrogen reserve, vacuolar storage proteins are inert, which further complicates their detection. However, vacuolar sorting signals (VSSs) from PSV proteins such as barley lectin (BL) are sufficient to target chimeric proteins to the vacuole (19). This interchangeable VSS could be used to develop a storage vacuole marker whose secretion in mutants would be easily detectable. By using an endogenous regulatory protein with activity in the apoplasm, we could monitor the response of the plant to isolate mutants. CLV3 is an extracellular ligand that negatively regulates meristem proliferation in the apoplasm (20). We have previously shown that the VSS of BL fused to CLV3 targets the resultant chimeric protein (VAC2) to the vacuole and renders it inactive. Moreover, saturation of the transport pathway by overexpressing VAC2 leads to its secretion and to reduced proliferation and even elimination of the meristem (20), demonstrating that the fusion protein is active in the apoplasm. These results suggest that reduced meristem proliferation could be used as an assay to isolate mutants that interfere with VAC2 trafficking and result in its secretion to the apoplasm. We have used this assay to show that the SNARE VTI12 is involved in trafficking of vacuolar storage proteins in vegetative and seed tissues. These results constitute an important step in elucidating trafficking to PSVs and also serve as a proof-of-principle for the validity of the VAC2 secretion assay to isolate mutants in this important plant pathway.
Results
VTI12 Is a SNARE Involved in Trafficking of VAC2.
SNARE proteins are essential to drive membrane fusion in exocytotic and vacuolar trafficking pathways in eukaryotes (21). Several SNARES have been localized to the late secretory pathway in Arabidopsis, and are candidates to regulate membrane fusion events in vacuolar trafficking pathways, and may therefore participate in transport of VAC2. Among these, VTI11, SYP22/SYP21, and SYP51 are members of a SNARE complex (SNvti11complex) found at the prevacuolar compartment (PVC) and the tonoplast that has been proposed to be involved in trafficking to the lytic vacuole (22, 23). VTI12, SYP41/SYP42, and SYP61 are members of another discrete SNARE complex (SNvti12complex) at the TGN (22, 23). No definitive function has been assigned to this complex, although some evidence implicates VTI12 in autophagy (24). VTI11 and VTI12 are homologous to a single yeast gene, Vti1, which is involved in trafficking to vacuoles through all pathways that have been described in that organism (25), supporting a role for their plant counterparts in vacuolar trafficking.
We have previously shown that SYP21, SYP22, SYP41, and SYP42 are essential for pollen development and that homozygous mutants cannot be recovered (16). In contrast, null mutants of VTI11 and VTI12 are viable, so their function in VAC2 trafficking can be tested. The vti11 mutants are agravitropic and show defects in leaf morphology and central lytic vacuole formation (26), whereas vti12 plants show no obvious developmental phenotype under normal growth conditions (24). We crossed single null mutants of vti11 and vti12, and the corresponding wild type (Wt) background ecotype (Col-0), into a marker line expressing VAC2. We used the L1 marker line [see Supporting Information (SI)] that is homozygous for a single insertion of VAC2 in the intergenic region between At3g46110 and At3g46120. This line expresses amounts of VAC2 that are close to the empirically determined saturation levels (SI and data not shown). Therefore, we anticipated that even weak inhibition of trafficking in the mutants would lead to secretion of VAC2 and consequent early termination of meristems. The F1 plants from the three crosses were identical and featured normal meristems. In the F2 populations, we found plants with terminated shoot and floral meristems in the cross with vti12 mutants but not in the crosses with vti11 mutants or Wt plants. Genotyping by PCR demonstrated that all of the plants showing determinate shoot meristem and early termination of floral meristems were homozygous vti12 mutants expressing VAC2 (Figs. 1A and 2). No effect of VAC2 on the shoot or floral meristem was observed in Wt or in vti11 (Figs. 1A and 2). We analyzed the expression of VAC2 to ensure that the phenotype in vti12 plants was not due to enhanced VAC2 levels. We could not detect the VAC2 protein by regular Western blot analysis, most likely because of the in planta processing of CLV3 (27), so we analyzed VAC2 expression by Northern blot. VAC2 expression was similar in all genotypes (SI), suggesting that secretion of VAC2 in vti12 was most likely due to defects in its transport.
Fig. 1.
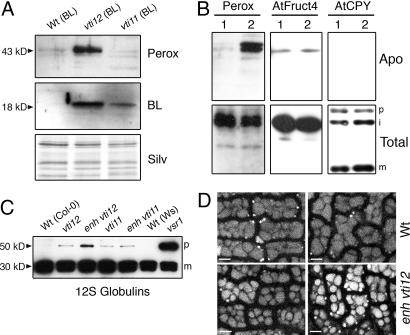
The vti12 mutant secretes VAC2 and BL. (A) Shown are plants homozygous for the VAC2 construct, derived from crosses of L1 with the wild type (Wt) ecotype Col-0, with vti12 and with vti11 plants. The genotype of the plants is shown above the figures. (B) The presence of BL in the apoplastic fluid (Apo) from Wt, vti12, and vti11 mutant lines homozygous for the BL transgene was analyzed by Western blot with anti-BL antibodies. Silver staining of the samples is shown as loading control. The accumulation of BL in a total fraction (Tot) from Wt is shown for comparison.
Fig. 2.
The effects of combinations of the vti12 and vti11 null alleles on trafficking of VAC2. Shown are F2 progenies from a cross between the L1 line and an enhanced vti11 plant (vti11/vti11 VTI12/vti12). The genotype of the F2 plants is shown above the figures. All plants are homozygous for the VAC2 insert. Arrowheads indicate terminated shoot meristems; arrows indicate flowers without carpels. (Inset) a vti12/vti12 VTI11/vti11 plant 5 months after germination.
To exclude the possibility that trafficking of the chimeric VAC2 construct was not representative of the trafficking of natural storage proteins, we analyzed transport of full-length BL. We crossed vti11 and vti12 plants to a BL-expressing line (6) and compared the accumulation of BL in apoplasmic protein samples from leaves of Wt, vti11, and vti12 plants. As shown in Fig. 1B, vti12 mutants accumulated BL in the extracellular space, whereas no BL was detected in Wt plants, indicating that VTI12 is involved in vacuolar trafficking of both BL and VAC2. We also detected weaker BL secretion in vti11 plants, which is consistent with the observation that in vti11 VTI12 assumes VTI11 functions (27) and thus may be partially depleted from its role in transport of BL.
VTI11 Conditionally Substitutes for VTI12 in VAC2 Trafficking.
Although VTI11 and VTI12 serve independent functions in Wt plants, they can replace each other under certain conditions (24): in vti11 mutants, VTI12 is transferred to the SNvti11 complex and partially substitutes for VTI11 function in the gravitropic response pathway. Conversely, in vti12 null mutants, VTI11 is transferred to the SNvti12 complex. Double vti11/vti12 mutants are embryo lethal, indicating that one or both of their functions are essential for viability of the plant and that they have some redundant roles (24). We predicted that in plants devoid of VTI12, VTI11 would take over its function in transport of VAC2. To test this hypothesis we created an allelic series of plants with various combinations of VTI11 and VTI12 mutant alleles. In plants homozygous for VAC2 there were increasing degrees of meristem termination that correlated with increasing loss of function of the SNvti12 complex. We found four classes of phenotypes that were associated with specific genotypes (Fig. 2): plants that showed no effect on the meristem had two functional copies of VTI12 or had one functional copy of VTI12 and were not null for VTI11 (Class 1); the weakest meristem phenotype, consisting of termination of several flower meristems before the production of carpels (arrowheads, Fig. 2), was observed in vti11 plants that were hemizygous for VTI12 (designated enhanced vti11 plants; Class 2); an intermediate effect was observed in vti12 plants with two functional copies of VTI11(Class 3). In these plants the primary meristems were terminated (Fig. 1A), but the secondary meristems gave rise to inflorescences where only some lateral meristems were terminated (arrows, Fig. 2), and <50% of the flowers had carpels and developed into siliques (arrowheads). The strongest phenotype was observed in vti12 plants hemizygous for VTI11 (designated enhanced vti12 plants), in which most meristems were terminated before elongation, and only a few lateral meristems gave rise to elongating stems with meristems terminated after the emergence of cauline leaves (Class 4). Flowers were seldom produced, and the few that were lacked carpels. Even after 5 months, these plants did not produce fertile flowers (Fig. 2 Inset). These experiments show that VTI12 was involved in VAC2 trafficking, whereas VTI11 was required only when the amount of VTI12 was limiting and VTI11 replaced its functions by transferring to the SNvti12 complex. We conclude from these results that trafficking of VAC2 required the TGN-localized SNvti12 complex, and was independent of the PVC-localized SNvti11, which may instead be involved in central lytic vacuole formation (26).
The Trafficking of Storage and Lytic Vacuole Markers Is Differentially Affected in vti12 and vti11.
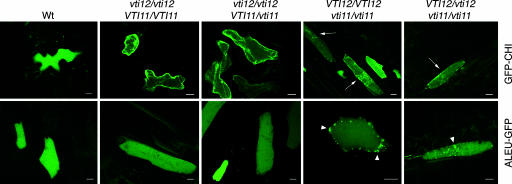
To determine whether the results with VAC2 and BL could be generalized to other vacuolar proteins, we studied the localization of tobacco chitinase and barley aleurain, which are markers of PSVs and LVs, respectively. Tobacco chitinase contains a VSS that, when fused to GFP (GFP-CHI), targets the fusion protein to PSVs, whereas the VSS from barley aleurain targets GFP (ALEU-GFP) to LVs (28, 29). These fluorescent reporters can be used to monitor in vivo trafficking through storage and lytic vacuole pathways. We analyzed the localization of the two markers by transient expression in leaf epidermal cells. Over 90% of Wt cells transformed with GFP-CHI showed a diffuse fluorescence throughout most of their cellular volume (Fig. 3), the expected pattern for a protein localized to the vacuole lumen in Arabidopsis epidermal cells, and a weak fluorescence in the ER. Nine percent of transformed Wt cells showed a predominant localization of GFP-CHI in the ER (data not shown). This additional localization of GFP-CHI in the ER has been reported for this construct (28, 30) and most likely reflects proteins in transit to the vacuole. In vti11 and enhanced vti11 mutants, >90% of the transformed cells showed diffuse vacuolar fluorescence, although in many cells (marked by arrows in Fig. 3) at a lower level than in Wt plants. In those cells showing lower vacuolar fluorescence, we also observed spherical compartments that may correspond to the small vacuole-like structures seen by electron microscopy in vti11 plants (26) and that are probably visualized by the GFP-CHI present in the ER network surrounding these vacuoles. Importantly, in vti12 mutants, most transformed cells (81% in vti12 and 92% in enhanced vti12 plants) showed very weak diffuse fluorescence, and, instead, GFP-CHI was observed predominantly in the ER (Fig. 3). These results indicate that GFP-CHI trafficking to the vacuole was hindered in vti12 mutants, and the underlying ER-localized GFP-CHI was revealed. Similarly, in Wt cells incubated in the light, the vacuole-localized GFP-CHI is degraded, and then the underlying ER-network pattern becomes evident (30). We did not observe accumulation of GFP-CHI in the apoplasm in vti12 mutants, possibly because the protein diffuses or is degraded in the extracellular space and does not accumulate to a significant concentration.
Fig. 3.
VTI12 is required for trafficking of GFP-CHI to the vacuole. Leaves from plants with designated genotypes were transiently transformed with GFP-CHI and ALEU-GFP constructs. GFP fluorescence was observed by CLSM. Transformed cells shown are representative of the patterns observed in the various genotypes. Arrows indicate cells showing weak diffuse vacuolar fluorescence and accumulation of small vacuoles; arrowheads indicate speckled structures accumulating ALEU-GFP; Wt, Col-0. (Scale bar: 10 μm.)
When transformed with ALEU-GFP, fluorescence was observed in the vacuole lumen in all transformed cells from Wt, vti12, and enhanced vti12 plants, indicating that ALEU-GFP transport to the vacuole does not require VTI12. In contrast, bright granular staining, in addition to the diffuse vacuolar fluorescence, was observed in vti11 and enhanced vti11 cells transformed with ALEU-GFP (arrowhead, Fig. 3). These punctate structures may correspond to Golgi or post-Golgi/prevacuolar compartments that have been shown to accumulate ALEU-GFP when transport is blocked by a dominant negative mutant version of m-Rabmc (4). VTI11 and m-Rabmc are both localized in the prevacuolar compartment and the Golgi apparatus, and their mutations may cause the retention of ALEU-GFP in these compartments. In contrast, in vti12 cells, we observed accumulation of GFP-CHI in the ER but not in punctate structures, even though VTI12 is also localized in the Golgi. Vacuolar storage proteins are sorted earlier in the secretory pathway than lytic vacuole proteins (8), in some cases, even in the ER (1, 10). Moreover, storage proteins may be transported to the PSV through a direct pathway from the ER that bypasses the Golgi (1, 10). The accumulation of GFP-CHI in the ER in vti12 cells may reflect the specialized role of this compartment in the sorting and transport of vacuolar storage cargo in plants.
VTI12 Is Involved in Transport of Endogenous Vacuolar Proteins.
Several classes of VSSs have been described in plants (13). Among these, the COOH-terminal class of VSSs (ctVSS) has only been found in storage vacuole proteins. In Arabidopsis, no proteins containing ctVSSs have been characterized (31), so the analysis of this class of vacuolar proteins in this model plant has relied on the expression of exogenous markers, such as BL and tobacco chitinase. Recently, we described a subgroup of Arabidopsis peroxidases (VacPerox) that contain putative ctVSSs and were found in enriched vacuolar fractions (32). We raised antibodies against an internal peptide that is specific to this subclass of peroxidases. Using immunoelectron microscopy, we localized VacPerox to the vacuole of Wt plants (SI), confirming that they are true vacuolar residents. Some labeling was also observed in the cytosol but not in the apoplasm. Accordingly, we did not observe substantial accumulation of VacPerox in apoplastic fluids isolated from Wt plants, but neither did we observe VacPerox in the apoplasm of vti mutants (data not shown), possibly because of redundancy between VTI11 and VTI12. We reasoned that defects in VacPerox trafficking might be detected in conditions closer to saturation, i.e., in plants overexpressing other vacuolar cargo, so we analyzed plants expressing BL and VAC2. We observed secretion of VacPerox in vti12 plants expressing BL, but importantly, not in Wt, nor in vti11 plants expressing BL (Fig. 4A). We then examined plants expressing VAC2 and detected secretion of VacPerox in enhanced vti12 plants, but not in Wt, vti11, vti12, or enhanced vti11 plants (Fig. 4B and data not shown). Two bands were occasionally observed reacting with the anti-VacPerox antibodies (Fig. 4B) and may represent different isoforms of the subgroup of vacuolar peroxidases. Importantly, both bands were observed in the apoplasm fraction of vti12 mutants. Moreover, there was specificity in the secretion of vacuolar cargo, because no secretion of AtCPY and AtFruct4, markers of LVs, was observed in vti12 or in enhanced vti12 plants expressing either VAC2 or BL (Fig. 4B and data not shown). These data show that the specific role of VTI12 was in transport of endogenous vacuolar proteins containing putative ctVSS signals and not in the transport of LV markers. In addition, the enhanced VacPerox secretion observed in BL- and VAC2-expressing plants indicates that all these cargo proteins use a common transport pathway, and, thus, VacPerox may serve as endogenous storage vacuole markers in Arabidopsis vegetative tissues.
Fig. 4.
Trafficking of endogenous vacuolar markers is altered in vti12 mutants. (A) Samples of apoplastic fluid collected from Wt, vti12, and vti11 plants homozygous for the BL transgene were analyzed with anti-VacPerox and anti-BL antibodies. The same samples were silver-stained to confirm equal loading. (B) Samples of apoplastic fluid (Apo) and total protein extracts (Total) were analyzed by Western blot with antibodies against VacPerox, AtFruct4, and AtCPY (46). Plants were derived from crosses with the L1 line and were homozygous for the VAC2 transgene. Lane 1, Wt plants; lane 2, vti11/VTI11 vti12/vti12 plants. The precursor (p, 60 kDa), intermediate (i, 48 kDa), and mature (m, 24 kDa) forms of AtCPY are marked at the sides of the figures. The estimated molecular masses of VacPerox and AtFruct4 in the gels are 43 and 52 kDa, respectively. (C) Total protein extracts from siliques were analyzed by Western blot with antibodies against 12S globulins. The genotype of the mother plant is shown above the figure. Siliques were harvested 18 d after anthesis. The precursor (p) and mature (m) forms are marked at the sides of the figures. (D) PSVs imaged by confocal microscopy. Shown are epidermal cells from cotyledons dissected from dried seeds of Col-0 and enhanced (enh)vti12 plants. (Scale bar: 5 μm.)
Seed Storage Protein Deposition Is Altered in vti12 Mutants.
12S globulins are a major class of storage proteins in Arabidopsis seeds. It has been shown that their normal deposition requires the putative vacuolar sorting receptor VSR1, which interacts with a C-terminal peptide from 12S globulins (12). Seeds of vsr1–1 mutants secrete a fraction of 12S globulins to the apoplasm and accumulate their precursors, possibly because the secreted pool is not processed. As shown in Fig. 4C, we also observed accumulation of 12S globulin precursors in siliques from vti12 plants, suggesting that partial secretion occurred. Precursor accumulation was higher in siliques from enhanced vti12 plants, even though, because of low transmission of the vti11 allele, only 45% of the embryos in those siliques were actually enhanced vti12 (as determined by genotyping the progeny, n = 132). However, even considering this, the levels of precursor accumulation are lower than those observed in vsr1–1 mutants, indicating that redundancy from VTI11 is more prominent in seeds than in vegetative tissues. Consistent with a higher contribution from VTI11 in seed storage protein transport, precursors of 12S globulins were observed in vti11 and enhanced vti11 mutants (Fig. 4C), albeit at lower levels than in enhanced vti12 siliques. Furthermore, three of the four members of the Arabidopsis VTI family, VTI11, VTI12, and VTI14, are induced during seed maturation (33), coinciding with the induced expression of seed storage genes, and may provide added redundancy to the trafficking step regulated by VTI12. An alternative model to explain the relatively low levels of precursor accumulation in enhanced vti12 mutants is that they may cosecrete both the storage proteins and their processing peptidases, leading to maturation of 12S globulins in the apoplasm.
To gain further evidence of the possible role of VTI12 in storage protein deposition in seeds, we analyzed the morphology of PSVs, which was altered in the vsr1–1 trafficking mutant (12). In enhanced vti12 embryos, we observed PSVs that were smaller than in Wt (Fig. 4D). Furthermore, the cellular space in enhanced vti12 embryos was not completely occupied by PSVs, which retained spherical shapes and clear separations between them. In contrast, Wt cells were tightly packed with PSVs, which had irregular shapes and narrower intervening spaces between them. Therefore, PSVs occupy a smaller fraction of cellular volume in enhanced vti12 embryos, suggesting that deposition of storage proteins is decreased.
Taken together, our results lend strong support to the role of VTI12 in mediating transport to PSVs in both vegetative and seed tissues, although in these latter tissues redundancy from VTI11 and VTI14 may make the system less dependent on VTI12. The identification of VTI12 provides important leads to elucidate the possible role of other components of the trafficking machinery to PSVs in plants, such as SYP41, SYP42, SYP61, YKT61, YKT62, and AtVPS45, all of which have been shown to form complexes with VTI12 (22, 23, 34).
Discussion
Numerous components of the vesicle trafficking machinery have been identified in eukaryotes, initially through genetic screens in yeast and biochemical cell-free reconstitution assays in animal systems (35). These findings revealed that the core molecular apparatus responsible for driving membrane fission and fusion and vesicle transport is conserved throughout kingdoms. Interestingly, the genomes of plants and mammals contain much larger sets of genes that encode vesicle trafficking components than the genomes of other eukaryotes, including yeast and multicellular organisms such as insects and nematodes (36). This expansion in the number of trafficking machinery components probably reflects novel and/or cell-specific functions. Although components of the trafficking machinery to LVs are well defined and appear to be conserved in yeast and multicellular eukaryotes, very little is known about trafficking to PSVs.
In this article, we report the development of a genetic assay designed to identify components of the machinery required for trafficking to the PSV in Arabidopsis. We present, as proof-of-principle, the results of a reverse genetics approach to test the role of two related v-SNARE proteins, VTI11 and VTI12, in trafficking to PSVs. Because of their differential localization and SNARE complex specificity, it had been proposed that VTI11 and VTI12 function in different membrane fusion steps (22, 24). Our results corroborate that hypothesis and, in addition, show that their functions are carried out in separate trafficking pathways. VTI12 is involved in trafficking of vacuolar storage proteins (this work), which is thought to be clathrin independent (8, 9), and in autophagy (27), whereas VTI11 has been associated with clathrin-dependent transport to the lytic vacuole (7, 22, 26, and this work). Although yeast contains a single VTI gene (Vti1), when VTI11 and VTI12 are expressed in vti1 mutant yeast cells, they retain differential activities in independent vacuolar trafficking pathways (37): VTI11 complements the defects in clathrin-dependent transport to vacuole in vti1 yeast cells, whereas VTI12 complements the defects in clathrin-independent/AP-3 dependent transport to the vacuole and also in the direct cytoplasm-to-vacuole transport, which is tightly linked to autophagy (38). The similarity in the pathways regulated by VTI11 and VTI12 in plants and yeast suggest that those routes may be evolutionarily related. In particular, the clathrin independent/AP-3 dependent pathway to the yeast vacuole may be related to the trafficking pathway to the plant storage vacuole. In this respect, AP-3 in animal cells is involved in trafficking to specialized lysosomal compartments, such as pigment granules, melanosomes, and platelet-dense bodies (39) that may coexist in the same cell with conventional lysosomes (40), because PSVs coexist with LVs in plant cells.
The expansion of the VTI family in higher eukaryotes (one gene in yeast compared with four genes in Arabidopsis and two genes in mammals) probably allowed their specialization in separate transport pathways to different types of vacuoles and/or lysosomes, which may have diverged from the multiple pathways to the single vacuole in yeast. Mammalian genomes contain two members of the VTI family, Vti1a and Vti1b. Vti1b localizes to late endosomes (21, 41) and interacts with SYX7 and SYX8 (42), the animal orthologues of SYP21/22 and SYP51, components of the plant SNvti11 complex, which is also localized at a late endosomal/prevacuolar compartment (22). In contrast, Vti1a localizes to the TGN and interacts with SYX6 and SYX16 (41), the animal orthologues of SYP61and SYP41/42, components of the TGN-localized SNvti12 complex (22). In addition, Vti1b, as does VTI11, complements the clathrin-dependent trafficking pathway in vti1 mutants yeast cells, whereas neither Vti1a nor VTI12 do (43, 44). Based on these strong analogies, we propose that plant and animal VTI proteins can be grouped in two functional classes: (i) The plant VTI11/animal Vti1b proteins form SNARE complexes with SYP2/SYX7 and SYP5/SYX8 type of syntaxins and may function in clathrin-dependent pathways to the vacuole; (ii) plant VTI12/animal Vti1a proteins define another class that forms SNARE complexes with SYP4/SYX16 and SYP6/SYX6 type of syntaxins and may function in alternative pathways to specialized vacuoles. It will be important to test whether in yeast VTI11/Vti1b and VTI12/Vti1a interact respectively with Pep12p and Tlg2p, the yeast counterparts of SYP2/SYX7 and SYP4/SYX16 syntaxins, which would explain their differential activities in this organism.
Very little is known about how SNAREs are localized to the different compartments where they perform their functions. The localization and function of VTI proteins may be determined indirectly by its interaction with specific syntaxins that would carry the sorting information (45). The identification of the residues responsible for their interactions with specific syntaxins or for their localization would help to resolve this issue. In this regard, Arabidopsis VTI11 and VTI12 constitute a very good experimental model because they are highly homologous (>60% identical), yet they have very different localization, SNARE partners, and function. In contrast, Vti1a and Vti1b proteins in mammals have <30% identity. Moreover, vti1 mutant yeast may be used as a convenient heterologous model to test the interaction, localization, and function of the modified VTI11 and VTI12 proteins. Recently, a mutated allele of VTI12, zip1, was shown to suppress the gravitropic defects of vti11 mutants (45). The zip1 allele had a single amino acid substitution that resulted in an altered localization and SNARE affinity of the VTI12 protein that now resembled that of VTI11. The zip1 mutation is neomorphic, because it is a change in an acidic residue that is strictly conserved in all VTI proteins from plants and animals. However, it may prove very informative to find the residues that define the functional differences between VTI11 and VTI12.
Materials and Methods
Plant Materials.
The Arabidopsis vti11 (zig1) and vti12 mutants were described (24) and are in the Col-0 background. The Arabidopsis vsr1–1 allele is in the Wassilewskija (Ws) background (12). Genotyping of the VTI11 and VTI12 loci was done as described (24).
Production of Antiserum Against VacPerox.
Antibodies were raised against a peptide (SFRTEKDAFGNANSARG) conserved in vacuolar peroxidases. Antibodies from an immunized rabbit (Invitrogen, San Diego, CA) were affinity-purified by transfer of the peptide to nitrocellulose membranes and subsequent immunoabsortion and elution of the antibody as described (6).
Isolation of Apoplastic Fluids.
Apoplastic fluid was obtained from 5-week-old plants. One gram of leaves was vacuum infiltrated in 50 ml of 50 mM Na-phosphate buffer, pH 7.0, and 150 mM NaCl for 15 min, breaking the vacuum every 3 min. After infiltration, leaves were blotted dry and the apoplastic fluid was collected by low-speed centrifugation (900 × g). Total proteins were extracted from leaves after apoplastic fluid collection. Aliquots from apoplastic and total protein fractions obtained from different plants were analyzed by SDS/PAGE and immunoblotting.
Transient Expression and Fluorescence Microscopy.
Five micrograms of plasmid DNA were used to coat gold particles for bombardment using a helium-driven particle accelerator (PDS-1000/He; Bio-Rad, Hercules, CA). Bombarded leaves were incubated for 24 h in the dark on solid media containing MS salts and 1% sucrose. Leaves were then mounted in water on glass slides with the adaxial epidermis facing the cover glass. GFP fluorescence was viewed with an Axiovert 200 confocal microscope (Zeiss, Thornwood, NY) coupled to the Bio-Rad Radiance 2100 laser scanning confocal imaging system with LaserSharp version 5 imaging software.
Storage Vacuole Visualization.
Seed covers were removed, and dissected embryos were mounted in water. PSV autofluorescence was excited at 488 nm by using an argon ion laser and subsequently detected through a 500LP nm emission filter.
Supplementary Material
Acknowledgments
We thank Prof. Lars Rask (Uppsala University, Uppsala, Sweden) for the rapeseed 12S globulin antibodies, Dr. Valentina Kovaleva (University of California, Riverside, CA) for electron micrographs, Prof. Jean-Marc Neuhaus (University of Neuchâtel, Neuchâtel, Switzerland) for ALEU-GFP and GFP-CHI expression plasmids, and Prof. Ikuko Hara-Nishimura (Kyoto University, Kyoto, Japan) for vsr1–1 seeds. This work was supported by Spanish Ministerio de Educación y Ciencia Grant BMC2003–08039 (to E.R.) and Department of Energy, Division of Energy Biosciences Grant DE-FG03–02ER15295/A000 (to N.V.R.).
Abbreviations
- PSV
protein storage vacuole
- LV
lytic vacuole
- BL
barley lectin
- VSS
vacuolar sorting signal.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611147104/DC1.
References
- 1.Herman EM, Larkins BA. Plant Cell. 1999;11:601–614. doi: 10.1105/tpc.11.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park M, Kim SJ, Vitale A, Hwang I. Plant Physiol. 2004;134:625–639. doi: 10.1104/pp.103.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paris N, Stanley CM, Jones RL, Rogers JC. Cell. 1996;85:563–572. doi: 10.1016/s0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- 4.Bolte S, Brown S, Satiat-Jeunemaitre B. J Cell Sci. 2004;117:943–954. doi: 10.1242/jcs.00920. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Lee D, Lee GJ, Hwang I. J Cell Biol. 2005;170:757–767. doi: 10.1083/jcb.200504112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed SU, Rojo E, Kovaleva V, Venkataraman S, Dombrowski JE, Matsuoka K, Raikhel NV. J Cell Biol. 2000;149:1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song J, Lee MH, Lee GJ, Yoo CM, Hwang I. Plant Cell. 2006;18:2258–2274. doi: 10.1105/tpc.105.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinz G, Hillmer S, Baumer M, Hohl II. Plant Cell. 1999;11:1509–1524. doi: 10.1105/tpc.11.8.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hohl I, Robinson DG, Chrispeels MJ, Hinz G. J Cell Sci. 1996;109(Pt 10):2539–50. doi: 10.1242/jcs.109.10.2539. [DOI] [PubMed] [Google Scholar]
- 10.Herman E, Schmidt M. Plant Physiol. 2004;136:3440–3446. doi: 10.1104/pp.104.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolliffe NA, Brown JC, Neumann U, Vicre M, Bachi A, Hawes C, Ceriotti A, Roberts LM, Frigerio L. Plant J. 2004;39:821–833. doi: 10.1111/j.1365-313X.2004.02167.x. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Fuji K, Tamura K, Kondo M, Nishimura M, Hara-Nishimura I. Proc Natl Acad Sci USA. 2003;100:16095–16100. doi: 10.1073/pnas.2530568100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitale A, Hinz G. Trends Plant Sci. 2005;10:316–323. doi: 10.1016/j.tplants.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Bowers K, Stevens TH. Biochim Biophys Acta. 2005;1744:438–454. doi: 10.1016/j.bbamcr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Rojo E, Gillmor CS, Kovaleva V, Somerville CR, Raikhel NV. Dev Cell. 2001;1:303–310. doi: 10.1016/s1534-5807(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 16.Sanderfoot AA, Pilgrim M, Adam L, Raikhel NV. Plant Cell. 2001;13:659–666. doi: 10.1105/tpc.13.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frigerio L, de Virgilio M, Prada A, Faoro F, Vitale A. Plant Cell. 1998;10:1031–1042. doi: 10.1105/tpc.10.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craig S, Goodchild DJ. Protoplasma. 1984;122:91–97. [Google Scholar]
- 19.Bednarek SY, Raikhel NV. Plant Cell. 1991;3:1195–1206. doi: 10.1105/tpc.3.11.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rojo E, Sharma VK, Kovaleva V, Raikhel NV, Fletcher JC. Plant Cell. 2002;14:969–977. doi: 10.1105/tpc.002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong W. Biochim Biophys Acta. 2005;1744:120–144. doi: 10.1016/j.bbamcr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Mol Biol Cell. 2001;12:3733–3743. doi: 10.1091/mbc.12.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassham DC, Sanderfoot AA, Kovaleva V, Zheng H, Raikhel NV. Mol Biol Cell. 2000;11:2251–2265. doi: 10.1091/mbc.11.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surpin M, Zheng H, Morita MT, Saito C, Avila E, Blakeslee JJ, Bandyopadhyay A, Kovaleva V, Carter D, Murphy A, et al. Plant Cell. 2003;15:2885–2899. doi: 10.1105/tpc.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer von Mollard G, Stevens TH. Mol Biol Cell. 1999;10:1719–1732. doi: 10.1091/mbc.10.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M. Plant Cell. 2002;14:47–56. doi: 10.1105/tpc.010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surpin M, Raikhel N. Nat Rev Mol Cell Biol. 2004;5:100–109. doi: 10.1038/nrm1311. [DOI] [PubMed] [Google Scholar]
- 28.Di Sansebastiano GP, Paris N, Marc-Martin S, Neuhaus JM. Plant Physiol. 2001;126:78–86. doi: 10.1104/pp.126.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maruyama N, Mun LC, Tatsuhara M, Sawada M, Ishimoto M, Utsumi S. Plant Cell. 2006;18:1253–1273. doi: 10.1105/tpc.105.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi SI, Watanabe M, Nishimura M, Hara-Nishimura I. Plant J. 2003;35:545–555. doi: 10.1046/j.1365-313x.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 31.Jolliffe NA, Craddock CP, Frigerio L. Biochem Soc Trans. 2005;33:1016–1018. doi: 10.1042/BST20051016. [DOI] [PubMed] [Google Scholar]
- 32.Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV. Plant Cell. 2004;16:3285–3303. doi: 10.1105/tpc.104.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Scholkopf B, Weigel D, Lohmann JU. Nat Genet. 2005;37:501–506. doi: 10.1038/ng1543. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Shin YK, Bassham DC. J Mol Biol. 2005;350:92–101. doi: 10.1016/j.jmb.2005.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Bonifacino JS, Glick BS. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 36.Sanderfoot AA, Assaad FF, Raikhel NV. Plant Physiol. 2000;124:1558–1569. doi: 10.1104/pp.124.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng H, von Mollard GF, Kovaleva V, Stevens TH, Raikhel NV. Mol Biol Cell. 1999;10:2251–2264. doi: 10.1091/mbc.10.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalfan WA, Klionsky DJ. Curr Opin Cell Biol. 2002;14:468–475. doi: 10.1016/s0955-0674(02)00343-5. [DOI] [PubMed] [Google Scholar]
- 39.Boehm M, Bonifacino JS. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- 40.Dell'Angelica EC. Curr Opin Cell Biol. 2004;16:458–464. doi: 10.1016/j.ceb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Kreykenbohm V, Wenzel D, Antonin W, Atlachkine V, von Mollard GF. Eur J Cell Biol. 2002;81:273–280. doi: 10.1078/0171-9335-00247. [DOI] [PubMed] [Google Scholar]
- 42.Antonin W, Holroyd C, Fasshauer D, Pabst S, Von Mollard GF, Jahn R. EMBO J. 2000;19:6453–6464. doi: 10.1093/emboj/19.23.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer von Mollard G, Stevens TH. J Biol Chem. 1998;273:2624–2630. doi: 10.1074/jbc.273.5.2624. [DOI] [PubMed] [Google Scholar]
- 44.Lupashin VV, Pokrovskaya ID, McNew JA, Waters MG. Mol Biol Cell. 1997;8:2659–2676. doi: 10.1091/mbc.8.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niihama M, Uemura T, Saito C, Nakano A, Sato MH, Tasaka M, Morita MT. Curr Biol. 2005;15:555–560. doi: 10.1016/j.cub.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 46.Rojo E, Zouhar J, Carter C, Kovaleva V, Raikhel NV. Proc Natl Acad Sci USA. 2003;100:7389–7394. doi: 10.1073/pnas.1230987100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.